Abstract
Epidermal structure is damaged by exposure to ultraviolet (UV) light but the molecular mechanisms governing structural repair are largely unknown. UVB (290-320 nm wavelengths) exposure prior to induction of differentiation reduced expression of differentiation-associated proteins, including Desmoglein 1 (Dsg1), Desmocollin 1 (Dsc1) and Keratins 1 and 10 (K1/K10) in a dose-dependent manner in normal human epidermal keratinocytes (NHEKs). The UVB- induced reduction in both Dsg1 transcript and protein was associated with reduced binding of the p63 transcription factor to previously unreported enhancer regulatory regions of the Dsg1 gene. Since Dsg1 promotes epidermal differentiation in addition to participating in cell-cell adhesion, the role of Dsg1 in aiding differentiation after UVB damage was tested. Compared to controls, depleting Dsg1 via shRNA resulted in further reduction of Dsc1 and K1/K10 expression in monolayer NHEK cultures and in abnormal epidermal architecture in organotypic skin models recovering from UVB exposure. Ectopic expression of Dsg1 in keratinocyte monolayers rescued the UVB-induced differentiation defect. Treatment of UVB-exposed monolayer or organotypic cultures with Trichostatin A, a histone deacetylase inhibitor, partially restored differentiation marker expression, suggesting a potential therapeutic strategy for reversing UV-induced impairment of epidermal differentiation after acute sun exposure.
Introduction
The epidermis is a multilayered structure that provides a barrier against environmental insults. However, all epidermal layers can be penetrated by ultraviolet (UV) light resulting in potentially mutagenic DNA damage (Courdavault et al., 2005; Pfeifer and Besaratinia, 2012) and changes in epidermal structure. UV-induced histological changes include hyperplasia, appearance of “sunburn cells” (pyknotic nuclei, eosinophilic cytoplasm, lacking expression of differentiation markers), disappearance of the granular layer, parakeratosis (aberrant persistence of nuclei in the stratum corneum), and acanthosis (thickening) and hyperkeratinization of the stratum corneum, all indicative of abnormal differentiation and altered barrier function (Bayerl et al., 1995; Bernerd and Asselineau, 1997; Lavker et al., 1995; Lorincz, 1960; Matsumura and Ananthaswamy, 2004; Rosario et al., 1979). The molecular pathways leading to repair of the epidermal structure and restoration of normal differentiation after UV exposure remain largely unknown.
Normal epidermal differentiation and barrier formation require the carefully choreographed expression of cytoskeletal, cell adhesion, and cell envelope proteins specific for each cell layer. UV exposure impairs the expression of later differentiation markers such as involucrin, loricrin, filaggrin, and transglutaminase, corresponding with the histologically observed reduction in the granular layer and disturbance of the stratum corneum (Bayerl et al., 1995; Bernerd and Asselineau, 1997; Del Bino et al., 2004; Gambichler et al., 2008; Kwon et al., 2008; Lee et al., 2005; Lee et al., 2002; Li et al., 2001; Rundhaug et al., 2005; Sesto et al., 2002; van der Vleuten et al., 1996). UV exposure also disrupts the epidermal permeability barrier and cell-cell communication by altering the arrangement of the tight junction proteins occludin and claudins-1 and -4 (Robert et al., 1999; Yuki et al., 2011), the lipid composition in upper epidermal layers (Holleran et al., 1997), and the localization of connexin 43 (Cx43) (Bellei et al., 2008; Provost et al., 2003).
Expression of both classic and desmosomal cadherins is also altered by UV exposure (Dusek et al., 2006; Gambichler et al., 2008; Hung et al., 2006; Li et al., 2001; Murakami et al., 2001; Rundhaug et al., 2005; Sesto et al., 2002). Of particular interest is Desmoglein 1 (Dsg1), one of several desmosomal cadherins that complex with armadillo proteins (i.e. Plakoglobin – Pg and Plakophilins – Pkps), and plakins (e.g. Desmoplakin – Dp) to anchor keratin intermediate filaments to the cell membrane. Dsg1 is first expressed as keratinocytes transit from the basal to the immediate suprabasal layer and becomes increasingly concentrated in desmosomes of the granular layer where it plays a critical role in intercellular adhesion (Green and Simpson, 2007). The Dsg1 cytoplasmic domain also promotes epidermal differentiation and proper epidermal morphogenesis (Getsios et al., 2009). Knocking down Dsg1 results in reduction of the granular layer in epidermal models and reduced expression of differentiation markers desmocollin 1 (Dsc1), loricrin, filaggrin, and keratin 10 (K10). Dsg1 mRNA transcripts have been reported as downregulated after UV exposure, one of many transcriptional changes that occur in UV- exposed keratinocytes (Murakami et al., 2001; Rundhaug et al., 2005). Further, exposure of well differentiated keratinocytes to UVC wavelengths (below 290 nm which do not reach the earth's surface) leads to cellular redistribution and caspase-dependent cleavage of Dsg1 protein (Duseket al., 2006). However, no study has yet examined the impacts of UVB wavelengths (290-320 nm that impact human skin) on the expression or function of Dsg1 protein in the epidermis.
Since Dsg1 promotes epidermal differentiation (Getsios et al., 2009), we examined the role of Dsg1 in governing recovery of keratinocyte differentiation following UVB exposure. UVB exposure resulted in reduced expression of Dsg1, Dsc1, K10, and K1 expression in a dose- dependent manner but did not reduce the predominantly basally-expressed Dsg3 protein or the adherens junction cadherin E-cadherin (Ecad). UVB-induced reduction of Dsg1 mRNA and protein was associated with decreased binding of the transcription factor p63 to previously unreported enhancer regulatory regions of the Dsg1 gene. Dsg1 silencing resulted in further reduction in Dsc1, K1, and K10 protein expression and in abnormal epidermal architecture after UVB exposure while ectopic Dsg1 expression led to recovery of the differentiation program. These findings establish Dsg1 is a specific regulator of the epidermal recovery process after assault by UVB light. Dsg1 may therefore be a therapeutic target for restoration of epidermal differentiation after sun exposure. Indeed, the histone deacetylase (HDAC) inhibitor Trichostatin A (TSA), which was previously shown to increase expression of desmosomal cadherins (Simpson et al., 2010a), partially rescued UVB-induced reduction in differentiation markers in both monolayer cells and organotypic epidermal models. HDAC inhibitors may therefore be useful treatments for enhancing epidermal differentiation after acute UVB exposure, with potential applications for skin cancer prevention.
