Abstract
Background and Purpose
Hyperbaric oxygen (HBO) has been reported to be neuroprotective and improved neurofunctional outcomes in acute stroke. However it is not clear if delayed HBO enhances endogenous neurogenesis and promotes neurofunctional recovery. The aim of this study is to evaluate the effects of delayed HBO therapy on neurogenesis and its potential mechanisms.
Methods
One hundred and eleven male Sprague-Dawley rats survived for 7 days from 2 hours of middle cerebral artery occlusion (MCAO) and reperfusion were used. Delayed and multiple HBO were administrated beginning at 7 days after MCAO and last for 42 days with three HBO free intervals (5 days each). Motor sensory deficits were measured by foot-fault test and learning and memory abilities were evaluated by Morris water maze. Neurogenesis was examined by double immunostaining of BrdU and Doublecortin, BrdU and NeuN at day 42. For mechanism studies, inhibitors for reactive oxygen species (ROS), HIF-1α and β-catenin were administrated and the levels of ROS, HIF-1α, β-catenin, LEF-1, TCF-1, neurogenin-1, Doublecortin and synapsin-1 were assessed by ELISA or western blot at day 14.
Results
Delayed HBO treatment promoted neurogenesis and improved neurofunctional recovery at day 42, and the improvements were reversed by inhibition of ROS and HIF-1α. Delayed HBO significantly increased ROS and HIF-1α, and up-regulated the expression of neurogenin-1, Doublecortin and synapsin-1. Inhibition of ROS, HIF-1α, removed the effects of delayed HBO.
Conclusions
Delayed HBO enhanced endogenous neurogenesis and improved neurofunctional recovery in the late-chronic phase of stroke possibly mediated by ROS/HIF-1α/β-catenin pathway. Delayed HBO may serve as an alternative treatment to improve long-term recovery of stroke survivors.
Keywords: Hyperbaric oxygen therapy, Reactive oxygen species, HIF-1α, neurogenesis, MCAO
Introduction
Stroke is a leading cause of long-term disability worldwide 1. Since most stroke patients go to hospitals at hours or days after the initial event, tPA as the only FDA approved treatment for ischemic stroke is applied to about 2-5% of stroke patients 1. For the chronicle recovery stage of stroke, few therapeutic options are available even though vigorous research have been conducted including stem cell treatment 1, 2. Recently, some preclinical studies demonstrated that hyperbaric oxygen (HBO) promoted neurogenesis 3, 4 and neurofunctional recovery 5, 6, possibly by the initial neuroprotective action in the treatment of acute stroke 7-9. However, in reality, HBO is applied mostly to chronic stroke patients not to reduce infarction but to improve long term neurological and neurobehavioral functions. The potential therapeutic effects of delayed and multiple HBO, as a clinical modality for stroke treatment, on neurogenesis and its mechanisms during stroke recovery stage have not been investigated. The aim of the current study is to evaluate the effects of delayed and multiple HBO on neurogenesis at the late-chronic phase when acute infarction is stabilized.
Previous studies have shown that the Wnt/β-catenin pathway is involved in adult neurogenesis after stroke10, 11. Wnts act mitogenically on progenitor cells, and the activation of β-cateninleads to the proliferation and differentiation of neural stem progenitor cells (NSPCs). Hypoxia inducible factor-1α (HIF-1α) can activate Wnt/β-catenin pathway and promotes neurogenesis in the adult nervous system 12, 13. It has been demonstrated that HBO exposure has the potential to increase the level of reactive oxygen species (ROS) and stabilize HIF-1α 14-16. Therefore, we hypothesized that HBO enhances the endogenous neurogenesis and promotes functional recovery through HIF-1α modulation of Wnt/β-catenin signaling.
Materials and Methods
All experiments were approved by the Institutional Animal Care and Use Committee of Loma Linda University.
Animal Model and Experimental Protocol
Middle cerebral artery occlusion (MCAO) in rats was performed as reported previously 17. One hundred and eleven male (275-325 g) Sprague-Dawley rats (Indianapolis, IN) survived for 7 days from 2 hours of MCAO were used. To examine whether delayed and multiple treatments with HBO promote functional recovery and neurogenesis, 2.5 atmospheres absolutes (ATA) HBO was administered starting at 7 days after MCAO for 3 sessions (n=7). Each session was 1.5 hr daily for consecutive 7 days followed with 5 days break. Doses of HBO were selected based on previous studies 18. MCAO rats treated with normal baric oxygen (NBO) (n=7) were used as controls.
For labeling proliferating cells, bromodeoxyuridine (BrdU, Sigma Chemical, 50 mg/kg) was injected intraperitoneally (i.p.) 1 hr before each HBO treatment. Neurobehavioral function was evaluated by foot-fault test (at day 1, day 15, day 27 and day 39) and memory and learning abilities were detected by Morris water maze (MWM) (from day 39 to day 42). All rats were euthanized and perfused at 42 days after stroke for immunochemistry.
