Abstract
BACKGROUND
We have previously reported that a DNA vaccine encoding prostatic acid phosphatase (PAP) could elicit PAP-specific T cells in patients with early recurrent prostate cancer. In the current pilot trial we sought to evaluate whether prolonged immunization with regular booster immunizations, or “personalized” schedules of immunization determined using real-time immune monitoring, could elicit persistent, antigen-specific T cells, and whether treatment was associated with changes in PSA doubling time (PSA DT).
METHODS
16 patients with castration-resistant, non-metastatic prostate cancer received six immunizations at two-week intervals, and then either quarterly (Arm 1) or as determined by multi-parameter immune monitoring (Arm 2).
RESULTS
Patients were on study a median of 16 months; four received 24 vaccinations. Only one event associated with treatment > grade 2 was observed. 6/16 (38%) remained metastasis-free at 2 years. PAP-specific T cells were elicited in 12/16 (75%), predominantly of a Th1 phenotype, which persisted in frequency and phenotype for at least one year. IFNγ-secreting T-cell responses measured by ELISPOT were detectable in 5/13 individuals at one year, and this was not statistically different between study arms. The overall median fold change in PSA DT from pre-treatment to post-treatment was 1.6 (range 0.6–7.0, p=0.036).
CONCLUSIONS
Repetitive immunization with a plasmid DNA vaccine was safe and elicited Th1-biased antigen-specific T cells that persisted over time. Modifications in the immunization schedule based on real-time immune monitoring did not increase the frequency of patients developing effector and memory T-cell responses with this DNA vaccine.
Keywords: DNA vaccine, prostate cancer, prostatic acid phosphatase, clinical trial, immune monitoring
INTRODUCTION
Despite several new agents recently approved for the treatment of advanced prostate cancer, this remains the most commonly diagnosed malignancy in the United States and the second leading cause of male cancer-related death (1). Approximately one third of patients will have recurrent disease after definitive surgery/radiation therapy, the first evidence of which is usually an increase in the serum PSA blood test. At present there is no standard treatment for patients with biochemical recurrence in the absence of radiographically apparent metastases, although androgen deprivation is commonly used. In these individuals, ultimately the disease recurs, heralded again by a rise in serum PSA before the tumor becomes radiographically detectable. No therapies have been approved for this castration-resistant “biochemically-relapsed” stage of disease on the basis of clinical benefit, and surveillance is generally recommended until metastases are identified. The baseline PSA, and rate of PSA change (doubling time, PSA DT), were found to be independently predictive of time to metastasis (which occurs with a median time of 30 months (2)) and overall survival (3). Thus, this non-metastatic castration-resistant prostate cancer (nmCRPC) represents a stage for which there is not a standard therapy, yet one for which prognostic criteria are available. Moreover, the relatively long period of time during which patients generally have no disease-related symptoms provides an opportunity to evaluate novel therapies, including anti-tumor vaccines, which may exert effects over many months.
Sipuleucel-T was approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with metastatic, castration-resistant prostate cancer based on an improvement in overall survival compared to placebo (4). This landmark event represents the first FDA approval of an anti-tumor vaccine for the treatment of existing cancer (4, 5). These findings demonstrate that anti-tumor vaccines can have activity and might be investigated in earlier stages of prostate cancer. Moreover, the specific findings with sipuleucel-T suggest that the target of this vaccine, prostatic acid phosphatase (PAP), is a rational vaccine target.
We have similarly been evaluating PAP-targeted vaccines, using plasmid DNA as the means of antigen delivery (6, 7). In a phase I trial in patients with non-castrate, non-metastatic prostate cancer we found vaccination six times at two-week intervals was safe and elicited PAP-specific CD4+ and CD8+ T cells with a Th1 phenotype (8). Moreover, PAP-specific T cells could be boosted with subsequent immunization, and the development of durable Th1-biased immune responses (detectable up to one year after treatment) appeared to be associated with favorable changes in PSA DT (9). These findings suggested that prolonged schedules of immunization (beyond six vaccinations) with the goal of eliciting/maintaining a Th1-biased PAP-specific cellular immune response might be further investigated.
We describe here the results of a randomized, pilot clinical trial (NCT00849121) in patients with nmCRPC to specifically evaluate different schedules of immunization with a DNA vaccine. The goal of this study was to determine whether ongoing immunization could elicit and maintain Th1-biased memory immune responses over many months, and if immune monitoring conducted in real-time could assist in “personalizing” a schedule of immunization able to maintain tumor antigen-specific effector and memory T cells. Secondary objectives were to determine whether the elicited immune response changed over time with repetitive immunization, and whether immunization was associated with changes in PSA DT. We report that immunization with plasmid DNA encoding PAP elicited Th1-biased PAP-specific T cells in the majority of patients, and responses elicited persisted over many months with ongoing booster immunizations. While a small study, there was no indication that changes to the vaccine schedule by real-time immune monitoring increased the percentage of patients who developed long-term durable immune responses.
MATERIALS AND METHODS
Study Agent and Regulatory Information
pTVG-HP is a plasmid DNA encoding the full-length human PAP (ACPP gene) cDNA downstream of a eukaryotic promoter (6). The study protocol was reviewed and approved by all local (University of Wisconsin Human Subjects’ Review Board), and federal (FDA, NIH Recombinant DNA Advisory Committee) entities. All patients gave written informed consent for participation.
Patient Population
Male patients with a histological diagnosis of prostate adenocarcinoma prostate and PSA recurrence following castration (surgical or ongoing luteinizing hormone-releasing hormone agonist therapy) were eligible, provided there was no evidence of suspected lymph node, bone or visceral metastatic disease on bone scans or CT scans. All patients had to have been previously treated with an anti-androgen, but with rising PSA on treatment and persistent rise in PSA after withdrawal. Patients were required to have an Eastern Cooperative Oncology Group performance score of < 2, and normal bone marrow, liver and renal function as defined by a WBC ≥ 3000/µL, hematocrit ≥ 30%, platelet count ≥ 100,000/µL, total bilirubin ≤ 2.0 mg/dL, and serum creatinine < 1.5 mg/dL or a creatinine clearance ≥ 60 mL/min. Patients were excluded if they had been treated with immunosuppressive therapy (chemotherapy, corticosteroids, or extensive radiation therapy) within six months of study entry, or were on concurrent medications with possible anti-cancer effects. Patients were required to have at least four serum PSA values, from the same clinical laboratory, over a 3- to 6-month period of time immediately prior to entry to calculate a pre-treatment PSA DT. For eligibility purposes, PSA DT was determined by linear regression, calculated using an online nomogram (http://nomograms.mskcc.org/Prostate/PsaDoublingTime.aspx). Patients were further excluded if they had a history of HIV, hepatitis B, or hepatitis C infection, or if they had received a prior anti-cancer vaccine.
