Abstract
Background
There is a critical need for safer and more effective pharmacological management of atrial fibrillation (AF) in the setting of heart failure (HF).
Methods and Results
This study investigates the electrophysiological, anti- and pro-arrhythmic effects of a clinically-relevant concentration of ranolazine (5 μM) in coronary-perfused right atrial and left ventricular preparations isolated from the hearts of HF dogs. HF was induced by ventricular tachypacing (2-6 weeks at 200-240 beats/min; n=17). Transmembrane action potentials were recorded using standard microelectrode techniques. In atria, ranolazine slightly prolonged action potential duration but very significantly depressed sodium channel current (INa)-dependent parameters causing a reduction of maximum rate of rise of the action potential upstroke, a prolongation of the effective refractory period secondary to development of post-repolarization refractoriness, an increase in diastolic threshold of excitation and atrial conduction time. Ranolazine did not significantly alter these parameters or promote arrhythmias in the ventricles. Ranolazine produced greater inhibition of peak INa in atrial cells isolated from HF vs. normal dogs. A single premature beat reproducibly induced self-terminating AF in 10/17 atria. Ranolazine (5 μM) suppressed induction of AF in 7/10 (70%) atria. In the remaining 3 atria, ranolazine reduced frequency and duration of AF.
Conclusions
Our results demonstrate more potent suppression of AF by ranolazine in the setting of HF than previously demonstrated in non-failing hearts and absence of ventricular proarrhythmia. The data suggest that ranolazine may be of benefit as an alternative to amiodarone and dofetilide in the management of AF in patients with HF.
Keywords: cardiac arrhythmias, electrophysiology, pharmacology, antiarrhythmic drugs, sodium channel blockers
Heart failure (HF) and atrial fibrillation (AF) frequently coexist, and can promote each other. Pharmacological options for rhythm control of patients with HF are limited to amiodarone and dofetilide in the United States and amiodarone in the European Union.1 These agents cause cardiac and extra-cardiac adverse effects in many cases. There is a critical need for safer and more effective pharmacological rhythm control treatments of AF in the setting of HF. Most clinically available antiarrhythmic agents are not recommended in patients with AF who also have HF, due to the risk of induction of ventricular proarrhythmia. This limitation can be minimized with the use of atrial-selective agents.2,3
The actions of ranolazine to produce atrial-selective electrophysiological effects, enabling it to suppress AF without inducing ventricular arrhythmias have been demonstrated in several “healthy” canine and porcine models.4,5 In the present study, we investigated the electrophysiological and anti-AF effects as well as ventricular proarrhythmic potential of a clinically-relevant and relatively low concentration of ranolazine (5 μM) in coronary-perfused right atrial (RA) and left ventricular (LV) preparations isolated from the hearts of dogs with HF.
Methods
This investigation conformed with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Pub. No 85-23, Revised 1996) and was approved by the Animal Care and Use Committee of the Masonic Medical Research Laboratory (MMRL). We used a well-established protocol involving long-term ventricular tachypacing (VTP, 200-240 beat/min for 2-6 weeks) to produce a HF model in dogs.6-8 HF in this well-characterized model has been confirmed by hemodynamic and histopathologic changes as well as other clinical signs, such as lethargy, dyspnea, and edema. This model recapitulates many features of clinical HF (including LV systolic and diastolic dysfunctions).
Pacemaker implantation for right VTP was performed at the Cornell University Hospital for Animals, Ithaca, NY, using previously described protocols.6-8 After recovering from the procedure (1-2 days), they were transported to MMRL. Within 2 days of arrival at MMRL the dogs were continuously paced at 200-240 bpm for a period of 2 or 6 weeks. The dogs were constantly monitored for clinical signs of HF and followed by a licensed veterinarian weekly. Pulse rate was monitored daily and a 12 lead electrocardiogram (ECG) was recorded weekly to ensure proper pacing.
After 2-6 weeks of VTP, the HF dogs (≥1 year old) were anticoagulated with heparin and anesthetized with pentobarbital (with an initial dose of 30-35 mg/kg, IV and, if needed, an additional dose of 15-20 mg/kg, IV was used). After loss of corneal reflex, the chest was opened via a left thoracotomy, the heart excised and placed in a cardioplegic solution consisting of cold (4°C) Tyrode's solution containing 8.5 mM [K+]o.
Arterially-perfused canine RA and LV wedge preparations
Experiments were performed using isolated coronary-perfused canine RA and LV preparations (~3×1.5×1 cm). Isolation and perfusion of the preparations were as previously described.4,8,9 Unfolded RA with attached rim of the right ventricle was cannulated and perfused through the ostium of the right coronary artery; the LV wedge was perfused through a diagonal branch of the left anterior descending coronary artery. Unperfused tissue was removed with a razor blade. Cut ventricular and atrial branches were ligated with silk thread. The preparations were then transferred to a temperature-controlled bath and arterially-perfused with Tyrode's solution by use of a roller pump. For both atrial and ventricular preparations, the composition of the Tyrode's solution was (in mM): NaCl 129, KCl 4, NaH2PO4 0.9, NaHCO3 20, CaCl2 1.8, MgSO4 0.5, and D-glucose 5.5, buffered with 95% O2 and 5% CO2 (37.0±0.5°C).