Results
Acute UVB exposure of keratinocytes prior to induction of differentiation results in decreased expression of differentiation-associated proteins
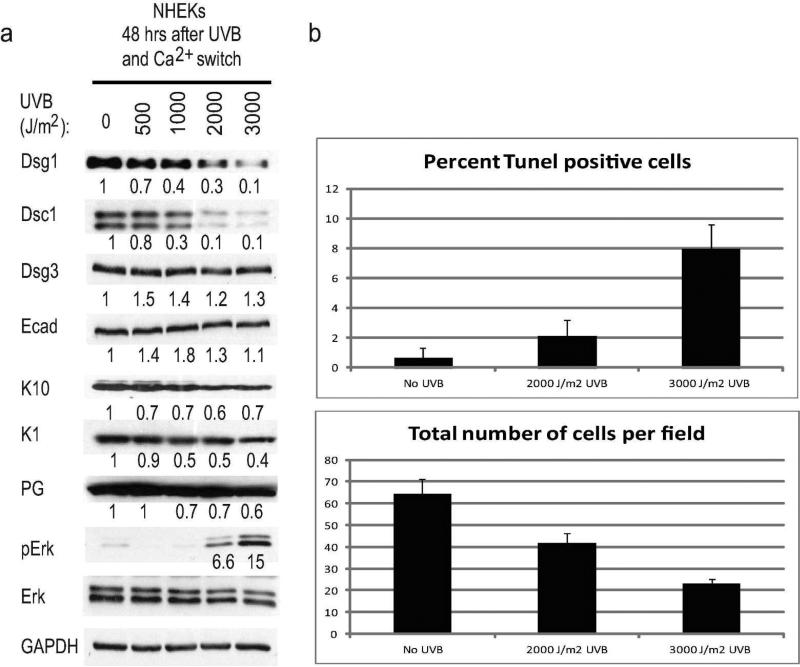
To examine the impact of UVB exposure on differentiation-dependent desmosomal cadherins and keratins in early epidermal differentiation, normal human epidermal keratinocytes (NHEKs) were exposed to increasing dosages of UVB prior to switching to high calcium medium to induce differentiation. A reduction in both Dsg1 and Dsc1 was observed when NHEKs were exposed to 1000, 2000 or 3000 J/m2 UVB prior to calcium switch, while other cadherins Dsg3 and Ecad were not affected (Fig. 1a). Differentiation-associated keratins K10 and K1 as well as Pg levels were also reduced after exposure to the higher UVB dosages. An increase in phosphorylated Erk (pErk) was observed at the same UVB dosages associated with decreased differentiation markers. Thus the viable cells remaining after acute UVB exposure exhibited an increase in pro-proliferative signaling (Dumesic et al., 2009).
Figure 1. Acute UVB exposure of keratinocytes prior to induction of differentiation results in decreased expression of differentiation-associated proteins.
a) Immunoblots showing differentiation-associated proteins reduced in NHEKs exposed to UVB prior to inducing differentiation. Numbers under immunoblots represent band intensity fold change from no UVB control after normalization to GAPDH. b) Level of cell death induced by UVB dosages utilized throughout this study assessed using either the TUNEL assay or by counting the total number of cells per microscopic field 48 hours after UVB exposure.
To determine the level of cell death induced by the acute UVB dosages, apoptotic cells were assessed using the Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay 48 hours after UVB exposure. The remaining viable cells per microscopic field were also counted. 2000 J/m2 UVB exposure resulted in less than 3% of cells undergoing apoptosis at the 48 hour time point and approximately 2/3 of the cells remaining compared to unexposed controls, while exposure to 3000 J/m2 UVB resulted in approximately 8% of cells undergoing apoptosis and 1/3 of the cells remaining (Fig. 1b).
The morphology of organotypic cultures exposed to the same UVB regimen after differentiation and stratification had occurred was also analyzed for comparison (Supplemental Fig. 1 exposed on day 6 and harvested on day 7 after lifting, see timeline). Acanthosis of the stratum corneum and increased intercellular spaces, particularly in the lower epidermal layers, were observed in the cultures exposed to 1000 J/m2 UVB. Sunburn cells were apparent in the cultures exposed to 1500 J/m2 and increased in number with the higher UVB dosages. The stratum corneum and granular layers, and the expression and distribution of Dsg1, Dsc1, and loricrin were disturbed in the cultures exposed to UVB dosages from 1500 to 3000 J/m2, consistent with defects in differentiation.
Dsg1 promotes epidermal differentiation and architectural recovery after UVB exposure
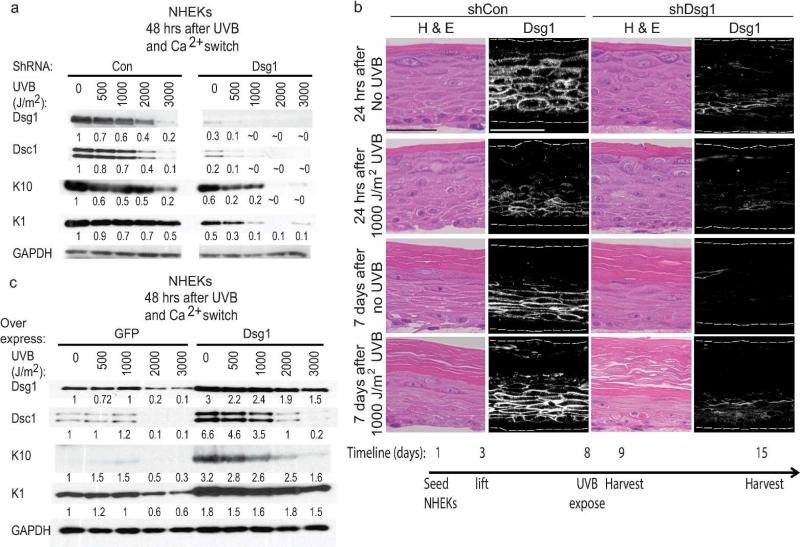
We previously demonstrated that Dsg1 promotes epidermal differentiation through attenuation of Erk1/2 signaling (Getsios et al., 2009). To address whether loss of Dsg1 exacerbates reduction in differentiation after UVB exposure, Dsg1 was depleted in NHEKs via shRNA. Knocking down Dsg1 resulted in more pronounced reductions in Dsc1, K10, and K1 at UVB dosages as low as 500 J/m2 and almost total loss of detectable Dsc1, K10, or K1 proteins in NHEKS exposed to 2000 or 3000 J/m2 UVB (Fig. 2a right). In control-infected NHEKs, as in uninfected NHEKs in Fig. 1, exposure to 2000 or 3000 J/m2 UVB resulted in reduction of Dsg1, Dsc1, K10, and K1 (Fig. 2a left). Thus Dsg1 loss exacerbated the reduction in keratinocyte differentiation markers in a UVB dose-dependent manner.