To examine the mechanisms of HBO on neurogenesis, ROS scavenger n-acetyl cysteine (NAC, Sigma-Aldrich Co., 150mg/kg, i.p.), HIF-1α inhibitor 2-methoxyestradiol (2ME2, Tocris Bioscience, 5mg/kg, i.p.), and β-catenin antagonist PKF115-584 (PKF, Tocris Bioscience, 5mg/kg, i.p.) was administered, respectively 1 hr before each HBO treatment. Neurogenesis, neurological function and the levels of ROS and proteins were measured at day 42 or day 14.
Brain Residual Volume
The brain residual volume was calculated as previously described19. The residual volume was measured from Nissl-stained coronal sections, and presented as a volume percentage by the following formula: (ipsilateral volume/contralateral volume) × 100%.
Foot-Fault Test
Foot-fault test (day 7, day 15, day 27, and day 39) was measured by an investigator who was blinded to the experimental groups as previously described19. The number of foot-faults was recorded and the foot faults number of left forelimb was used for the statistical analysis.
Morris Water Maze
At 39 days after MCAO, Morris water maze was performed in a blinded setup as previously described20. In brief, it consisted of three trials (cued, spatial, and probe) done over four consecutive days. Spatial learning was measured by the time and distance taken to find the platform in cued and spatial trials, and spatial memory was measured in the probe trial.
Immunohistochemistry
Immunofluorescent staining for brain tissue was performed on fixed frozen ultrathin sections as previously described21. Primary antibodies used were: BrdU (Santa Cruz Biotechnology), NeuN (Millipore), Doublecortin (DCX, Santa Cruz Biotechnology), and Synapsin-1 (abcam). Five random microscope fields (20×) in the peri-infarction area of the brain coronal section were imaged by Olympus-BX51. The number of positive cells was calculated as the mean of the numbers obtained from the five pictures.
ELISA for Reactive Oxygen Species
To measure ROS generation, a ROS-HRP conjugate ELISA kit (MyBioSource, Inc.) was used as described previously22. The brain samples were collected at 6 hours after the last treatment of consecutive 7-days HBO. The supernatant of the samples was incubated together with ROS-HRP conjugate in pre-coated plate, and then incubated with a substrate for HRP enzyme. Finally, the absorbance was measured spectrophotometrically at 450 nm in a microplate reader (Bio-Rad iMark).
Western Blot Analysis
The brain samples were collected at 24 hours after the last treatment of consecutive 7-days HBO. Western blotting was performed as described previously17. Primary antibodies used were: HIF-1α, T cell Factor-1 (TCF-1, Cell Signaling), Lymphoid enhancer-binding factor-1 (LEF-1, Cell Signaling), β-catenin (abcam), Neurogenin-1 (Santa Cruz Biotechnology), Synapsin-1 and DCX.
Statistical Analysis
Parametric data in different groups were compared using a one-way analysis of variance (ANOVA) followed by the Turkey method. The data were presented as means± SEM. Survival was analyzed by Wilcoxon test. In all statistical analysis, a value of p< 0.05 represents statistical significance.
Results
Delayed HBO Had no Effects on Brain Morphology but Improved Neurological Deficits in Long-term
At 42 days after MCAO, extensive atrophy of the ipsilateral brain tissue was observed in the MCAO group; delayed HBO has no effects on brain morphology and brain tissue loss (Figure 1A, 1B). Delayed HBO did not show improvement on foot faults at day 15 and day 27 (Figure 1C); however, it significantly decreased the foot faults at day 39 (Figure 1C). Figure 1D showed delayed HBO improved spatial learning and memory in Morris Water Maze by decreasing the latency to reach the platform 6 weeks after MCAO. Delayed HBO had no effects on survival and body weight in long-term (data no shown).
Figure 1.
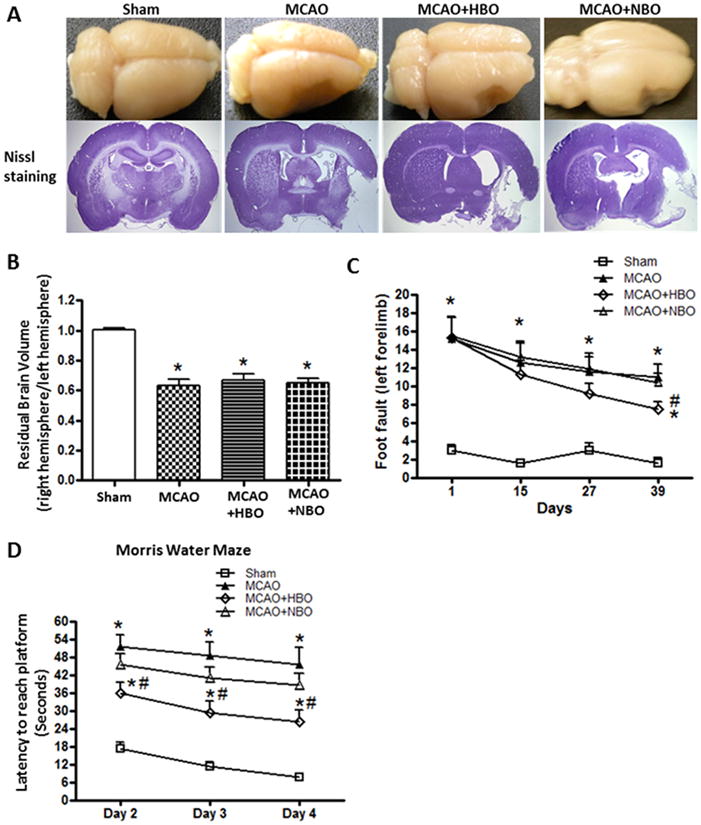
Delayed HBO had no effects on brain morphology but improved neurological functions 6 weeks after MCAO.