Transmembrane action potential (AP) recordings were obtained either differentially or referenced to ground using floating glass microelectrodes (2.7 M KCl, 10-25 MΩ DC resistance) connected to a high input impedance amplification system. A pseudo-ECG was recorded using two electrodes consisting of Ag/AgCl half cells placed in the Tyrode's solution bathing the preparation, 1.0 to 1.2 cm from the two opposite sides of the atrial or ventricular coronary-perfused preparations. Diastolic threshold of excitation (DTE) was determined by increasing stimulus intensity in 0.01 mA steps. Effective refractory period (ERP) was measured by delivering premature stimuli after every 10th regular beat at a pacing cycle length (CL) of 500 (with 5 ms resolution; stimulation with a 2xDTE amplitude). Post-repolarization refractoriness (PRR) was recognized when ERP exceeded 90% action potential duration (APD90) in the ventricle and APD70 in atria. Note that at baseline ventricular ERP coincides with APD90, whereas atrial ERP generally coincides with APD70-75.4 Conduction velocity was measured from the duration of “QRS” in ventricles and “P wave” on the pseudo–ECG at a level of representing 30% of QRS or P wave amplitude in isolated preparations. In contracting perfused preparations, a large variability in maximum rate of rise of the AP upstroke (Vmax) measurements is normally encountered at any given condition, primarily due to variability in the amplitude of phase 0 of the AP which strongly determines Vmax values. Vmax changes were determined by comparing the largest Vmax recorded before and after addition of ranolazine. The shortest S1-S1 permitting 1:1 activation was measured by progressively shortening pacing CL starting from a CL of 500 ms (at DTEx2 determined at a CL=500 ms).
The equilibration period for the preparations once placed in the bath was 30-60 min. The electrophysiological parameters were measured at a pacing CL of 500 ms before and after addition of ranolazine (5 μM) to the coronary perfusate. Tissues were exposed to ranolazine for ≥15 min before start of data collection. Electrophysiological data from atrial preparations were largely obtained from the endocardial pectinate muscle areas. Ventricular AP data were obtained from immediate subendocardial region and DTE and ERP from the endocardial surface. Programmed electrical stimulation (PES, a single premature stimulation, with amplitude of stimulation of 2xDTE) was used to induce atrial and ventricular arrhythmias. In some atrial preparations, rapid atrial pacing (CL=80-100 ms for 3-10 sec) was also used to induce atrial arrhythmias.
Single isolated myocytes
Single myocytes were obtained by enzymatic dissociation of the right atrium obtained from normal and tachypaced hearts.7 Whole cell sodium channel current (INa) was recorded in low external Na+ at 15°C to ensure adequate voltage control.10 Experiments were performed using a MultiClamp 700A (Molecular Devices, Sunnyvale, CA). Command voltages were delivered, and data were acquired via a DigiData 1322 computer interface using the pCLAMP 9 program suite (Molecular Devices) with data stored on computer hard disk. Patch pipettes were pulled from borosilicate glass (1.5-mm outer diameter and 1.1-mm inner diameter) on a model PP-830 vertical puller (Narishige Instruments, East Meadow, NY). The electrode resistance was 0.9–2.0 MΩ when filled with the internal solution (see below). The membrane was ruptured by applying negative pressure and series resistance compensated by 70–80%. Whole cell current data were acquired at 10–50 kHz and filtered at 5 kHz. Currents were normalized to cell capacitance.
The external solution used in the voltage-clamp experiments contained (in mM): 2 CaCl2, 10 glucose, 1 MgCl2, 40 NaCl, 120 N-methyl-D-glucamine, and 10 HEPES, with pH adjusted to 7.4 with HCl. CdCl2 (300 μM) was added to the external solution to block Ca2+ current and to partially reduce INa. The pipette solution contained (in mM): 1 MgCl2, 5 NaCl, 145 Cs-aspartate, 10 HEPES, 5 EGTA, and 5 MgATP. pH was adjusted to 7.1 with CsOH. Peak INa was evaluated using a square depolarization pulse to -10 mV for 100 ms from at holding potential of -140 mV to -20 mV for 5 seconds, applied once every 10 seconds. In addition, recordings of INa were made at least 5 min after rupture to minimize the effects of the time-dependent negative shift of steady-state inactivation that occurs in conventional voltage-clamp experiments.
Drugs
Ranolazine (Gilead Sciences, Foster City, CA), dissolved in distilled water, was prepared fresh as a stock of 5 mM before each experiment.
Statistics
Statistical analysis was performed using a paired and unpaired Student's t-test and one-way repeated measures or multiple-comparison ANOVA followed by Bonferroni's test, as appropriate. All data are expressed as means±SD. Statistical significance was assumed at P<0.05.