Figure 2. Dsg1 promotes epidermal differentiation and architectural recovery after UVB exposure.
a) Immunoblots showed that shRNA-mediated depletion of Dsg1 further reduced Dsc1, K10, and K1 compared to control NHEKs exposed to UVB prior to calcium switch. Numbers represent band intensity fold change from unexposed shCon-infected cells after normalization to GAPDH. b) Dsg1 depletion altered the architecture of UVB-exposed organotypic models compared to controls (H&E). Organotypic models were grown for 6 days before mock exposure or irradiation with 1000 J/m2 UVB then harvested 1 or 7 days later. Bars = 50 μm. c) Dsc1, K10, and K1 proteins were expressed at higher levels when Dsg1 was ectopically expressed in UVB-exposed differentiating NHEKs compared to controls. Numbers represent band intensity fold change from unexposed GFP-infected cells after normalization to GAPDH.
To understand the impact of Dsg1 loss on the recovery of proper epidermal architecture after UVB exposure, organotypic cultures were grown from NHEKs infected with control or Dsg1 shRNA. The cultures were exposed to 1000 J/m2 UVB on day 6 after lifting to the air-liquid interface and then allowed to recover for either 24 hours or 7 days (see timeline). In control-infected organotypic cultures, the number of cells staining positive for Dsg1 was reduced 24 hours after UVB exposure compared to unexposed cultures, but Dsg1 expression was restored by the later time point (Fig 2b, left panels). Seven days after UVB exposure in the Dsg1-depleted organotypic cultures, the thickness of the viable epithelial portion was reduced compared to controls. A thickened, disorganized stratum corneum accumulated, exhibiting remnants of cell nuclei (Fig. 2b, right panels). Dsg1 was therefore important for recovery of proper epidermal model architecture after UVB exposure.
To determine whether increasing Dsg1 expression would help restore expression of other differentiation-associated proteins after UVB exposure, NHEKs were infected with viruses expressing either GFP control or ectopic Dsg1. Ectopic Dsg1 expression resulted in increased Dsc1, K10, and K1 expression -8 hours after UVB exposure compared to controls (Fig. 2c). The data indicated that Dsg1 was important for regulating expression of other differentiation markers after UVB exposure.
UVB-induced delay in differentiation and reduced Dsg1 mRNA levels are associated with decreased binding of p63 to regulatory regions upstream of the Dsg1 gene
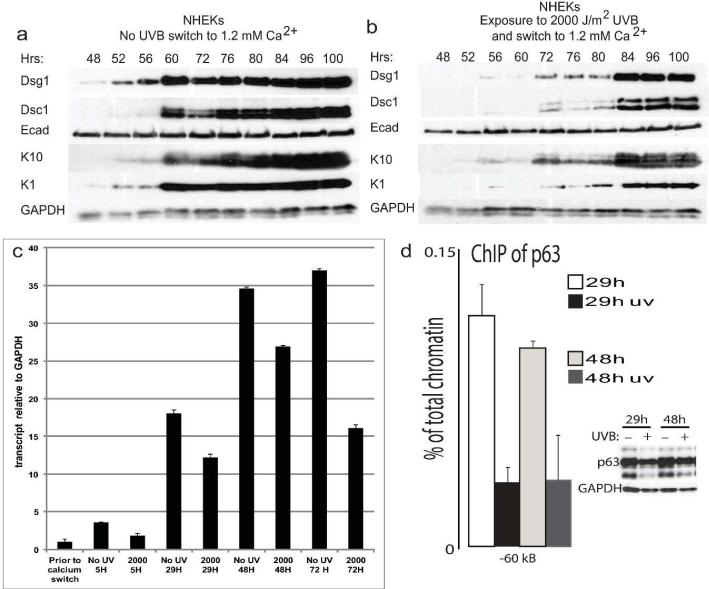
The effects of UVB exposure on expression of differentiation markers could be at the level of protein stability, gene transcription, or both. To test whether UVB exposure resulted in a delay in induction of differentiation marker expression, NHEKs were either unexposed (Fig. 3a) or exposed to 2000 J/m2 UVB (Fig. 3b) immediately prior to calcium switch to induce differentiation and harvested at 4 or 12 hour intervals beginning at 48 hours. While Dsg1 protein was detectable as early as 48 hours after calcium switch in the unexposed cells, it remained difficult to detect until 72 hours in the UVB-exposed NHEKs. Other differentiation markers Dsc1, K10, and K1 were detectable by 60 hours in the unexposed cells while remaining difficult to detect until 72 hours in the UVB-exposed NHEKs. Quantitative RT-PCR revealed that Dsg1 transcript levels were reduced as early as 5 hours and remained reduced 72 hours after calcium switch in UVB-exposed NHEKs compared to unexposed controls.
Figure 3. UVB-induced delay in differentiation and reduced Dsg1 mRNA levels are associated with decreased binding of p63 to regulatory regions upstream of the Dsg1 gene.
a-b) Initiation of Dsg1, Dsc1, K1, and K10 protein expression was delayed following exposure to UVB compared to unexposed control NHEKs. c) Dsg1 transcript levels remained reduced up to 72 hours in NHEKs following UVB exposure (2000 J/m2 prior to calcium switch to induce differentiation) compared to unexposed controls. d) The binding of p63 to an enhancer regulatory region -60kB from the Dsg1 gene was reduced following UVB exposure. NHEKs were unexposed or exposed to 2000 J/m2 UVB prior to calcium switch then harvested 29 or 48 hrs later and processed for ChIP. Immunoblot shows total p63 levels after UVB exposure.
