Abstract
Rationale
Chronic food restriction (FR) increases behavioral responsiveness to drugs of abuse and associated environments. Pre- and postsynaptic neuroadaptations have been identified in the mesoaccumbens dopamine pathway of FR subjects but the mechanistic basis of increased drug reward magnitude remains unclear.
Objectives
Effects of FR on basal and D-amphetamine-induced trafficking of AMPA receptor subunits to the nucleus accumbens (NAc) postsynaptic density (PSD) were examined, and AMPA receptor involvement in augmentation of D-amphetamine reward was tested.
Materials and methods
FR and ad libitum fed (AL) rats were injected with D-amphetamine (2.5 mg/kg, i.p.) or vehicle. Brains were harvested and subcellular fractionation and Western analyses were used to assess AMPA receptor abundance in NAc homogenate and PSD fractions. A follow-up experiment used a curve-shift protocol of intracranial self-stimulation to assess the effect of 1-naphthylacetyl spermine (1-NASPM), a blocker of Ca2+-permeable AMPA receptors, on rewarding effects of D-amphetamine microinjected in NAc shell.
Results
FR increased GluA1 in the PSD, and D-amphetamine increased p-Ser845-GluA1, GluA1, GluA2, but not GluA3, with a greater effect in FR than AL rats. D-amphetamine lowered reward thresholds, with greater effects in FR than AL rats, and 1-NASPM selectively reversed the enhancing effect of FR.
Conclusions
Results suggest that FR leads to increased synaptic incorporation of GluA1 homomers to potentiate rewarding effects of appetitive stimuli and, as a maladaptive byproduct, D-amphetamine. The D-amphetamine-induced increase in synaptic p-Ser845-GluA1, GluA1, and GluA2 may contribute to the rewarding effect of D-amphetamine, but may also be a mechanism of synaptic strengthening and behavior modification.
Keywords: Food restriction, Nucleus accumbens, Reward, Self-stimulation, D-amphetamine, AMPA receptors, GluA1, Postsynaptic density, 1-NASPM
Animal models and human neuroimaging increasingly support a scheme in which drugs of abuse target and subvert the neurocircuitry that mediates appetitive motivation and reward (Kelley and Berridge 2002; Cardinal and Everitt 2004; DiChiara 2005; Volkow et al. 2008). This relationship is evident in the high comorbidity of disordered eating and drug abuse (Krahn et al. 1992; Wiederman and Prior 1996; Pisetsky et al. 2008; Root et al. 2010), the low prevalence of substance use among obese individuals (Warren et al. 2005; Simon et al. 2006), and the increased vulnerability to use, relapse, and drug-induced psychopathology among those who are dieting or have low body mass index (French et al. 1994; Austin and Gortmaker 2001; Cheskin et al. 2005; Rosse et al. 2005). A related and emerging public health concern is the possible switch to alcohol and drug abuse in gastric bypass patients (Ivezaj et al. 2012; Conason et al. 2013).
Animal studies indicate that food restriction (FR) increases sensitivity to drugs of abuse in self-administration (Carroll and Meisch 1984), conditioned place preference (Bell et al. 1997; Stuber et al. 2002; Liu et al. 2011; Zheng et al. 2012), motor activity (Deroche et al. 1995; Carr et al. 2003), and electrical brain stimulation reward paradigms (Cabeza de Vaca and Carr 1998; Cabeza de Vaca et al. 2004). In addition, FR interacts with episodic access to palatable food to generate enduring binge-like patterns of intake (Hagan and Moss 1997; Avena et al. 2008; Consoli et al. 2009). Most recently, it was observed that FR increases the incentive effects of an environment paired with cocaine during a prior ad libitum fed state (Zheng et al. 2012) and enhances cue-induced reinstatement of heroin seeking (D’Cunha et al. 2013). Consequently, food restriction may increase vulnerability to both initial use and relapse, and may do so by harnessing neuroadaptations that evolved to confer a selective advantage in an ecology of food scarcity (Carr 2011).
A link between drug use and ingestive behavior is the common involvement of the mesoaccumbens dopamine (DA) pathway (Wise and Bozarth 1985; Pontieri et al. 1995; Hajnal et al. 2004; Palmiter 2007; Kenny 2011). A variety of neuroadaptations in the mesoaccumbens pathway are associated with the augmentation of drug reward in FR rats. Most of the evidence indicates that basal and evoked DA release are diminished, but postsynaptic intracellular signaling, gene expression and behavioral responses downstream of D1 DA receptor stimulation are upregulated (Pothos et al. 1995; Carr et al. 2003; Haberny et al. 2004; Haberny and Carr 2005a, b; Pan et al. 2006; Stamp et al. 2008; Carr et al. 2010; Stouffer et al. 2012).