Results
Isolated coronary-perfused atrial and ventricular preparations
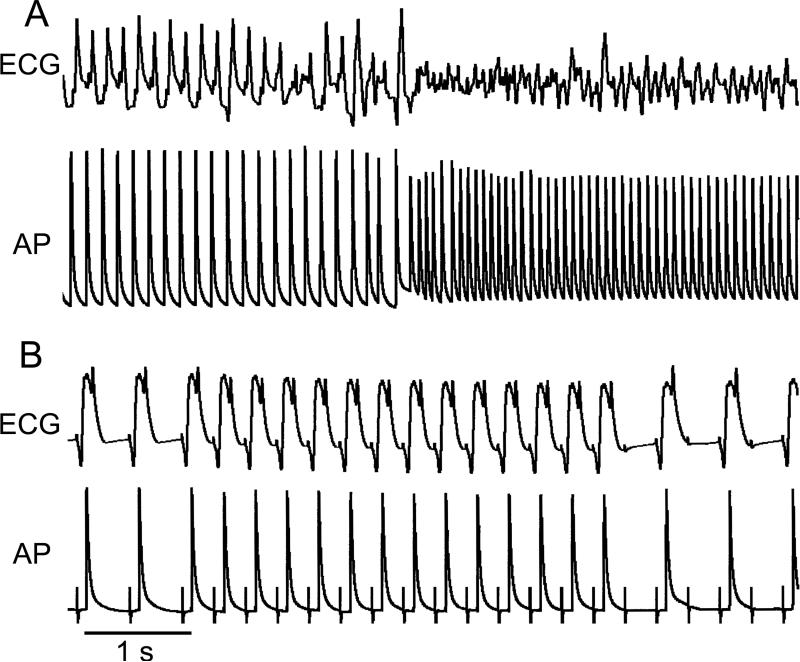
A single premature beat reproducibly induced self-terminating AF and/or atrial flutter (AFl) in 10 out of 17 atrial HF preparations (Figure 1; Table 1). Electrical and structural characteristics of atria that did and did not develop AF have been reported previously.8 The average duration of the vulnerable period, defined as the range of diastolic intervals during which a single extrastimulus could induce AF, was 36±23 ms (n=10). The addition of ranolazine (5 μM) to the coronary perfusate completely prevented the induction of arrhythmias in 7 of the 10 atria. In the remaining atrial preparations (n=3), the arrhythmia generally became slower and briefer and the duration of vulnerable period was reduced (Table 1). Ranolazine also prevented the induction of AF/AFl by rapid pacing in 4 out of 5 tested atrial preparations (80%; Figure 1B). The anti-AF/AFl effects of ranolazine were associated with a reduction of excitability, which prolonged PRR, thereby preventing development of closely coupled premature beats (that initiate AF/AFl) or the maintenance of rapid activation (as illustrated in Figure 1B).
Figure 1.
Sustained atrial flutter/fibrillation (A) induced by a single premature beat recorded in a coronary-perfused right atrial preparation isolated from a dog with heart failure. (B) Ranolazine (5 μM) terminated the arrhythmia and depressed excitability resulting in failure of 1:1 activation at a cycle length of 300 ms, thus preventing re-induction of the arrhythmia. Pacing artifacts are seen on the action potential (AP) tracing. ECG, electrocardiogram.
Table 1.
Potent anti-arrhythmic effect of ranolazine (5 μM) in coronary-perfused canine right atrial preparations isolated from dogs with heart failure
| AF/AFl incidence | AF/AFl duration (sec) | Vulnerable window (ms) | |
|---|---|---|---|
| Control | 10/17 atria | 109±178 | 36±23 |
| Ranolazine | 3/10 atria | 4.0±4.3* | 13±6 |
The longest atrial fibrillation/atrial flutter (AF/AFl) episode recorded in each atrium was taken for calculation of AF/AFl duration.
P<0.05 vs. control (paired t-test, three atria that develop AF both before and after ranolazine).
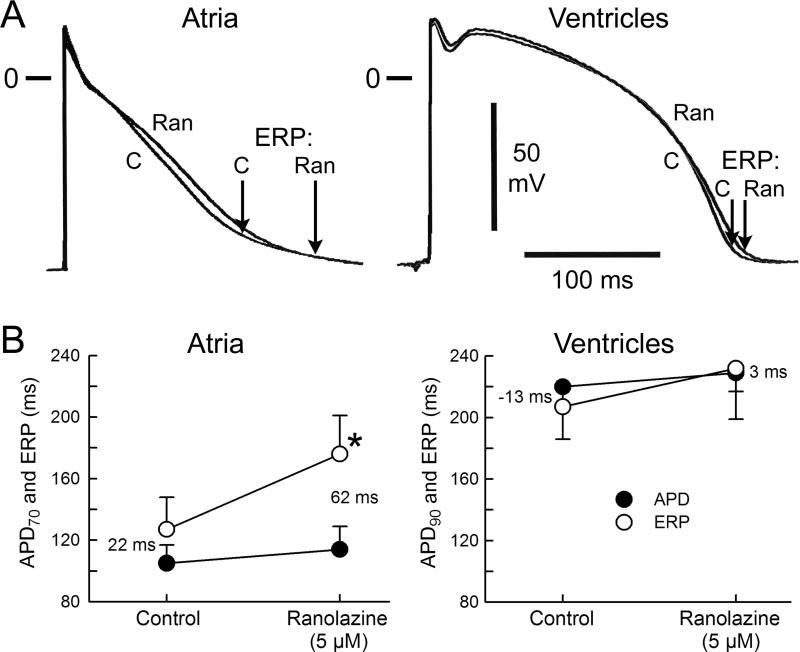
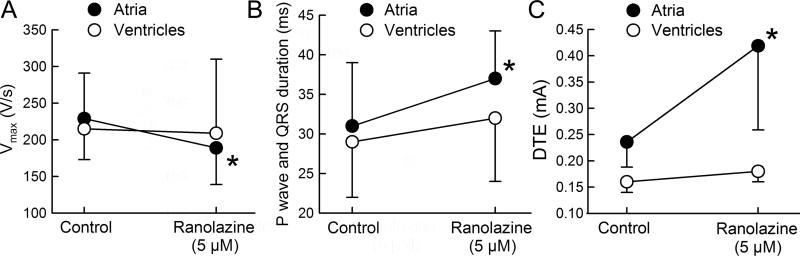
The actions of ranolazine on electrophysiological parameters were determined in the atrial preparations that reproducibly developed AF/AFl (n=10). Figures 2 and 3 summarize the effect of ranolazine at RA sites displaying a short ERP. These were the sites of briefest refractoriness where a premature stimulus was successful in inducing AF (commonly in the region of the pectinate muscles). Ranolazine (5 μM) caused little change in APD70 but significantly prolonged the ERP (Figure 2). The ERP prolongation was largely due to an increase in PRR (Figure 2). Vmax values were significantly reduced, “P wave” duration was prolonged, and DTE was significantly increased following exposure to 5 μM ranolazine (Figure 3). The shortest S1-S1 permitting 1:1 activation increased significantly as well (from 134±15 ms in control to 243±48 ms after ranolazine; p<0.001; n=9). There was no statistically-significant difference in ranolazine-induced prolongation of ERP and PRR between atria that developed AF (n=3) and those that did not (n=7) (ΔERP was 56±15 and 46±13 ms, p=0.27; and ΔPRR was 65±9 and 61±28 ms, p=0.80, respectively. CL=500 ms). It is noteworthy, however, that even in those preparations that remained inducible, ranolazine significantly reduced AF duration (Table 1).