The master regulator of epidermal differentiation, p63 (Koster, 2010), was recently shown to bind to a mouse Dsg1 gene regulatory region to induce Dsg1 transcription (Ferone et al., 2013). Thus, we reasoned that p63-induced transcription of Dsg1 may be impacted by exposure of NHEKs to UVB. To identify p63 binding sites in the human Dsg1 genomic locus, previously generated ChIP-seq data obtained in human keratinocytes (Kouwenhoven et al., 2010) were analyzed. Three p63-binding regions (-25Kb, -60Kb, -70Kb) were identified upstream of the gene and corresponded to genomic regions enriched in positive histone marks associated with active transcription, and to clusters of DNase hypersensitive sites (Supplemental Fig. 2a) (Bernstein et al., 2005; Sabo et al., 2006). Using Chromatin Immunoprecipitation (ChIP) to test binding of p63 to these putative regions resulted in the finding that p63 preferentially bound to the regulatory region 60kB from the human Dsg1 gene (Fig. 3d) and bound to a lesser extent to the other regions (Supplemental Fig. 2b). p63 binding to these regulatory regions was decreased following exposure of NHEKs to 2000 J/m2 UVB (Fig. 3d and Supplemental Fig. 2b), correlating with the UVB-induced reduction in Dsg1 transcripts.
Treatment of cultures with the HDAC inhibitor TSA increases differentiation marker expression after UVB exposure
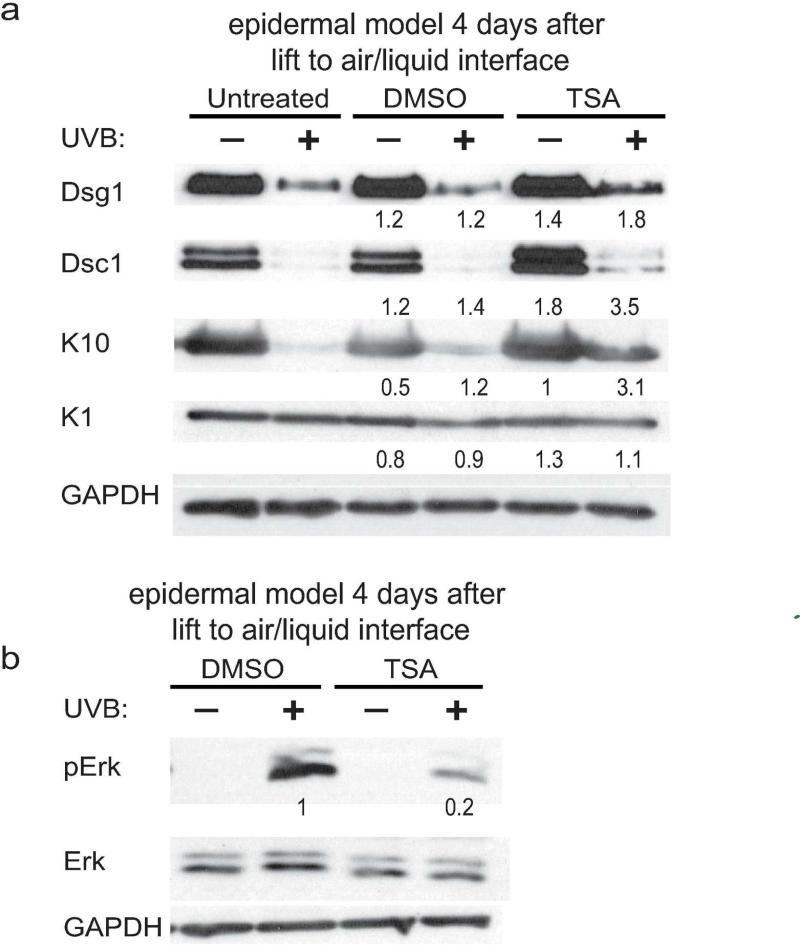
Since previous studies showed that treatment of cells with the HDAC inhibitor TSA increases expression of desmosomal cadherins (Simpson et al., 2010a) experiments were conducted to determine if TSA treatment after UVB exposure would rescue Dsg1 expression. Indeed, TSA treatment increased Dsg1 and Dsc1 expression in monolayer cultures (Supplemental Fig. 3) and Dsg1, Dsc1, and K10 expression in organotypic epidermal cultures (Fig. 4a) after UVB exposure compared to UVB-exposed untreated or DMSO treated controls. This was not through an increase in p63 binding to the Dsg1 gene regulatory regions (data not shown). TSA treatment also reduced UVB-induced phosphorylation of Erk compared to DMSO-treated controls (Fig 4b).
Figure 4. Treatment of epidermal models with the HDAC inhibitor TSA increases differentiation marker expression after UVB exposure.
a) Organotypic models were unexposed or exposed to 1500 J/m2 UVB prior to lifting to the air-liquid interface, left untreated or treated with DMSO or TSA for 4 hours daily starting 24 hours later, then harvested 4 days after lifting. Numbers represent band intensity fold change comparing unexposed treated and untreated cultures and comparing UVB-exposed treated and untreated cultures after normalization to GAPDH. Immunoblots revealed that TSA treatment helped restore Dsg1, Dsc1, and K10 expression after UVB exposure. b) TSA treatment reduces phosphorylated Erk in UVB- exposed organotypic cultures compared to DMSO treated controls.