The DA innervation of nucleus accumbens (NAc) is convergent with several limbic forebrain glutamate inputs (Groenewegen et al. 1999), and the integration of DA- and glutamate-coded signals regulates medium spiny neuron (MSN) activity (Moyer et al. 2007; Surmeier et al. 2007), goal-directed behavior, reward-related learning, and addiction (Kelley 2004; Dalley et al. 2005; Hyman et al. 2006). Changes in glutamatergic AMPA receptor abundance in the synaptic membrane mediate dynamic tuning of synaptic transmission as well as enduring forms of synaptic plasticity (Choquet 2010; Kessels and Malinow 2009). AMPARs are co-expressed with DA receptors in NAc neurons (Bernard et al. 1997; Glass et al. 2008), and most are either GluA1/GluA2 or GluA2/GluA3 heteromers (Reimers et al. 2011). In cultured MSNs, D1 DA receptor agonist rapidly increases GluA1 surface expression in a PKA-dependent manner (Chao et al. 2002; Mangiavacchi and Wolf 2004). It is therefore of interest that FR was recently shown to increase phosphorylation of GluA1 on Ser845, the PKA site, in response to a D1 DA receptor agonist, sucrose consumption, and exposure to a cocaine-paired environment (Carr et al. 2010; Liu et al. 2011; Zheng et al. 2013). Phosphorylation on Ser845 increases GluA1 peak currents and channel open probability, stabilizes the receptor in the membrane, and facilitates synaptic insertion (Roche et al. 1996; Shi et al. 2001; Esteban et al. 2003; Oh et al. 2006; Ehlers et al. 2007; Man et al. 2007; He et al. 2009; He et al. 2011). Moreover, there is evidence that phosphorylation regulates behavioral responsiveness to D-amphetamine; hyperlocomotion was blocked by NAc overexpression of an alanine mutant GluA1 that prevents Ser845 phosphorylation (Li et al. 2011).
In addition to potentiating stimulus-induced Ser845 phosphorylation, FR may increase basal GluA1 abundance in the NAc postsynaptic density (PSD); in a study of sucrose consumption, the FR control group with access to tap water displayed higher levels of GluA1 than the AL control group (Peng et al. 2011). Moreover, involvement of this AMPAR subunit in reward modulation was supported by the finding that, within NAc shell, 1-NASPM, an antagonist of Ca2+-permeable AMPARs, decreased the rewarding effect of a D1 DA receptor agonist in FR rats (Carr et al. 2010). The purpose of the present study was therefore to determine whether FR increases D-amphetamine-induced Ser845 phosphorylation, trafficking of GluA1-containing AMPARs to the NAc post-synaptic density, and 1-NASPM-reversible augmentation of D-amphetamine reward.
Materials and Methods
Subjects
Adult male Sprague–Dawley rats (Taconic Farms, Germantown, NY) received 40–50 % of the ad libitum intake of LabDiet 5001 (10–12 g) provided as a single meal daily, until body weight declined by 20 % (∼2 weeks). Experiments were initiated after FR rats had been stabilized for one week at their target body weight (i.e., ∼3 weeks after implementation of FR). Rats with ad libitum (AL) access to chow for the same period as paired FR rats served as controls. All rats had ad libitum access to water. In the biochemical experiment, 48 subjects were assigned to each diet condition. Twenty-four rats in each condition were injected with D-amphetamine (2.5 mg/kg, i.p.) and 24 with saline vehicle 30-min prior to sacrifice by brief exposure to CO2 and decapitation by guillotine. In the behavioral experiment, 24 AL and FR subjects were tested. Experiments were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory animals and were approved by the New York University School of Medicine Institutional Animal Care and Use Committee.
Subcellular fractionation
NAc was dissected from fresh brain on ice and three per treatment condition were pooled for fractionation. The whole cell homogenate (WC) and PSD fraction were obtained as reported previously (Peng et al. 2011). Briefly, protease inhibitor cocktail and PMSF were added to 0.32 M sucrose containing 1 mM NaHCO3, 1 mM MgCl2, and 0.5 mM CaCl2 (Solution A). Brain tissue was rinsed, homogenized and diluted to 10 % weight/volume in Solution A. After being well mixed, 50 μl of the WC were stored at −80 °C until use. Following preparation of a synaptosomal fraction, the PSD fraction was obtained using an equal volume solution containing 1 % Triton X-100, 0.32 M sucrose, and 12 mM Tris, pH 8.1, which was added to the resuspended sample. The mixture was rocked at 4 °C for 15 min, followed by centrifugation at 13,800g for 30 min. After centrifugation, the upper liquid was discarded and the pellet resuspended in a solution of 25 mM Tris, pH 7.4 with 2 % SDS and stored at −80 °C until use. This method of PSD purification yields a fraction enriched in proteins that are preferentially localized or novel to the PSD (Jordan et al. 2004). In the present study, PSD fractions were confirmed to contain abundant PSD95 relative to the WC.
Western blotting
Gel electrophoresis and Western blotting were as described (e.g., Peng et al. 2011) using rabbit polyclonal anti-phospho-Ser845-GluA1 (1:1,500; AB5849, Millipore, Temecula, CA), mouse monoclonal anti-GluA1 (1:1,500; MAB2263, Millipore), rabbit polyclonal anti-GluA2 (1:1,000; PA1-4659, Thermo Scientific, Rockford, IL), rabbit polyclonal anti-PSD95 (1:1,000; AB9708, Millipore), mouse monoclonal anti-GluA3 (1:500; MAB5416, Millipore), and mouse monoclonal anti-α-tubulin (1:5,000; T6199, Sigma-Aldrich, St. Louis, MO). Immunoblots were analyzed using NIH Image J software. Following densitometry, intensities of bands corresponding to GluA1, GluA2, GluA3, and p-Ser845-GluA1 for each sample were divided by intensities of the corresponding α-tubulin bands. Results were expressed in comparison to the normalized control, defined as the AL group that received the control treatment. Results were analyzed by two-way ANOVA, with significant interaction effects followed by comparison of cell means of interest using the error term from the ANOVA in the denominator of a t-statistic.
