Abstract
Background
Alterations in gastrointestinal (GI) permeability and immune measures are present in some patients with irritable bowel syndrome (IBS) but the relationship to symptoms is poorly defined. In adults with IBS, we compared permeability, unstimulated peripheral blood monocyte (PBMC) interleukin-10 (IL-10) levels, IBS life interference, and gastrointestinal (GI) and psychological distress symptoms.
Methods
In 88 women and 18 men with IBS, GI permeability was quantitated as percent recovery of urinary sucrose and the lactulose/mannitol (L/M) ratio. IL-10 was measured in supernatants from 72-hour incubated, unstimulated PBMCs. Participants completed a 4-week daily diary recording IBS life interference on daily activities and work, IBS symptoms, and psychological distress symptoms. They also completed the Brief Symptom Inventory and Composite International Diagnostic Interview.
Results
The L/M ratio but not percent sucrose recovery was significantly correlated with IBS interference with activities and work and retrospectively measured anxiety and depression. Unstimulated PBMC production of IL-10 correlated significantly with IBS interference with daily work, IBS symptom score, and abdominal pain. We identified a subgroup of IBS subjects with higher IL-10 and/or higher L/M ratio who had substantially higher IBS interference and IBS symptom scores.
Conclusions
Our findings suggest a distinct subgroup of IBS patients with alterations in gut barrier function. This subgroup is characterized by increased GI permeability and/or increased PBMC production of IL-10. These physiologic alterations reflect more severe IBS as measured by interference of IBS with daily activities and daily IBS symptoms.
Keywords: permeability, cytokine, symptoms, irritable bowel syndrome
INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic, intermittent condition characterized by altered bowel habits and abdominal pain or discomfort. Currently, no specific pathophysiologic factor(s) account for all the symptoms reported by patients with IBS. In the United States (U.S.) as well as other western countries, more women than men seek health care services for IBS. In the U.S. the estimate of the mean annual cost of IBS management including physician visits per patient ranges from $530 to $8750 [1, 2]. Patients with IBS also report higher rates of depression and anxiety disorders as well as reduced quality of life [3].
The current approach to the diagnosis of IBS uses symptom-based criteria [4]. Individuals may report a range of symptom severity (mild-sporadic to severe-disabling), symptom frequency (episodic, daily), and stool pattern characteristics (diarrhea, constipation, mixed diarrhea-constipation) as well as non-gastrointestinal (GI) symptoms (e.g., anxiety and depression). More than one pathophysiologic mechanism may underlie IBS [5]. Dysregulation of the autonomic nervous system, hypothalamic-pituitary-adrenal axis, increased pain sensitivity, imbalanced gut microbial population, and altered enteric physiology are factors that may contribute to the pathophysiology of IBS in at least some patients [4, 6].
Recent attention has focused on the contribution of, and to some extent, the potential interplay among increased GI permeability, altered immune function, low grade GI inflammation, and psychological distress in IBS [6, 7]. In recent reviews, it has been suggested that in some patients increased permeability (“leaky gut”) results in activation of the mucosal immune response leading to low grade GI mucosal inflammation and mucosal alterations in pro- and anti-inflammatory cytokine levels [8–10]. Alterations in serum cytokine levels also have been reported by a number of research groups although there are some inconsistencies, perhaps related in part, to differences in methodology [11, 12]. Of recent interest is the antiinflammatory cytokine interleukin-10 (IL-10). A recent metaanalysis found an association between the G allele of the IL-10 gene, particularly in those with the heterozygote genotype (GA) and susceptibility to Crohn’s disease [13]. IL-10 limits the immune response, preventing damage to the host [14, 15]. There also is evidence that increased GI permeability in humans and animals and psychological distress may be interrelated [16, 17].
Although GI permeability and immune function (e.g., cytokines) have been examined in a number of investigations in IBS patients, our study extends this work by investigating these parameters simultaneously in the same individuals. Further, few studies have related the results to symptoms and/or psychological distress. We extend previous findings by using prospective methods of symptom recording in concert with GI permeability assessments and an immune measure in adults with IBS. Thus, the goal of this study was to test the relationship of GI permeability and peripheral blood monocyte (PBMC) production of IL-10 with measures of IBS interference with activities and work, IBS symptoms, and psychological distress (both prospective and retrospective).
MATERIALS AND METHODS
Design and Setting
Baseline data from a randomized controlled trial of a comprehensive symptom management program were used (Clinicaltrials.gov identifier: NCT00907790). Subjects were recruited through community advertisements (e.g., flyers, community papers, city buses).
Subjects
Men and women had to be between 18 – 70 years of age, have a diagnosis of IBS made by a healthcare provider, and had to report current IBS symptoms based on the Rome III criteria for research studies [4]. Subjects were excluded if they had a history of coexisting GI pathology (e.g., IBD, celiac disease) or surgery (e.g., open abdominal surgery or midline abdominal incisions), renal, or reproductive pathology (e.g., endometriosis, prostate cancer). In addition, other comorbidities or medication use were exclusions, based on the guiding principle of whether the disorder or medications could confound the measurement of GI permeability, immune markers, as well as the symptoms of IBS or compromise the subject’s ability to complete the study. Examples of excluded comorbid conditions included moderate fibromyalgia, type 1 or 2 diabetes mellitus, infectious diseases (e.g., hepatitis B or C, HIV), symptoms of dementia, untreated sleep apnea/hypopnea, severe depression, and current substance abuse. Examples of medications that led to exclusion included the regular use of antibiotics, anticholinergics, cholestyramine, narcotics, colchicine, docusate, enema preparations, iron supplements, or laxatives. Human subjects institutional review approval was obtained prior to enrolling participants (February 2008 – January 2012) and written informed consent was obtained from the participants.
