Abstract
The cyclin D1 gene encodes the regulatory subunit of a holoenyzme that phosphorylates the retinoblastoma protein (pRb) and nuclear respiratory factor (NRF1) proteins. The abundance of cyclin D1 determines estrogen-dependent gene expression in the mammary gland of mice. Using estradiol (E2) and an E2-dendrimer conjugate which is excluded from the nucleus, we demonstrate E2 delays the DNA damage response (DDR) via an extranuclear mechanism. The E2-induced DDR required extranuclear cyclin D1 which bound ERα at the cytoplasmic membrane and augmented AKT phosphorylation (Ser473) and γH2AX foci formation. In the nucleus E2 inhibited, whereas cyclin D1 enhanced, homology directed DNA repair. Cyclin D1 was recruited to γH2AX foci by E2 and induced Rad51 expression. Cyclin D1 governs an essential role in the E2-dependent DNA-damage response via a novel extranuclear function. The dissociable cytoplasmic function to delay the E2 mediated-DDR together with the nuclear enhancement of DNA repair uncovers a novel extranuclear function of cyclin D1 that may contribute to the role of E2 in breast tumorigenesis.
Keywords: Cyclin D1, DNA damage repair, Estrogen, Cell-cycle
INTRODUCTION
Genomic integrity is monitored by cellular networks governing the DNA damage response (DDR). Defects in DNA damage signaling or repair contribute to degenerative diseases and cancer. The DDR involves a DNA damage signaling arm, which includes sensors, transducers (ATM, ATR), mediators, and effectors. The transducer kinases include Ataxia Telangiectasia-Mutated (ATM) and Ataxia Telangiectasia and Rad3-related (ATR). The ATM signaling mediators (p53BP1, MDC, BRCA1) and ATR mediators (TopBP1, Claspin) in turn activate effectors including CHK1 and CHK2 (1). ATR activity requires association with ATRIP and TopBP1 proteins. The downstream substrates of ATR and ATM include BRCA1, BRCA2, NBS1, and CHK2. ATR activation results from replication stress, whereas ATR and ATM both play a role in cellular response to double stranded DNA breaks. The DDR induces assemblage of nuclear repair foci. Phosphorylation of histone H2AX on Serine 139 produces γH2AX, which recruits proteins that sense or signal the presence of DNA damage. DDR activation occurs early in human tumor cells in which activation of γH2AX occurs early, often in the pre-invasive stages of human tumor correlating with the presence of senescence markers (2). Activated oncogenes, including c-Myc, Ras, Mos, CDC25, E2F1, cyclin D1 and cyclin E (3–6), induce double-stranded DNA breaks (DSB) and the DDR. DNA damage is a common feature of premalignant lesions, including breast ductal carcinoma in situ (7, 8).
In human breast cancer, estrogen receptor alpha (ERα) bound to its ligand estradiol (E2) contributes important survival and proliferative effects. The major adjuvant therapy for human breast cancer involves anti-estrogen therapy for the ~70% of ERα expressing human tumors. E2 is known to delay the assembly and prolongs the resolution of γH2AX and Rad51 foci through inhibition of ATR kinase signaling (9). As E2/ERα increased chromosomal damage after irradiation, it has been suggested that E2-mediated restraint of ATR activation may be a novel estrogen action that promotes breast malignancy. Estrogen serves as a ligand for ERα inducing nuclear receptor activity and also participates in an acute cytoplasmic membrane-associated activity (Reviewed in (10)). The ERα regulates nuclear gene expression via binding to both canonical DNA and non-canonical DNA sequences in the promoter of target genes. Extranuclear pools of ERα have been identified in the plasma membranes (11). The ability to distinguish nuclear from extranuclear ERα signaling has been enabled through the generation of 17β-estradiol dendrimer conjugates (EDC) which are localized to the extranuclear compartment (12, 13). The contribution of nuclear vs. extranuclear effects of E2 is important in providing optimal patient treatment as, for example, the vascular protection and the protection of cortical bone mass are mediated at least in part via non-genomic E2 signaling (14, 15).
The cyclin D1 gene is commonly overexpressed in human breast cancer correlating with chromosomal instability in the tumors (16, 17). The luminal B breast cancers, which overexpress cyclin D1, associated with chromosomal instability and poor prognosis, are uniformly ERα positive (17). Immunoneutralizing antibody and antisense cyclin D1 experiments demonstrated that the abundance of cyclin D1 is rate-limiting in estradiol-induced DNA synthesis and oncogene-induced contact-independent breast tumor growth in mice (18, 19). Cyclin D1 expression and promoter activity is induced by E2 and cyclin D1 associates with the ERα in the nucleus to enhance ligand independent transactivation (20). Genetic deletion studies of cyclin D1 in the mouse demonstrated a role for cyclin D1 in E2-mediated gene expression in the mammary gland (21). In these studies, cyclin D1 was required for the induction of a gene module involved in E2-dependent DNA damage signaling (21). Previous studies have implicated cyclin D1 in the DDR in response to ultraviolet (UV) and γ irradiation (22, 23). Cyclin D1 associates with and conveys functional interactions with BRCA1 (24), a mediator of the DNA damage signaling pathway and repair of double-stranded DNA breaks and with BRCA2 (25), which is known to be recruited sequentially by BRCA1 to DNA damage foci through the BRCA2 binding protein PALB2. The assembly of γH2AX foci in response to UV irradiation is enhanced by cyclin D1 which has shown to bind Rad51 (6). Cyclin D1b with a different carboxyl terminus from cyclin D1a has a defective binding ability to Rad51 (6). The 1–155 amino acids of cyclin D1 has also been shown to be necessary for its binding to Rad51 (25). Given the important role for cyclin D1 in estrogen-dependent signaling in vivo, we sought to determine whether cyclin D1 participated in the E2-dependent DNA-damage response. As the DDR may contribute to mutagenesis, we determined the functional significance of E2 and cyclin D1 on homologous DNA damage repair. Through deploying E2 dendrimer conjugates, we defined the role of nuclear vs extranuclear 17β-estradiol in the DDR and define a novel extranuclear function of cyclin D1 in ERα signaling.
MATERIALS AND METHODS
Plasmids
Tet-On TRIPZ inducible lentiviral human cyclin D1 shRNAs were purchased from Thermo Scientific. The retroviral vector encoding ERα was constructed as follows. The cDNA encoding human (ERα) was excised from HEGO vector with EcoRI digestion and subsequently inserted into MSCV-IRES-GFP retroviral vector. The direction of cDNA and the protein expression was confirmed by restriction digestion and Western blot, respectively. Rad51-luciferase plasmid (pGL3) was from Dr. Peter M. Glazer (26). Cherry-lacR-NLS-ATM and Cherry-lacR-NLS-NBS1 expression plasmids were from Dr. Tom Misteli (27). Cherry-lacR-NLS-cyclin D1 plasmid was previously described (6). The plasmid encoding ERα E domain ECFP-mem fusion protein (in pECFP-Mem vector, targeting the E domain/ligand-binding domain completely to the plasma membrane) was provided by Dr. Ellis R. Levin (28). I-SceI (pCAGGS-I-SceI, called pCβASce), GFP (pCAGGS-NZEGFP), and empty vector (pCAGGS-BSKX) were obtained from Dr. Jeremy M. Stark (29). The plasmids encoding FLAG-ATM or FLAG-ATR (in pCDNA3 vector) were purchased from Addgene.