Study Design
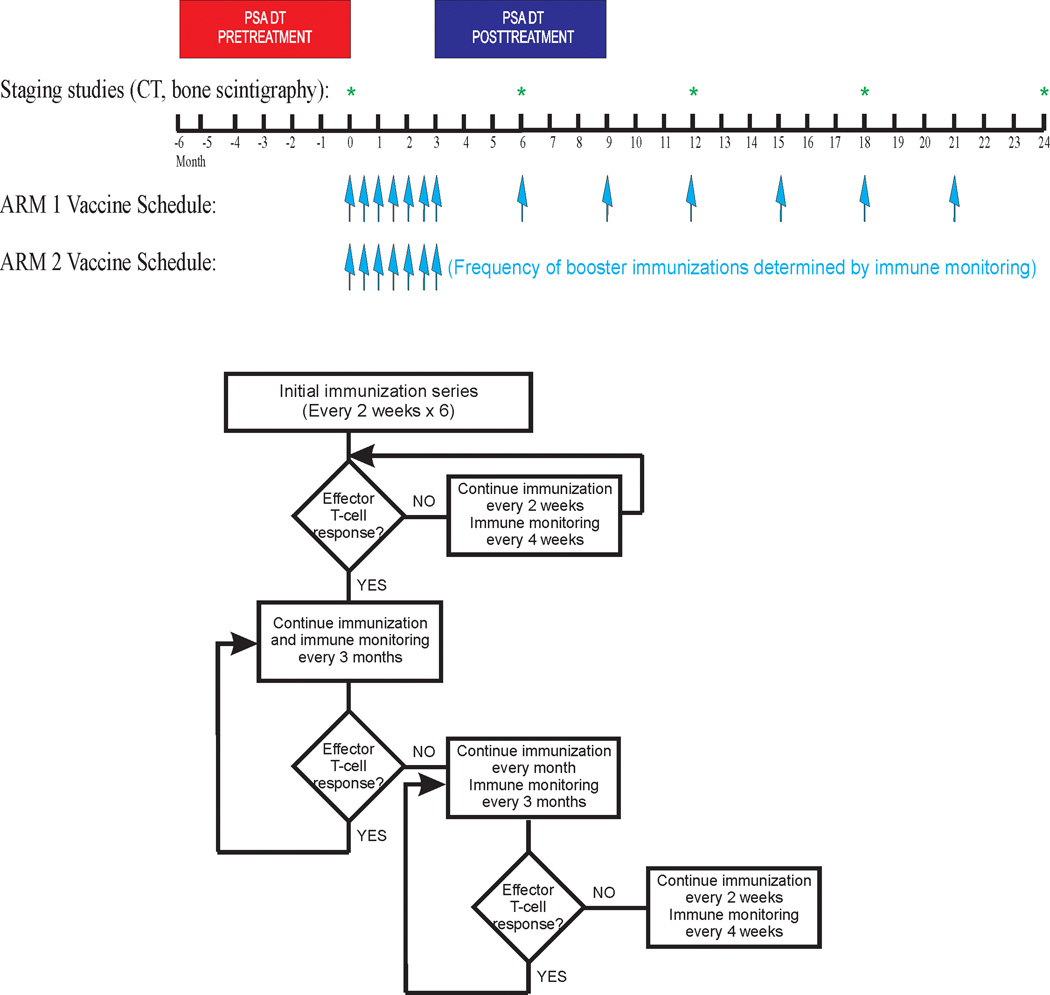
This study was an open-label, single institution, two-arm pilot trial evaluating different schedules of administration (Figure 1). Specifically, subjects in Arm 1 received immunizations at 2-week intervals for six total doses, and then every 3 months beginning two weeks after the last immunization, for a maximum of two years. Subjects in Arm 2 received immunizations at 2-week intervals for six total doses, and then immune monitoring (defined below) was conducted to determine the subsequent schedule; patients without a PAP-specific immune response continued immunizations at 2-week intervals with immune monitoring every month until there was evidence of a PAP-specific immune response. Subjects with a detectable immune response decreased the frequency of immunization to a 3-month interval of dosing, with immune monitoring accompanying each immunization. If an immune response was lost with subsequent visits, the schedule was changed to monthly vaccination. If an immune response was again not detectable after three monthly vaccinations, the schedule was changed to biweekly. Subjects received a maximum of 24 immunizations, or two years of treatment, whichever came first. Patients came off study at the time of detecting metastatic disease (radiographic progression), at any time of undue toxicity, or at the discretion of the patient or treating physician that other therapies for prostate cancer were warranted. 30 patients were planned for accrual. Based on the prior phase I/II study (8), it was anticipated that the immune response rate in Arm 1 would be 20–40%. The goal was to detect a difference of at least 50% in the immune response rates between the two arms. The proposed sample size of 15 patients per arm would have been sufficient to detect a difference of 50% in the immune response rates between arms with at least 80% power at the two-sided 0.05 significance level.
Figure 1. Trial Schema.
Trial schema with schedules of vaccine administration, and time periods for PSA DT and clinical staging evaluation, indicated. Also shown is the flow chart for determining the immunization schedule used in Arm 2.
Study Procedures
Each immunization consisted of 100 µg pTVG-HP plasmid DNA, co-administered with 200 µg GM-CSF (Sargramostim, Genzyme, Cambridge, MA), and delivered as an intradermal injection with a 28-gauge needle and syringe. Patients underwent a leukapheresis procedure within two weeks prior to the first immunization and at one year on trial. Patients also received a tetanus immunization immediately following the baseline leukapheresis. Blood tests were performed every 1–3 months and included CBC, creatinine, SGOT, alkaline phosphatase, amylase, LDH, serum PAP, and serum PSA. Anti-nuclear antibody (ANA) testing and urinalysis was performed prior to study entry, at 12 weeks, and at end of study. Serum testosterone was performed at baseline to confirm that patients were functionally castrate (testosterone levels < 50 ng/mL). All toxicities were graded according to the NCI Common Terminology Criteria Grading System, version 3.
Immunological Monitoring
For each time point, measures of antigen-specific immune response were performed with fresh (not cryopreserved) peripheral blood mononuclear cells (PBMC). Cryopreserved cells from the pre-treatment specimen were also assessed at each time point to evaluate the reproducibility of the methods over time, and as a benchmark for each time point evaluated. A PAP-specific immune response elicited after immunization (defined below) detected in at least two of these three tests defined an immune response used for altering the immunization schedule for patients treated in Arm 2 (Figure 1).
ELISPOT
Studies were conducted as previously described, using monoclonal capture antibodies specific for IFNγ (Thermo Scientific, Pittsburgh, PA) or granzyme B (GeneTex Inc., Irvine, CA) (9). For these analyses, all antigens and sera used were from the same lots to control for possible variation over time. Plates were then washed, developed with biotinylated detection antibodies for either IFNγ (Thermo Scientific) or granzyme B (GeneTex Inc), and spots enumerated (9). A response resulting from immunization was defined as a PAP-specific response detectable post-treatment that was both significant (compared to media only control), at least 3-fold higher than the pre-treatment value, and with a frequency > 1:100,000 PBMC.