Surgical procedures
Several days after arrival in the central animal facility, rats were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and were stereotaxically implanted with a 0.25-mm-diameter monopolar stimulating electrode (Plastics One, Roanoke, VA) in the lateral hypothalamic medial forebrain bundle (skull flat coordinates: 3.0 mm posterior to bregma, 1.6 mm lateral to the sagittal suture, and 8.5 mm ventral to skull surface). An anterior ipsilateral stainless steel skull screw served as ground. Rats were also implanted with two chronically indwelling guide cannulae (26 gauge) which were placed bilaterally 2.0 mm dorsal to injection sites in the NAc medial shell (1.6 mm anterior to bregma; 2.1 mm lateral to the sagittal suture, tips angled 8° toward the midline, 5.8 mm ventral to skull surface). Cannula patency was maintained with occlusion stylets. The electrode, ground, cannulae, and three additional mounting screws were then permanently secured to the skull by flowing dental acrylic around them. Postsurgical analgesia was achieved by administration of banamine (2.0 mg/kg, s.c.) following recovery from anesthesia and the morning after.
Lateral hypothalamic self-stimulation
Lateral hypothalamic self-stimulation (LHSS) was used to obtain a learning-free measure of drug reward magnitude. Subjects lever pressed to obtain 1-s trains of 0.1 ms duration cathodal pulses via the indwelling electrode. Brain stimulation frequency was varied systematically over 1-min trials, generating a rate-frequency curve analogous to the pharmacological dose-response curve. Two threshold parameters were determined: M-50, the pulse frequency that elicits half the maximum reinforcement rate, and x-axis intercept, the lowest frequency at which stimulation is rewarding. Drugs of abuse produce a decrease in reward threshold, expressed as a parallel left shift of the rate-frequency curve. Training and stabilization of baseline performance began ∼1 week after surgery and FR was initiated ∼1 week into training. Testing began once the diet had been in place for a minimum of 3 weeks. Test sessions included a pre-injection LHSS rate-frequency test of 28 min duration, followed by bilateral microinjection of D-amphetamine (5.0 μg, bilaterally in 0.5 μl) in the presence or absence of 1-NASPM (25.0 μg). Microinjection was followed, 5 min later, by a post-injection LHSS rate-frequency test. Tests were conducted a minimum of 72 h apart. Half of subjects in each diet group were first tested with co-microinjection of 1-NASPM and half without. The % changes in post-injection relative to pre-injection tests for each parameter were calculated as before (e.g., Cabeza de Vaca and Carr 1998). A second pair of test sessions followed to confirm the prior finding that neither saline nor 1-NASPM microinjected in the absence of D-amphetamine-altered LHSS thresholds in AL or FR rats (Carr et al. 2010). Results were analyzed by mixed two-way ANOVA (diet×drug treatment). Based on prior results obtained with intra-NAc D-amphetamine (Carr et al. 2009) and SKF-82958 in the presence and absence of 1-NASPM (Carr et al. 2010), significant interaction effects were followed by planned comparison of cell means using the error term from the ANOVA in the denominator of a one-tailed t-statistic.
Intracerebral microinjection procedures
Solutions were loaded into two 30-cm lengths of PE-50 tubing attached at one end to 5-μl Hamilton syringes filled with distilled water and at the other end to 33-gauge injector cannulae, which extended 2.0 mm beyond the implanted guides. The 0.5 μl injection volumes were delivered manually over a period of 90 s at a rate of 0.05 μl/10 s. One minute following completion of the microinjection, injector needles were removed from guides, stylets were replaced, and animals were returned to test chambers for an additional 4 min prior to behavioral testing.
Drugs and dose selection
D-amphetamine (Sigma-Aldrich) was dissolved in sterile 0.9 % saline and injected intraperitoneally in the biochemical experiment, and microinjected into NAc medial shell in the behavioral experiment. 1-Naphthylacetyl spermine (1-NASPM; Sigma-Aldrich) was dissolved in sterile 0.9 % saline and microinjected into NAc medial shell alone or in combination with D-amphetamine in the behavioral experiment.
In the biochemical experiment, the D-amphetamine dose of 2.5 mg/kg, and the 30-min interval to brain harvesting were chosen based on two considerations. First, it had been reported that at 30-min, this dose of D-amphetamine produced a nearly significant increase in NAc surface expression of GluA1 in AL rats (Nelson et al. 2009); it was predicted that any D-amphetamine-induced AMPA receptor trafficking, measured here in the PSD, would be augmented and therefore significant in FR rats. Second, in order to associate AMPAR findings with behavioral effects, it was necessary to probe at a time that coincided with post-injection testing of D-amphetamine reward magnitude. In the current behavioral experiment, the 5.0 μg dose of D-amphetamine was administered based on a previous observation that, when microinjected into NAc shell, it lowered LHSS thresholds with a significantly greater effect in FR than AL rats (Carr et al. 2009). Finally, the 25.0 μg dose of 1-NASPM, used to challenge the effect of D-amphetamine, was chosen based on effectiveness of a somewhat higher dose in blocking cue-induced reinstatement of cocaine seeking in subjects with increased NAc surface expression of GluA1 (Conrad et al. 2008), and effectiveness of this specific dose in reversing the enhancing effect of FR on the reward magnitude of SKF-82958 (Carr et al. 2010).
Histology
Rats were briefly exposed to CO2 and decapitated by guillotine. Brains were removed and fixed in 10 % buffered formalin for 48 h. Frozen 40 μm coronal sections were cut on a Reichert-Jung 2800 cryostat, thaw-mounted on gelatin-coated slides and stained with cresyl violet. Microinjection sites were determined by visual inspection under an Olympus SZ40 microscope. Only subjects whose cannulae were accurately placed within the NAc medial shell, including the shell/core and shell/olfactory tubercle borders were included in data analysis.