Cell Culture, Reagents, and Cellular Treatment
MCF-7 and HEK 293T cell lines were from the American Type Culture Collection (Manassas, VA). The NIH 2/4 stable cell line that contains 256 repeats of the lac operator sequence (lacO) stably integrated on chromosome 3 was obtained from Dr. Tom Misteli (30). MCF-7 DR-GFP stable cells and U2OS DR-GFP stable cells were provided by Dr. Maris Jasin (31) and Dr. Jeremy M. Stark (29).
Retroviral production and infection of NIH2/4 cells with MSCV-ERα-IRES-GFP or vector control were as described before (32). Fluorescence-activated cell sorting (FACS) (FACStar Plus; BD Biosciences, San Jose, CA) sorted GFP+ cells were used for subsequent analysis. The FACS sorted cells were transiently transfected with Cherry-lacR-NLS-ATM, Cherry-lacR-NLS-NBS1, Cherry-lacR-NLS-cyclin D1, or empty vector Cherry-lacR-NLS using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions and treated with 10 nM E2. 24 hrs later cells were processed for γH2AX immunostaining.
Lentiviral production and infection of U2OS DR-GFP cells with three individual TRIPZ-cyclin D1 shRNAs or vector control were performed by following the manufacturer’s instruction (Thermo Scientific) in culture medium with 10% tetracycline-free FBS. To induce cyclin D1 shRNA expression in the Tet-On TRIPZ transduced U2OS DR-GFP cells, 2 μg/ml doxycycline (Research Products International Corp. Mount Prospect, IL) was added to the medium.
17β-estradiol (E2) dendrimer conjugate (EDC) and dendrimer control were prepared as previously described (12, 33). MCF-7 cells were cultured in phenol red-free DMEM with 10% charcoal/dextran–treated FBS and 2 mM glutamine for 48 hrs before treatment with 10 nM E2 or EDC equivalent to 10 nM E2. Ultraviolet (UV) exposure (100 J/m2) was performed using a Spectrolinker XL-1000 Crosslinker (Spectronics Corporation. Westbury, NY).
Luciferase reporter assays
Luciferase assays were performed as previously described (32).
siRNA knockdown of endogenous cyclin D1 in MCF-7 cell
For suppression of endogenous cyclin D1 expression in MCF-7 breast cancer cell lines, 3 siRNAs (Hs_CCND_1, Hs_CCND_2, Hs_CCND_3) that specifically target human cyclin D1 mRNA were purchased from Qiagen.
Western blot
Western blot analyses were conducted as described previously (32).
Immunostaining
Immunoflurescence was conducted as described previously (6).
Homologous recombination directed DNA repair assay (HDR)
MCF-7 DR-GFP and U2OS DR-GFP cells harbor a chromosomally integrated copy of DR-GFP reporter. The DR-GFP reporter contains an inactive expression cassette for GFP that is interrupted by a recognition site for the rare-cutting endonuclease I-SceI. When I-SceI is expressed in DR-GFP expressing cells, it induces DSBs within the SceGFP fragment providing a signal for homologous recombination and leads to restoration of the functional GFP (31) (Figure 7A). To analyze the efficiency of HDR, MCF-7 DR-GFP and U2OS DR-GFP cells were transiently transfected with pCβA-Sce using the Nucleofector kit (Lonza) following the manufacturer’s recommendations. The cells were cultured for 3 days to allow completion of repair, then the percentage of GFP-positive (GFP+) cells were analyzed by FACS analysis. The GFP expression plasmid in the same backbone, pCAGGS-NZEGFP was used as a transfection efficiency control. The empty vector pCAGGS-BSKX was used as a negative control.
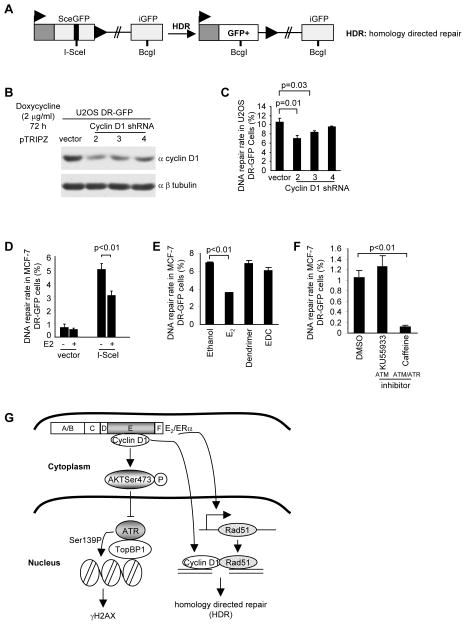
Figure 7. Estradiol inhibits homology-directed DNA damage repair (HDR).
(A) Schematic representation of the HDR reporter system in which I-SceI- induced DNA breaks are repaired by HDR, resulting in GFP fusion protein production. (B) U2OS-GFP stable cell lines were transduced with 3 distinct doxycycline inducible cyclin D1 shRNA and Western blot conducted after 72 hours of doxycycline treatment with the antibodies as indicated. (C) HDR rate of U2OS DR-GFP cells expressing doxycycline inducible cyclin D1 shRNA shown as mean ±SEM for N>3 separate experiments in which double stranded DNA breaks were introduced by I-SceI. (D, E, F) Homology directed DNA repair in MCF-7 cells expressing the Sce-GFP repair reporter plasmid shown in A. Cells were transduced with an I-SceI expression plasmid and the repair rate assessed after 72 hours of E2 (10−8M) treatment (D), E2 (10−8M) and EDC (E), and specific ATM inhibitor (10 mM KU55933), ATM/ATR inhibitor (5 mM Caffeine), or vehicle DMSO control (F). Data are mean ±SEM for 3 separate transfections. (G) Schematic representation of model by which cyclin D1 augments AKT signaling associated with ERα in the cytoplasmic membrane thereby participating in E2-dependent signaling; and cyclin D1 augments homology directed DNA damage repair, in part through binding to DNA at γH2AX foci and through inducing Rad51 transcription and abundance.
A more detailed Materials and Methods are included in supplemental material.