Discussion
From these studies we conclude that Dsg1 promotes differentiation and structural repair of UVB-damaged epidermis. Further, our work shows that a clinically relevant HDAC inhibitor, TSA, increases Dsg1 expression and helps restore epidermal differentiation following acute UVB exposure. This work is important, as cumulative sun exposure and childhood sunburns significantly correspond with increased risk of skin carcinogenesis in adulthood (English et al., 1998), and UV-induced alterations in epidermal structural proteins may contribute to retention of mutated cells within the skin structure that later become cancerous. For example, loss of Dsg1 and differentiation-associated proteins filaggrin and occludin, have been associated with a reduction in UV-mediated apoptosis (Dusek et al., 2006; Mildner et al., 2010; Rachow et al., 2013). Filaggrin loss also results in increased UV-induced DNA damage (Mildner et al., 2010). In the present study increased Erk phosphorylation was observed at the same UVB dosages where differentiation markers were reduced, consistent with the idea that surviving cells that do not undergo differentiation activate Erk pathway-mediated pro-proliferative signaling (Dumesicet al., 2009). Treatment of organotypic cultures with TSA reduced UVB-induced phosphorylation of Erk while promoting expression of Dsg1 and associated differentiation proteins. We propose that therapeutically increasing expression of Dsg1 to promote restoration of differentiation markers (Getsios et al., 2009) as well as to repress pro-oncogenic EGFR/MAPK/ras signaling (Getsios et al., 2009; Hammers and Stanley, 2013; Harmon et al., 2013) may help prevent skin carcinogenesis.
In addition to skin cancer, UV exposure can initiate or exacerbate the pathology of other human skin diseases. For example, UV exposure induces acantholysis in the uninvolved skin of patients with pemphigus foliaceus, pemphigus vulgaris, and pemphigus erythematosus, disorders caused by auto-antibodies against Dsg1 or Dsg3 (Cram and Winkelmann, 1965; Igawa et al., 2004; Jacobs, 1965; Kawana and Nishiyama, 1990; Makino et al., 2013; Reis et al., 2000). This was posited to be due to increased IgG deposits in intercellular spaces within the UV-induced lesions (Cram and Fukuyama, 1972). However, Dsg1 levels or localization were not examined to determine whether UV exposure exacerbates pemphigus antibody-induced depletion of Dsg1 from desmosomes. Studies are warranted to understand how decreases in Dsg1, Dsc1, K1, and K10 following UVB exposure may contribute to the pathologies of these and other more common epidermal diseases like psoriasis and eczema. There are reports of psoriasis patients developing pemphigus lesions following phototherapy and the underlying mechanisms remain unexplained (Kwon et al., 2011; Sanchez-Palacios and Chan, 2004).
Therapeutically stabilizing Dsg1 in UV-exposed skin through use of HDAC inhibitors or other drugs could reduce symptoms of patients suffering from several skin diseases as well as potentially preventing skin carcinogenesis. HDAC inhibitors are in clinical trials as anti-cancer agents. Two have been approved by the Food and Drug Administration for use against cutaneous T cell lymphomas, and several are being explored for treating psoriasis (Khan and La Thangue, 2012; Shuttleworth et al., 2010). Therefore, use of these agents to help restore epidermal differentiation after acute sun exposure may be feasible.
Materials and Methods
Cell culture and retroviral transduction
NHEKs were isolated from neonatal foreskin by the Northwestern University Skin Disease Research Center (NUSDRC) as described (Halbert et al., 1992). Cells were propagated in M154 medium supplemented with human keratinocyte growth supplement (HKGS, Life Technologies, Grand Island, NY, USA), 1,000× gentamycin/amphotericin B solution (Life Technologies), and 0.07 mM CaCl2 (low calcium). Confluent keratinocyte monolayers were induced to differentiate by addition of 1.2 mM CaCl2 (high calcium) in M154 in the absence of HKGS. LZRS-GFP, LZRS-Flag Dsg1, LZRS-miR Dsg1, and LZRS-miR Lamin (control) were generated as previously described (Getsios et al., 2004; Getsios et al., 2009). Keratinocytes were transduced with retroviral supernatants produced from Phoenix cells (provided by G. Nolan, Stanford University, Stanford, CA) as previously described (Getsios et al., 2004; Simpson et al., 2010a). For organotypic cultures, keratinocytes were seeded on collagen/fibroblast matrices and grown submerged in E medium supplemented with 5 ng/ml epidermal growth factor (EGF) for two days, then grown at the air–medium interface in E medium without EGF according to published protocols (Simpson et al., 2010a; Simpson et al., 2010b).
UVB exposure
Cells (in sufficient Phosphate Buffered Saline - PBS - to minimally cover the culture plate surface) or organotypic cultures (raised to the air/liquid interface) were irradiated under two TL20W/01 narrow band UVB bulbs with peak irradiance at 311 nm wavelength (Solarc Systems, Inc, Barrie, ON, Canada). The exposure time required to obtain UVB doses (J/m2) was calculated by measuring μW/cm2 using an ILT1400A photometer (International Light Technologies, Peabody, MA) calibrated by the manufacturer. The photometer is equipped with detectors to measure UVB and UVC and no UVC was emitted from the UVB bulbs. For a clinical comparison of UVB dosages used throughout this study, depending upon skin type (age, pigmentation, thickness), 4000 J/m2 UVB is considered 1 Minimal Erythemal Dose (MED) (van der Vleuten et al., 1996).
Antibodies and reagents
Mouse monoclonal antibodies used: P124 (anti–Dsg1 extracellular domain; Progen, Heidelberg, Germany); 27B2 (anti–Dsg1 cytodomain; Invitrogen), U100 (anti-Dsc1; Progen), HECD1 (anti– E-cadherin; Takara, Kyoto, Japan) and 4A4 (anti-p63; Santa Cruz Biotechnology, Dallas, TX). Rabbit polyclonal antibodies used: K1, K10, and loricrin (gifts from J. Segre, National Human Genome Research Institute, Bethesda, MD), 1905 (anti-Dsg3; gift from J. Stanley, University of Pennsylvania, Philadelphia, PA), C33E10 (anti-pERK; Cell Signaling Technology, Danvers, MA), anti-Erk1/2 (Promega, Madison, WI), H-137 (anti-p63; Santa Cruz Biotechnology) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase; Sigma-Aldrich). Chicken polyclonal antibody used: Pg (1407; Aves Laboratories, Tigard, OR). Secondary antibodies for immunoblotting were goat anti–mouse, –rabbit, and –chicken peroxidase (Rockland; KPL, Gaithersburg, MD). Secondary antibodies for immunofluorescence microscopy were goat anti– mouse, –rabbit, and –chicken linked to fluorophores of 488 nm and 568 nm (Alexa Fluor; Invitrogen).