Brain photos and brain slices with Nissl staining (A), statistical analyses of residual brain volume (B), foot-fault test (C), and learning curve in Morris Water Maze (D) at 42 days after MCAO. Delayed and multiple HBO had no effect on brain morphology and residual brain volume but improved the long term neurological functions at 6 weeks after MCAO. Sham n=5; MCAO n=5; MCAO+HBO n=7; MCAO+NBO n=6. *p<0.05 vs. Sham; #p<0.05 vs. MCAO.
Delayed HBO Improved Neurological Functions depends on ROS/HIF-1α
The improvement of foot faults by delayed HBO was reversed by ROS scavenger NAC and HIF-1α inhibitor 2ME2 at day 39 after MCAO (Figure 2A). There were spatial learning and memory deficits at 6 weeks after MCAO. The animals in MCAO group need more time to reach the platform (Figure 2B), had a significantly greater distance moved from the target (Figure 2C), showed less time in the probe quadrants (Figure 2D). Delayed HBO significantly reduced the latency for platform (Figure 2B), decreased total distance the moved (Figure 2C) and improved the amount of time in the probe quadrant (Figure 2C), and the improvement were reversed by NAC and 2ME2.
Figure 2.
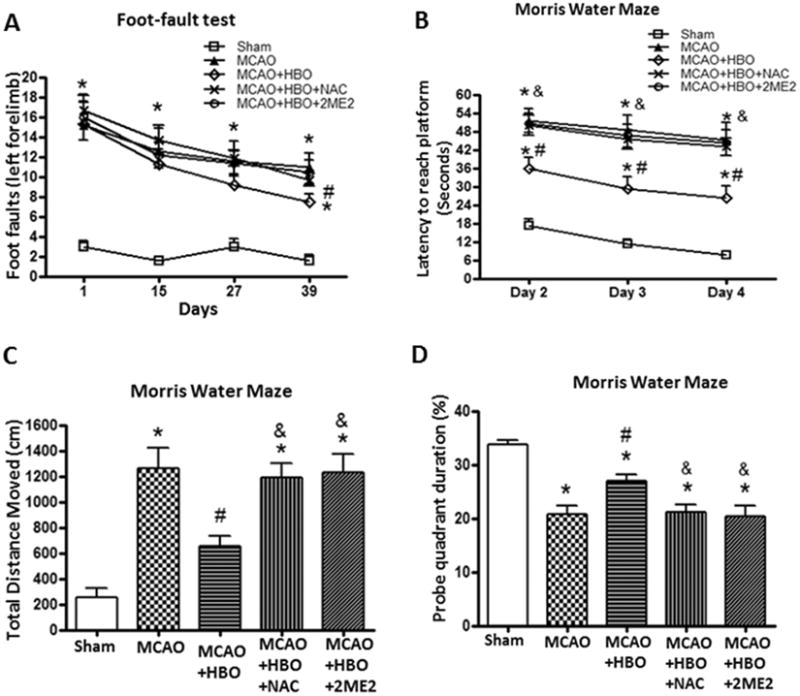
Delayed HBO improved neurological functions depend on ROS/HIF-1α.
Statistical analysis of foot-fault test (A) and Morris Water Maze (B, C, D). Delayed and multiple HBO significantly improved the performance of foot-fault test and increased the spatial memory and learning abilities at 6 weeks after MCAO. The improvement of neurological functions was reversed by ROS scavenger NAC and HIF-1α inhibitor 2ME2. Sham, n=5; MCAO, n=5; MCAO+HBO, n=7; MCAO+HBO+NAC, n=7; MCAO+HBO+2ME2, n=6. *p<0.05 vs. Sham; #p<0.05 vs. MCAO; & p<0.05 vs MCAO+HBO.