Results
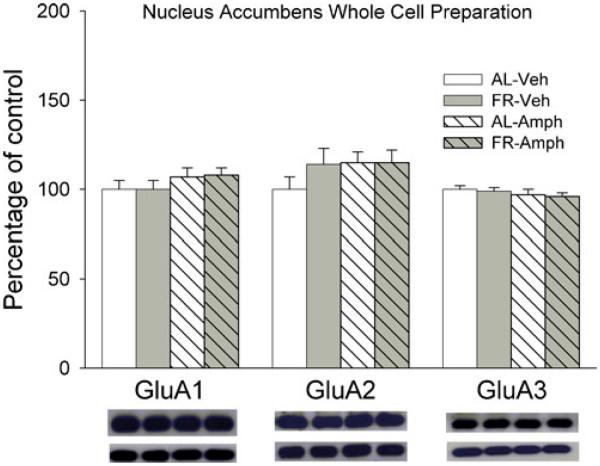
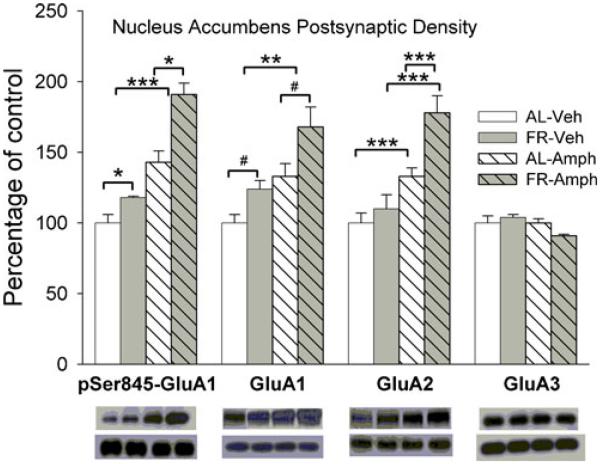
Neither FR, D-amphetamine nor their combination altered levels of GluA1, GluA2, or GluA3 in the NAc whole cell homogenate (Fig. 1), suggesting that treatments did not alter synthesis or degradation of AMPAR proteins. However, in the NAc postsynaptic density fraction (Fig. 2), FR increased levels of p-Ser845-GluA1 (Fdiet;1,28=6.13, p<.025) and GluA1 (Fdiet;1,28=5.2, p<.05). The planned comparison of GluA1 abundance in vehicle-treated groups confirmed greater abundance in FR relative to AL rats (t(14)=1.95, p<.05). D-amphetamine increased levels of p-Ser845-GluA1 (Fdrug;1,28=15.5, p<.001) and GluA1(Fdrug;1,28=8.78, p<.01) across diet conditions. A significant interaction between diet and drug treatment (Fdiet;1,28=6.84, p<.025; Fdrug;1,28=17.7, p<.001; Fdiet×drug;1,28=3.97, p=.05), followed by comparison of cell means, revealed an increased GluA2 abundance in D-amphetamine-treated groups relative to corresponding vehicle-treated groups (tAL(28)=3.8, p<.001; tFR(28)=5.2, p<.001) and a greater effect of D-amphetamine in FR relative to AL rats (tFR vs AL(28)=4.5, p<.001). There were no effects of FR or D-amphetamine on levels of GluA3 or PSD95.
Fig. 1.
Effects of food restriction and D-amphetamine (2.5 mg/kg, i.p.) on GluA1, GluA2, and GluA3 abundance in nucleus accumbens whole cell homogenate. Results (mean±s.e.m.) are expressed in comparison to the normalized control, defined as the ad libitum fed group injected with vehicle. Representative immunoblots for target proteins, with corresponding α-tubulin beneath, are included in the same sequence as the data bars immediately above. n=24 per group, with 3 NAc pooled per tube for fractionation
Fig. 2.
Effects of food restriction and D-amphetamine (2.5 mg/kg, i.p.) on p-Ser845-GluA1, GluA1, GluA2, and GluA3 abundance in nucleus accumbens postsynaptic density. Results (mean±s.e.m.) are expressed in comparison to the normalized control, defined as the ad libitum fed group injected with vehicle. Representative immunoblots for target proteins, with corresponding α-tubulin beneath, are included in the same sequence as the data bars immediately above. p-Ser845-GluA1, GluA1, GluA2, and GluA3 were identified as bands at ∼100, 110, 100, and 110 kDA, respectively. n=24 per group, with 3 NAc pooled per tube for fractionation. #p<.05, *p<.025, **p<.01, ***p<.001
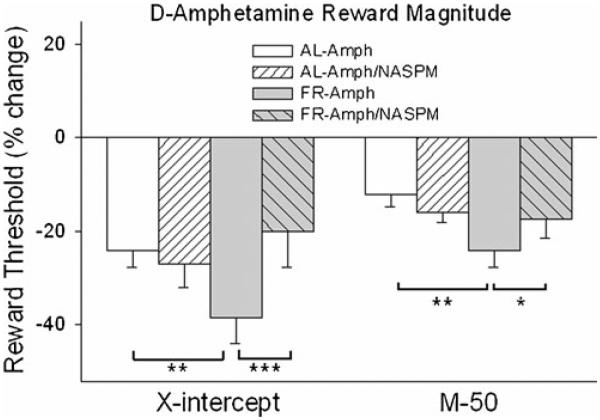
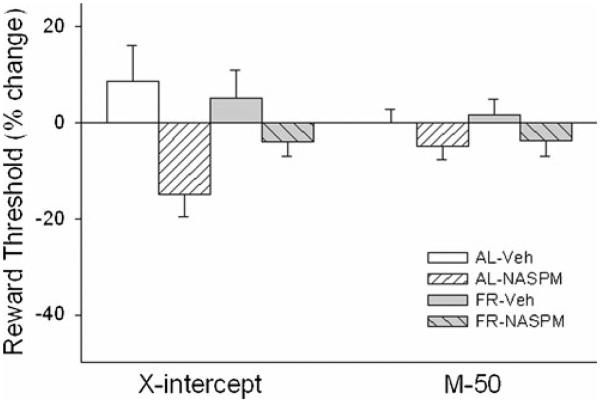
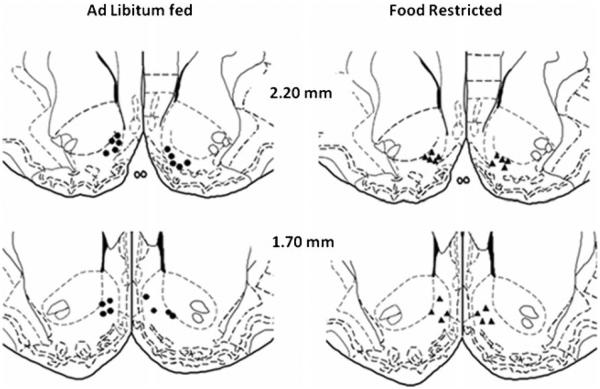
NAc medial shell microinjection of D-amphetamine produced a greater rewarding effect in FR than AL rats on both the M-50 (Fdiet×drug;1,16=4.26, p=.05; tFR vs AL (16)=3.1, p<.01) and x-axis intercept (Fdiet×drug;1,16=7.41, p<.025; tFR vs AL (16)=2.67, p<.01) measures of reward threshold (Fig. 3). Co-microinjection of 1-NASPM decreased these rewarding effects in FR but not AL rats (M-50, tAmph vs Amph/NASPM (16) = 1.89, p <.05; x-intercept, tAmph vs Amph/NASPM (16)=3.56, p<.005). Threshold measures were unaffected by saline vehicle or 1-NASPM microinjection in the absence of D-amphetamine (Fig. 4). Schematic diagrams indicating microinjection sites are provided in Fig. 5.
Fig. 3.
Effects of D-amphetamine (5.0 μg/ 0.5 μl), microinjected bilaterally in nucleus accumbens medial shell in the presence and absence of 1-NASPM (25.0 μg), on two measures of threshold (left x-axis intercept; right M-50) in the curve-shift protocol of LHSS. n=9 per group. *p<.05, **p<.01, ***p<.005