Immunoblot analysis of proteins
Whole cell or organotypic culture lysates were collected in Urea-SDS buffer (8M Urea/ 1% Sodium dodecyl sulfate/ 60 mM Tris pH 6.8/ 5% β-mercaptoethanol/ 10% glycerol) and sonicated. Samples separated by SDS-PAGE were transferred to nitrocellulose, blocked in 5% milk/PBS, and probed with primary antibody in milk for 1 hour at room temperature. Secondary, HRP-conjugated antibodies diluted 1:5000 in milk were added to blots after washing with PBS. Protein bands were visualized using enhanced chemiluminescence and exposure to X-ray film.
Quantitative real-time PCR
RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. Total RNA concentrations were equalized between samples, and cDNA prepared using the Superscript III First Strand kit (Invitrogen). Quantitative PCR was performed using SYBR Green PCR master mix (Applied Biosystems) and gene-specific primers in a StepOnePlus instrument (Applied Biosystems). Calculations for relative mRNA levels were performed using the PPCt method, normalized to GAPDH. Primers used: Dsg1F TCCATAGTTGATCGAGAGGTCAC, Dsg1R CTGCGTCAGTAGCATTGAGTATC, GAPDHF ACATCGCTCAGACACCATG, GAPDHR TGTAGTTGAGGTCAATGAAGGG.
ChIP
NHEKs were fixed with 1% formaldehyde and ChIP was performed using anti-p63 antibodies with rabbit IgG antibodies as a negative control. ChIP was performed as previously described (Antonini et al., 2010). Real-time PCR was performed using the SYBR Green PCR master mix (Applied Biosystems) in an ABI PRISM 7500.
Primers used: -25kbDsg1F TCTCTCAACCTGCACTCAATCTG, -25kbDsg1R GGGAGGCTTCTCTGCGATTA, -60kbDsg1F GGGCAATGACATCCCTTGTT, -60kbDsg1R GGTGTGTTCTGCAAGTTCCACTT, -70kbDsg1F TTAAGCAAAACTAATGGACCACAGA -70kbDsg1R GCTCATGCATGTTCATATACAAACC.
Histology, indirect immunofluorescence microscopy
Organotypic cultures fixed in 10% neutral buffered formalin were embedded in paraffin blocks, cut into 5 μm sections, and H&E stained by the NUSDRC. For indirect immunofluorescence microscopy, slides were baked at 60°C, de-paraffinized by xylenes, dehydrated with ethanol, rehydrated in PBS and permeablized by 0.5% Triton X-100 in PBS. Antigen retrieval was performed by incubation in 0.01 M Citrate buffer (pH 6.0) or 0.5 M Tris buffer (pH 8.0) at 95°C for 15 minutes. Sections were blocked in 1% BSA/0.05% Tween/PBS for 30 minutes at 37°C. Primary antibody incubation was carried out overnight at 4°C in blocking buffer followed by washing in PBS. Secondary antibody incubation was carried out at 37°C for 45 minutes followed by washing in PBS. Sections were stained with 4′,6-Diamidino-2-phenylindole (DAPI - Sigma- Aldrich) at a final concentration of 5 ng/μl at room temperature for 2 minutes followed by washing in PBS and water. Cover slips were mounted with ProLong Gold Antifade Reagent (Life Technologies). Images were obtained with a 40x PL Fluotar, NA 1.0 objective on a Leica DMR microscope using a charge-coupled device camera (Orca 100, model C4742-95, Hamamatsu, Bridgewater, NJ) and MetaMorph 6.1 software (MDS Analytical Technologies, Union City, CA) for fluorescence or a Leica DFC320 digital camera and Photoshop software (Adobe Systems, Mountain View, CA) for H&E images.
TUNEL assay
Cells undergoing apoptosis 48 hours following UVB exposure were detected using the in situ Cell Death Detection Kit (Roche, Penzberg, Germany) according to the manufacturer's protocol. The total cells per microscopic field were detected using DAPI.
HDAC Inhibition
On the day of lifting to the air-liquid interface organotypic models were left unexposed or exposed to 1500 J/m2 UVB on ice. Beginning the next day organotypic cultures were left untreated or treated with Dimethyl sulfoxide (DMSO) (Sigma-Aldrich) or TSA (1 μmol/L, Sigma-Aldrich) for 4 hours daily. Cultures were harvested on day 4 after lifting. Confluent cells were unexposed or exposed to 2000 J/m2 UVB immediately prior to switching to 1.2 mM CaCl2 containing medium. The next day cells were either left untreated or treated with DMSO or TSA in fresh high calcium-containing medium and harvested 72 hours after calcium switch.
Supplementary Material
Acknowledgements
This work was supported by NIH R01 AR041836 and CA122151 with partial support from AR43380 to KJG. Additional support was provided by the JL Mayberry endowment to KJG. The financial support of Telethon, Italy (GGP09230), of the European ERA-Net Research Program on Rare Diseases (E-Rare-2; Skindev), and of the Italian Association for Cancer Research (AIRC; IG5348) to CM is gratefully acknowledged. JLJ's salary was supported by a Research Career Development Award through the Dermatology Foundation and salary and some research supplies were supported by a NIH/NCI Ruth L. Kirschstein Training Grant through Northwestern University's Robert H. Lurie Comprehensive Cancer Center (T32 CA070085-14) “Signal Transduction in Cancer”. ATA's stipend and some research supplies were supported through the Northwestern University Lurie Comprehensive Cancer Center's Continuing Umbrella of Research Experience (CURE) Summer Program for Underserved Students, a supplement to P30 CA060553. Additionally, this research was supported in part by resources provided by the Northwestern University Skin Disease Research Center (NIH/NIAMS 5P30AR057216-02). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the Northwestern University Skin Disease Research Center or the NIH/NIAMS.
Abbreviations used
- Dsc
desmocollin
- Dsg
desmoglein
- Ecad
E-cadherin
- HDAC
histone deacetylase
- K1/10
Keratin 1 or 10
- Lor
loricrin
- NHEK
normal human epidermal keratinocyte
- Pg
plakoglobin
- TSA
trichostatin A
- UV
ultraviolet
Footnotes
Conflicts of Interest:
The authors state no conflict of interest.