T-cell proliferation
PBMC were labeled in vitro with PKH26 dye (Sigma, St. Louis, MO) and cultured at 2 × 105 cells/well in 96-well round bottom microtiter plates (Corning, Corning, NY) using the same antigen-stimulating conditions as above. After 7 days of culture at 37°C/5% CO2cells were stained (CD4-V450, CD8-FITC, CD45RO-APC, CCR7-PECy7) and enumerated by flow cytometry (LSRII, Becton Dickinson, Franklin Lakes, NJ). The precursor frequency of antigen-specific CD4+ and CD8+ lymphocytes was determined among PKH26-diluted CD4+ or CD8+ events (ModFit software, Verity Software House, Topsham, ME), and subtracting the mean precursor frequency of proliferating cells under media-only conditions. Results are presented as the mean and standard deviation of antigen-specific proliferative precursors per 106 CD4+ or CD8+ T cells using triplicate assessments for each antigen-stimulation condition. Antigen-specificity was defined as above using a two-tailed t-test. A response resulting from immunization was defined as a PAP-specific response detectable post-treatment that was both significant (compared to media only control), at least 3-fold higher than the pre-treatment value, and with a frequency > 1:100,000 CD4+ or CD8+ T cells.
Other Immunologic Evaluation
Antigen-specific IgG
IgG specific for PAP, PSA or tetanus toxoid were evaluated by indirect ELISA, as previously described (10).
Antigen-specific cytokine staining and release
Cryopreserved PBMC obtained from different time points were thawed and cultured in T-cell medium with 2 µg/mL PAP protein, PSA protein, PHA, or anti-CD3- (BioLegend, San Diego, CA) and anti-CD28- (BioLegend) coated latex beads (Invitrogen) for 72 hours at 37°C/5%CO2. Culture supernatants were assessed for IFNγ, granzyme B, IL-2, IL-4, IL-6, IL-10, IL-17, or TGFβ by direct ELISA using standard methods (11). In similar studies, cells were treated after 18 hours in culture with monensin, and cultured an additional 4 hours prior to cell surface staining (CD3-V500, CD4-PE-Cy5.5, CD8-eFluor625), cell permeabilization, and staining for intracellular cytokines (IL-2-APC, IL-4-PE, IL-6-PE-CF594, IL-10-AF488, IL-17-PacBlue, IFNγ-PerCP-Cy5.5, and TNFα-PE-Cy7) using standard methods (BD cytofix/cytoperm kit). Results are reported as % of individual populations expressing specific cytokines as compared with fluorescently-labeled isotype controls for each cytokine, and subtracting the % of populations in media-only conditions.
Clinical Response Evaluation
Staging studies (CT of abdomen/pelvis and bone scintigraphy) were performed every 6 months, or as clinically indicated. PSA values (same clinical laboratory) were collected at 1-to-3-month intervals. A minimum of four PSA values collected over a 6-month period of time (minimum of 3 months), including the screening value, was used to determine the pre-treatment PSA DT, and all values collected from the 6-month period beginning at study week 12 (months 3–9 on study) for the post-treatment PSA DT. PSA DT was calculated as log(2) divided by the slope parameter estimate of the linear regression model of the log-transformed PSA values on time. For analysis purposes, negative PSA DT estimates and high positive PSA DT estimates (>36 months) were censored at 36 months. On-treatment PSA DT and PAP DT were calculated using all available serum PSA or PAP values from the same clinical laboratory from day 1 to the time off study.
Statistical Analysis
Categorical data were summarized as proportions and percentages. Continuous data were summarized and reported as medians and ranges. Profile plots were generated to display changes in PSA values over time. Changes in PSA DT from the pre-treatment to the post-treatment period were assessed by calculating fold changes in PSA DT. The standard errors of the fold-changes in PSA DT were estimated using Taylor’s expansion method. Fold changes in PSA DT from the pre-treatment to the post-treatment period were evaluated using a one-sample t-test. Analogously, comparison of PSA DT fold changes between arms was performed utilizing a two-sample t-test. Since the distribution of PSA DT fold changes was skewed, the fold change values were log-transformed before conducting the comparisons. To account for variability in the PSA DT estimates within each subject, these analyses were weighted using the inverse of the standard errors of the fold change estimates. Fold change in PSA DT were displayed in graphical format using a waterfall plot. The associations between time to radiographic progression and immune response, PSA DT or PAP DT were evaluated using Kaplan-Meier analysis and the stratified log-rank test was used to determine differences between groups. The results of these analyses were summarized in terms of hazard ratios (HR). Weighted logistic regression analysis was conducted to evaluate the association between each marker of immune response and fold change in PSA DT. A two-sided significance level of p < 0.05 was used for all tests and comparisons. Statistical analyses were conducted using SAS software version 9.2 (SAS Institute Inc., Carey, NC).
RESULTS
Patient population and course of study
17 patients were enrolled in this trial between 2009 and 2012 at the University of Wisconsin Carbone Cancer Center. As shown in Table I, the median age of participants was 74 years (range 47–89 years), the median serum PSA at the time of study entry for all participants was 5.6 ng/mL (range 2.3 – 54.4 ng/mL), and the median pre-treatment PSA DT for all patients was 3.0 months (range 1.4 – 5.5 months). Grade 1 injection site reactions were common, experienced by all subjects. One subject experienced a grade 3 allergic reaction, angioedema, within 30 minutes of his 11th vaccine administration. The angioedema responded to treatment with an oral antihistamine, and the patient was removed from further treatment, but followed until he met a study endpoint. As depicted in the Supplemental Table, no other events greater than grade 2, at least possibly attributed to treatment, were observed, and there were no dosing delays due to adverse events. In addition to the one patient who came off study for an adverse event, seven patients came off study for evidence of disease progression, one of these at the final 24 month time point, and two patients came off study for physician discretion due to rising PSA. 12/16 (75%) evaluable patients, who received at least six immunizations, were progression-free at one year and 6/16 (38%) remained metastasis-free at 2 years. Four patients in Arm 2 received 24 immunizations total. Patients were on study a median of 16 months. While the original goal was to enroll 30 patients, given the long period of treatment and observation the trial was closed early due to slow accrual and funding constraints (Supplemental Figure 1).
Table I. Demographics.
Demographics for all patients enrolled.