Eligibility Assessment
The appraisal of eligibility included four steps. First, we assessed eligibility during a telephone screening. If the person qualified, questionnaires were mailed to subjects, completed at home, and returned at the first visit. At this visit, consent was obtained, questionnaires were reviewed, and two additional questionnaires were completed with the research nurse. Within the next 3 weeks, the subjects completed a computerized mental health assessment, the Composite International Diagnostic Interview (CIDI), over the telephone with a trained nurse. Each evening for 4 weeks they completed a symptom diary. An experienced gastroenterologist reviewed the assessment data. GI permeability testing and assay of cytokine production were completed once the subject started the daily symptom diary. A 5 ml blood sample was obtained for cytokine measures.
Measures
GI Permeability testing
Subjects were instructed to refrain from non-steroidal antiinflammatory drug use for at least 2 weeks prior to testing. Similarly, they were to refrain from alcohol ingestion for at least 2 days prior to testing.
After the evening meal, subjects fasted for 4 hours, urinated, then drank a 127.5 mL solution containing sucrose (10 g), lactulose (5 g) and mannitol (1 g) followed by 240 mL water.
For the next 24 hours a plastic “hat” was placed over the toilet seat to capture the subject’s urine. Each urine sample was placed into a container containing either thimerosal or chlorhexidine to inhibit bacterial growth. Urine containers were kept in the subject’s freezer until they were returned to the laboratory. Samples were then frozen at −70°C until analyzed as we have previously described [18, 19]. Sucrose results were based on the time from ingestion through the first morning urine upon arising. Lactulose and manntiol results were based on the complete 24 hour urine collection. The lactulose/mannitol (L/M) ratio was calculated using the fractional excretion of each sugar [20].
IL-10 Production
Blood samples were collected in a sodium citrate CPT Vacutainer® (Becton Dickinson, Franklin Lakes, NJ) and analyzed as described previously [21]. In brief, peripheral blood mononuclear cells (PBMCs) were isolated by centrifuging the Vacutainer® for 25 min at 1800 relative centrifugal force (RCF). Cells were harvested, washed with phosphate buffered saline, and counted with a hemocytometer. Cell viability was assessed by trypan blue staining following which they were resuspended to 1×106 cells/mL in culture media (Dulbecco’s modified Eagle medium containing 25 mmol/L glucose, 10% fetal calf serum, 1% nonessential amino acids, 50 U/mL penicillin, and 50 μg/mL streptomycin (Invitrogen, Grand Island, NY). Plates were incubated unstimulated for 72 hours at 37°C in a 5% CO2 humidified atmosphere. The supernatants were harvested and stored at −70°C until tested for IL-10 by ELISA (R&D Systems, Minneapolis, MN).
Life Interference
Two questions from the Work Productivity and Activity Impairment Questionnaire (WPAI) for IBS were included on the daily diary [22]. They were, “How much do your IBS symptoms affect your ability to carry out normal daily activities, other than work?” and “How much did your IBS symptoms affect your productivity while you were working.” The latter was answered only on days on when the subject went to work or school. Days on which work or school were missed due to IBS were counted as the worst possible productivity loss. Both items were rated as 1 (not at all), 2 (some), 3 (quite a lot) or 4 (very much). The percent of days that IBS symptoms interfered with daily activities or work (some to very much; i.e., > 1) was used to summarize each of these items for analysis.
Daily IBS Symptoms and Stooling
IBS symptoms were measured for 4 weeks with a previously described daily diary which recorded: abdominal pain or discomfort, abdominal pain or discomfort up to 2 hours after eating, abdominal distension, bloating, intestinal gas, constipation, diarrhea, and urgency [23]. Each symptom was rated each evening on a scale of 0 (not present), 1 (mild), 2 (moderate), 3 (severe) or 4 (very severe). The score for each individual symptom was expressed as the percent of days the symptom was present (mild or greater; i.e., ≥ 1) over all diary days. The percent of days the symptom was present for each of the eight GI symptoms were then averaged to create the Composite IBS Symptom Score.
Subjects rated the consistency of each stool based on an image of the stool and a discription as 0 (no stool), 1 (very hard), 2 (hard), 3 (formed), 4 (loose) or 5 (watery) as previously described.[19]. These days were summarized as the percent of days with at least one hard or very hard stool (ratings 1–2) and the percent of days with at least one loose or watery stool (ratings 4–5) over the 4 weeks of the diary.
Daily Psychological Distress Symptoms
Daily anxiety and depression were measured for 4 weeks and rated as previously described on a scale of 0 (not present), 1 (mild), 2 (moderate), 3 (severe) or 4 (very severe) [23]. The score for each individual symptom was summarized as the percent of days the symptom was present (mild or greater; i.e., ≥ 1) over the 4 weeks.
Brief Symptom Inventory (BSI)
This questionnaire includes 53 items that represent symptoms of nine psychological disorders: anxiety, phobic anxiety, obsessive-compulsive, depression, somatization, interpersonal sensitivity, hostility, paranoid ideation, and psychoticism [24]. The participant is asked to consider how distressed or bothered they felt during the past 7 days, and then rate the symptoms from 0 (not at all), 1 (a little bit), 2 (moderately), 3 (quite a bit) or 4 (extremely). Validity and reliability are based on adult psychiatric outpatients and adult non-patients [24]. Included were the anxiety and depression subscales and mean score for all items (Global Symptom Index [GSI]). Higher values reflect greater psychological distress.