Fig. 4.
Effects of saline vehicle and 1-NASPM (25.0 μg) microinjected bilaterally in nucleus accumbens medial shell on two measures of threshold (left x-axis intercept; right M-50) in the curve-shift protocol of LHSS. n=9 per group
Fig. 5.
Schematic diagrams adapted from Paxinos and Watson (1998), indicating microinjection sites in NAc shell. Closed circles in the left panel and closed triangles in the right panel indicate sites in AL and FR rats, respectively
Discussion
Three main findings were obtained in this study. First, FR subjects receiving acute injection of saline vehicle displayed elevated levels of GluA1, but not GluA2 or GluA3, in the NAc PSD relative to AL subjects receiving the same treatment. This result is consistent with the previous finding that FR subjects with brief access to tap water, as a control for sucrose solution, displayed elevated levels of GluA1, but not GluA2, in the NAc PSD (Peng et al. 2011). Most NAc AMPARs are either GluA1/GluA2 or GluA2/GluA3 heteromers (Reimers et al. 2011). GluA2-lacking AMPARs, which are Ca2+-permeable, make up only ∼7 % of the total (Reimers et al. 2011). Yet, it appears that FR is associated with increased synaptic incorporation of homomeric GluA1. This effect is reminiscent of the synaptic incorporation of GluA1 in primary visual cortex following visual sensory deprivation (Goel et al. 2006), and the cross-modal compensatory delivery of GluA1 into barrel cortex synapses to sharpen the functional whisker-barrel map (Jitsuki et al. 2011). AMPARs are the main excitatory postsynaptic glutamate receptors, and their trafficking is an established mechanism for regulating neuronal excitability (Lee 2012) and synaptic homeostasis following sustained inactivity (Man 2011; Lee 2012; Shepherd 2012). Consequently, the mechanism underlying increased synaptic GluA1 in Nac of FR subjects may be tied, at least in part, to diminished DA transmission during FR, and the deprivation of input via D1 receptors which exist in a low affinity state and require high DA concentrations for activation. When MSNs receive strong glutamatergic input, D1 stimulation facilitates the transition from a hyperpolarized downstate to the upstate where membrane potential is near spike threshold (Surmeier et al. 2007). Decreased D1 signaling during FR may therefore decrease excitatory activity and contribute to a compensatory synaptic accumulation of GluA1.
The second finding of this study is that acute administration of D-amphetamine rapidly delivered AMPARs into the NAc PSD. The dose and interval to brain harvesting were based on the study of Nelson et al. (2009) who, using a protein cross-linking method, observed a ∼10 % increase in surface expression that approached statistical significance. A more robust increase was seen 2 h after D-amphetamine administration, but that latency to measurement would have fallen outside the time frame of behavioral testing in the present and previous comparisons of AL and FR subjects. In both diet groups, D-amphetamine increased levels of GluA1 and GluA2, but not GluA3, with an overall greater effect in FR than AL rats. In light of the high prevalence of GluA1/GluA2 heteromers in NAc, and their well demonstrated activity-dependent trafficking into synapses in hippocampal models (Barry and Ziff 2002), it is likely that D-amphetamine delivered GluA1/GluA2 heteromers into the PSD.