| N (Mean +/− SD) |
Arm 1 (N=8) |
Arm 2 (N=9) |
|
|---|---|---|---|
| Age (mean and range, years) | 17 | 76.1 (47–89) | 67.7 (60–76) |
| Race: | |||
| Caucasian | 17 | 8 | 9 |
| Gleason Score: | |||
| Unknown | 1 | 1 | 0 |
| < 7 | 3 | 2 | 1 |
| 7 | 8 | 2 | 6 |
| 8 | 1 | 1 | 0 |
| 9 | 4 | 2 | 2 |
| Primary treatment: | |||
| Prostatectomy | 11 | 5 | 6 |
| Radiation therapy | 3 | 1 | 2 |
| Androgen deprivation | 3 | 2 | 1 |
| Salvage radiation therapy | 8 | 3 | 5 |
| Pre-treatment: | |||
| Baseline serum PSA (ng/mL) | (12.7 +/− 16) | 16.7 (3.06 – 54.4) | 9.1 (2.3 – 25.4) |
| Baseline PSA doubling time (mos) | (3.45 +/− 1.47) | 4.20 (2.22 – 5.28) | 2.78 (1.36 – 5.48) |
Immunological Evaluation – Immune Monitoring to Determine Timing of Immunization
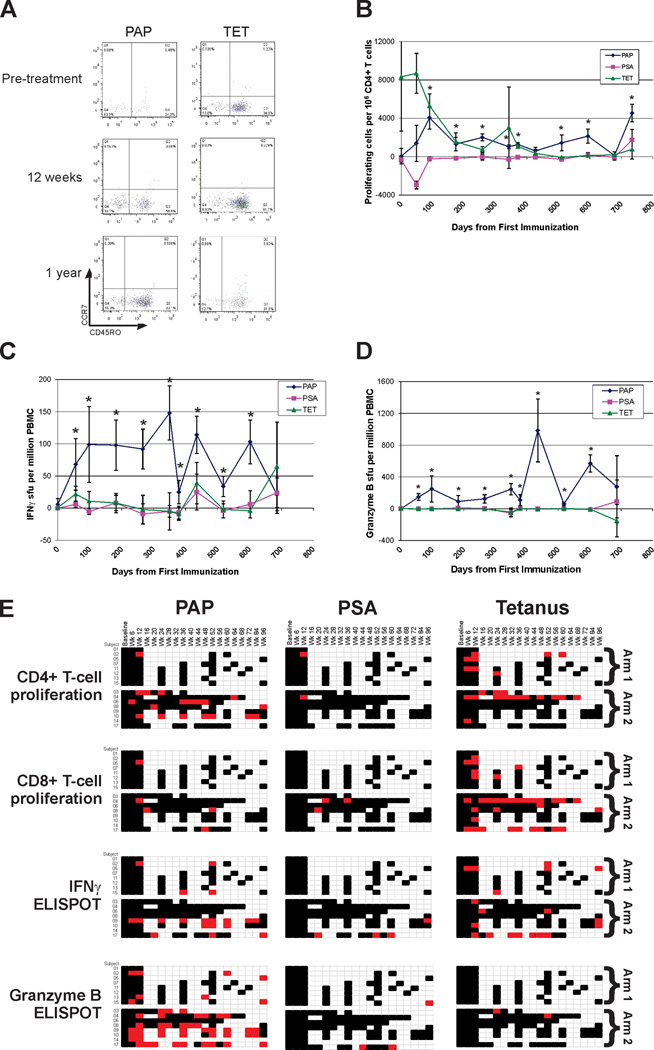
The primary immunological goal of this study was to determine whether real-time immune monitoring could be used to define a personalized schedule of immunization, able to elicit and maintain a PAP-specific cellular immune response. Patients were randomly assigned to one of two arms, with immunizations delivered on an empiric fixed schedule (Arm 1) or as determined by immune monitoring (Arm 2, Figure 1). All subjects received a tetanus booster immunization prior to beginning the immunization series, providing a test of an individual’s immune competence (although tetanus responses are predominantly humoral (IgG) and CD4-biased (8)). Responses to PSA, a non-target prostate-specific protein, were concurrently evaluated as a negative control. Patients had PBMC collected by leukapheresis at baseline and at one year for multiple analyses, and by peripheral blood draw at immune monitoring time points. An example of these immune analyses, performed over time for two particular individuals, is shown in Figure 2 (and Supplemental Figure 2A). As demonstrated, PAP antigen-specific T-cell proliferation (Figure 2A), IFNγ release (Figure 2B), and granzyme B release (Figure 2C) could be identified following immunization that persisted over the period of treatment. In multiple patients, antigen-specific proliferating cells were predominantly of an effector (CCR7−CD45RO−) and effector memory (CCR7−CD45RO+) phenotype (illustrated in Figure 2D). At each time point, cryopreserved pre-treatment cells were evaluated to assess reproducibility (Supplemental Figure 2C). A summary of all real-time immune monitoring assessments, for the 16 evaluable subjects who received at least six immunizations, is shown in Figure 2E. As demonstrated, PAP-specific cellular responses were augmented and detected in most individuals (12/16, 75%) by one or more methods and were persistent over time, up to two years in 5/6 (83%) individuals who remained on study. Responses to PSA were infrequent, as expected. Granzyme B-secreting responses to PAP were elicited in the majority (11/16, 69%) of individuals and persisted over time, and this type of response was essentially not detected to tetanus. IFNγ-secreting responses determined by ELISPOT were detectable for at least one year in five individuals (two patients in Arm 1, and three patients in Arm 2). Using purified CD4+ or CD8+ T cells, granzyme B ELISPOT responses were found to consist of both CD4+ and CD8+ T cells, while PAP-specific IFNγ ELISPOT responses were predominantly CD8-mediated (data not shown). While immune responses were elicited to PAP in all patients in the immune monitoring arm (Arm 2) by one or more methods, responses by two or more methods at any one time point were not detectable in 3/8 (38%) individuals (subjects 3, 6 and 8), despite two of these individuals (#6 and #8) receiving 24 immunizations at 2-week intervals. Two subjects (#4 and #17) were found to have an immune response after immunization that was subsequently lost, and had their immunization schedule changed to biweekly as per protocol.
Figure 2. DNA immunization elicits PAP-specific cellular immune responses.
Panel A: Antigen-specific CD4+PKH26low (proliferating) cells were evaluated for concurrent CCR7 and CD45RO expression. Panel B: Antigen-specific CD4+ T cell proliferation was evaluated in real-time over multiple time points for one patient (#10) and displayed as precursor antigen-specific CD4+ T cells per 106 CD4+ T cells. Asterisks denote a positive immune response, defined as a statistically significant antigen-specific response (compared to media alone), with a frequency of at least 1:100,000, and at least 3-fold higher than the baseline (pre-treatment) value. Panel C: ELISPOT was used to determine the frequency of antigen-specific IFNγ-secreting T-cells over multiple time points. Shown are results for one patient (#9) obtained in real-time at each time point. Asterisks denote positive immune responses elicited, defined as above. Panel D: ELISPOT was also used to determine the frequency of antigen-specific granzyme B-secreting T-cells over multiple time points. Shown are results for patient #9 obtained in real-time at the same time points as in panel C. Asterisks denote positive immune responses elicited, defined as above. Panel E: Shown are the comprehensive summary data from immune monitoring by evaluation for PAP-, PSA- or tetanus-specific immune responses as assessed by CD4+ or CD8+ T-cell proliferation, IFNγ secretion by ELISPOT, or granzyme B secretion by ELISPOT. A positive immune response (red) was defined as an antigen-specific response (statistically higher than the media-only control, p<0.05 by t-test) that was at least 3-fold higher than background and with a frequency >1:100,000 cells. Black squares indicate a time point where an assessment was performed, but in which there was no evidence of an antigen-specific immune response.