Composite International Diagnostic Interview (CIDI)
This instrument is a structured diagnostic interview administered via a computer to derive diagnoses of DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th edition) and ICD-10 (International Classification of Disease, 10th revision) mental disorders. The CIDI mood-disorders module (depression as single and recurrent episode, dysthymia and mania), anxiety-disorders module (panic disorder with or without agoraphobia, agoraphobia, generalized anxiety disorder, obsessive-compulsive disorder, phobias including social phobia, and posttraumatic stress disorder) [25].
Statistical analysis
T-tests and chi square tests were used to test for gender differences in demographic variables, permeability, IL-10 levels, IBS interference, and symptoms. Pearson correlations were used to test for the association of IBS interference and symptoms with permeability and IL-10. Based on these correlations and the corresponding scatter plots, post hoc analyses categorized subjects as high versus low L/M ratio and high versus low IL-10. T-tests were used to test for differences in mean IBS interference and symptoms between those with an high versus low L/M ratio, or high versus low IL-10. Scatterplots were used to examine the association of both permeability and IL-10 production to IBS life interference and IBS symptoms simultaneously. Additional plots and tables are included in the online supplemental materials for the benefit of researchers who would like to see more details of the results. Data are shown as Mean (Standard Deviation).
RESULTS
Sample Description (Table 1)
Table 1.
Demographic Characteristics, Irritable Bowel Symptom (IBS) Subtypes and Symptoms, Stool Ratings, and Psychological Distress by Gender
| Women (n = 88) | Men (n = 18) | P value | |
|---|---|---|---|
| Demographics | |||
| Age | 39 (15)* | 41 (17) | NS# |
| White | 73 (83%) | 18 (100%) | .069 |
| College education | 64 (74%) | 10 (56%) | .158 |
| IBS subtypes (Rome III criteria) | .177 | ||
| Diarrhea | 25 (28%) | 10 (56%) | |
| Constipation | 12 (14%) | 0 | |
| Mixed | 48 (54%) | 8 (44%) | |
| Un-subtyped | 3 (3%) | 0 | |
| Stool ratings (% of days) (daily diary) | |||
| Hard | 36.1 (27.2) | 14.5 (16.7) | .002 |
| Very hard | 11.8 (16.9) | 1.7 (2.8) | .014 |
| Loose | 47.1 (29.2) | 66.1 (24.7) | .012 |
| Very loose | 15.2 (23.2) | 18.6 (15.3) | .NS |
| IBS symptoms (daily diary)a | |||
| Composite IBS symptom score | 62.8 (19.5) | 41.0 (21.9) | <.001 |
| Abdominal pain | 79.4 (23.7) | 62.9 (29.8) | .011 |
| Psychological distress symptoms (daily diary) | |||
| Anxiety | 50.6 (31.3) | 48.2 (30.0) | NS# |
| Depression | 30.3 (30.0) | 32.6 (36.6) | NS |
| Brief Symptom Inventory | |||
| Global Symptom Index | .53 (.44) | .55 (.51) | NS |
| Anxiety | .68 (.69) | .69 (.74) | NS |
| Depression | .48 (.50) | .67 (.87) | NS |
Mean (standard deviation)
NS = P>0.2
Percent days with symptom reported reported (≥ mild)
Of the 106 subjects, most were female, White, and had a college degree. IBS-mixed was the most predominant bowel subtype (53%) followed by IBS-diarrhea (33%), and IBS-constipation (11%) in the women and men. A small number (3; 3%) did not have sufficient frequency of hard or loose stools to meet criteria for any of these three. Ten out of the 106 subjects (9.4%) reported having post-infections IBS. The Composite IBS symptom score and frequency of abdominal pain were higher in women than in men.
Psychological Distress
Psychological distress as measured by either the daily diary or the BSI did not differ by sex (Table 1).
GI Permeability and IL-10 production by PBMCs
Complete urine collections for permeability were obtained in 94 subjects (men, 17; women, 77). Mean values were as follows: overnight sucrose 9.4 ± 8.9 mg; 24 hr lactulose 20.4 mg ± 16.5, mannitol 356 mg ± 165; and L/M ratio 0.011 ± 0.008. The mean PBMC IL-10 production was 164 ± 195 pg/mL. There were no statistically significant differences between women and men in sucrose, lactulose, or mannitol recoveries, L/M ratios, or PBMC IL-10 production (Supplemental Table 1).
Correlations of permeability measures and PBMC IL-10 levels with IBS life interference and symptoms are shown in Supplemental Table 2. Sucrose recovery was not correlated with any of the clinical variables. The L/M ratio was significantly correlated with IBS interference with activities and work and with psychological distress, anxiety, and depression by BSI (Supplemental Table 2). PBMC production of IL-10 was significantly correlated with IBS interference with daily work, composite IBS symptom score, and abdominal pain (Supplemental Table 2). GI permeability and IL-10 production were correlated (r = 0.23, P = 0.03).
Figures 1A shows the association of IBS interference with urinary L/M ratio. The relationship follows roughly a step function, with low mean IBS interference to the left, and higher IBS interference to the right. The vertical line at 0.015 was chosen as a round number approximately in the middle of the transition from low to high mean IBS interference. The relationship of IBS interference with IL-10 production in Figure 1B shows a similar pattern. The vertical line at 250 pg/mL was again chosen to be approximately in the middle of the transition from low to high. Hence, these two thresholds were used to dichotomize L/M ratio and IL-10 for the remaining analyses.
Figure 1.