Figure 2.
Ranolazine (Ran) caused little change in action potential duration (APD) of atrial and ventricular tissues, but caused a greater prolongation of effective refactory period (ERP) in atrial vs. ventricular preparations isolated from the hearts of dogs with heart failure. Shown are typical AP tracings (A) and avarage data (B) on the effect of ranolazine on APD and ERP. Ranolazine-induced atrial-selective ERP prolongation secondary to accentuation of post-repolarization refractoriness (depicted by the numbers). Of note, ERP corresponds to APD70-75 in atria and to APD90 in ventricles. C, control; Cycle length=500 ms. n=6-10. *p<0.05 vs control (paired t-test). Means±SD.
Figure 3.
Ranolazine significantly reduced maximum rate of rise of the action potential upstroke (Vmax; A) and conduction velocity (B; estimated from the duration of the P wave and QRS duration) as well as increased diastolic threshold of excitability (DTE; C) in atrial but not in ventricular preparations isolated from dogs with heart failure. Cycle length=500 ms. n=6-10. *p<0.05 vs control (pairted t-test). Means±SD.
In contrast to its effects in atria, ranolazine (5 μM) did not significantly affect APD, ERP, Vmax, QRS duration, and DTE in coronary-perfused LV wedge preparations isolated from dogs with HF (Figures 2 and 3). Ranolazine did not promote arrhythmias in these ventricular preparations (0/8 tested). In the absence of drug, PES induced polymorphic ventricular tachycardia in 3 out of 17 LV wedge preparations isolated from dogs with HF and all arrhythmic episodes lasted less than 5 seconds. Ranolazine (5 μM) prevented the induction of arrhythmias in 2 of 3 ventricular wedge preparations.
Isolated atrial myocytes
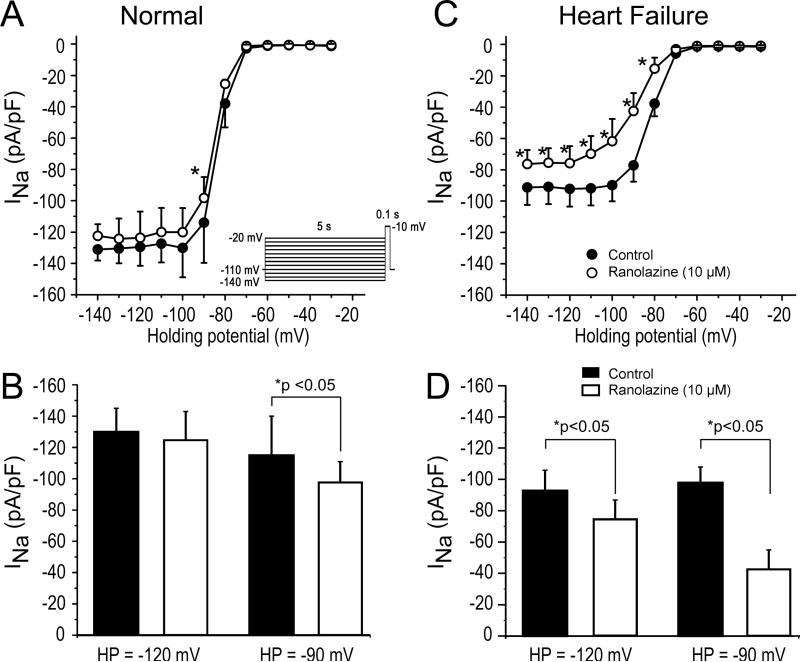
We next investigated the effect of ranolazine to inhibit peak INa in atrial myocytes isolated from HF vs. normal dogs. In the absence of drug, INa density was smaller in cells isolated from HF dogs (Figure 4A-D). Ranolazine (10 μM) produced significantly greater inhibition of peak INa in atrial cells isolated from the dogs with HF. The efficacy of ranolazine-induced block of INa was greater at -90 mV than at -120 mV in both normal and HF atrial cells.
Figure 4.
Ranolazine causes a significantly greater reduction of peak cardiac sodium channel current (INa) in atrial myocytes isolated from heart failure (HF) vs. normal dogs. A: Relation showing magnitude of INa (at -10 mV) as a function of voltage for normal atrial cells (filled circles). Application of ranolazine (open circles) resulted in minor tonic block of INa. B: Bar graph highlighting the differences in INa magnitude at 2 different holding potentials (HPs) in the absence and presence of ranolazine. C: Relation showing size of INa (at -10 mV) as a function of voltage for HF atrial cells (filled circles). Application of ranolazine (open circles) resulted in significant tonic block of INa. D: Bar graph highlighting the differences in INa magnitude in HF atrial cells. The size of INa in HF atrial cells was reduced compared normal atrial cells and application of ranolazine resulted in greater tonic block. *p<0.05. n=6-8 (oneway repeated measures or multiple-comparison ANOVA followed by Bonferroni's test).