Immunological Evaluation – Changes in Immune Response over Time
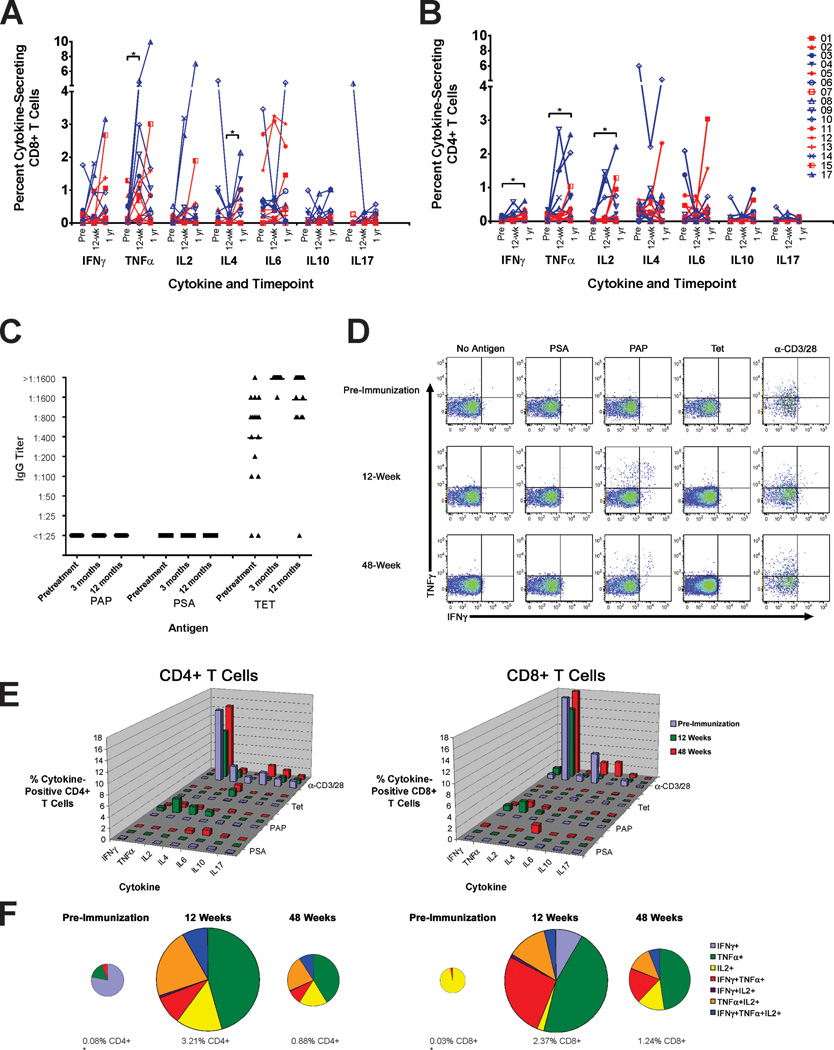
We next questioned whether the character or magnitude of the immune response changed over time, particularly in patients receiving multiple biweekly immunizations. As suggested in Figure 2E, PAP-specific immune responses elicited were frequently detected at later time points, and these tended to be of a Th1/cytolytic (IFNγ- and granzyme B-secreting) phenotype. To further address this, the cytokine profile of PAP-specific T cells was assessed by intracellular cytokine analysis for concurrent antigen-specific expression of IFNγ, TNFα, IL-2, IL-4, IL-6, IL-10, and/or IL-17. As shown in Figure 3A and 3B, CD4+ and CD8+ T cell responses to PAP tended to be Th1-biased (expressing IFNγ, TNFα and/or IL-2). IL-4 and IL-6 expression was also observed in some patients. These responses persisted beyond 12 weeks, and the type of immune response (Th1 versus Th2 versus Th17) did not appreciably change over time. Notably, patients #4, 6, 8, and 17, who received the greatest number of immunizations (n=24), did not develop increased IL-4- or IL-10-biased responses with multiple immunizations. Th1-biased responses to tetanus and PSA were only occasionally detected (Supplemental Figure 3). Similar results were obtained by ELISA using culture supernatants assessed for cytokines following antigen stimulation (Supplemental Figure 4). While antibody responses to tetanus toxoid were common (and amplified following booster immunization), IgG responses to PAP were not detected in any individual, even after 24 immunizations, further consistent with a Th1-biased immune response (Figure 3C), and as we had previously observed (8). CD4+ and CD8+ cells expressing more than one Th1 cytokine (IFNγ, TNFα and/or IL-2) were detected (Figure 3D), and in several patients (patients 9, 10, 13, 15, 17) were found to persist for at least one year. An example of this analysis demonstrated that multifunctional Th1-biased cells remained present one year after beginning immunization (patient #9, Figure 3E,F).
Figure 3. Immune responses to PAP tend to be Th1-biased and persist over time.
Intracellular cytokine staining was performed with PBMC samples collected pretreatment, after 12 weeks, and at one year (3 patients did not have one-year samples). The frequency of CD8+ (panel A) or CD4+ (panel B) T cells expressing IFNγ, TNFα, IL-2, IL-4, IL-6, IL-10, or IL-17 was assessed. Asterisks show significant changes from pre-treatment (paired t-test). Panel C: Sera at these same time points were assessed for IgG responses to PAP, PSA, or tetanus toxoid (TET). Antibody titers are shown. Panel D: Intracellular cytokine staining permitted the concurrent evaluation of cells expressing more than one Th1 cytokine. Shown is an example (subject 9) of CD8+ gated T cells expressing IFNγ and/or TNFα in response to antigen stimulation at the time points indicated. Panel E: Single cytokine CD4+ and CD8+ intracellular cytokine analysis at these same time points (pretreatment, 12 weeks, and one year) for subject 9. Panel F: Shown are the fraction of PAP-specific CD4+ T cells (left plots) or CD8+ T cells (right plots) expressing one or more Th1 (IFNγ, TNFα, and/or IL-2) cytokines. The pie charts demonstrate the frequency of individual cell populations, and numbers below the pie charts indicate the percentage of PAP-specific Th1+ events among total CD8+ or CD4+ T cells. The size of the plots is proportional to the percentage of PAP-specific CD4+ or CD8+ T cells over time (* = not to scale).