References
- Antonini D, Russo MT, De Rosa L, et al. Transcriptional repression of miR-34 family contributes to p63-mediated cell cycle progression in epidermal cells. J Invest Dermatol. 2010;130:1249–57. doi: 10.1038/jid.2009.438. [DOI] [PubMed] [Google Scholar]
- Bayerl C, Taake S, Moll I, et al. Characterization of sunburn cells after exposure to ultraviolet light. Photodermatol Photoimmunol Photomed. 1995;11:149–54. doi: 10.1111/j.1600-0781.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Bellei B, Mastrofrancesco A, Briganti S, et al. Ultraviolet A induced modulation of gap junctional intercellular communication by P38 MAPK activation in human keratinocytes. Exp Dermatol. 2008;17:115–24. doi: 10.1111/j.1600-0625.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- Bernerd F, Asselineau D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev Biol. 1997;183:123–38. doi: 10.1006/dbio.1996.8465. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Kamal M, Lindblad-Toh K, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–81. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Courdavault S, Baudouin C, Charveron M, et al. Repair of the three main types of bipyrimidine DNA photoproducts in human keratinocytes exposed to UVB and UVA radiations. DNA Repair (Amst) 2005;4:836–44. doi: 10.1016/j.dnarep.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Cram DL, Fukuyama K. Immunohistochemistry of ultraviolet-induced pemphigus and pemphigoid lesions. Arch Dermatol. 1972;106:819–24. [PubMed] [Google Scholar]
- Cram DL, Winkelmann RK. Ultraviolet-induced acantholysis in pemphigus. Arch Dermatol. 1965;92:7–13. [PubMed] [Google Scholar]
- Del Bino S, Vioux C, Rossio-Pasquier P, et al. Ultraviolet B induces hyperproliferation and modification of epidermal differentiation in normal human skin grafted on to nude mice. Br J Dermatol. 2004;150:658–67. doi: 10.1111/j.0007-0963.2004.05886.x. [DOI] [PubMed] [Google Scholar]
- Dumesic PA, Scholl FA, Barragan DI, et al. Erk1/2 MAP kinases are required for epidermal G2/M progression. J Cell Biol. 2009;185:409–22. doi: 10.1083/jcb.200804038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek RL, Getsios S, Chen F, et al. The differentiation-dependent desmosomal cadherin desmoglein 1 is a novel caspase-3 target that regulates apoptosis in keratinocytes. J Biol Chem. 2006;281:3614–24. doi: 10.1074/jbc.M508258200. [DOI] [PubMed] [Google Scholar]
- English DR, Armstrong BK, Kricker A, et al. Case-control study of sun exposure and squamous cell carcinoma of the skin. Int J Cancer. 1998;77:347–53. doi: 10.1002/(sici)1097-0215(19980729)77:3<347::aid-ijc7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Ferone G, Mollo MR, Thomason HA, et al. p63 control of desmosome gene expression and adhesion is compromised in AEC syndrome. Hum Mol Genet. 2013;22:531–43. doi: 10.1093/hmg/dds464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambichler T, Rotterdam S, Tigges C, et al. Impact of ultraviolet radiation on the expression of marker proteins of gap and adhesion junctions in human epidermis. Photodermatol Photoimmunol Photomed. 2008;24:318–21. doi: 10.1111/j.1600-0781.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- Getsios S, Amargo EV, Dusek RL, et al. Coordinated expression of desmoglein 1 and desmocollin 1 regulates intercellular adhesion. Differentiation. 2004;72:419–33. doi: 10.1111/j.1432-0436.2004.07208008.x. [DOI] [PubMed] [Google Scholar]
- Getsios S, Simpson CL, Kojima S, et al. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J Cell Biol. 2009;185:1243–58. doi: 10.1083/jcb.200809044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499–515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Demers GW, Galloway DA. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activity in human epithelial cells. J Virol. 1992;66:2125–34. doi: 10.1128/jvi.66.4.2125-2134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers CM, Stanley JR. Desmoglein-1, differentiation, and disease. J Clin Invest. 2013;123:1419–22. doi: 10.1172/JCI69071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon RM, Simpson CL, Johnson JL, et al. Desmoglein-1/Erbin interaction suppresses ERK activation to support epidermal differentiation. J Clin Invest. 2013;123:1556–70. doi: 10.1172/JCI65220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran WM, Uchida Y, Halkier-Sorensen L, et al. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatol Photoimmunol Photomed. 1997;13:117–28. doi: 10.1111/j.1600-0781.1997.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Hung CF, Chiang HS, Lo HM, et al. E-cadherin and its downstream catenins are proteolytically cleaved in human HaCaT keratinocytes exposed to UVB. Exp Dermatol. 2006;15:315–21. doi: 10.1111/j.0906-6705.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- Igawa K, Matsunaga T, Nishioka K. Involvement of UV-irradiation in pemphigus foliaceus. J Eur Acad Dermatol Venereol. 2004;18:216–7. doi: 10.1111/j.1468-3083.2004.00900.x. [DOI] [PubMed] [Google Scholar]
- Jacobs SE. Pemphigus Erythematosus and Ultraviolet Light. A Case Report. Arch Dermatol. 1965;91:139–41. doi: 10.1001/archderm.1965.01600080047007. [DOI] [PubMed] [Google Scholar]
- Kawana S, Nishiyama S. Involvement of membrane attack complex of complement in UV-B-induced acantholysis in pemphigus. Arch Dermatol. 1990;126:623–6. [PubMed] [Google Scholar]
- Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90:85–94. doi: 10.1038/icb.2011.100. [DOI] [PubMed] [Google Scholar]