The reduction in peak INa due to ranolazine may be due to a shift in Na+ channel availability. Therefore, we next evaluated steady-state inactivation using a standard pre-pulse test pulse protocol in the absence and presence of 10 μM ranolazine. Peak current after a 5 s prepulse was normalized to the maximum current and plotted as a function of the prepulse voltage, and a Boltzman function was fitted to the data (Figure 4E). For atrial cells, V1/2 in absence and presence of ranolazine was -84.5±0.15 and -87.1±0.18 mV, respectively (p<0.05); for ventricular cells, V1/2 in absence and presence of ranolazine was -77.0±0.34 and -77.4±0.20 mV (p=0.32).
Discussion
Our data demonstrate that ranolazine at a relatively low concentration (5 μM), well within the therapeutic range of the drug, causes marked atrial-selective electrophysiological effects and is very effective in preventing induction of AF/AFl in coronary-perfused atria isolated from the heart of dogs with VTP-induced HF. Moreover, ranolazine did not induce ventricular arrhythmias in the setting of HF. Our experimental data suggest that ranolazine may be safe and effective for rhythm control management of AF in patients with HF.
Atrial selective electrophysiologic effect of ranolazine in canine HF heart
Previous in vitro and in vivo studies have demonstrated atrial-selective effects of ranolazine (5-10 μM) to block peak INa and depress peak INa-mediated parameters in “healthy” cardiac preparations.4,5,10 The present study demonstrates that a relatively low concentration of ranolazine (5 μM) has a more potent atrial-selective effect to prolong ERP and PRR and to depress INa in a diseased model of non-ischemic dilated cardiomyopathy induced by 2-6 weeks of VTP compared to healthy controls.4,11
Ranolazine (5 μM) did not significantly prolong repolarization in either atria and ventricles isolated from the hearts dogs with HF, an effect generally consistent with that observed in healthy canine atria and ventricles.4,11 This effect appears to be due to the fact that ranolazine blocks both depolarizing late INa and repolarizing rapidly activating K+ currents (IKr), which act to abbreviate and prolong APD, respectively.
Ranolazine (5-10 μM) is known to produce APD abbreviation in ventricular cells when APD is significantly prolonged (APD90 ≥500 ms; in the conditions of long QT syndromes and ischemic HF at slow pacing rates).12,13 It is noteworthy that late INa is increased in ischemic HF model.12 Whether late INa is altered in non-ischemic HF, used in the present study, remains to be established.
Antiarrhythmic potential and safety of ranolazine in heart failure
Ranolazine, originally introduced as an anti-anginal agent, has a significant anti-arrhythmic potential in both ventricles and atria.9,13 In atria, the primary antiarrhythmic action is most likely due to block of peak INa , whereas in the ventricles it is due to block of late INa.13,14 A low prevalence of ventricular arrhythmias in our current study (3 out of 17 wedges) precluded us from a proper evaluation of anti-arrhythmic potential of ranolazine. The limited data obtained indicate that ranolazine (5 μM) may be quite effective in preventing ventricular arrhythmias in the setting of HF.
An important result of our study is that ranolazine did not induce ventricular proarrhythmia in our canine HF model. The lack of proarrhythmic effect in the ventricles is likely due to the relatively minor effects of ranolazine on peak INa mediated parameters in the ventricles of dogs with HF. The high propensity of ventricles for development of INa blocker-induced proarrhythmia is well recognized, but generally limited to agents that dissociate slowly from the cardiac sodium channel.3,15 Ranolazine has rapid unbinding kinetics.4,10 The lack of effect of ranolazine to induce major QT prolongation or Torsade de Pointes (TdP) is consistent with the results of previous clinical and experimental investigations.13,16,17 Indeed, ranolazine has been repeatedly demonstrated to suppress early afterdepolarizations and TdP in various experimental models.9,18,19
Ranolazine was more effective in preventing the induction of AF in atria from dogs with HF than from healthy dogs. At a concentration of 5 μM, ranolazine prevents the induction of persistent AF in our vagal model in 29% of atria.11 In comparison, this concentration of the drug prevented induction of AF/AFl in 70% of atria from dogs with HF. This greater effect of ranolazine to suppress AF in the HF model than in the non-HF acetylcholine (ACh) AF model is consistent with the greater effect of 5 μM ranolazine to alter sodium channel-mediated parameters in the former than in the latter AF model (Table 2). It is also consistent with a greater efficacy of ranolazine to inhibit peak INa in HF vs. healthy atrial cells (Figure 4). ACh markedly abbreviates APD and ERP and hyperpolarizes the resting membrane potential (RMP). Both of these factors significantly reduce the efficacy of ranolazine and of similar sodium channel blockers to inhibit peak INa.4,10 As a consequence, the effectiveness of INa blockers may be underestimated in the ACh-mediated AF model. In the HF atria, RMP is more likely to be depolarized, which augments ranolazine's effectiveness to inhibit peak INa.10
Table 2.