Delayed HBO Promoted Neurogenesis and Synaptogenesis depends on ROS/HIF-1α after MCAO in Long-term
In the HBO treatment group, numerous DCX (a marker of neuronal precursor cells) positive cells in the ipsilateral SGZ (Figure 3A) were observed at 6 weeks after MCAO, which showed the activation of endogenous neurogenesis. Some DCX positive cells showed the trend of their migration to the infarct region (Figure 3A). Delayed HBO promoted neurogenesis and synaptogenesis after MCAO in long-term (Figure 3B, 3C, 3D). Delayed HBO increased numbers of newborn neuronal precursor cells in both SGZ and SVZ compared to the MCAO group (Figure 3B, 3C). There are more newborn neuronal precursor cells observed in SVZ than in SGZ. In the cortex, there were few positive cells of double staining of BrdU and NeuN, and low expression of synapsin-1 (a specific marker of synapses) in the peri-infarct region after MCAO (Figure 3C). Delayed HBO increased the newborn neurons and promoted the expression of Synapsin-1 compared with the MCAO group (Figure 3B, 3D). The promotion of neurogenesis and synaptogenesis by HBO was removed by ROS scavenger NAC and HIF-1α inhibitor 2ME2.
Figure 3.
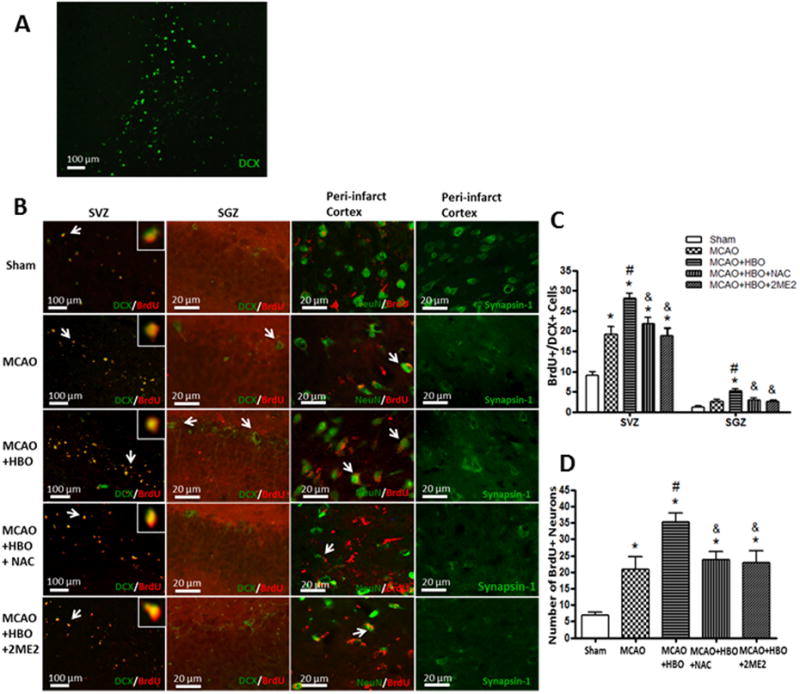
Delayed HBO increased neurogenesis and synaptogenesis through ROS/HIF-1α.
Representative immunofluorescence staining of newborn neuronal precursor cells, newborn neurons and synapses at 6 weeks after MCAO. (A) immunofluorescence staining of DCX in ipsilateral SGZ after HBO treatment in MCAO rats; (B) Double immunofluorescence staining of BrdU and DCX, BrdU and NeuN, and immunofluorescence staining of synapsin-1; (C) and (D)Statistic analysis of BrdU+/DCX+ cells in SVZ and SGZ, BrdU+/NeuN+ cells in peri-infarct cortex. Delayed and multiple HBO promoted neurogenesis and synaptogenesis after MCAO, and the promotion was removed by NAC and 2ME2. Sham, n=5; MCAO, n=5; MCAO+HBO, n=7; MCAO+HBO+NAC, n=7; MCAO+HBO+2ME2, n=6. *p<0.05 vs. Sham; #p<0.05 vs. MCAO; & p<0.05 vs MCAO+HBO.
Delayed HBO Activated β-catenin Pathway by Up-regulating ROS and HIF-1α
After the consecutive 7-days HBO treatments, the levels of ROS and HIF-1α significantly increased when compared to MCAO group (Figure 4A and 4B); ROS scavenger NAC effectively removed the ROS produced by HBO and inhibited the up-regulation of HIF-1α(Figure 4A and 4B). Delayed HBO increased the accumulation of β-catenin and enhanced the expression of its associated co-regulators TCF-1 and LEF-1 after consecutive 7-days HBO exposure in MCAO rats (Figure 5). Administration of NAC and 2ME2 before each HBO remarkably abolished the up-regulation of β-catenin, LEF-1 and TCF-1 (Figure 5B, 5C and 5D).
Figure 4.