RESULTS
Cyclin D1 is required for E2-mediated delay in the DNA damage response
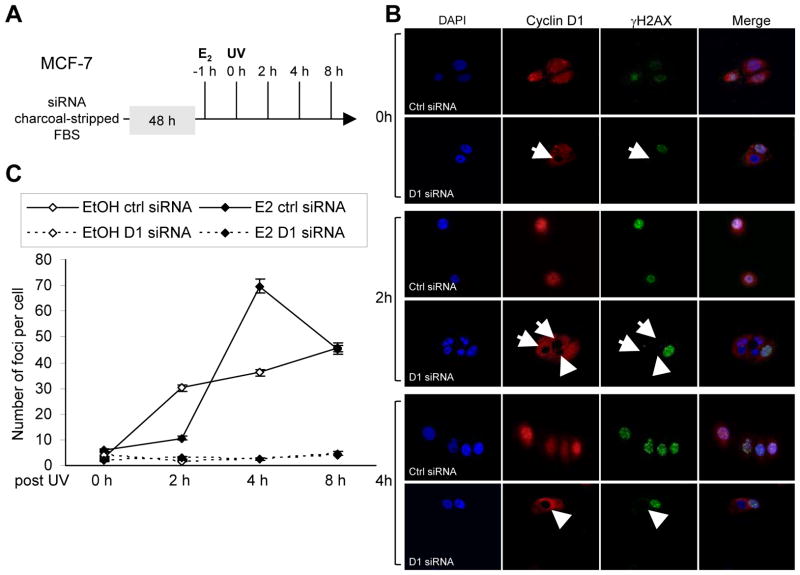
Previous studies had demonstrated a role for cyclin D1 in enhancing the DNA damage response (6). To examine the functional interaction between endogenous cyclin D1 and estrogen in mediating γH2AX, MCF-7 cells were treated with either control siRNA or cyclin D1 siRNA and subsequently with E2 (10 nM) (Fig. 1A). Upon UV irradiation (100 J/m2), γH2AX was induced and the presence of foci was observed at 2 hrs (Fig. 1B). Quantitation of the number of γH2AX foci was shown in Figure 1C. E2 treatment delayed the number of irradiation-induced γH2AX foci for 2 hrs. At 4 hrs, E2-treated cells had significantly more γH2AX foci (Fig. 1C). Cyclin D1 siRNA abrogated the formation of γH2AX at 4 hrs (Figure 1B Arrows in Col. 3, Row 6). The delayed induction of DDR by E2, assessed by γH2AX foci number, was reduced by cyclin D1 siRNA (Fig. 1B, 1C). Collectively, these studies demonstrate that cyclin D1 is required for the E2-mediated delayed induction of γH2AX foci.
Figure 1. Endogenous cyclin D1 mediates E2-dependent DNA damage signaling in human breast cancer cell lines MCF-7 cells.
(A) Schematic representation of experimental protocol. (B) Confocal microscopy of immunofluorescence for cyclin D1 (red) and γH2AX (green) and nuclear staining with DAPI in MCF-7 cells after UV (100 J/M2). The cyclin D1 siRNA transduced cells are indicated. (C) Quantitation of γH2AX foci as number of foci per cell. Confocal microscopy of γH2AX immunofluorescence in MCF-7 cells after UV exposure either in the absence or presence of estradiol (10−8M) was used to determine data shown as mean ±SEM for N>50 cells.
Extranuclear E2-dependent DDR is mediated via cyclin D1
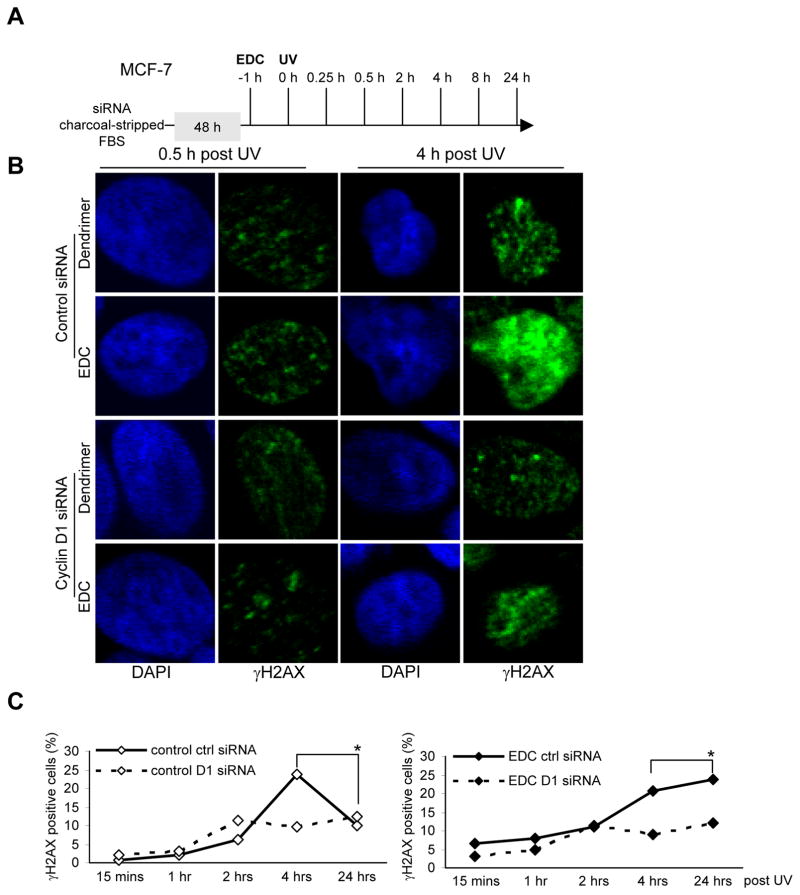
The non-genomic extranuclear actions of the ERα are more rapid than the genomic and are unaffected by inhibitors of RNA or protein synthesis. Large abiotic non-degradable polyamidoamine (PAMAM) dendrimer macromolecules conjugated to estrogen remain outside the nucleus, providing optimal ligand access to ERα and conveying a binding affinity comparable to that of E2 (12, 33). EDC stimulates ERK, SHC, and Src phosphorylation, activating non-genomic activities at concentrations that do not alter the transcription of nuclear estrogen target genes (12, 13). EDC is therefore useful in studying non-genomic effects of estrogen action in target cells (15). To determine whether cyclin D1 contributes to non-genomic estrogen-mediated signaling, MCF-7 cells were treated with UV irradiation in the presence or absence of EDC, compared with the control dendrimer. Immunohistochemical analysis of γH2AX foci was conducted (Fig. 2). After UV induced DDR, the number of γH2AX-positive cells was increased at 4 and 8 hrs. Treatment with EDC resulted in the continued increase in γH2AX at 24 hrs (Fig. 2C, * right vs. left). Cyclin D1 siRNA reduced basal UV-induced γH2AX staining and reduced both the EDC and E2-induced γH2AX at 24 hrs by ~50% (Fig. 2C, Supplemental Fig. 1). Thus, endogenous cyclin D1 participates in the persistence of EDC-dependent γH2AX.
Figure 2. Endogenous cyclin D1 mediates the delayed DNA damage signaling mediated by extranuclear estrogen signaling.
(A) Experimental protocol in which cells transduced with cyclin D1 siRNA or control siRNA were treated with the 17β-estradiol (E2) dendrimer conjugate (EDC) which is localized to the cytoplasm and incapable of stimulating nuclear actions of ERα. (B) Cells were then exposed to DNA damage by UV (100 J/M2) for time points as indicated. H2AX phosphorylation of Ser139 was detected by immunofluorescence and (C) the percentage of γH2AX positive cells collated as mean ±SEM.