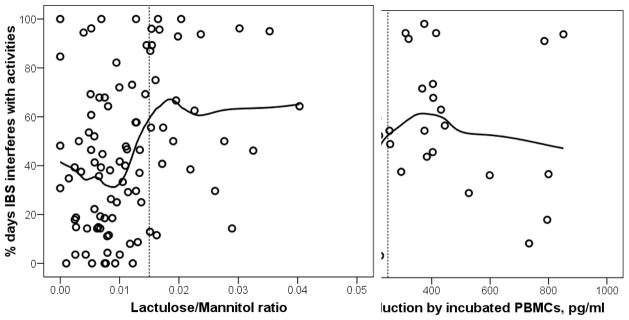
Scatterplots showing the relationship between IBS interference (percent of days on a 28 day daily diary when symptoms interfered with activities) and: gut permeability as measured by lactulose/mannitol ratio (Left Panel); production of IL-10 by non-stimulated peripheral blood mononuclear cells incubated for 72 hours (Right Panel). The vertical lines at 0.015 in A and at 250 pg/ml in B were chosen to be approximately in the middle of the transition from low to high mean IBS interference. Tables 2 and 3 are based on these thresholds.
As seen in Table 2, the 25 subjects with higher L/M ratio had greater IBS interference with daily activities and work, and higher IBS symptom and abdominal pain scores compared to the 69 subjects with a lower L/M ratio. There were no differences based on bowel pattern. A difference in psychological symptoms was seen on the BSI, but not on the daily diary (Table 2).
Table 2.
Comparison of Interference, Irritable Bowel Syndrome (IBS) and Psychological Distress Symptoms in IBS Subjects with Lower or Higher Lactulose/Mannitol Ratio
| Lower (≤ 0.015) (n = 69) Mean (SD) |
Higher (>0.015) (n = 25) Mean (SD) |
P value | |
|---|---|---|---|
| IBS symptoms interfered witha | |||
| Daily activities | 38.7 (28.7) | 64.8 (29.4)* | <.000 |
| Work | 37.4 (30.2) | 62.9 (28.0) | .001 |
| IBS symptoms (daily diary)b | |||
| Composite IBS symptom score | 56.1 (23.1) | 66.9 (15.3) | .032 |
| Abdominal pain/discomfort | 73.2 (26.8) | 84.8 (15.4) | .044 |
| IBS subtypes (Rome III criteria) | .060 | ||
| Undetermined | 1 (1%) | 2 (8%) | |
| Constipation | 6 (9%) | 2 (8%) | |
| Diarrhea | 29 (42%) | 4 (16%) | |
| Mixed | 33 (48%) | 17 (68%) | |
| Psychological distress symptoms (daily diary) | |||
| Anxiety | 48.2 (30.7) | 53.8 (32.6) | NS# |
| Depression | 30.8 (31.0) | 32.3 (31.1) | NS |
| Brief Symptom Inventory | |||
| Global Symptom Index | .48 (.42) | .69 (.23) | .052 |
| Anxiety | .58 (.67) | .92 (.78) | .046 |
| Depression | .48 (.59) | .63 (.58) | NS |
Mean (standard deviation)
NS = P>0.2
≥ some
percent of days symptom reported ≥ mild
Controlling for age and sex did not affect the analyses
Similar results are seen in Table 3, with higher interference with activities and composite IBS symptom scores and abdominal pain in those with IL10 > 250 pg/mL. Psychological distress symptom differences (i.e., anxiety and depression higher in those with higher IL-10 production) were found but only on the BSI (Table 3).
Table 3.
Comparison of Interference, and Irritable Bowel Syndrome (IBS) and Psychological Distress Symptoms in IBS Subjects with Lower and Higher PBMCa IL-10 Production
| Lower (IL-10< 250pg/ml) (n = 81) Mean (SD) |
Higher (IL-10> 250pg/ml) (n = 22) Mean (SD) |
P value | |
|---|---|---|---|
| IBS symptoms interfered withb | |||
| Daily activities | 40.6 (29.9)* | 60.7 (27.2) | .005 |
| Work | 40.3 (30.8) | 54.1 (29.6) | .077 |
| IBS symptoms (daily diary)c | |||
| Composite IBS symptom score | 56.0 (21.1) | 71.0 (19.0) | .003 |
| Abdominal pain/discomfort | 73.2 (26.8) | 89.1 (15.3) | .009 |
| IBS subtypes (Rome III criteria) | |||
| Undetermined | 2 (20) | 1 (4) | NS |
| Constipation | 20 (25) | 5 (23) | NS |
| Diarrhea | 50 (62) | 10 (46) | NS |
| Mixed | 9(11) | 6 (27) | NS |
| Psychological distress symptoms (daily diary)c | |||
| Anxiety | 50.5 (31.3) | 51.0 (32.0) | NS |
| Depression | 30.7 (31.7) | 33.3 (30.4) | NS |
| Psychological symptoms (BSI) | |||
| Global Severity Index | 0.49 (0.42) | 0.74 (0.53) | .020 |
| Anxiety | 0.63 (0.65) | 0.92 (0.85) | .093 |
| Depression | 0.48 (0.56) | 0.68 (0.67) | .155 |
PBMC = peripheral blood mononuclear cell
percent of days IBS interfered activities (≥ some) ± standard deviation
percent of days symptom reported (≥ mild) ± standard deviation
BSI = Brief Symptom Inventory
NS = P > .20
Controlling for age and sex did not affect the analyses
Figure 2A shows the association of IBS life interference with permeability and IL-10 jointly. Life interference was split into three categories with approximately equal numbers of subjects, namely low (<25% of days with symptoms), medium (25–50% of days with symptoms), and high (>50% days with symptoms). Those with low life interference are almost all (81%) in the bottom left of the plot, having both low permeability and low IL-10 levels. In contrast, over half (57%) of those with high (>50%) life interference are characterized by having increased GI permeability and/or IL-10 production, compared to only 27% of those with low or medium life interference (odds ratio = 3.8, P = 0.007).