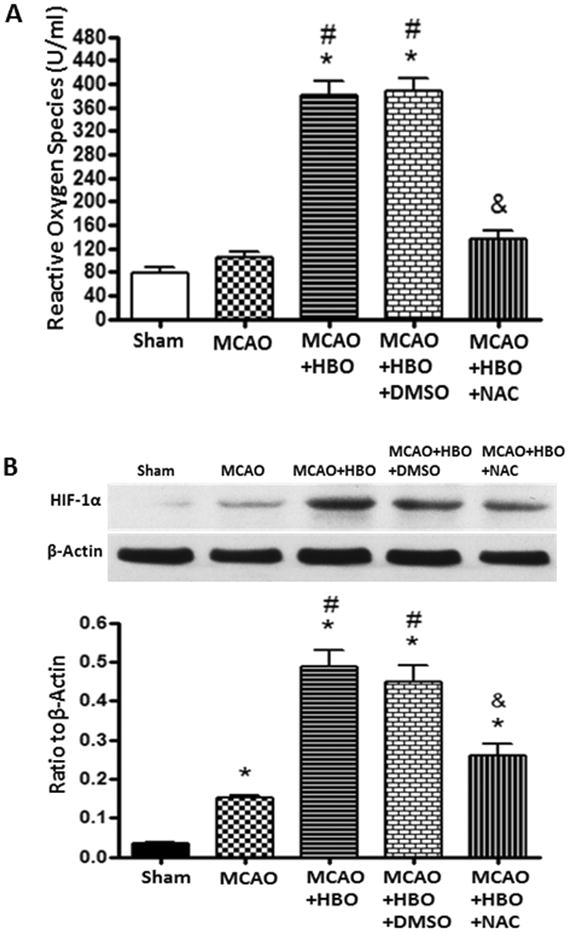
Delayed HBO increased the levels of ROS and HIF-1α 6 hours after the first 7days treatment.
Statistic analysis of ROS levels (A) and expression of HIF-1α (B) after consecutive 7-days HBO treatments. In the chronic phase of MCAO, no increase of ROS and a slight but significant increase of HIF-1α expression were observed. Consecutive 7-days HBO treatments increased the levels of ROS and enhanced the expression of HIF-1α. The effect of HBO was abolished by NAC. N=6 for each group. *p<0.05 vs. Sham; #p<0.05 vs. MCAO; &p<0.05 vs. MCAO+HBO.
Figure 5.
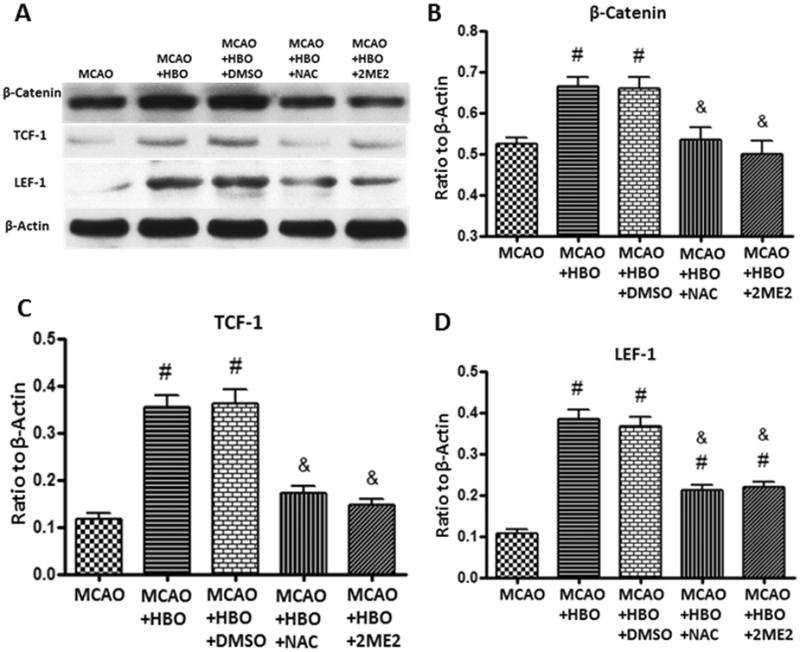
HBO enhanced β-catenin pathway 24 hours after the first 7days treatment.
Representative western blots (A) and quantitative analysis of β-catenin (B), TCF-1 (C) and LEF-1 (D) in ischemic hemisphere after consecutive 7-days HBO treatments. Delayed and multiple HBO increased the expression of β-catenin, TCF-1 and LEF-1 and these effects were abolished by ROS and HIF-1α inhibitors NAC and 2ME2. N=6 for each group. #p<0.05 vs. MCAO; &p<0.05 vs. MCAO+HBO.
Delayed HBO Enhanced Neurogenesis through ROS/ HIF-1α/ β-catenin Signaling Pathway
To test a role of HIF-1α/β-catenin signaling pathway in cell differentiation after MCAO and HBO, we detected the expression of neurogenin-1 (a gene implicated in neuronal differentiation and one of the downstream transcription factors of β-catenin), DCX and synapsin-1 by western blot after the consecutive 7-days treatments. Delayed HBO significantly increased the expression of neurogenin-1, DCX and synapsin-1 (Figure 6). Administration of NAC, PKF and 2ME2 reversed the effects of HBO (Figure 6).
Figure 6.