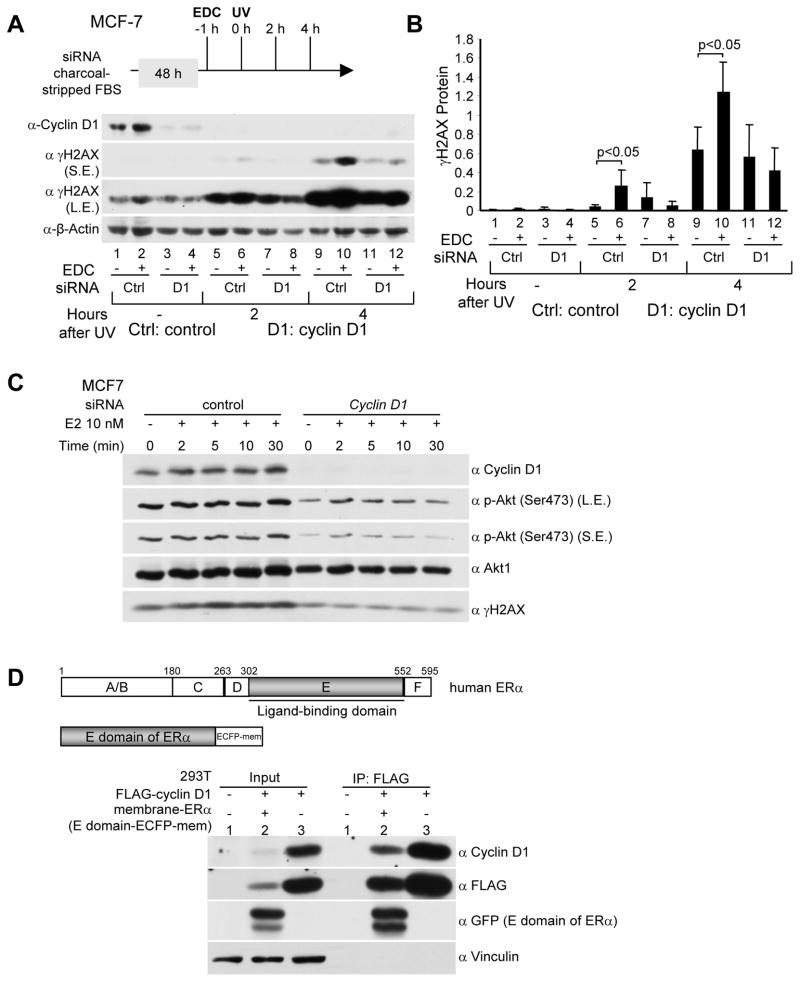
In order to examine further the role of extranuclear E2 signaling to the DDR in breast cancer cells, Western blot was conducted of MCF-7 cells transduced with cyclin D1 siRNA and treated with UV irradiation and either EDC or control dendrimer (Fig. 3). EDC treatment enhanced γH2AX in the basal state (Fig. 3A). Cyclin D1 siRNA reduced cyclin D1 abundance (Lane 3 vs 1). EDC induced cyclin D1 abundance and γH2AX (Lane 2 vs 1). Endogenous cyclin D1 was required for the EDC-induced γH2AX (Lanes 4 vs 2). Two hrs after UV irradiation, γH2AX was induced (Fig. 3A Lane 5 vs. 1). Cyclin D1 siRNA reduced UV-induced γH2AX at 2 and 4 hrs (Fig. 3A, Lane 7 vs. 5 and Lane 11 vs. 9). EDC enhanced UV irradiation induced γH2AX. Cyclin D1 siRNA reduced γH2AX (Fig. 3A, Lanes 8 vs. 6 and Lane 12 vs. 10). Quantitation of multiplicate experiments confirmed the importance of cyclin D1 in EDC-mediated induction of γH2AX (Fig. 3B). E2 is known to inhibit ATR signaling through rapid induction of PI3K/AKT activity (9) to thereby delay the assembly and prolong the resolution of γH2AX foci. As cyclin D1 was required for E2-mediated delay in γH2AX resolution, we determined a potential role for cyclin D1 in AKT activation by E2. MCF-7 cells transduced with cyclin D1 siRNA or scrambled siRNA control, demonstrated the E2-mediated induction of AKT phosphorylation (Ser473) (Fig. 3C). Cyclin D1 siRNA reduced cyclin D1 abundance with a commensurate reduction in phosphorylated AKT (Ser473) (Fig. 3C). Prior studies had identified nuclear cyclin D1/ERα interactions. As E2-mediated induction of PI3K/AKT signaling involves a membrane associated form of ERα, we determined whether the membrane associated ERα binds cyclin D1. 293T cells transfected with an expression vector encoding ERα (E domain) linked to ECFP membrane and cyclin D1 were subjected to immunoprecipitation with an α-FLAG antibody directed to the N-terminus of cyclin D1, with sequential western blot for the GFP fusion protein of the ERα E domain (Fig. 3D). Cyclin D1 co-precipitated cytoplasmic membrane-associated ERα (Fig. 3D). Together these studies identify a novel extra-nuclear function of cyclin D1 to augment E2-dependent AKT phosphorylation at Ser473.
Figure 3. Endogenous cyclin D1 mediates the cytoplasmic membrane mediated E2-dependent DNA damage response.
(A) Western blot analysis of MCF-7 cells treated with cyclin D1 siRNA and EDC (estrogen-dendrimer conjugate) at a dose equivalent to 10−8M E2. Western blot analysis was conducted using antibodies directed to γH2AX, cyclin D1 or β-actin as a protein loading control (SE, short exposure, LE, long exposure). (B) Quantitation of relative γH2AX protein abundance shown as mean ±SEM for N>3 separate experiments. (C) Endogenous cyclin D1 enhances E2-induced Akt phophorylation (Ser473) in MCF-7 cells exposed to UV. MCF-7 cells were transfected with cyclin D1 siRNAs or control siRNA. 48 hrs later cells were treated with 10 nM E2 for time points as indicated. Then cells were exposed to UV (100 J/m2). Specific antibodies to phospho-Serine 473 Akt1/2/3, total Akt1, and γH2AX were used in Western blot analysis. (D) Membrane E domain of ERa binds to cyclin D1 in 293T cells. 293T cells were co-transfected with FLAG-cyclin D1 (in CMV10 vector) and the E domain of ERα (in ECFP-Mem vector targeting the E domain to the plasma membrane). 48 hrs later immunoprecipitation and Western blot analysis were conducted to detect membrane-ERα and FLAG-cyclin D1 interaction.