More potent effect of ranolazine (Ran) to reduce atrial fibrillation (AF) inducibility and depress sodium channel-dependent parameters in heart failure (HF) vs. vagal non-HF models of AF
| AF Model | ΔVmax | ΔVmax 500-300 ms | ΔERP | Δ PRR | ΔS1 –S1 | Reduction of AF/AFl inducibility |
|---|---|---|---|---|---|---|
| Ran 5 μM in HF Model | −18%* | −32%* | +49 ms† (+39%) | +40 ms† | +109 ms† (+81%) | 70% |
| Ran 5 μM in Vagal Non-HF Model | −4% | −9% | +20 ms (38%) | + 11 ms | +38 ms (+68%) | 29% |
| Ran 10 μM in Vagal Non-HF Model | −7% | −15% | +57 ms (+98%) | +47 ms | +108 ms (+148%) | 80% |
Shown are changes of average values. Data from non-HF atria are from Burashnikov et al.4, 11 S1-S1 = the shortest S1-S1 pacing interval permitting a 1:1 activation. Pacing cycle length (CL) = 500 ms (except for the shortest S1–S1). Vagal non-HF Model = control atria exposed to acetylcholine (1.0 μM). Vmax 500-300 ms = maximum rate of rise of the action potential upstroke (Vmax) changes in % following acceleration of pacing rate from a CL of 500 to 300 ms.
p<0.05 vs Ran 5 μM and μM 10 in Vagal non-HF model.
p<0.05 vs Ran 5 μM in Vagal non-HF model (unpaired t-test).
N=10 for each. ERP, effective refractory period; PRR, post-repolarization refractoriness.
ACh-mediated and HF-mediated AF models are quite distinct. In healthy atria, ACh markedly abbreviates atrial APD and ERP allowing for induction of AF by a single premature beat or burst pacing in 100% of atria. The induced arrhythmia is commonly persistent. In the HF model of AF, all that is needed to induce in AF, in the majority of cases, is a single premature beat, but however most episodes are non-sustained. Although the ACh model is representative of vagally-induced AF, the VTP-induced HF model is more clinically relevant. The anti-arrhythmic efficacy of 5 μM ranolazine in the HF AF model is similar to that of the ischemia/reperfusion-mediated AF model.4 In the ischemic model, 5 μM ranolazine prevented AF induction in 60% of atria (3/5). In all models of AF tested, it seems clear that ranolazine's action is due largely to atrial-selective inhibition of peak INa.
Rate-dependent induction of PRR contributed prominently to the anti-AF effectiveness of ranolazine (Table 2). Interestingly, whereas slowly dissociating INa blockers such as propafenone induce significant PRR in both atria and ventricles,20,21 rapidly dissociating INa blockers like ranolazine and amiodarone are atrial-selective in their ability to induce PRR.4,22
Frommeyer et al. reported that ranolazine (10 μM) effectively suppresses AF in Langendorff-perfused hearts isolated from control and VTP-induced HF rabbits. However, AF was induced by pretreatment with ACh+Isoproterenol and burst-pacing.23 In these rabbit AF models, as in our studies, anti-AF efficacy of ranolazine was associated with induction of PRR. There are a number of important differences between this study and our current investigation. First, we investigated the anti-arrhythmic effect of ranolazine on AF induced by a single premature beat in the setting of HF without the need to use ACh+isoproterenol or burst-pacing. It is noteworthy that ACh, isoproterenol, and burst pacing can each induce AF in control hearts. Second, in contrast to our study, Frommeyer et al. did not report the electrophysiological and anti-AF effects of ranolazine in the absence of ACh+isoproterenol,23 confounding assessment of effect of ranolazine in the setting of HF. Third, we used a more clinically relevant concentration of ranolazine (i.e., 5 μM vs. 10 μM used by Frommeyer et al.23). Fourth, we evaluated the electrophysiological effects of ranolazine in both atria and ventricles, evaluating its proarrhythmic potential. Finally, we directly measured the efficacy of ranolazine to inhibit INa in atrial myocytes isolated from control and HF dogs.
There are important differences in hemodynamic, structural, and electrophysiological parameters in a dog's ventricular tachypaced for 2-3 weeks vs. 5-6 weeks. Our recent study, demonstrated greater AF vulnerability associated with moderate vs. severe electro-structural atrial remodeling.8 Ranolazine prevented AF induction in 71% of atria isolated from the “2-3 week tachypaced” group and in 66% of atria from the “5-6 week tachypaced” group.
Study Limitations
HF patients commonly present with a constellation of intra- and extra-cardiac diseases, with various underlying, often overlapping, etiologies (e.g., coronary artery diseases and hypertension), and the disease commonly develops gradually. In this respect, although the canine VTP-induced HF model recapitulates major HF clinical features, it does not reproduce the full spectrum of clinical phenotypes, being largely a non-ischemic dilated cardiomyopathy HF model. The electrophysiological data were obtained from isolated cardiac preparations which do not entirely recapitulate in vivo conditions. Our measurements were obtained from the right atrium and it is possible that these data may not apply to the left atrium.