- Koster MI. p63 in skin development and ectodermal dysplasias. J Invest Dermatol. 2010;130:2352–8. doi: 10.1038/jid.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouwenhoven EN, van Heeringen SJ, Tena JJ, et al. Genome-wide profiling of p63 DNA-binding sites identifies an element that regulates gene expression during limb development in the 7q21 SHFM1 locus. PLoS Genet. 2010;6:e1001065. doi: 10.1371/journal.pgen.1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HH, Kwon IH, Chung JH, et al. Pemphigus Foliaceus Associated with Psoriasis during the Course of Narrow-Band UVB Therapy: A Simple Coincidence? Ann Dermatol. 2011;23:S281–4. doi: 10.5021/ad.2011.23.S3.S281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OS, Yoo HG, Han JH, et al. Photoaging-associated changes in epidermal proliferative cell fractions in vivo. Arch Dermatol Res. 2008;300:47–52. doi: 10.1007/s00403-007-0812-3. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Gerberick GF, Veres D, et al. Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J Am Acad Dermatol. 1995;32:53–62. doi: 10.1016/0190-9622(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Lee DS, Quan G, Choi JY, et al. Chronic ultraviolet radiation modulates epidermal differentiation as it up-regulates transglutaminase 1 and its substrates. Photodermatol Photoimmunol Photomed. 2005;21:45–52. doi: 10.1111/j.1600-0781.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, An HT, Chung JH, et al. Acute effects of UVB radiation on the proliferation and differentiation of keratinocytes. Photodermatol Photoimmunol Photomed. 2002;18:253–61. doi: 10.1034/j.1600-0781.2002.02755.x. [DOI] [PubMed] [Google Scholar]
- Li D, Turi TG, Schuck A, et al. Rays and arrays: the transcriptional program in the response of human epidermal keratinocytes to UVB illumination. FASEB J. 2001;15:2533–5. doi: 10.1096/fj.01-0172fje. [DOI] [PubMed] [Google Scholar]
- Lorincz AL. Physiological and pathological changes in skin from sunburn and suntan. JAMA. 1960;173:1227–31. doi: 10.1001/jama.1960.73020290008011. [DOI] [PubMed] [Google Scholar]
- Makino T, Seki Y, Hara H, et al. Induction of Skin Lesions by Ultraviolet B Irradiation in a Case of Pemphigus Erythematosus. Acta dermato-venereologica. 2013 doi: 10.2340/00015555-1781. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Mildner M, Jin J, Eckhart L, et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol. 2010;130:2286–94. doi: 10.1038/jid.2010.115. [DOI] [PubMed] [Google Scholar]
- Murakami T, Fujimoto M, Ohtsuki M, et al. Expression profiling of cancer-related genes in human keratinocytes following non-lethal ultraviolet B irradiation. J Dermatol Sci. 2001;27:121–9. doi: 10.1016/s0923-1811(01)00124-4. [DOI] [PubMed] [Google Scholar]
- Pfeifer GP, Besaratinia A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci. 2012;11:90–7. doi: 10.1039/c1pp05144j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost N, Moreau M, Leturque A, et al. Ultraviolet A radiation transiently disrupts gap junctional communication in human keratinocytes. Am J Physiol Cell Physiol. 2003;284:C51–9. doi: 10.1152/ajpcell.00205.2002. [DOI] [PubMed] [Google Scholar]
- Rachow S, Zorn-Kruppa M, Ohnemus U, et al. Occludin is involved in adhesion, apoptosis, differentiation and ca(2+)-homeostasis of human keratinocytes: implications for tumorigenesis. PLoS One. 2013;8:e55116. doi: 10.1371/journal.pone.0055116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis VM, Toledo RP, Lopez A, et al. UVB-induced acantholysis in endemic Pemphigus foliaceus (Fogo selvagem) and Pemphigus vulgaris. J Am Acad Dermatol. 2000;42:571–6. [PubMed] [Google Scholar]
- Robert M, Bissonauth V, Ross G, et al. Harmful effects of UVA on the structure and barrier function of engineered human cutaneous tissues. Int J Radiat Biol. 1999;75:317–26. doi: 10.1080/095530099140492. [DOI] [PubMed] [Google Scholar]
- Rosario R, Mark GJ, Parrish JA, et al. Histological changes produced in skin by equally erythemogenic doses of UV-A, UV-B, UV-C and UV-A with psoralens. Br J Dermatol. 1979;101:299–308. doi: 10.1111/j.1365-2133.1979.tb05623.x. [DOI] [PubMed] [Google Scholar]
- Rundhaug JE, Hawkins KA, Pavone A, et al. SAGE profiling of UV-induced mouse skin squamous cell carcinomas, comparison with acute UV irradiation effects. Mol Carcinog. 2005;42:40–52. doi: 10.1002/mc.20064. [DOI] [PubMed] [Google Scholar]
- Sabo PJ, Kuehn MS, Thurman R, et al. Genome-scale mapping of DNase I sensitivity in vivo using tiling DNA microarrays. Nat Methods. 2006;3:511–8. doi: 10.1038/nmeth890. [DOI] [PubMed] [Google Scholar]
- Sanchez-Palacios C, Chan LS. Development of pemphigus herpetiformis in a patient with psoriasis receiving UV-light treatment. J Cutan Pathol. 2004;31:346–9. doi: 10.1111/j.0303-6987.2004.0188.x. [DOI] [PubMed] [Google Scholar]
- Sesto A, Navarro M, Burslem F, et al. Analysis of the ultraviolet B response in primary human keratinocytes using oligonucleotide microarrays. Proc Natl Acad Sci U S A. 2002;99:2965–70. doi: 10.1073/pnas.052678999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth SJ, Bailey SG, Townsend PA. Histone Deacetylase inhibitors: new promise in the treatment of immune and inflammatory diseases. Curr Drug Targets. 2010;11:1430–8. doi: 10.2174/1389450111009011430. [DOI] [PubMed] [Google Scholar]
- Simpson CL, Kojima S, Cooper-Whitehair V, et al. Plakoglobin rescues adhesive defects induced by ectodomain truncation of the desmosomal cadherin desmoglein 1: implications for exfoliative toxin-mediated skin blistering. Am J Pathol. 2010a;177:2921–37. doi: 10.2353/ajpath.2010.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CL, Kojima S, Getsios S. RNA interference in keratinocytes and an organotypic model of human epidermis. Methods Mol Biol. 2010b;585:127–46. doi: 10.1007/978-1-60761-380-0_10. [DOI] [PubMed] [Google Scholar]
- van der Vleuten CJ, Kroot EJ, de Jong EM, et al. The immunohistochemical effects of a single challenge with an intermediate dose of ultraviolet B on normal human skin. Arch Dermatol Res. 1996;288:510–6. doi: 10.1007/BF02505246. [DOI] [PubMed] [Google Scholar]
- Yuki T, Hachiya A, Kusaka A, et al. Characterization of tight junctions and their disruption by UVB in human epidermis and cultured keratinocytes. J Invest Dermatol. 2011;131:744–52. doi: 10.1038/jid.2010.385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.