The third finding of this study was the selective decrease of D-amphetamine reward by 1-NASPM microinjection in the NAc medial shell of FR rats. D-amphetamine decreased the minimum frequency at which brain stimulation became rewarding (x-axis intercept) and the frequency supporting 50 % of the maximal reinforcement rate (M-50). Most importantly, both threshold-lowering effects were augmented by FR, and the augmenting effect was blocked by 1-NASPM, a synthetic analogue of Joro Spider toxin that selectively blocks Ca2+-permeable AMPARs (Tsubokawa et al. 1995; Koike et al. 1997). The biochemical results of this study, suggesting that this type of AMPAR may be driven into the PSD by FR rather than by D-amphetamine, suggests that a basal increase in GluA1, rather than enhanced D-amphetamine-induced trafficking of AMPARs to the PSD, is the mechanistic underpinning of increased drug reward magnitude in FR subjects. Several findings support this scheme. Unlike D-amphetamine, acute administration of cocaine and D1 DA receptor agonists do not increase surface expression of GluA1 in NAc of AL rats (albeit, FR rats were not tested; Ferrario et al. 2011). Yet, amphetamine, cocaine, D1 agonists, and cocaine-paired environmental stimuli are all subject to the enhancing effect of FR, and parsimony would point to a common underlying mechanism. In fact, the enhanced rewarding effect of the D1 agonist, SKF-82958, in FR rats was blocked by microinjection of 1-NASPM in NAc shell (Carr et al. 2010). Because the phenotype(s) of NAc MSNs in which FR increases PSD GluA1 abundance cannot be discerned here, the pharmacologic evidence implicating Ca2+-permeable AMPARs in the enhanced rewarding effect of a D1 agonist is important. Unlike the D2 receptor, D1 requires high extracellular DA concentrations for activation and, when activated, can facilitate MSN transition from the hyperpolarized downstate to near threshold for firing. Increased GluA1 abundance would be expected to increase responsiveness of NAc neurons to excitatory inputs (Conrad et al. 2008) that potentially remove extracellular Mg2+ blockade of NMDAR-associated channels (Ozawa et al. 1998), and facilitate transition to the upstate (Wolf et al. 2005). Consequently, the present findings point to mechanisms via which FR may promote the occurrence of upstates and enhance responsiveness to DA. Recent optogenetic stimulation studies in D1- and D2-Cre BAC transgenic mice indicate that excitation of D1-expressing MSNs facilitates, while excitation of D2-expressing MSNs inhibits, cocaine’s rewarding and stimulant effects (Lobo et al. 2010). Thus, FR-induced upregulation of D1 DA receptor function and increased synaptic abundance of GluA1 may combine to increase ventral striatal and behavioral responsiveness to appetitive reward stimuli and their pharmacological proxies.
FR and D-amphetamine both increased levels of p-Ser845-GluA1 in the NAc PSD. Phosphorylation of GluA1 on Ser845 is necessary for maintaining Ca2+-permeable AMPARs on the surface (He et al. 2009) and may therefore contribute to the 1-NASPM-reversible augmentation of D-amphetamine reward in FR rats. However, phosphorylation on Ser845 also facilitates trafficking of GluA1-containing AMPARs to the perisynaptic membrane as the first step toward synaptic incorporation (Boehm et al. 2006). It is therefore possible that enhanced behavioral responses to reward stimuli in FR rats are mediated not only by an increase in basal synaptic GluA1 but also an upregulation of stimulus-induced phosphorylation and synaptic delivery. AMPA receptors in NAc have previously been implicated in sensitization, craving, and relapse to cocaine seeking (Cornish and Kalivas 2000; Boudreau and Wolf 2005; Conrad et al. 2008; Famous et al. 2008). In light of the documented involvement of AMPAR trafficking in synaptic strengthening that underlies acquisition of aversive behavior (Whitlock et al. 2006; Nedelescu et al. 2010), upregulation of stimulus-induced AMPAR trafficking by FR may play a role in the enhanced acquisition and ingraining of appetitive behavior.
Results of the present study suggest that FR upregulates basal and stimulus-induced trafficking of GluA1-containing AMPARs to the NAc PSD. In the wild, these mechanisms would be expected to enhance behavioral responsiveness to appetitive reward stimuli and facilitate synaptic plasticity that mediates the learning of survival behavior. However, in modern societies, severe dieting is prevalent in a context of abundant appetitive rewards. As such, the present findings offer a new mechanistic focus for research aimed at clarifying the basis of dieting as a risk factor for development and reinstatement of maladaptive reward-directed behavior, including drug addiction.
Acknowledgements
This research was supported by DA003956 (K.D.C.) and 5T32 DA007254 (X-X. P) from the National Institute on Drug Abuse, and NS061920 from the National Institute on Neurological Disorders and Stroke (E.B.Z.).