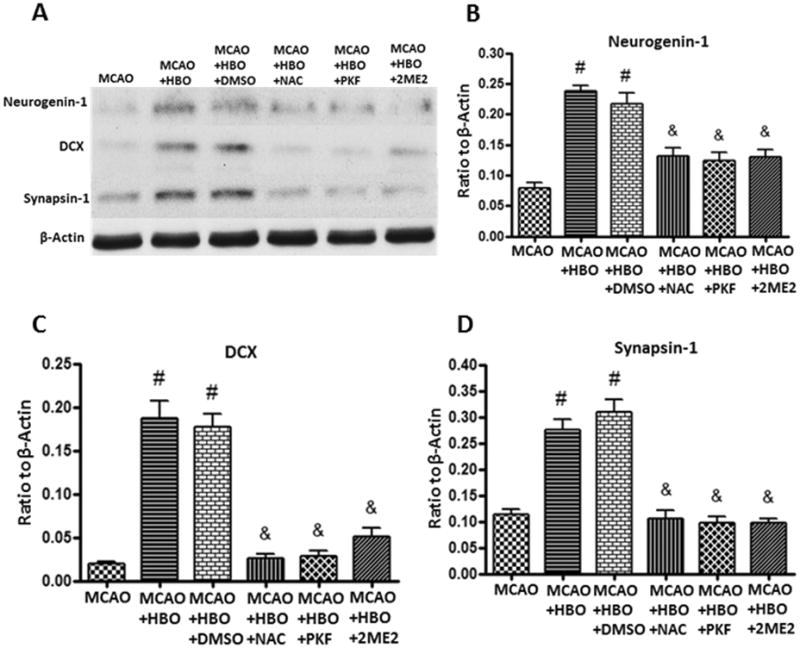
HBO promotes neurogenesis and synapgenesis by increase the expression of neurogenin-1 through ROS / HIF-1α/ β-catenin.
Representative western blots (A) and quantitative analysis of Neurogenin-1 (B), DCX (C) and Synapsin-1 (D) after consecutive 7-days HBO treatments. HBO up-regulated the expression of Neurogenin-1, DCX and Synapsin-1 and these effects were abolished by ROS/HIF-1α/β-catenin inhibitors NAC, 2ME2, and PKF. N=6 for each group. #p<0.05 vs. MCAO; &p<0.05 vs. MCAO+HBO.
Discussion
HBO has been tested in animal models of stroke and neuroprotective effects have been observed7-9. Most of these studies used one application of HBO and HBO was applied within a few hours after acute ischemic stroke. These experimental studies provided important information but were mismatched with clinical HBO modalities that HBO is applied in the delayed phase of stroke, most times days or weeks after the initial stroke, and multiple applications are used for treatment. Therefore, we adapted clinical modality of HBO and started HBO treatment at 7 days after stroke, when acute infarction was stabilized, and multiple HBO treatments were used with several intervals between each group of treatments to prevent oxygen toxicity. Our goals were to test firstly if delayed and multiple HBO improved functional recovery without affecting the initial infarction, and secondly potential mechanisms of the delayed HBO on neurogenesis. We observed that delayed HBO amplified significantly neurogenesis, represented by the proliferative responses of neural stem cells in the SVZ and SGZ, and improved the neurological deficits. It seemed that delayed HBO enhanced the endogenous neurogenesis through ROS/HIF-1α/β-catenin signaling pathway and inhibition of ROS/HIF-1α/β-catenin abolished the effect of HBO. This observation indicated that delayed HBO might serve as an alternative therapeutic strategy to improve the quality of life in post-stroke patients by promoting neurogenesis. The observations in the present study are consistent with one of our previous observation in a permanent MCAO rat model that delayed HBO therapy improved functional recovery18. In this previous study, HBO was applied either at 3 hrs or 48 hrs after occlusion of MCA and the effect of HBO were mediated by cAMP responsive element binding protein. The present study is a step further that HBO was tested at 7 days after the initial stroke which is a clinical relevant application.
When adult rodents are subjected to MCAO, the resulting infarction injury stimulates a low level of endogenous neurogenesis in the SVZ and SGZ of the affected side23-25. The newly generated cells then migrate towards the ischemic boundary and differentiate into neurons, which may subsequently improve neurological outcomes. In this study, we observed that delayed and multiple HBO treatments enhanced the differentiation of NSPCs to neuronal precursor cells in SVZ and SGZ, which may develop to mature neurons in the ischemic cortex and promote synaptogenesis. We also observed that delayed and multiple HBO improved the sensory-motor deficits, spatial memory and learning abilities at 6 weeks after the initial ischemic stroke. It may be the increased neurons and synapses, which underlie changes in N-methyl-D-aspartate (NMDA) receptor level and affect long-term potentiation (LTP) that we did not detect, play an important role in neurobehavioral recovery after multiple HBO treatment in MCAO rats. Since HBO was administered at 7 days after the acute stroke, and HBO did not reduce infarction and nor it improved the brain morphology, our results suggest that delayed HBO may improve neurological outcomes by promoting neurogenesis. This observation is consistent with a recent study that the human brain undergoes post-ischemic neurogenesis26.