Figure 2.
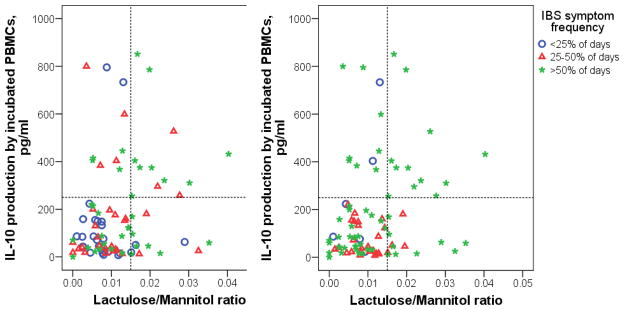
Scatterplots showing the joint relationship of IL-10 production by incubated peripheral blood mononuclear cell (PBMC) and lactulose/mannitol ratio to clinical measures shown as colored symbols indicating: IBS interference (percent of days on a 28 day daily diary when symptoms interfered with activities) (Left Panel); Composite IBS symptom score defined by averaging the percent of days with each of the symptoms: abdominal pain or discomfort, abdominal pain or discomfort up to 2 hours after eating, abdominal distension, bloating, intestinal gas, constipation, diarrhea, and urgency (Right Panel). Subjects with low interference and low IBS symptom score predominate in the lower left corner of these plots, while subjects with higher IBS interference and higher IBS symptom score predominate in the rest of the plot, among those with either higher IL-10 or higher L/M ratio. See Table 4 for additional related results.
Figure 2B is similar to Figure 2A except that subjects are classified based on the Composite IBS Symptom Score (the averaged percent of days symptoms were ≥ mild) using the cutoffs of <25%, 25 – 50%, and >50% of days. The pattern is similar to Figure 1 where almost all of those with low IBS symptoms are in the lower left (with low permeability and IL-10 production). Fifty-one percent of those with high IBS symptom score (>50%) are characterized by having increased GI permeability and/or IL-10 production, compared to only 18% of those with low or medium IBS symptom scores (odds ratio = 4.8, P = 0.002). This joint relationship suggests the definition of a composite category, namely those with elevated permeability and/or elevated IL-10 production. A comparison of those who fit in this category versus those who do not shows very strong differences in clinical variables, as seen in Table 4.
Table 4.
Increased Lactulose/Mannitol Ratio and/or PBMCa IL-10 Production and Life Interference, and Irritable Bowel Syndrome and Psychological Distress Symptoms
| Neither n = 56 Mean (SD) |
Either or Both n = 35 Mean (SD) |
P | |
|---|---|---|---|
| IBS symptoms interfered withb | |||
| Daily activities | 37.0 (28.7) | 60.2 (29.0) | .000 |
| Work | 36.7 (30.3) | 56.6 (30.3) | .004 |
| IBS Symptoms (daily diary)c | |||
| Composite IBS symptom score | 53.9 (22.2) | 67.5 (18.5) | .003 |
| Abdominal pain/discomfort | 70.1 (27.4) | 86.3 (16.3) | .002 |
| IBS subtypes (Rome III criteria) | .045 | ||
| Undetermined | 1 (2) | 2 (6) | |
| Constipation | 6 (11) | 2 (6) | |
| Diarrhea | 25 (45) | 7 (20) | |
| Mixed | 24 (433) | 24 (69) | |
| Psychological distress symptoms (daily diary)c | |||
| Anxiety | 48.8 (31.1) | 52.3 (32.4) | .549 |
| Depression | 31.1 (30.9) | 33.2 (31.1) | .754 |
| Psychological distress (BSI) | |||
| Global severity index | .47 (.41) | .68 (.53) | .040 |
| Anxiety | .57 (.65) | .86 (.80) | .065 |
| Depression | .47 (.55) | .63 (.65) | NS |
PBMC = peripheral blood mononuclear cell
percent of days IBS interfered activities (≥ some)
percent of days symptom reported (≥ mild) ± standard deviation
BSI = Brief Symptom Inventory
NS = P > .20
Controlling for age and sex did not affect the analyses
DISCUSSION
There is increasing interest in the contribution of altered GI barrier function (permeability) and immune function in the pathophysiology of IBS and the generation of intestinal symptoms as well as psychological distress [9, 26]. To our knowledge, our study is the first to investigate prospectively GI permeability, immune function, IBS life interference with activities, and IBS and psychological distress symptoms in the same cohort of IBS participants.
Findings from our study indicate that among men and women with IBS recruited from the community, those with increased GI permeability are more likely to have increased life interference with activities as compared to those with normal permeability (Table 2). Our finding of an association between permeability and composite IBS symptom score and abdominal pain/discomfort (Table 2) extends previous work. Zhou et al. [27] studied patients with diarrhea-predominant IBS and found that increased GI permeability (L/M ratio) was related to retrospective report of GI symptoms as well as the results of visceral and thermal sensitivity testing [27]. Similarly, in a study of patients with postinfectious IBS, increased GI permeability was associated with increased stool frequency using retrospective symptom data [28]. In contrast, using 51Cr-EDTA to measure GI permeability, Dunlop et al. found no relationship between retrospective abdominal pain reports and GI permeability in adults with postinfectious IBS [29].