Endogenous cyclin D1 is required for E2-induced Rad51 abundance
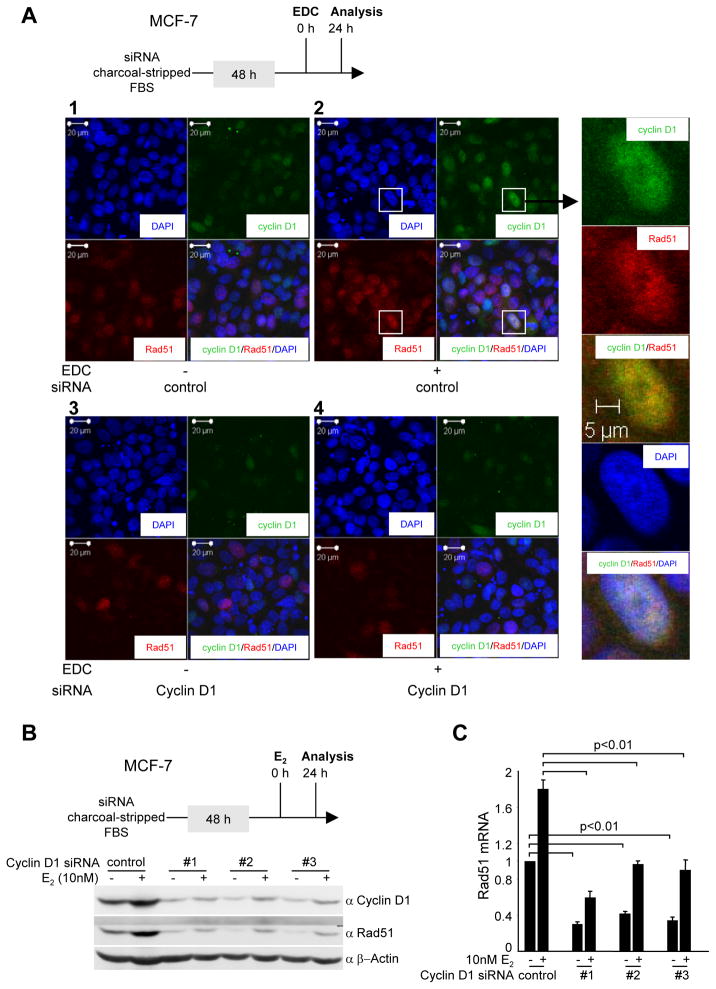
An important regulatory event that determines the type of DNA repair used in the cell is the process of double-stranded break repair. The Rad51 nucleofilament mediates homology search in the sister-chromatin followed by strand invasion. We therefore examined the role of EDC and cyclin D1 in the regulation of Rad51 abundance (Fig. 4). MCF-7 cells were treated with EDC or control. EDC treatment increased cyclin D1 and Rad51 abundance (Fig. 4A, 2 vs. 1). Cyclin D1 siRNA reduced cyclin D1 and Rad51 abundance induced by EDC (Fig 4A, 4 vs. 2). Three separate cyclin D1 siRNA were used to reduce cyclin D1 abundance by Western blot and each siRNA reduced E2 enhancement of Rad51 abundance (Fig. 4B). Rad51 mRNA induction by E2, as well as basal levels, were also reduced by cyclin D1 siRNA (Fig. 4C).
Figure 4. Cyclin D1 is required for extranuclear E2-mediated expression and recruitment of Rad51 to nuclear foci in response to DNA damage.
(A) Schematic depicting experimental procedure. Immunofluorescence staining for cyclin D1 and Rad51 with the nucleus identified by DAPI staining. (B) Western blot of MCF-7 cells transduced with three distinct cyclin D1 siRNA and subjected to treatment with E2 (10−8M). (C) Quantitation of Rad51 mRNA abundance determined by QT-PCR. Cells were treated with 10−8M E2 for 24 hours.
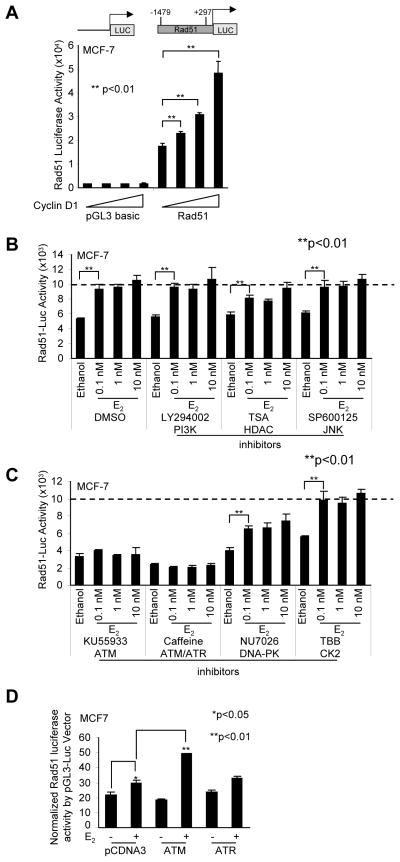
As Rad51 abundance may be transcriptionally induced, we deployed the Rad51 promoter linked to a luciferase reporter gene (Fig. 5A). In transient expression studies, co-expression of a cyclin D1 expression vector enhanced Rad51 promoter activity in a dose-dependent manner (Fig. 5A). There was no effect of cyclin D1 co-expression on the luciferase reporter backbone pGL3-luc (Fig. 5A). E2 induced Rad51 promoter activity 1.5-fold. The addition of inhibitors for the PI3K, HDAC and JNK signaling did not significantly reduce E2-mediated induction of Rad51 promoter (Fig. 5B). In contrast, inhibitors of the ATM (KU55933) and ATM/ATR pathways (caffeine) abrogated E2-mediated induction of the Rad51 promoter (Fig. 5C). The DNA-PK inhibition NU7026 and the CK2 inhibitor (TBB) did not significantly reduce the E2 induction of Rad51 (Fig. 5C). These studies suggest E2 induces Rad51 transcription via ATM. In order to determine precisely the role of ATM and ATR in regulating Rad51 promoter activity, expression vectors encoding either ATM or ATR were introduced into cultured cells with the Rad51 promoter reporter gene. Co-expression of ATM was sufficient to induce Rad51 promoter activity in the presence of E2 (Fig. 5D). These studies suggest cyclin D1 governs two apparently distinct functions in the DDR: the first to participate in an extranuclear signaling that augments AKT signaling, and delay the resolution of γH2AX foci; and the second to augment expression of Rad51, a protein participating in DNA damage repair.
Figure 5. Cyclin D1 and E2 induces the Rad51 promoter.
(A) Schematic representation of the Rad51 promoter linked to a luciferase reporter gene. MCF-7 cells were transfected with the Rad51-Luc or the control pGL3-luc reporter together with an expression vector encoding cyclin D1. The data are shown as mean ±SEM for N>5 separate transfections. P<0.01 (B) The Rad51-luc reporter gene was transfected into MCF-7 cells and treated with E2 and a series of kinase inhibitors: PI3K inhibitor (100 nM LY294002), HDAC inhibitor (100 nM Trichostatin A (TSA)), JNK inhibitor (5 μM SP600125), ATM inhibitor (10 μM KU55933), ATM/ATR inhibitor (5 mM Caffeine), DNA-PK inhibitor (10 μM NU7026), casein kinase-2 (CK2) inhibitor (100 μM TBB), or vehicle (DMSO) control. (D) ATM is a co-activator of E2-stimulated induction of the Rad51 promoter in MCF-7 cells. MCF-7 cells were transfected with the Rad51-luc or the control pGL3-luc reporter together with the expression vector encoding ATM, ATR, or vector control pCDNA3 and treated with 10 nM E2 or vehicle ethanol control. Data are mean ±SEM for 3 separate transfections.
E2 enhances the DDR induced by cyclin D1 tethered to chromatin
In order to examine further the mechanism by which E2 regulated cyclin D1-dependent DNA damage signaling we deployed a DNA-repair factor chromatin recruitment assay (27). The recruitment of DDR factors into chromatin can trigger and amplify the DDR signal via an ATM- and DNA-PK-dependent mechanism (27). Like the DDR factors, cyclin D1 is recruited to chromatin in these assays, requiring the cyclin D1 carboxyl terminus (6). The role of E2 in regulating the DDR induced by the DDR factor recruitment to chromatin is unknown.