The potential effect of HBO on neurogenesis was investigated by using inhibitors for ROS/HIF-1α/β-catenin pathways. HBO has been reported to enhance the production of ROS through mitochondrial respiration27, which is consistent with this study that a significantly enhanced production of ROS were observed after consecutive 7-days HBO treatments in MCAO rats. It was also noticed that sub-lethal elevation of ROS influenced multiple aspects of neural differentiation and function, and ROS has been shown to be essential for the nerve growth factor-induced differentiation of PC12 cells28. In hippocampal neurons, high levels of superoxide modulated neuronal plasticity29. ROS has also been shown to modulate differentiation of mesencephalic precursors30 and neural crest stem cells31. The mechanisms of ROS affecting differentiation of mammalian NSPCs are not well understood. There are evidence that elevated ROS production promoted the inactivation of prolyl-hydroxylase domain (PHD)-containing enzyme and stabilized HIF-1α32, 33, which plays a central role in regulating the balance between self-renewal and differentiation of NSPCs under non-hypoxic conditions and stroke16, 34. There is a report that suggested HIF-1a activated Wnt/β-catenin signaling in embryonic and neural stem cells, and its deletion reduced proliferation and neurogenesis in the dentate gyrus of the hippocampus12. The results indicated that HIF-1α modulated Wnt/β-catenin signaling by enhancing β-catenin activation and directly increasing the transcription of LEF-1/TCF-1genes. In another study, activation of Wnt/β-catenin signaling increased the expression of neurogenin-1, a gene implicated in neuronal differentiation, and enhanced neurogenesis35. In the present study, we observed that HBO stabilized HIF-1α and increased the level of β-catenin, LEF-1 and TCF-1, which up-regulated the expression of neurogenin-1, DCX and Synapsin-1. Scavenging ROS and inhibition of HIF-1α or β-catenin prevented the neurogenesis effects of HBO.
In conclusion, delayed and multiple HBO treatments starting at 7 days after MCAO, improved motor function, spatial learning and memory, evaluated by foot-fault test and Morris water maze. This novel observation suggests that delayed and multiple HBO in a clinical relevant modality may have potentials to improve long term outcome and this action seems related to neurogenesis mediated by ROS/HIF-1α/β-catenin signaling pathway.
Acknowledgments
Sources of Funding: This study was supported partially by a grant from NIH NS43338 to JHZ.
Footnotes
Disclosures: None.
References
- 1.Brainin M, Teuschl Y, Kalra L. Acute treatment and long-term management of stroke in developing countries. Lancet Neurol. 2007;6:553–561. doi: 10.1016/S1474-4422(07)70005-4. [DOI] [PubMed] [Google Scholar]
- 2.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang YJ, Wang XL, Yu XH, Wang X, Xie M, Liu CT. Hyperbaric oxygen induces endogenous neural stem cells to proliferate and differentiate in hypoxic-ischemic brain damage in neonatal rats. Undersea Hyperb Med. 2008;35:113–129. [PubMed] [Google Scholar]
- 4.Lee YS, Chio CC, Chang CP, Wang LC, Chiang PM, Niu KC, et al. Long course hyperbaric oxygen stimulates neurogenesis and attenuates inflammation after ischemic stroke. Mediat Inflamm. 2013:512978. doi: 10.1155/2013/512978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efrati S, Fishlev G, Bechor Y, Volkov O, Bergan J, Kliakhandler K, et al. Hyperbaric oxygen induces late neuroplasticity in post stroke patients--randomized, prospective trial. PLos One. 2013;8:e53716. doi: 10.1371/journal.pone.0053716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harch PG, Andrews SR, Fogarty EF, Amen D, Pezzullo JC, Lucarini J, et al. A phase i study of low-pressure hyperbaric oxygen therapy for blast-induced post-concussion syndrome and post-traumatic stress disorder. J Neurotraum. 2012;29:168–185. doi: 10.1089/neu.2011.1895. [DOI] [PubMed] [Google Scholar]
- 7.Huang ZX, Kang ZM, Gu GJ, Peng GN, Yun L, Tao HY, et al. Therapeutic effects of hyperbaric oxygen in a rat model of endothelin-1-induced focal cerebral ischemia. Brain Res. 2007;1153:204–213. doi: 10.1016/j.brainres.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 8.Yin D, Zhou C, Kusaka I, Calvert JW, Parent AD, Nanda A, et al. Inhibition of apoptosis by hyperbaric oxygen in a rat focal cerebral ischemic model. J Cerebr Blood Flow Metabol. 2003;23:855–864. doi: 10.1097/01.WCB.0000073946.29308.55. [DOI] [PubMed] [Google Scholar]
- 9.Mink RB, Dutka AJ. Hyperbaric oxygen after global cerebral ischemia in rabbits reduces brain vascular permeability and blood flow. Stroke. 1995;26:2307–2312. doi: 10.1161/01.str.26.12.2307. [DOI] [PubMed] [Google Scholar]