The intestinal epithelium is a vital barrier that protects the GI tract from the luminal constituents [30]. Our data fit with observations demonstrating that the intestinal epithelium also plays an important role in sensineural processing affecting both motor and sensory activities of the gut [31]. Increased mucosal permeability resulting in mechanosensitivity may explain, in part, the visceral hypersensitivity that leads to increased GI symptoms in IBS [10]. GI permeability also was positively correlated with PMBC production of the antiinflammatory cytokine IL-10 providing further support for a relationship between changes in GI permeability and altered immune function [9, 26].
We speculate that the relationships between IL-10 and IBS symptoms in our study may be in response to luminal inflammatory stimulation (such as from the gut microbiota), which may prime an antiinflammatory response (i.e., IL-10) to limit a pro-inflammatory cytokine release. It has been shown in humans that the administration of probiotics can increase the expression of IL-10 linearly [21]. This would fit with the described relationship between an altered GI microbial population composition in IBS and GI symptoms [32]. The altered microbial population may be responsible for the low grade mucosal inflammation found in some patients with IBS [33]. Whether an altered GI microbial population and low-grade inflammation as reflected by other markers explains our findings related to GI permeability and IL-10 production awaits further studies.
Cytokines play important roles in regulating the extent of immune response including the type and duration of response to intestinal pathogens [14, 34]. As noted by Hughes et al., the role of immune activation in IBS is controversial with reports showing higher levels of circulating pro- (e.g., IL-6, TNF-alpha) and anti-inflammatory (e.g., IL-10) cytokines as well as no differences between IBS patients and healthy controls [35–37]. These differing results may be related to differences in methodologies among studies (see below). The levels (both plasma and PBMC production) may be linked to mood as well as other factors. For example, measuring plasma levels of IL-10, Kindt et al. demonstrated a weak but significant correlation between plasma IL-10 levels and depression scores but not with anxiety [37].
How cytokines contribute to the pathophysiology of IBS is complex and may not be the key element in IBS pathophysiology in all IBS patients. In one study, supernatant (24-hour incubated PBMCs) from IBS-D patients sensitized rat colonic afferents to mechanical stimuli [38]. However, when colonic sensory afferents were incubated with only IL-10 there were no changes in afferent nerve firing as opposed to what was observed with proinflammatory cytokines (TNF-α, IL-1β). These findings suggest that PBMC IL-10 production may be in response to another pathophysiologic process linked to proinflammatory states, increased permeability, and/or central perception of pain or discomfort. Despite finding higher retrospective recall of psychological distress (global severity index and anxiety) in those with IL-10 levels greater than 250 pg/mL, there were no differences in daily prospective psychological distress symptoms (Table 3). However, our results indicated a positive relationship between IL-10 production and daily interference with activities as well as composite IBS symptom score and abdominal pain (Figures 1 and 2, S2 Table). These results are similar in part to the findings of Liebregts et al. who found positive correlations between 24-hour incubation PBMC production of IL-10 and upper abdominal pain (r = 0.58), abdominal cramps (r = 0.58), nausea (r = 0.44), and vomiting (r = 0.61) in patients with functional dyspepsia [39]. However, our findings are not consistent with the study by Hughes et al. [38]. They used 24-hour incubated PBMCs from adults with IBS-D and found elevations in IL-10 levels along with pro-inflammatory cytokines (TNF-α, IL-6, IL-1beta) relative to controls but no relationship of IL-10 to retrospective measures of pain intensity and frequency. Our differing results may be related to the IBS subtypes in our study versus those in the study of Hughes et al. [38].
Unfortunately, we did not have a control group to compare the IL-10 values we obtained in our IBS subjects. A recently published study by Hua and colleagues [40] measured 24-hour unstimulated and stimulated (lipopolysaccharide [LPS]) PBMCs from children with IBS and healthy controls. They found a nonsignificant reduction in IL-10 production in unstimulated PBMCs and significantly lower levels in stimulated PBMCs in all IBS bowel pattern groups. Another study in adults using LPS-stimulated PBMCs found a trend (P = 0.06) towards decreased IL-10 production after 72-hour incubation in IBS as compared to controls [37].
IL-10 can be produced by almost every type of cell in the immune system [14]. In IBS patients it has been measured in serum, colonic mucosa via biopsy, and with isolated PBMCs either stimulated or unstimulated; incubated for either 24 and 72 hr. These variations in methodological approaches make direct comparisons challenging. When serum levels are measured by ELISA and compared between IBS patients and healthy controls, consistent findings at least linking symptoms to IL-10 levels emerge. Chang et al. found no differences in levels in adults with IBS but did find that lower serum IL-10 levels correlated with higher ratings of IBS symptoms [35]. Schumlson et al. reported lower serum levels of IL-10 in IBS patients, particularly those with IBS-D as compared to controls [36].
Given the correlation between GI permeability and IL-10 production, and the fact that only a proportion of subjects had increased permeability, we asked if individuals with increased GI permeability and/or high IL-10 production represented a subset of patients with the most severe symptoms. Conversely, we questioned whether subjects in whom GI permeability and IL-10 production results were low would represent a group with less severe symptoms. Figures 1 and 2 suggest this to be the case. As seen in Figure 1, subjects with high interference with activities had increased GI permeability and/or IL-10 (using what appeared to be a natural cutoff of 250 pg/mL; Figures 1 and 2, S2 Table). Similarly, in Figure 2 those with the highest composite IBS symptom scores make up the majority of those with increased GI permeability and/or IL-10. In both Figures 1 and 2, those with the least symptoms cluster in the lower left of the graph, having normal GI permeability and low IL-10.