Clinical Evaluation
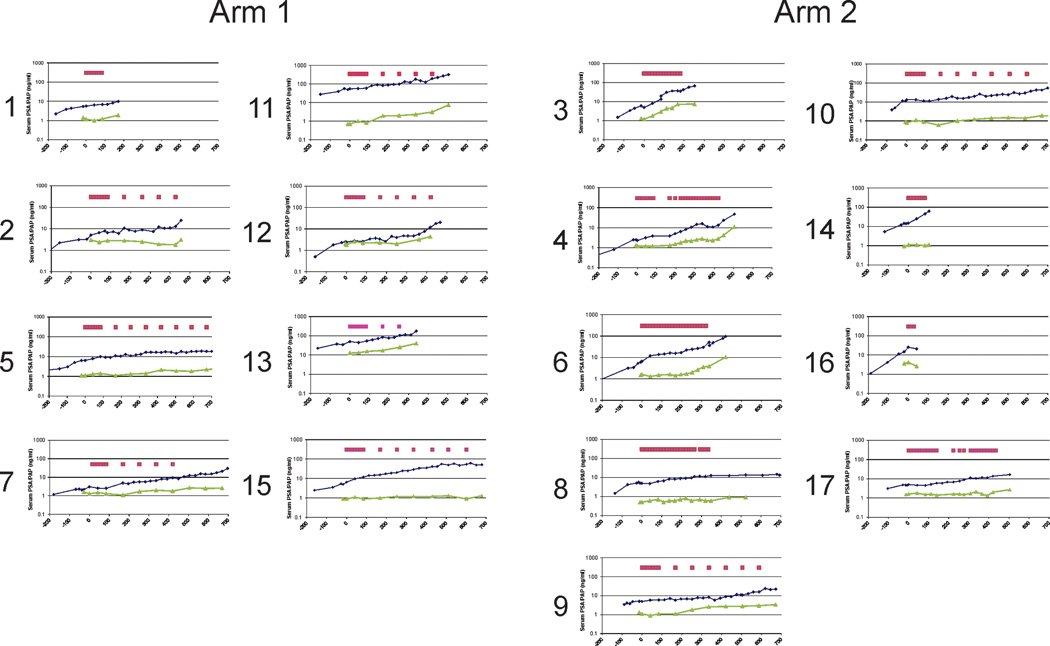
As described above, patients enrolled had no radiographic evidence of metastatic disease, and remained on study until progression or 2 years, whichever came first. Serum PSA values were collected in a protocol-specified fashion to determine PSA doubling times (DT) and changes in PSA DT from pre- to post-treatment. While PSA DT is a known prognostic marker for men with this stage of disease (2, 3), changes in PSA DT have not been validated as a measure associated with clinical benefit. Hence our use of changes in PSA DT must be viewed as exploratory. Serum PSA values, and serum PAP values, with respect to time points of vaccination are shown for all subjects in Figure 4. Overall, PSA values increased over time, although some patients exhibited stable PSA over many months (e.g. subjects 5, 8, 9, and 10). Of note, several subjects demonstrated stable serum PAP levels over many months (e.g. subjects 2, 8, 10, 15, 17), in some cases despite rising serum PSA (e.g. subjects 2, 15, 17). Serum PAP values were not available pre-treatment. 7/17 (41%) patients experienced at least a 2-fold increase in PSA DT (subjects 2, 5, 6, 8, 9, 10, and 12). Overall, the median fold change in PSA DT from pre-treatment to post-treatment was 1.6 (range 0.6–7.0, p=0.036). Stratified by study arm, the median fold change in PSA DT was 1.21 (range 1.13–5.55, p=0.027) for Arm 1, and 1.72 (range 0.57–7.02, p=0.293) for Arm 2. There was no statistically significant difference in the PSA DT fold change detected between Arm 1 and Arm 2 (p=0.764).
Figure 4. Serum PSA and PAP.
Shown are the serum PSA (blue) and serum PAP (green) values for all patients on trial, including the PSA values collected for individuals in the pre-treatment period. The magenta boxes indicate the time points of immunization.
Association of Immune Response with Exploratory Clinical Parameters
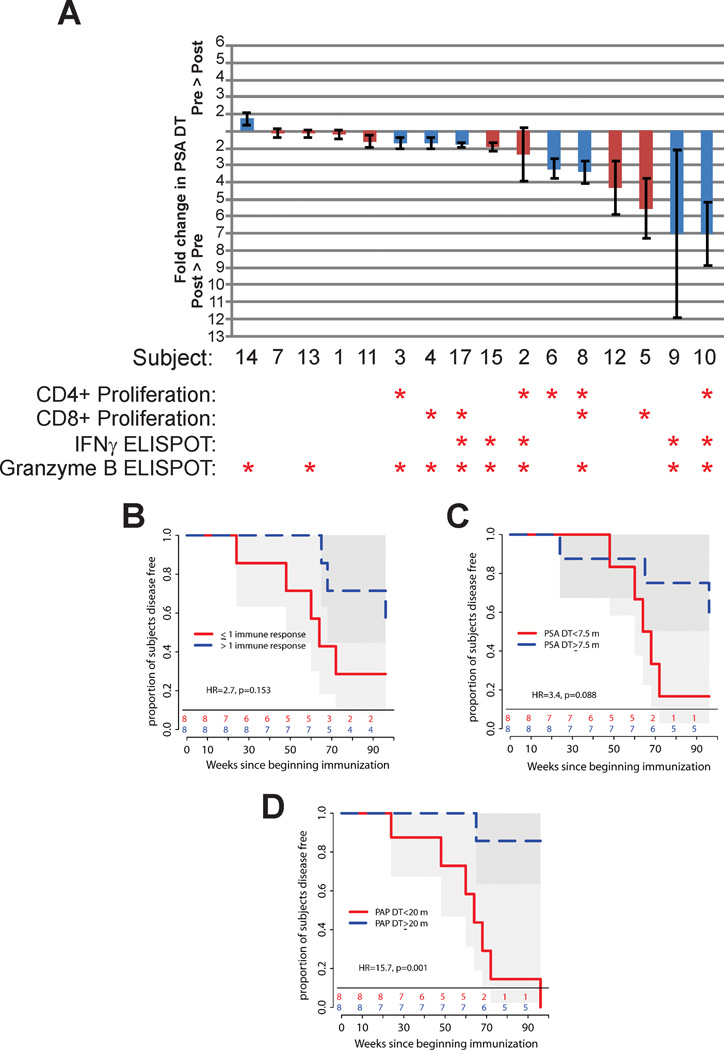
Ultimately we wished to determine whether the development of a biological response from vaccination was associated with favorable changes in serum PSA kinetics or time to disease progression. Immune responses to PAP, identified by the different measures of immune response in Figure 2, are depicted in Figure 5A with respect to changes observed in PSA DT. As shown, all measures of immune response tended to be more common in patients who experienced an increase in PSA DT. However, except for CD4 T-cell proliferation (odds ratio 2.3, p=0.049), these associations were not statistically significant. A trend toward increased time to disease progression was observed in patients who developed more than one measure of immune response for PAP, however this was not statistically significant (HR=2.7, p=0.153, Figure 5B). Similarly, and as expected based on prior studies demonstrating PSA DT to be a predictor for time to radiographic progression in this population (3), a trend toward increased time to progression was observed in patients who had higher on-study PSA DT (HR=3.4, p=0.088, Figure 5C). Curiously, patients who had a higher on-study doubling time in serum PAP had a significantly prolonged time to progression (HR=15.7, p=0.001, Figure 5D). While these findings must be viewed as exploratory, given the small number of patients, they suggest that changes in serum PAP kinetics might be evaluated as a future biomarker for response to DNA immunization targeting PAP.
Figure 5. Biomarker associations with changes in PSA DT and time to disease progression.
Shown are the fold changes in PSA DT from the pre-treatment period to the post-treatment period as a waterfall plot (panel A). Red columns indicate patients treated in Arm 1, and blue columns for patients in Arm 2. The detection of an immune response by one of the measures described is indicated with an asterisk. The time to radiographic disease progression was evaluated using Kaplan-Meier analysis with respect to the development of more than one measure of immune response to PAP (panel B), PSA DT (using all on-study serum PSA values to determine an on-study PSA DT, panel C), or PAP DT (using all on-study serum PAP values to determine an on-study PAP DT, panel D).