References
- Austin SB, Gortmaker SL. Dieting and smoking initiation in early adolescent girls and boys: a prospective study. Am J Public Health. 2001;91:446–450. doi: 10.2105/ajph.91.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MR, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology. 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J, Kang M-G, Johnson RC, Esteban J, Rl H, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Krahne L, Carr KD. A progressive ratio schedule of self-stimulation testing reveals profound augmentation of d-amphetamine reward by food restriction but no effect of a “sensitizing” regimen of d-amphetamine. Psychopharmacology. 2004;175:106–113. doi: 10.1007/s00213-003-1768-4. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Carr KD. Food scarcity, neuroadaptations, and the pathogenic potential of dieting in an unnatural ecology: compulsive eating and drug abuse. Physiol Behav. 2011;104:162–167. doi: 10.1016/j.physbeh.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Carr KD, Cabeza de Vaca S, Sun Y, Chau LS. Reward-potentiating effects of D-1 dopamine receptor agonist and AMPA GluR1 antagonist in nucleus accumbens shell are increased by food restriction; possible relevance to enhancement of adaptive and maladaptive reward-directed behavior. Psychopharmacology. 2009;202:731–743. doi: 10.1007/s00213-008-1355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Chau LS, de Vaca C, Gustafson K, Stouffer M, Tukey DS, Restituito S, Ziff EB. AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neuroscience. 2010;165:1074–1086. doi: 10.1016/j.neuroscience.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Increased drug-reinforced behavior due to food deprivation. Adv Behav Pharmacol. 1984;4:47–88. [Google Scholar]
- Chao SZ, Ariano MA, Peterson DA, Wolf ME. D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J Neurochem. 2002;83:704–712. doi: 10.1046/j.1471-4159.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- Cheskin LJ, Hess JM, Henningfield J, Gorelick DA. Calorie restriction increases cigarette use in adult smokers. Psychopharmacology. 2005;179:430–436. doi: 10.1007/s00213-004-2037-x. [DOI] [PubMed] [Google Scholar]
- Choquet D. Fast AMPAR trafficking for a high-frequency synaptic transmission. Eur J Neurosci. 2010;32:250–260. doi: 10.1111/j.1460-9568.2010.07350.x. [DOI] [PubMed] [Google Scholar]
- Conason A, Teixeira J, Hsu CH, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg. 2013;148:145–150. doi: 10.1001/2013.jamasurg.265. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L-J, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli D, Contarino A, Tabarin A, Drago F. Binge-like eating in mice. Int J Eat Disord. 2009;42:402–408. doi: 10.1002/eat.20637. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DEH, Armstrong HC, Corlett PR, Chudasama Y, Robbins TW. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102:6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cunha TM, Sedki F, Macri J, Casola C, Shalev U. The effects of chronic food restriction on cue-induced heroin seeking in abstinent male rats. Psychopharmacology. 2013;225:241–250. doi: 10.1007/s00213-012-2810-1. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids: I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Dopamine in disturbances of food and drug motivated behavior: a case of homology? Physiol Behav. 2005;86:9–10. doi: 10.1016/j.physbeh.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wolf ME. Effects of acute cocaine or dopamine receptor agonists on AMPA receptor distribution in the rat nucleus accumbens. Synapse. 2011;65:54–63. doi: 10.1002/syn.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SA, Perry CL, Leon GR, Fulkerson JA. Weight concerns, dieting behavior, and smoking initiation among adolescents: a prospective study. Am J Public Health. 1994;84:1818–1820. doi: 10.2105/ajph.84.11.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Lane DA, Colago EEO, Chan J, Schlussman SD, Zhou Y, Kreek MJ, Pickel VM. Chronic administration of morphine is associated with a decrease in surface AMPA GluR1 receptor subunit in dopamine D1 receptor expressing neurons in the shell and non-D1 receptor expressing neurons in the core of the rat nucleus accumbens. Exp Neurol. 2008;210:750–761. doi: 10.1016/j.expneurol.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci. 2006;9:1001–1003. doi: 10.1038/nn1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Comparison of basal and D-1 dopamine receptor agonist-stimulated neuropeptide gene expression in caudate-putamen and nucleus accumbens of ad libitum fed and food-restricted rats. Mol Brain Res. 2005a;141:121–127. doi: 10.1016/j.molbrainres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Food restriction increases NMDA receptor-mediated CaMK II and NMDA receptor/ERK 1/2-mediated CREB phosphorylation in nucleus accumbens upon D-1 dopamine receptor stimulation in rats. Neuroscience. 2005b;132:1035–1043. doi: 10.1016/j.neuroscience.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Haberny S, Berman Y, Meller E, Carr KD. Chronic food restriction increases D-1 dopamine receptor agonist-induced ERK1/2 MAP kinase and CREB phosphorylation in caudate-putamen and nucleus accumbens. Neuroscience. 2004;125:289–298. doi: 10.1016/j.neuroscience.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord. 1997;22:411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol - Reg Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Goel A, Ciarkowski CE, Song L, Lee HK. Brain area specific regulation of synaptic AMPA receptors by phosphorylation. Commun Integr Biol. 2011;4:569–572. doi: 10.4161/cib.4.5.15890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Ann Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ivezaj V, Saules KK, Wiedemann AA. “I didn’t see this coming.”: why are postbariatric patients in substance abuse treatment? Patients’ perceptions of etiology and future recommendations. Obes Surg. 2012;22:1308–1314. doi: 10.1007/s11695-012-0668-2. [DOI] [PubMed] [Google Scholar]
- Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, Sano A, Yuzaki M, Zukin RS, Ziff EB, Kessels HW, Takahashi T. Serotonin mediates cross-modal reorganization of cortical circuits. Neuron. 2011;69:780–792. doi: 10.1016/j.neuron.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA. Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics. 2004;3:857–871. doi: 10.1074/mcp.M400045-MCP200. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3303. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M, Iino M, Ozawa S. Blocking effect of 1-naphthyl acetyl spermine on Ca2+-permeable AMPA receptors in cultured rat hippocampal neurons. Neurosci Res. 1997;29:27–36. doi: 10.1016/s0168-0102(97)00067-9. [DOI] [PubMed] [Google Scholar]