Taken together, these findings suggest a subgroup of IBS patients with greater symptoms associated with increased GI permeability and/or elevated PBMC IL-10. We speculate that these patients may have an IBS etiology related to abnormal gut permeability and/or immune alteration. The remaining IBS patients have normal GI permeability and/or no or little peripheral indication of gut inflammation. In these latter patients, other etiologies may be important. For example, poor coping skills and/or altered pain sensitivity may contribute to symptom expression to a greater degree than in those with increased GI permeability or IL-10 production. One might anticipate that these two groups of patients might benefit from different therapies; treatment to correct the abnormal permeability and/or immune dysfunction (e.g., probiotics) whereas another intervention (e.g., psychological treatment) may be of more benefit in the group with normal GI permeability/immune function. Such speculations await evaluation in future prospective studies.
The current study has limitations. The sample included only those with IBS who volunteered for an intervention trial. Exclusion criteria reduced the likelihood that subjects with severe psychopathology or other chronic inflammatory conditions participated but did not separate those who may have had postinfectious IBS. In addition, because the study was part of an intervention trial, there was no healthy control group. The focus on only one antiinflammatory cytokine limits our ability to characterize fully the relationship of immune activation with IBS symptoms.
Interestingly, we found differences in permeability and IL-10 and psychological distress only on the retrospective measure (BSI) used. However, the BSI scores for anxiety and depression were positively (r = 0.59 and 0.60, respectively) related to diary items for these two symptoms. Because different time periods were rated (subjects began keeping the diary after giving their retrospective rating of the past week), perfect agreement would not be expected. There is the potential for recall bias to influence responses to psychological tools.
Strengths of our study include the relatively large sample size and that all measurements were on the same individuals. Subjects were well screened prior to enrollment. Additionally, we used prospective, long term (one month) diary data. Recent literature on pediatric IBS and functional abdominal pain patients support the use of prospective diaries as more reliable than recall [41]. In adults, Engsbro et al. demonstrated discordance between retrospective, questionnaire-based and prospective, diary-based IBS Rome subtypes with a tendency to report more symptoms on retrospective questionnaires [42]. However, even when using prospective diaries, the timing of when patients record symptoms may be an important variable. In one study IBS patients reported more symptoms on a diary completed at the end of the day as compared to five assessments a day for 14 days [43]. We also limited the number of symptom comparisons we made (activity interference and Composite IBS Symptom Score) to reduce the chance of a type 1 error. Thus, exploratory comparisons that may be susceptible to such errors were included only in the supplementary material.
In summary, our results suggest that in adults with IBS, increased GI permeability is associated with life interference with daily activities and a trend toward increased IBS symptoms as measured by a Composite IBS Symptom Score. Increased production of IL-10 by PBMCs appears to correlate with interference with activities as well as with a Composite IBS Symptom Score. There appear to be subgroups of patients with IBS, one in whom increased GI permeability and/or IL-10 may play a significant role in life interference with activities and GI symptom expression.
Supplementary Material
Acknowledgments
This study was supported in part by R01 NR004142 and R01 NR05337 from the National Institutes of Health, the Daffy’s Foundation, the USDA/ARS under Cooperative Agreement No. 6250-51000-043, and P30 DK56338 which funds the Texas Medical Center Digestive Disease Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work is a publication of the University of Washington and the USDA/ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital. The contents do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
References
- 1.Maxion-Bergemann S, Thielecke F, Abel F, Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24(1):21–37. doi: 10.2165/00019053-200624010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal N, Spiegel BM. The effect of irritable bowel syndrome on health-related quality of life and health care expenditures. Gastroenterology clinics of North America. 2011;40(1):11–79. doi: 10.1016/j.gtc.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Graham DP, Savas L, White D, El-Serag R, Laday-Smith S, Tan G, et al. Irritable bowel syndrome symptoms and health related quality of life in female veterans. Aliment Pharmacol Ther. 2010;31(2):261–73. doi: 10.1111/j.1365-2036.2009.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drossman D, Corazziari E, Delvaux M, Spiller R, Talley N, Thompson W, et al. Rome III: The functional gastrointestinal disorders. 3. McLean, VA: Degnon Associates, Inc; 2006. [Google Scholar]
- 5.Qin HY, Wu JC, Tong XD, Sung JJ, Xu HX, Bian ZX. Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. Journal of gastroenterology. 2011;46(2):164–74. doi: 10.1007/s00535-010-0321-6. [DOI] [PubMed] [Google Scholar]
- 6.Stasi C, Rosselli M, Bellini M, Laffi G, Milani S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. Journal of gastroenterology. 2012;47(11):1177–85. doi: 10.1007/s00535-012-0627-7. [DOI] [PubMed] [Google Scholar]
- 7.Adam B, Tsopelas C, Liebregts T, Bartholomeusz FD, Holtmann G. Host immune response determines visceral hyperalgesia in a rat model of post-inflammatory irritable bowel syndrome. Journal of gastroenterology. 2013;48(10):1119–27. doi: 10.1007/s00535-012-0729-2. [DOI] [PubMed] [Google Scholar]
- 8.Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. Journal of gastroenterology. 2011;46(4):421–31. doi: 10.1007/s00535-011-0379-9. [DOI] [PubMed] [Google Scholar]