DISCUSSION
A common theme emerging from the evaluation of anti-tumor vaccines and T-cell checkpoint inhibitors is that it can be difficult to recognize immediate clinical benefit, since radiographic progression can occur before regression or stable disease is observed (12, 13). This has led to conclusions that longer term clinical endpoints are necessary for evaluating these therapies (4, 14). Results from animal models would further suggest that immune-based treatments may have their greatest role early in the course of disease, prior to the development of tumor-mediated immune suppression or evasion mechanisms (15). For these reasons, we sought to evaluate the immunological efficacy of a DNA vaccine, and evaluate different schedules of vaccination, in patients with early castration-resistant disease, a stage of disease with a long natural history and without standard treatment options (2). This population enabled us to evaluate the development and durability of immune responses elicited over many months. In our experience, this is also a rarer population, as fewer patients elect to begin androgen deprivation therapy in the absence of metastatic disease. As a result of this, our trial closed early due to slow accrual. Nonetheless, we report here that prolonged DNA vaccination, up to 24 times and/or 2 years, was without significant toxicity in this population and could elicit and amplify Th1-biased antigen-specific immune responses which persisted with repetitive immunization.
One of the primary objectives of our study was to determine whether the frequency of antigen-specific effector and memory T cells could be augmented by real-time immune monitoring, using this information to adjust the schedule of immunization. We were particularly interested in the development of IFNγ-secreting T-cell responses, given results from a previous phase I trial (9). While the study population was small, we did not observe a higher frequency of IFNγ ELISPOT responses in the immune monitoring arm (3/8) relative to the fixed schedule arm (2/8, p=0.59) at one year. Moreover, some immune responses were not detectable after the initial series but became detectable at later time points. The observation that some immune responses developed over time regardless of the immunization schedule, and IFNγ-secreting T-cell responses in particular were not augmented in more individuals treated in the variable arm, suggested that using real-time immune monitoring to personalize the vaccination schedule was not necessary and was not particularly informative for managing individual schedules.
Plasmid DNA has been used as an antigen delivery vehicle both for anti-tumor immunization, in which the goal is to elicit a cytolytic cellular response, and for the treatment of autoimmune disease, in which the goal is to elicit a tolerant response (16, 17). Given this, we were particularly keen to determine whether multiple repetitive immunizations could skew an immune response towards a Th2 or tolerant phenotype. This was not observed, including in four individuals who received 24 immunizations. Notably, antibody responses, common after treatment with another vaccine (sipuleucel-T) targeting this same PAP antigen (4), were not elicited following this DNA immunization (Figure 3C). At present it remains unknown whether the type of immune response elicited is related to the particular antigen targeted, potentially due to preexisting inflammatory immune responses that are amplified with immunization. Alternatively, the Th1 bias that we observed could be due to the route of immunization and the adjuvant used. We used an intradermal route of immunization and GM-CSF as an adjuvant, a route and adjuvant previously investigated in other models for the specific development of Th1-biased immune responses (18–21). GM-CSF, however, has pleiotropic effects, and its efficacy as an adjuvant has been questioned in the context of peptide-based vaccines (22). While preclinical models support its use with DNA vaccines as an adjuvant (23), the role of GM-CSF, and whether it promotes or inhibits the development of Th1 immunity in humans, will be a goal of future trials. In addition, it remains unknown whether the frequency or magnitude of the immune responses we observed could be further augmented by the use of different delivery methods, such as electroporation or particle bombardment being investigated by others (24–27).
We had previously observed in a phase I trial that six immunizations with plasmid DNA encoding PAP was sufficient to elicit a PAP-specific cellular immune response in some individuals with prostate cancer, but not others, and that immune responses tended to develop over time with repetitive immunization (8). Consequently, an additional goal of the current trial was to determine whether ongoing immunization was necessary to elicit responses in some individuals that did not develop an immune response after six immunizations only. Notably, in two patients who did not have an immune response at the end of the initial 12-week immunization series, continuous immunization at 2-week intervals did not lead to higher magnitude immune responses. Potential reasons for the inability to effectively immunize these individuals remain unknown and were not addressed in our study. This could be due to differences in these individuals even prior to beginning treatment, perhaps suggesting that some individuals are completely tolerant to this antigen. It is also conceivable that plasmid DNA immunization is ineffective in some individuals irrespective of the target antigen. It is further conceivable that immune responses were elicited but at levels undetectable in the peripheral blood. While no baseline parameters (e.g. Gleason score) or prior therapy appeared to be associated with the development of immune response (data not shown), the identification of differences leading some patients to develop measurable immune responses, and others not, will be an important area for future investigation.
In our study, PSA protein was included as a prostate-specific negative control for the immunological assessments. Curiously, we did identify Th1 biased responses elicited to this protein, in one patient detectable at multiple time points after immunization (Figure 2, subject 17). We have previously reported that Th1-biased T-cell responses to PSA can occur in patients with prostate cancer (11). However, the finding that these were not detectable at baseline but augmented after immunization suggests that antigen spread might have occurred in at least these individuals. Antigen spread to non-target tumor-associated proteins has been observed following different anti-tumor vaccine treatments, and in some circumstances has been associated with favorable outcomes from vaccination (28). Using antibody responses to prostate-derived proteins, we have previously identified antibody responses to non-targeted proteins elicited following DNA immunization (29). The identification of T-cell antigen spread to other prostate–associated proteins, and whether this is associated with better clinical outcome, will be a direction for future studies.
Ultimately a goal of our study was to determine whether the development of PAP-specific long-term immunity was associated with improved clinical outcome. However, our desire to evaluate vaccination in the setting of minimal residual disease with a long natural history necessarily confounds the analysis of clinical benefit; this population of patients did not have radiographically apparent disease that could be monitored – the only measure of tumor response was by changes in serum tumor markers. Hence an exploratory objective of our trial was to evaluate biomarkers that might be associated with prolonged time to disease progression. While PSA DT is a known prognostic factor in this stage of disease (2), and changes in PSA DT have been associated with prolonged time to disease progression in non-castrate patients with prostate cancer (30), this has not been prospectively validated in clinical trials and awaits validation in trials currently underway. Notwithstanding, those patients who demonstrated an increase in PSA DT tended to have multiple measures of PAP-specific immunity that were detectable over multiple time points. This is consistent with our previous findings in patients with non-castrate PSA-recurrent prostate cancer (9). We also observed that the four patients who experienced the greatest change in PSA DT (patients 5, 9, 10, 12; two of whom were on the immune monitoring arm) were effectively treated with the same fixed quarterly-booster schedule. This observation, with the demonstration of Th1-biased immune responses being elicited using this schedule, have led us to select this schedule for further evaluation in a larger, randomized phase II clinical trial (NCT01341652).
In the current trial we also evaluated the time to disease progression by standard radiographic criteria. The development of immune responses to PAP by more than one measure, and a longer on-trial PSA DT, were associated with an increased time to radiographic progression; however, these associations were not statistically significant. Intriguingly, a longer on-trial PAP doubling time was significantly associated with a prolonged time to disease progression, potentially due to immunological targeting of tumor cells expressing PAP. As there was no evidence of PAP antibody responses by immunization, it is unlikely that serum levels were affected by antibody-mediated clearance of PAP. Consequently, it is possible that serum PAP kinetics might serve as a simple biomarker for an effective anti-tumor cytolytic response following PAP-targeted immunization. At present it is unknown whether changes in serum PAP might generally be evaluated as a prognostic biomarker, or whether changes observed in this trial are specifically related to targeting the PAP antigen. It will be of interest to determine whether serum PAP kinetics are affected by other anti-prostate tumor immunization approaches such as Prostvac-VF (targeting PSA) or sipuleucel-T (targeting PAP). The evaluation of serum PAP and immune biomarkers demonstrating treatment effect will be further explored in randomized phase II trials.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
We previously showed that a DNA vaccine targeting prostatic acid phosphatase (PAP) can elicit antigen-specific T-cell immune responses in patients with early recurrent prostate cancer, and these responses were associated with favorable changes in PSA doubling time (DT). In this pilot clinical trial we evaluated whether repetitive immunization up to two years could elicit durable effector and memory T cell immune responses, and whether the frequency of these immune responses could be augmented using ‘personalized’ immunization schedules determined by multi-parameter real-time immune monitoring, compared with an empiric fixed schedule of immunization. Repetitive immunizations elicited Th1-biased immune responses in 12/16 (75%) patients, and these remain Th1-biased even after multiple immunizations. Individualized schedules of vaccination determined by immune monitoring did not appear advantageous as measured by either the development of Th1-biased immune responses or changes in PSA DT.