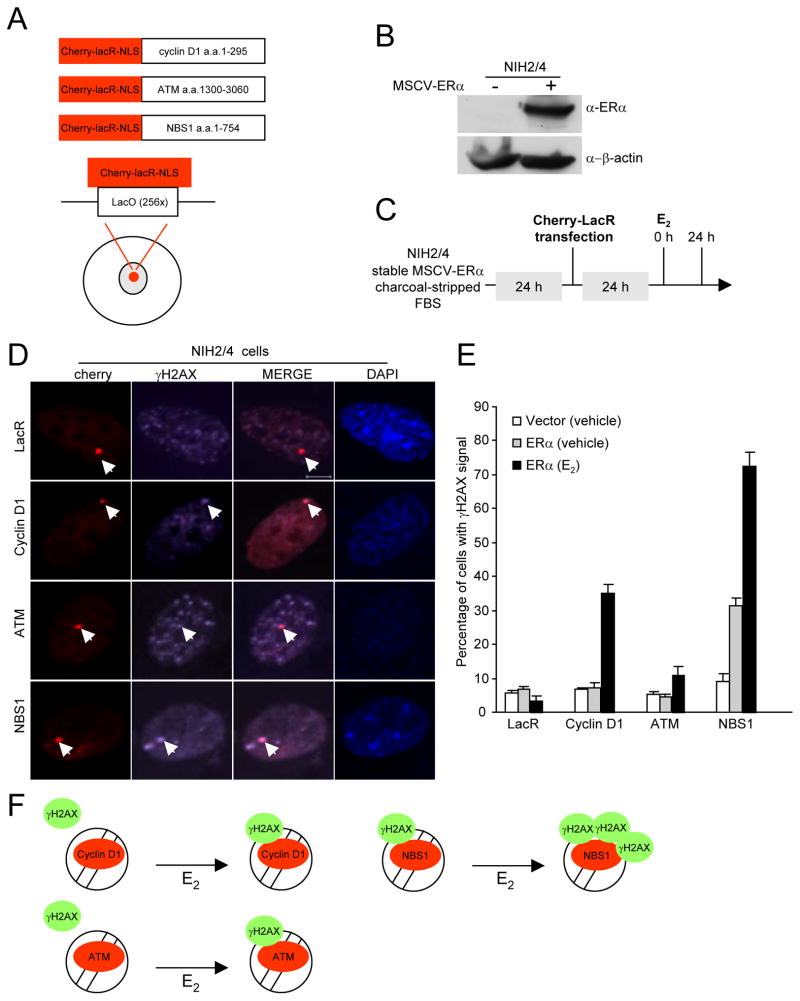
DNA repair factors or cyclin D1 fused to the Escherichia coli lac-repressor (LacR) were tagged with the cherry-red fluorescent protein. An NIH 3T3 cell line that contains 256 repeats of the LacO site, stably integrated into chromosome 3 (NIH2/4) (30) was transduced with retroviral vectors encoding either ERα or control vector. The NIH2/4-ERα cells were used to analyze the role of E2 in recruitment of cyclin D1 and DDR fusion proteins to DNA (Fig. 6B). The presence of ERα was readily detectable by Western blot (Fig. 6B). The fusion protein accumulated at the LacO multimer site as distinct nuclear foci (Fig. 6D). NBS1 recruitment to the LacO site was sufficient to induce the DDR and activate γH2AX and form foci at the LacO site (Fig. 6D, E, LacR ERα (vehicle) vs. NBS1 ERα (vehicle)). In the absence of E2 cyclin D1 and ATM were not sufficient to induce γH2AX foci at the LacO site (Fig. 6E, LacR ERα (vehicle) vs. cyclin D1 ERα (vehicle) and LacR ERα (vehicle) vs. ATM ERα (vehicle)). E2 treatment in NIH2/4-ERα cells enhanced NBS1 and ATM recruitment-mediated γH2AX foci (Fig. 6E, NBS1 ERα (vehicle) vs. NBS1 ERα (E2) and ATM ERα (vehicle) vs. ATM ERα (E2)). The presence of ERα and E2 enhanced cyclin D1-mediated γH2AX foci formation at the LacO site (Fig. 6E, cyclin D1 ERα (vehicle) vs. cyclin D1 ERα (E2)). Thus E2 enhances cyclin D1 recruitment to γH2AX foci. Secondly these studies demonstrate that liganded ERα enhances γH2AX and the formation of DNA repair foci induced by cyclin D1 recruitment into chromatin (Fig. 6F).
Figure 6. Estradiol enhances the DNA damage response of repair factor recruitment to DNA.
(A) Schematic representation of the chimeric fusion proteins in which the DNA damage signaling repair factors, or cyclin D1, were linked to the cherry-lacR-NLS (red). (B)Western blot NIH 2/4 cells and NIH 2/4 cells transduced with an ERα expression plasmid. Immuno-blotting was conducted with antibodies as indicated. (C) Schematic representation of the cell treatment protocol. (D) Confocal immunofluorescence microscopy of NIH 2/4 cells transiently transfected with the DNA damage repair factor or cyclin D1 fused to cherry-lacR-NLS (red). Phosphorylation of γH2AX indicates activation of the DDR. (E) The percentage of cells with γH2AX signal was determined at 24 hours after treatment (E2 10−8M). The data are mean ±SEM for N>50 cells and N>3 separate experiments. (F) Models of E2 on cyclin D1, ATM and NBS1 chromatin recruitment-mediated γH2AX foci.
Endogenous cyclin D1 facilitates and E2 inhibits homology-directed DNA damage repair
Double-stranded breaks generated by ionizing radiation are repaired by either homologous or non-homologous end-joining. DSB and repair can be simulated in mammalian cells by using the homing endonuclease I-SceI. Recombination repair can be monitored through the ligation-mediated formation of GFP protein from a reporter plasmid (DR-GFP) (Fig. 7A). As a form of positive control, we deployed a previously described homology directed repair (HDR) U2OS cell line, and assessed the role of endogenous cyclin D1 in the repair process by transducing the cells with three separate cyclin D1 shRNA under control of a tetracycline inducible promoter (Fig. 7B). Cyclin D1 shRNA reduced the abundance of cyclin D1 by Western blot (Fig. 7B) and reduced the I-SceI induced DNA damage repair in U2OS cells (Fig. 7C). In MCF-7 cells, the addition of E2 reduced the rate of HDR by ~30% (Fig. 7D). Thus endogenous cyclin D1 facilitates homologous DNA repair in MCF-7 cells. To determine whether both the DNA damage signaling (the formation of γH2AX foci), and the DNA damage repair process were both regulated by extranuclear E2 signaling, we compared the role of E2 vs EDC on the assays of homologous DNA repair. In contrast with the DNA damage signaling, the repair of damaged DNA repair was regulated by E2 and not by extranuclear ERα, assessed by addition of the E2 dendrimer (EDC) (Fig. 7E). Furthermore the use of ATM vs ATM/ATR inhibitors demonstrated the DNA repair process was dependent upon both ATM and ATR (Fig. 7F). Together these data are consistent with a model in which cyclin D1 conducts distinct functions. The membrane associated ERα bound cyclin D1 is associated with the augmentation of E2 mediated induction of AKT phosphorylation (Ser473) (Fig. 7G). In contrast with the DNA damage signaling, cyclin D1 facilitates homologous DNA repair, being recruited to γH2AX foci by E2 and inducing Rad51 abundance.