Clinical implications
Sinus rhythm is preferable in patients with HF and a history of AF, provided that the anti-AF therapeutic approach is safe and effective. Current pharmacological rhythm control management of patients with HF and AF is limited to amiodarone and dofetilide. While these agents are generally safer than other antiarrhythmics, they nevertheless cause significant adverse effects in some patients, due to development of prolonged QT intervals and TdP in the case of dofetilide but rarely in the case of amiodarone, and due to extra-cardiac toxicity in the case of amiodarone.1 Due to a high risk of induction of ventricular arrhythmias in patients with structural heart diseases, propafenone and flecainide (potent INa blockers and effective anti-AF drugs) are contraindicated in patients with HF. Available evidence indicates that ranolazine is safe for both short and long-term administration in patients with HF.13,17 Interestingly, ranolazine's ion channel profile is similar to that of amiodarone9 and amiodarone, like ranolazine, is also an atrial-selective peak INa inhibitor.22,24 In the present study, we demonstrate that ranolazine is also an atrial-selective sodium channel blocker in the setting of HF. This similarity notwithstanding, ranolazine does not have any of the severe extra-cardiac adverse effects of amiodarone. Propafenone and flecainide are not atrial-selective in their action to block sodium channel activity.3,21 The anti-AF efficacy of ranolazine in HF patients is poorly studied, but available evidence indicate that this drug can be an effective anti-AF agent in patients with HF.25,26 Although the clinical antifibrillatory effect of long-term ranolazine therapy in patients has not been tested, our data suggest that ranolazine may be a better alternative to amiodarone or dofetilide in patients with HF, when taking into account both safety and anti-AF efficacy.
In an experimental vagal model of AF, we have shown a synergistic action of ranolazine and amiodarone as well as ranolazine and dronedarone to suppress AF and to atrial-selectively depress sodium channel-dependent pararamters.11,27 Future studies might be directed at assessing the effectiveness of the combination of ranolazine together with low dose amiodarone or low-dose dronedarone for the management of AF in the setting of HF.
Secondary to its inhibition of late INa and direct effect on the myofilaments, ranolazine has been shown to ameliorate diastolic dysfunction in several HF animal and human in vitro studies.28-31 Ranolazine's effect to improve LV diastolic dysfunction in HF patients with preserved left ventricular ejection fraction (LVEF) (often referred to as diastolic HF) is being tested in a clinical trial.32 In most patients with HF, systolic and diastolic abnormalities coexist. Diastolic dysfunction promotes AF and appears to contribute to the disease phenotype at all levels of LVEF impairment.33 Interestingly, the prevalence of AF is significantly greater in patients with HF with preserved than reduced LVEF.34 Ranolazine may be particularly useful in the management of patients with HF who have diastolic dysfunction and AF, because it has both anti-HF and anti-AF actions. As with all experimental models, the clinical relevance of our experimental data is clearly speculative, but reasonable in light of this model's demonstrated value in predicting clinical outcomes (The Harmony Trial; Late-Breaking Clinical Trials III. The Heart Rhythm Society Meeting 2014).
Conclusions
Our results indicate that ranolazine possesses potent anti-AF efficacy and does not promote ventricular proarrhythmia in a canine HF model. Ranolazine produces more potent atrial-selective depression of peak INa-mediated parameters in atria isolated from dogs with HF than those form normal animals. Rate-dependent inhibition of peak INa leading to development of post-repolarization refractoriness appears to be the primary mechanism underlying the anti-AF efficacy of ranolazine in HF-mediated model of AF. In view of the clinical safety of ranolazine in patients with structural heart disease,16,17 our results suggest a need for studies specifically designed to evaluate the clinical utility of ranolazine for rhythm control of AF in patients with HF.
Acknowledgments
We gratefully acknowledge the expert technical assistance of Judy Hefferon and Robert Goodrow.
Sources of Funding
This work was supported by a grant from Gilead Science, Foster City, CA; National Institutes of Health [HL47678 to CA]; New York State Stem Cell Science: NYSTEM [C026424 to CA]; and the Masons of New York, Florida, Massachusetts, Connecticut, Maryland and Rhode Island.
Footnotes
Disclosures
Dr. Antzelevitch is a consultant for and received research support from Gilead Sciences. Dr. Belardinelli is an employee of Gilead Sciences. The other authors have no conflicts of interest to disclose. Pacemakers used for tachypacing were a kind gift from Medtronic, Inc., Minneapolis, MN.
References
- 1.Kirchhof P, Curtis AB, Skanes AC, Gillis AM, Samuel WL, John CA. Atrial fibrillation guidelines across the Atlantic: a comparison of the current recommendations of the European Society of Cardiology/European Heart Rhythm Association/European Association of Cardiothoracic Surgeons, the American College of Cardiology Foundation/American Heart Association/Heart Rhythm Society, and the Canadian Cardiovascular Society. Eur Heart J. 2013;34:1471–1474. doi: 10.1093/eurheartj/ehs446. [DOI] [PubMed] [Google Scholar]
- 2.Ehrlich JR, Nattel S. Novel approaches for pharmacological management of atrial fibrillation. Drugs. 2009;69:757–774. doi: 10.2165/00003495-200969070-00001. [DOI] [PubMed] [Google Scholar]