ACKNOWLEDGMENTS
This work was supported by NIH/NCI (R21 CA132267 and P30 CA014520), and by the NIH NCRR Clinical and Translational Science Award (CTSA) program (1UL1RR025011). We thank Dr. Lawrence Fong for helpful suggestions and critical review of the manuscript. We are grateful for the assistance of the research staff of the UWHC infusion center, UW pharmacy research center, clinical research coordinators and staff, treating physicians, and the participation of the patients and families.
Footnotes
Conflicts of Interest
Douglas G. McNeel has ownership interest, receives research support, and serves as consultant to Madison Vaccines, Inc. which has licensed intellectual property related to this content. None of the other authors have relevant potential conflicts of interest.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–2925. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 3.Smith MR, Saad F, Oudard S, Shore N, Fizazi K, Sieber P, et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J Clin Oncol. 2013;31:3800–3806. doi: 10.1200/JCO.2012.44.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson LE, Frye TP, Arnot AR, Marquette C, Couture LA, Gendron-Fitzpatrick A, et al. Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP) . Vaccine. 2006;24:293–303. doi: 10.1016/j.vaccine.2005.07.074. [DOI] [PubMed] [Google Scholar]
- 7.Johnson LE, Frye TP, Chinnasamy N, Chinnasamy D, McNeel DG. Plasmid DNA vaccine encoding prostatic acid phosphatase is effective in eliciting autologous antigen-specific CD8+ T cells. Cancer Immunol Immunother. 2007;56:885–895. doi: 10.1007/s00262-006-0241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker JT, Olson BM, Johnson LE, Davies JG, Dunphy EJ, McNeel DG. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J Immunother. 2010;33:639–647. doi: 10.1097/CJI.0b013e3181dda23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNeel DG, Nguyen LD, Storer BE, Vessella R, Lange PH, Disis ML. Antibody immunity to prostate cancer-associated antigens can be detected in the serum of patients with prostate cancer. J Urol. 2000;164:1825–1829. [PubMed] [Google Scholar]
- 11.McNeel DG, Nguyen LD, Ellis WJ, Higano CS, Lange PH, Disis ML. Naturally occurring prostate cancer antigen-specific T cell responses of a Th1 phenotype can be detected in patients with prostate cancer. Prostate. 2001;47:222–229. doi: 10.1002/pros.1066. [DOI] [PubMed] [Google Scholar]
- 12.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 13.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulley JL, Drake CG. Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin Cancer Res. 2011;17:3884–3891. doi: 10.1158/1078-0432.CCR-10-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garren H, Robinson WH, Krasulova E, Havrdova E, Nadj C, Selmaj K, et al. Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Annals of neurology. 2008;63:611–620. doi: 10.1002/ana.21370. [DOI] [PubMed] [Google Scholar]
- 17.Colluru VT, Johnson LE, Olson BM, McNeel DG. Preclinical and clinical development of DNA vaccines for prostate cancer. Urol Oncol. 2013 doi: 10.1016/j.urolonc.2013.09.014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledgerwood JE, Hu Z, Gordon IJ, Yamshchikov G, Enama ME, Plummer S, et al. Influenza virus h5 DNA vaccination is immunogenic by intramuscular and intradermal routes in humans. Clinical and vaccine immunology : CVI. 2012;19:1792–1797. doi: 10.1128/CVI.05663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staff C, Mozaffari F, Haller BK, Wahren B, Liljefors M. A Phase I safety study of plasmid DNA immunization targeting carcinoembryonic antigen in colorectal cancer patients. Vaccine. 2011;29:6817–6822. doi: 10.1016/j.vaccine.2010.12.063. [DOI] [PubMed] [Google Scholar]
- 20.Kwissa M, Kroger A, Hauser H, Reimann J, Schirmbeck R. Cytokine-facilitated priming of CD8(+) T cell responses by DNA vaccination. J Mol Med. 2003;81:91–101. doi: 10.1007/s00109-002-0395-6. [DOI] [PubMed] [Google Scholar]
- 21.Hess G, Kreiter F, Kosters W, Deusch K. The effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on hepatitis B vaccination in haemodialysis patients. J Viral Hepat. 1996;3:149–153. doi: 10.1111/j.1365-2893.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 22.Slingluff CL, Jr, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–7044. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Disis ML, Shiota FM, McNeel DG, Knutson KL. Soluble cytokines can act as effective adjuvants in plasmid DNA vaccines targeting self tumor antigens. Immunobiology. 2003;207:179–186. doi: 10.1078/0171-2985-00230. [DOI] [PubMed] [Google Scholar]
- 24.Roos AK, Moreno S, Leder C, Pavlenko M, King A, Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther. 2006;13:320–327. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000;164:4635–4640. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- 26.Ferraro B, Cisper NJ, Talbott KT, Philipson-Weiner L, Lucke CE, Khan AS, et al. Co-delivery of PSA and PSMA DNA vaccines with electroporation induces potent immune responses. Human vaccines. 2011;7(Suppl):120–127. doi: 10.4161/hv.7.0.14574. [DOI] [PubMed] [Google Scholar]
- 27.Roy MJ, Wu MS, Barr LJ, Fuller JT, Tussey LG, Speller S, et al. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine. 2000;19:764–778. doi: 10.1016/s0264-410x(00)00302-9. [DOI] [PubMed] [Google Scholar]
- 28.Lally KM, Mocellin S, Ohnmacht GA, Nielsen MB, Bettinotti M, Panelli MC, et al. Unmasking cryptic epitopes after loss of immunodominant tumor antigen expression through epitope spreading. Int J Cancer. 2001;93:841–847. doi: 10.1002/ijc.1420. [DOI] [PubMed] [Google Scholar]
- 29.Smith HA, Maricque BB, Eberhardt J, Petersen B, Gulley JL, Schlom J, et al. IgG responses to tissue-associated antigens as biomarkers of immunological treatment efficacy. J Biomed Biotech. 2011;2011:454861. doi: 10.1155/2011/454861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonarakis ES, Zahurak ML, Lin J, Keizman D, Carducci MA, Eisenberger MA. Changes in PSA kinetics predict metastasis- free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents: combined analysis of 4 phase II trials. Cancer. 2012;118:1533–1542. doi: 10.1002/cncr.26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.