DISCUSSION
The current studies provide new evidence for cyclin D1 to enhance both the activity of the DNA damage signaling pathway and homology-directed DNA repair. First, UV-induced γH2AX was reduced by cyclin D1 siRNA. Second, the estradiol-mediated DNA damage response (DDR) was attenuated by cyclin D1 siRNA. A reduction in cyclin D1 reduced γH2AX foci. Importantly, these studies demonstrate that cyclin D1 binds to the cytoplamic membrane associated ERα which in turn governs γH2AX, a function distinct from the previously defined nuclear ERα/cyclin D1 interactions. Third, cyclin D1 was required for both basal and E2-induced expression of one of the key components of the DDR, Rad51. And finally cyclin D1 siRNA reduced homology directed DNA repair. Collectively, these observations demonstrate cyclin D1 mediates UV- and estrogen-induced DNA damage repair signaling via a novel mechanism in breast cancer epithelial cells.
Cyclin D1 participates in a non-genomic ERα function. In the current studies, E2 induced a sustained DDR. Exposure to estrogen or its catechol metabolites results in oxidative DNA damage and DNA strand breaks, however catechol metabolites function independently of ERα and E2 induces only low levels of oxidative stress (34–36). ERα-mediated transcription requires the decantenating activity of Topoisomerase IIβ, in which transient double strand breaks are generated (37, 38). E2 has been shown to induce double strand breaks and γH2AX foci (39) with careful kinetic analysis showing that E2 delays the resolution of the DDR essentially extending the response (9). In the current studies, the induction of the DDR by E2 was abrogated by cyclin D1 siRNA demonstrating the importance of cyclin D1 in maintaining the DDR. E2 functions by genomic and non-genomic signaling (10). Extranuclear signaling by the estrogen receptor can be dissected using estrogen-dendrimer conjugates (EDCs). The large abiotic nondegradable poly(amido)amine molecule is conjugated to estrogen via a stable covalent bond and has been shown to activate only extranuclear signaling (12, 13). Herein, cyclin D1 was required for EDC-induced γH2AX. Furthermore, cyclin D1 binds to ERα at the cytoplasmic membrane. Thus, cyclin D1 participates in an extranuclear ERα signaling pathway. As prior studies have demonstrated, cyclin D1 functions within the nuclear estrogen signaling pathway (24). Together, these studies suggest cyclin D1 functions in both extranuclear and nuclear estrogen signaling pathways (23). Consistent with the finding that endogenous cyclin D1 mediates UV-induced γH2AX, the relative abundance of cyclin D1 also governed the abundance of genes involved in the DDR in vivo (21). Using cyclin D1−/− mammary gland or cyclin D1−/− liver cells, gene expression analysis, determined through microarray, identified a subset of genes involved in the DDR.
Herein, E2-enhanced the γH2AX foci formation induced by recruitment of cyclin D1, ATM or NBS1 to chromatin. E2 inhibited homologous DNA repair. The finding that E2 inhibits HDR is consistent with our prior studies that E2 and BRCA1 function are mutually antagonistic (40). Herein endogenous cyclin D1 enhanced the HDR. Our recent studies provided evidence for a direct physical interaction between cyclin D1 and the distal end of the DNA damage repair complex (24). The RAD50/MRE11/NBS1 complex functionally interacts with BRCA1 to regulate repair of DNA double strand breaks. Cyclin D1 bound to BRCA1 via the carboxyl terminal domain of BRCA1 (21). The carboxyl terminus of BRCA1 binds cyclin D1 (21). The BRCA1 gene product is important for DNA repair of both chromosomal double stranded breaks by homologous recombination and transcription coupled repair (41, 42). Cyclin D1 colocalizes with BRCA1 by imunohistochemistry in breast cancer epithelial cells and co-associated by immune-precipitation (24). Chromatin immunoprecipitation (ChIP) assays have demonstrated dynamic interactions of ERα, cyclin D1 and BRCA1 at target estrogen response elements after E2 treatment. The current findings are consistent with prior observations. In ChIP assays BRCA1 inhibited E2-induced ERα recruitment, consistent with functional antagonism between BRCA1 and ERα signaling. At an ERα binding site, the BRCA1/ERα complex is displaced by cyclin D1, to form a cyclin D1/ERα complex (24). Cyclin D1 is also recruited in ChIP assays to BRCA1 binding sites, such as the Areg gene (21). Importantly, and consistent with the current studies demonstrating a role for cyclin D1 in promoting HDR, cyclin D1 genetic knockout demonstrated that cyclin D1 functioned as a scaffold factor for assembly of BRCA1 complex (21). The distinct functions of cytoplasmic vs nuclear cyclin D1 in the DNA damage response remains to be further explored in vivo.
E2 is known to delay the assembly and prolong the resolution of the DDR. E2 inhibition of DNA damage-induced check point activity might allow the passage of unrepaired mutations, which may in turn enhance mutation acquisition. The finding that cyclin D1 was required for the E2 induced delay in the assembly of the DDR is consistent with studies in which cyclin D1 was required for tumorigenesis by carcinogens and oncogenes. Oncogenes induce both double stranded DNA break (DSB) and the DDR (3–6). Cyclin D1 is a limiting step in cellular transformation by oncogenes (Ras, Notch, Stat3 and ErbB2 (43, 18)). DNA damage and cyclin D1 expression are both features of pre-invasive breast cancer (7, 8). Cyclin D1 overexpression is associated with induction of expression of genes involved in DNA replication and DNA damage checkpoint control (MCM3, MCM4, and several H2A histone family members) (44). Conversely, inducible mammary epithelial cell targeted cyclin D1 anti-sense transgenic mice showed reduced abundance of DNA damage repair signaling proteins, including MCM2, CDC20, and Rad51 (45). Together, these studies rise the possibility that cyclin D1 may participate in the induction of gene expression governing the DDR in early tumorigenesis.
Supplementary Material
Acknowledgments
This work was supported in part by R01CA70896, R01CA75503, R01CA86072 (R.G.P.), and R37DK015556 (J.A.K.). The Kimmel Cancer Center was supported by the NIH Cancer Center Core Grant P30CA56036 (R.G.P). This project is funded in part from the Dr. Ralph and Marian C. Falk Medical Research Trust and a grant from Pennsylvania Department of Health (R.G.P.). The Department specifically disclaims responsibility for analyses, interpretations or conclusions. Michael P. Lisanti and his laboratory were supported by the resources of the Thomas Jefferson University. We thank Dr. S. McMahon for constructive advice and comments on the manuscript.
Footnotes
For reprints: director@kimmelcancercenter.org
Conflicts of Interest: R.G.P. holds ownership interests in, and serves as CSO/Founder of the biopharmaceutical companies ProstaGene, LLC and AAA Phoenix, Inc. R.G.P. additionally holds ownership interests (value unknown) for several submitted patent applications.