- 10.Lei ZN, Zhang LM, Sun FY. Beta-catenin sirna inhibits ischemia-induced striatal neurogenesis in adult rat brain following a transient middle cerebral artery occlusion. Neurosci Lett. 2008;435:108–112. doi: 10.1016/j.neulet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 11.Morris DC, Zhang ZG, Wang Y, Zhang RL, Gregg S, Liu XS, et al. Wnt expression in the adult rat subventricular zone after stroke. Neurosci Lett. 2007;418:170–174. doi: 10.1016/j.neulet.2007.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazumdar J, O'Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, et al. O2 regulates stem cells through wnt/beta-catenin signalling. Nat Cell Biol. 2010;12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui XP, Xing Y, Chen JM, Dong SW, Ying DJ, Yew DT. Wnt/beta-catenin is involved in the proliferation of hippocampal neural stem cells induced by hypoxia. Ir J Med Sci. 2011;180:387–393. doi: 10.1007/s11845-010-0566-3. [DOI] [PubMed] [Google Scholar]
- 14.Peng Z, Ren P, Kang Z, Du J, Lian Q, Liu Y, et al. Up-regulated hif-1alpha is involved in the hypoxic tolerance induced by hyperbaric oxygen preconditioning. Brain Res. 2008;1212:71–78. doi: 10.1016/j.brainres.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Salhanick SD, Belikoff B, Orlow D, Holt D, Reenstra W, Buras JA. Hyperbaric oxygen reduces acetaminophen toxicity and increases hif-1alpha expression. Acad Emerg Med. 2006;13:707–714. doi: 10.1197/j.aem.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Milosevic J, Adler I, Manaenko A, Schwarz SC, Walkinshaw G, Arend M, et al. Non-hypoxic stabilization of hypoxia-inducible factor alpha (hif-alpha): Relevance in neural progenitor/stem cells. Neurotox Res. 2009;15:367–380. doi: 10.1007/s12640-009-9043-z. [DOI] [PubMed] [Google Scholar]
- 17.Hu Q, Ma Q, Zhan Y, He Z, Tang J, Zhou C, et al. Isoflurane enhanced hemorrhagic transformation by impairing antioxidant enzymes in hyperglycemic rats with middle cerebral artery occlusion. Stroke. 2011;42:1750–1756. doi: 10.1161/STROKEAHA.110.603142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mu J, Ostrowski RP, Soejima Y, Rolland WB, Krafft PR, Tang J, et al. Delayed hyperbaric oxygen therapy induces cell proliferation through stabilization of camp responsive element binding protein in the rat model of mcao-induced ischemic brain injury. Neurobiol Dis. 2013;51:133–143. doi: 10.1016/j.nbd.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, et al. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/akt pathway. Stroke. 2010;41:1521–1527. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lekic T, Hartman R, Rojas H, Manaenko A, Chen W, Ayer R, et al. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J Neurotraum. 2010;27:627–637. doi: 10.1089/neu.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Q, Chen C, Yan J, Yang X, Shi X, Zhao J, et al. Therapeutic application of gene silencing mmp-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp Neurol. 2009;216:35–46. doi: 10.1016/j.expneurol.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Sugimoto K, Fujisawa T, Shindo N, Minato S, Kamada Y, et al. Novel effect of ezetimibe to inhibit the development of non-alcoholic fatty liver disease in Fatty Liver Shionogi. Hepatol Res. 2014;44:102–113. doi: 10.1111/hepr.12092. [DOI] [PubMed] [Google Scholar]
- 23.Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 25.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 26.Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conconi MT, Baiguera S, Guidolin D, Furlan C, Menti AM, Vigolo S, et al. Effects of hyperbaric oxygen on proliferative and apoptotic activities and reactive oxygen species generation in mouse fibroblast 3t3/j2 cell line. J Investig Med. 2003;51:227–232. doi: 10.1136/jim-51-04-24. [DOI] [PubMed] [Google Scholar]
- 28.Katoh S, Mitsui Y, Kitani K, Suzuki T. Hyperoxia induces the differentiated neuronal phenotype of pc12 cells by producing reactive oxygen species. Biochem Biophys Res Comm. 1997;241:347–351. doi: 10.1006/bbrc.1997.7514. [DOI] [PubMed] [Google Scholar]
- 29.Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: Selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JY, Chang MY, Park CH, Kim HY, Kim JH, Son H, et al. Ascorbate-induced differentiation of embryonic cortical precursors into neurons and astrocytes. J Neurosci Res. 2003;73:156–165. doi: 10.1002/jnr.10647. [DOI] [PubMed] [Google Scholar]
- 31.Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon MC. Mitochondrial reactive oxygen species are required for hypoxic hif alpha stabilization. Adv Exp Med Biol. 2006;588:165–170. doi: 10.1007/978-0-387-34817-9_15. [DOI] [PubMed] [Google Scholar]
- 33.Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, et al. The qo site of the mitochondrial complex iii is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang B, Qu Y, Wang D, Mu D. Targeting hypoxia inducible factor-1alpha: A novel mechanism of ginsenoside rg1 for brain repair after hypoxia/ischemia brain damage. CNS Neurol Disord Drug Targets. 2011;10:235–238. doi: 10.2174/187152711794480456. [DOI] [PubMed] [Google Scholar]
- 35.Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, et al. The wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]