- Krahn D, Kurth C, Demitrack M, Drewnowski A. The relationship of dieting severity and bulimic behaviors to alcohol and other drug use in young women. J Subst Abuse. 1992;4:341–353. doi: 10.1016/0899-3289(92)90041-u. [DOI] [PubMed] [Google Scholar]
- Lee HK. Ca-permeable AMPA receptors in homeostatic synaptic plasticity. Front Mol Neurosci. 2012;5(17):1–11. doi: 10.3389/fnmol.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Herrera S, Bubula N, Nikitina E, Palmer AA, Hanck DA, Loweth JA, Vezina P. Casein kinase 1 enables nucleus accumbens amphetamine-induced locomotion by regulating AMPA receptor phosphorylation. J Neurochem. 2011;118:237–247. doi: 10.1111/j.1471-4159.2011.07308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zheng D, Peng XX, Cabeza de Vaca S, Carr KD. Enhanced cocaine-conditioned place preference and associated brain regional levels of BDNF, p-ERK1/2 and p-Ser845-GluA1 in food-restricted rats. Brain Res. 2011;1400:31–41. doi: 10.1016/j.brainres.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY. GluA2-lacking, calcium-permeable AMPA receptors— inducers of plasticity? Curr Opin Neurobiol. 2011;21:291–298. doi: 10.1016/j.conb.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Moyer JT, Wolf JA, Finkel LH. Effects of dopaminergic modulation on the integrative properties of the ventral striatal medium spiny neuron. J Neurophysiol. 2007;98:3731–3748. doi: 10.1152/jn.00335.2007. [DOI] [PubMed] [Google Scholar]
- Nedelescu H, Kelso CM, Lázaro-Muñoz G, Purpura M, Cain CK, Ledoux JE, Aoki C. Endogenous GluR1-containing AMPA receptors translocate to asymmetric synapses in the lateral amygdala during the early phase of fear memory formation: an electron microscopic immunocytochemical study. J Comp Neurol. 2010;518:4723–4739. doi: 10.1002/cne.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CL, Milovanovic M, Wetter JB, Ford KA, Wolf ME. Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J Neurochem. 2009;109:35–51. doi: 10.1111/j.1471-4159.2009.05911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pan Y, Haberny LY, Berman Y, Meller E, Carr KD. Synthesis, protein levels and phosphorylation state of tyrosine hydroxylase in mesoaccumbens and nigrostriatal dopamine pathways of chronically food-restricted rats. Brain Res. 2006;1122:135–142. doi: 10.1016/j.brainres.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Peng XX, Ziff EB, Carr KD. Effects of food restriction and sucrose intake on synaptic delivery of AMPA receptors in nucleus accumbens. Synapse. 2011;65:1024–1031. doi: 10.1002/syn.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisetsky EM, Chao YM, Dierker LC, May AM, Striegel-Moore RH. Disordered eating and substance use in high-school students: results from the Youth Risk Behavior Surveillance System. Int J Eat Disord. 2008;41:464–470. doi: 10.1002/eat.20520. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Root TL, Pinheiro AP, Thornton L, Strober M, Fernandez-Aranda F, Brandt H, Crawford S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, Klump KL, La Via M, Mitchell J, Woodside DB, Rotondo A, Berrettini WH, Kaye WH, Bulik CM. Substance use disorders in women with anorexia nervosa. Int J Eat Disord. 2010;43:14–21. doi: 10.1002/eat.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse R, Deutsch S, Chilton M. Cocaine addicts prone to cocaine-induced psychosis have lower body mass index than cocaine addicts resistant to cocaine-induced psychosis—implications for the cocaine model of psychosis proneness. Isr J Psychiatry Relat Sci. 2005;42:45–50. [PubMed] [Google Scholar]
- Shepherd JD. Memory, plasticity and sleep—a role for calcium permeable AMPA receptors? Front Mol Neurosci. 2012;5(49):1–5. doi: 10.3389/fnmol.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp JA, Mashoodh R, van Kampen JM, Robertson HA. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and DeltaFosB expression in the nucleus accumbens of the rat. Brain Res. 2008;1204:94–101. doi: 10.1016/j.brainres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Stouffer M, Carr KD, Rice ME. Insulin-induced increase in striatal dopamine release are enhanced by chronic food restriction and blunted by an obesogenic diet. Soc Neurosci Abstr. 2012;283:06. [Google Scholar]
- Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse. 2002;46:83–90. doi: 10.1002/syn.10120. [DOI] [PubMed] [Google Scholar]
- Surmeier J, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Tsubokawa H, Oguro K, Masuzawa T, Nakaima T, Kawai N. Effects of a spider toxin and its analogue on glutamate-activated currents in the hippocampal CA1 neuron after ischemia. J Neurophysiol. 1995;74:218–225. doi: 10.1152/jn.1995.74.1.218. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Brit. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M, Frost-Pineda K, Gold M. Body mass index and marijuana use. J Addict Dis. 2005;24:95–100. doi: 10.1300/J069v24n03_08. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wiederman MW, Pryor T. Substance abuse and impulsive behaviors among adolescents with eating disorders. Addict Behav. 1996;21:269–272. doi: 10.1016/0306-4603(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med. 1985;3:445–460. [PubMed] [Google Scholar]
- Wolf JA, Moyer JT, Maciej T, Lazarewicz DC, Benoit-Marand M, O’Donnell P, Finkel LH. NMDA/AMPA ratio impacts state transitions and entrainment to oscillations in a computational model of the nucleus accumbens medium spiny projection neuron. J Neurosci. 2005;25:9080–9095. doi: 10.1523/JNEUROSCI.2220-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Cabeza de Vaca S, Carr KD. Food restriction increases acquisition, persistence and drug prime-induced expression of a cocaine-conditioned place preference in rats. Pharmacol Biochem Behav. 2012;100:538–544. doi: 10.1016/j.pbb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Liu S, Cabeza de Vaca S, Carr KD. Effects of time of feeding on psychostimulant reward, conditioned place preference, metabolic hormone levels, and nucleus accumbens biochemical measures in food-restricted rats. Psychopharmacology. 2013;227:307–320. doi: 10.1007/s00213-013-2981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]