There are no conflicts of interests associated with this manuscript.
References
- 1.Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25:409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 3.Denko NC, Giaccia AJ, Stringer JR, Stambrook PJ. The human Ha-ras oncogene induces genomic instability in murine fibroblasts within one cell cycle. Proc Natl Acad Sci U S A. 1994;91:5124–5128. doi: 10.1073/pnas.91.11.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 5.Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Jiao X, Wang C, Shirley LA, Elsaleh H, Dahl O, et al. Alternative cyclin d1 splice forms differentially regulate the DNA damage response. Cancer Res. 2010;70:8802–8811. doi: 10.1158/0008-5472.CAN-10-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 8.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 9.Pedram A, Razandi M, Evinger AJ, Lee E, Levin ER. Estrogen Inhibits ATR Signaling to Cell Cycle Checkpoints and DNA Repair. Mol Biol Cell. 2009;20:3374–3389. doi: 10.1091/mbc.E09-01-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin ER. Minireview: Extranuclear Steroid Receptors: Roles in Modulation of Cell Functions. Mol Endocrinol. 2010 doi: 10.1210/me.2010-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin ER, Pietras RJ. Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res Treat. 2008;108:351–361. doi: 10.1007/s10549-007-9618-4. [DOI] [PubMed] [Google Scholar]
- 12.Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, et al. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 13.Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and Extranuclear Pathway Inputs in the Regulation of Global Gene Expression by Estrogen Receptors. Mol Endocrinol. 2008 doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartell SM, Han L, Kim HN, Kim SH, Katzenellenbogen JA, Katzenellenbogen BS, et al. Non-nuclear-initiated actions of the estrogen receptor protect cortical bone mass. Mol Endocrinol. 2013;27:649–656. doi: 10.1210/me.2012-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold A, Papanikolaou A. Cyclin d1 in breast cancer pathogenesis. J Clin Oncol. 2005;23:4215–4224. doi: 10.1200/JCO.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 17.Casimiro MC, Crosariol M, Loro E, Ertel A, Yu Z, Dampier W, et al. ChIP sequencing of cyclin D1 reveals a transcriptional role in chromosomal instability in mice. J Clin Invest. 2012;122:833–843. doi: 10.1172/JCI60256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee RJ, Albanese C, Fu M, D’Amico M, Lin B, Watanabe G, et al. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol. 2000;20:672–683. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalides R, van Veelen N, Hart A, Loftus B, Wientjens E, Balm A. Overexpression of cyclin D1 correlates with recurrence in a group of forty-seven operable squamous cell carcinomas of the head and neck. Cancer Res. 1995;55:975–978. [PubMed] [Google Scholar]
- 20.Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488–3498. doi: 10.1101/gad.12.22.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casimiro MC, Wang C, Li Z, Di Sante G, Willmart NE, Addya S, et al. Cyclin D1 determines estrogen signaling in the mammary gland in vivo. Mol Endocrinol. 2013;27:1415–1428. doi: 10.1210/me.2013-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albanese C, D’Amico M, Reutens AT, Fu M, Watanabe G, Lee RJ, et al. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J Biol Chem. 1999;274:34186–34195. doi: 10.1074/jbc.274.48.34186. [DOI] [PubMed] [Google Scholar]
- 23.Shimura T, Ochiai Y, Noma N, Oikawa T, Sano Y, Fukumoto M. Cyclin D1 overexpression perturbs DNA replication and induces replication-associated DNA double-strand breaks in acquired radioresistant cells. Cell Cycle. 2013;12:773–782. doi: 10.4161/cc.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Fan S, Li Z, Fu M, Rao M, Ma Y, et al. Cyclin D1 antagonizes BRCA1 repression of estrogen receptor alpha activity. Cancer Res. 2005;65:6557–6567. doi: 10.1158/0008-5472.CAN-05-0486. [DOI] [PubMed] [Google Scholar]
- 25.Jirawatnotai S, Hu Y, Michowski W, Elias JE, Becks L, Bienvenu F, et al. A function for cyclin D1 in DNA repair uncovered by protein interactome analyses in human cancers. Nature. 2011;474:230–234. doi: 10.1038/nature10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bindra RS, Schaffer PJ, Meng A, Woo J, Maseide K, Roth ME, et al. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24:8504–8518. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soutoglou E, Misteli T. Activation of the cellular DNA damage response in the absence of DNA lesions. Science. 2008;320:1507–1510. doi: 10.1126/science.1159051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunn A, Stark JM. I-SceI-based assays to examine distinct repair outcomes of mammalian chromosomal double strand breaks. Methods Mol Biol. 2012;920:379–391. doi: 10.1007/978-1-61779-998-3_27. [DOI] [PubMed] [Google Scholar]
- 30.Soutoglou E, Dorn JF, Sengupta K, Jasin M, Nussenzweig A, Ried T, et al. Positional stability of single double-strand breaks in mammalian cells. Nat Cell Biol. 2007;9:675–682. doi: 10.1038/ncb1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13:2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Wang C, Jiao X, Lu Y, Fu M, Quong AA, et al. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Mol Cell Biol. 2006;26:4240–4256. doi: 10.1128/MCB.02124-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SH, Katzenellenbogen JA. Hormone-PAMAM dendrimer conjugates: polymer dynamics and tether structure affect ligand access to receptors. Angew Chem Int Ed Engl. 2006;45:7243–7248. doi: 10.1002/anie.200601923. [DOI] [PubMed] [Google Scholar]
- 34.Roy D, Liehr JG. Estrogen, DNA damage and mutations. Mutat Res. 1999;424:107–115. doi: 10.1016/s0027-5107(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 35.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21:40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 36.Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78:161–170. doi: 10.1016/j.steroids.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, et al. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science. 2006;312:1798–1802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- 38.Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 2009;9:327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson LM, Lees-Miller SP. Estrogen receptor alpha-mediated transcription induces cell cycle-dependent DNA double-strand breaks. Carcinogenesis. 2011;32:279–285. doi: 10.1093/carcin/bgq255. [DOI] [PubMed] [Google Scholar]
- 40.Fan S, Wang J, Yuan R, Ma Y, Meng Q, Erdos MR, et al. BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science. 1999;284:1354–1356. doi: 10.1126/science.284.5418.1354. [DOI] [PubMed] [Google Scholar]
- 41.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 42.Zhong Q, Chen CF, Li S, Chen Y, Wang CC, Xiao J, et al. Association of BRCA1 with the Rad50-Mre11-p95 complex and the DNA Damage response. Science. 1999;285:747–750. doi: 10.1126/science.285.5428.747. [DOI] [PubMed] [Google Scholar]
- 43.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 44.Fu M, Wang C, Rao M, Wu X, Bouras T, Zhang X, et al. Cyclin D1 represses p300 transactivation through a cyclin-dependent kinase-independent mechanism. J Biol Chem. 2005;280:29728–29742. doi: 10.1074/jbc.M503188200. [DOI] [PubMed] [Google Scholar]
- 45.Sakamaki T, Casimiro MC, Ju X, Quong AA, Katiyar S, Liu M, et al. Cyclin D1 determines mitochondrial function in vivo. Mol Cell Biol. 2006;26:5449–5469. doi: 10.1128/MCB.02074-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.