- 3.Burashnikov A, Antzelevitch C. Novel pharmacological targets for the rhythm control management of atrial fibrillation. Pharmacol Ther. 2011;132:300–313. doi: 10.1016/j.pharmthera.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar K, Nearing BD, Carvas M, Nascimento BC, Acar M, Belardinelli L, Verrier RL. Ranolazine exerts potent effects on atrial electrical properties and abbreviates atrial fibrillation duration in the intact porcine heart. J Cardiovasc Electrophysiol. 2009;20:796–802. doi: 10.1111/j.1540-8167.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 6.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 7.Cordeiro JM, Calloe K, Moise NS, Kornreich B, Giannandrea D, Di Diego JM, Olesen SP, Antzelevitch C. Physiological consequences of transient outward K+ current activation during heart failure in the canine left ventricle. J Mol Cell Cardiol. 2012;52:1291–1298. doi: 10.1016/j.yjmcc.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burashnikov A, Di Diego JM, Sicouri S, Doss MX, Sachinidis A, Barajas-Martinez H, Hu D, Minoura Y, Moise NS, Kornreich BG, Chi L, Belardinelli L, Antzelevitch C. A temporal window of vulnerability for development of atrial fibrillation with advancing heart failure. Eur J Heart Fail. 2014;16:271–280. doi: 10.1002/ejhf.28. [DOI] [PubMed] [Google Scholar]
- 9.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas GP. Electrophysiologic effects of ranolazine: a novel anti-anginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zygmunt AC, Nesterenko VV, Rajamani S, Hu D, Barajas-Martinez H, Belardinelli L, Antzelevitch C. Mechanisms of atrial-selective block of sodium channel by ranolazine I. Experimental analysis of the use-dependent block. Am J Physiol Heart Circ Physiol. 2011;301:H1606–H1614. doi: 10.1152/ajpheart.00242.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burashnikov A, Sicouri S, Di Diego JM, Belardinelli L, Antzelevitch C. Synergistic effect of the combination of dronedarone and ranolazine to suppress atrial fibrillation. J Am Coll Cardiol. 2010;56:1216–1224. doi: 10.1016/j.jacc.2010.08.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17:S161–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antzelevitch C, Burashnikov A, Sicouri S, Belardinelli L. Electrophysiological basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011;8:1281–1290. doi: 10.1016/j.hrthm.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92(Suppl 4):iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CAST Investigators Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 16.Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, Molhoek P, Verheugt FW, Gersh BJ, McCabe CH, Braunwald E. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 17.Koren MJ, Crager MR, Sweeney M. Long-term safety of a novel antianginal agent in patients with severe chronic stable angina: the Ranolazine Open Label Experience (ROLE). J Am Coll Cardiol. 2007;49:1027–1034. doi: 10.1016/j.jacc.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004;310:599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 19.Antoons G, Oros A, Beekman JDM, Engelen MA, Houtman MJC, Belardinelli L, Stengl M, Vos MA. Late Na+ current inhibition by ranolazine reduces torsades de pointes in the chronic atrioventricular block dog model. J Am Coll Cardiol. 2010;55:801–809. doi: 10.1016/j.jacc.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Kirchhof PF, Fabritz CL, Franz MR. Postrepolarization refractoriness versus conduction slowing caused by class I antiarrhythmic drugs: antiarrhythmic and proarrhythmic effects. Circulation. 1998;97:2567–2574. doi: 10.1161/01.cir.97.25.2567. [DOI] [PubMed] [Google Scholar]
- 21.Burashnikov A, Belardinelli L, Antzelevitch C. Atrial-selective sodium channel block strategy to suppress atrial fibrillation. Ranolazine versus propafenone. J Pharmacol Exp Ther. 2012;340:161–168. doi: 10.1124/jpet.111.186395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burashnikov A, Di Diego JM, Sicouri S, Ferreiro M, Carlsson L, Antzelevitch C. Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm. 2008;5:1735–1742. doi: 10.1016/j.hrthm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frommeyer G, Schmidt M, Clauss C, Kaese S, Stypmann J, Pott C, Eckardt L, Milberg P. Further insights into the underlying electrophysiological mechanisms for reduction of atrial fibrillation by ranolazine in an experimental model of chronic heart failure. Eur J Heart Fail. 2012;14:1322–1331. doi: 10.1093/eurjhf/hfs163. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, Morishima M, Kato S, Ueda N, Honjo H, Kamiya K. Atrial selectivity in Na+ channel blockade by acute amiodarone. Cardiovasc Res. 2013;98:136–144. doi: 10.1093/cvr/cvt007. [DOI] [PubMed] [Google Scholar]
- 25.Murdock DK, Reiffel JA, Kaliebe J, Larrian G. The conversion of paroxysmal or initial onset atrial fibrillation with oral ranolazine: implications for a new “pill-in-pocket” approach in structural heart disease. J Atr Fibrillation. 2010;2:705–710. [Google Scholar]
- 26.Miles RH, Passman R, Murdock DK. Comparison of effectiveness and safety of ranolazine versus amiodarone for preventing atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2011;108:673–676. doi: 10.1016/j.amjcard.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Sicouri S, Burashnikov A, Belardinelli L, Antzelevitch C. Synergistic electrophysiologic and antiarrhythmic effects of the combination of ranolazine and chronic amiodarone in canine atria. Circ Arrhythm Electrophysiol. 2010;3:88–95. doi: 10.1161/CIRCEP.109.886275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belardinelli L, Shryock JC, Fraser H. The mechanism of ranolazine action to reduce ischemia-induced diastolic dysfunction. Eur Heart J Suppl. 2006;8:A10–A13. [Google Scholar]
- 29.Sossalla S, Maier LS. Role of ranolazine in angina, heart failure, arrhythmias, and diabetes. Pharmacol Ther. 2012;133:311–323. doi: 10.1016/j.pharmthera.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Undrovinas NA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci. 2010;60:245–257. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, Taglieri DM, Gu L, Kumar P, Pokhrel N, Zeng D, Belardinelli L, Sorescu D, Solaro RJ, Dudley SC., Jr. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012;110:841–850. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander JLC, Wachter R, Edelmann F, Hasenfuss G, Jacobshagen C. RAnoLazIne for the treatment of diastolic Heart Failure in patients with preserved ejection fraction: The RALI-DHF proof-of-concept study. JACC Heart Failure. 2013;2:115–122. doi: 10.1016/j.jchf.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg MA, Gottdiener JS, Heckbert SR, Mukamal KJ. Echocardiographic diastolic parameters and risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012;33:904–912. doi: 10.1093/eurheartj/ehr378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]