- 9.Matricon J, Meleine M, Gelot A, Piche T, Dapoigny M, Muller E, et al. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36(11–12):1009–31. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303(7):G775–85. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 11.Scully P, McKernan DP, Keohane J, Groeger D, Shanahan F, Dinan TG, et al. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol. 2010;105(10):2235–43. doi: 10.1038/ajg.2010.159. [DOI] [PubMed] [Google Scholar]
- 12.Ortiz-Lucas M, Saz-Peiro P, Sebastian-Domingo JJ. Irritable bowel syndrome immune hypothesis. Part one: the role of lymphocytes and mast cells. Rev Esp Enferm Dig. 2010;102(11):637–47. doi: 10.4321/s1130-01082010001100004. [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Lei X, Liu Q, Wang Y. Interleukin-10-1082A/G polymorphism and inflammatory bowel disease susceptibility: a meta-analysis based on 17,585 subjects. Cytokine. 2013;61(1):146–53. doi: 10.1016/j.cyto.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nature immunology. 2012;13(10):925–31. doi: 10.1038/ni.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nature immunology. 2012;13(8):722–8. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barreau F, Ferrier L, Fioramonti J, Bueno L. New insights in the etiology and pathophysiology of irritable bowel syndrome: contribution of neonatal stress models. Pediatr Res. 2007;62(3):240–5. doi: 10.1203/PDR.0b013e3180db2949. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Kan EM, Lu J, Cao Y, Wong RK, Keshavarzian A, et al. Combat-training increases intestinal permeability, immune activation and gastrointestinal symptoms in soldiers. Aliment Pharmacol Ther. 2013;37(8):799–809. doi: 10.1111/apt.12269. [DOI] [PubMed] [Google Scholar]
- 18.Catassi C, Pierani P, Natalini G, Gabrielli O, Coppa GV, Giorgi PL. Clinical application of a simple HPLC method for the sugar intestinal permeability test. J Pediatr Gastroenterol Nutr. 1991;12(2):209–12. doi: 10.1097/00005176-199102000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Shulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou CN, Smith EO. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatr Res. 1998;44:519–23. doi: 10.1203/00006450-199810000-00009. [DOI] [PubMed] [Google Scholar]
- 20.McOmber ME, Ou CN, Shulman RJ. Effects of timing, sex, and age on site-specific gastrointestinal permeability testing in children and adults. J Pediatr Gastroenterol Nutr. 2010;50(3):269–75. doi: 10.1097/MPG.0b013e3181aa3aa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541–51. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 22.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 23.Jarrett ME, Cain KC, Burr RL, Hertig VL, Rosen SN, Heitkemper MM. Comprehensive self-management for irritable bowel syndrome: randomized trial of in-person vs. combined in-person and telephone sessions. Am J Gastroenterol. 2009;104(12):3004–14. doi: 10.1038/ajg.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derogatis L. BSI: Brief Symptom Inventory; Administration, Scoring and Procedures Manual. 4. Minneapolis: National Computer Systems; 1993. [Google Scholar]
- 25.Andrews G, Peters L. The psychometric properties of the Composite International Diagnostic Interview. Soc Psychiatry Psychiatr Epidemiol. 1998;33(2):80–8. doi: 10.1007/s001270050026. [DOI] [PubMed] [Google Scholar]
- 26.Camilleri M, Lasch K, Zhou W. The Confluence of Increased Permeability, Inflammation, and Pain in Irritable Bowel Syndrome. Am J Physiol Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146(1–2):41–6. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall JK, Thabane M, Garg AX, Clark W, Meddings J, Collins SM. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20(11–12):1317–22. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 29.Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101(6):1288–94. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 30.Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13(5):321–35. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 31.Kaji I, Yasuoka Y, Karaki S, Kuwahara A. Activation of TRPA1 by luminal stimuli induces EP4-mediated anion secretion in human and rat colon. Am J Physiol Gastrointest Liver Physiol. 2012;302(7):G690–701. doi: 10.1152/ajpgi.00289.2011. [DOI] [PubMed] [Google Scholar]
- 32.Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141(5):1782–91. doi: 10.1053/j.gastro.2011.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langhorst J, Junge A, Rueffer A, Wehkamp J, Foell D, Michalsen A, et al. Elevated human beta-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104(2):404–10. doi: 10.1038/ajg.2008.86. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cellular and molecular life sciences: CMLS. 2013;70(4):631–59. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang L, Adeyemo M, Karagiannides I, Videlock EJ, Bowe C, Shih W, et al. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol. 2012;107(2):262–72. doi: 10.1038/ajg.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmulson M, Pulido-London D, Rodriguez O, Morales-Rochlin N, Martinez-Garcia R, Gutierrez-Ruiz MC, et al. Lower serum IL-10 is an independent predictor of IBS among volunteers in Mexico. Am J Gastroenterol. 2012;107(5):747–53. doi: 10.1038/ajg.2011.484. [DOI] [PubMed] [Google Scholar]
- 37.Kindt S, Van Oudenhove L, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, et al. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21(4):389–98. doi: 10.1111/j.1365-2982.2008.01220.x. [DOI] [PubMed] [Google Scholar]
- 38.Hughes PA, Harrington AM, Castro J, Liebregts T, Adam B, Grasby DJ, et al. Sensory neuro-immune interactions differ between Irritable Bowel Syndrome subtypes. Gut. 2012 doi: 10.1136/gutjnl-2011-301856. [DOI] [PubMed] [Google Scholar]
- 39.Liebregts T, Adam B, Bredack C, Roth A, Heinzel S, Lester S, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132(3):913–20. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 40.Hua MC, Lai MW, Kuo ML, Yao TC, Huang JL, Chen SM. Decreased interleukin-10 secretion by peripheral blood mononuclear cells in children with irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2011;52(4):376–81. doi: 10.1097/MPG.0b013e3181fd9816. [DOI] [PubMed] [Google Scholar]
- 41.Chogle A, Sztainberg M, Bass L, Youssef NN, Miranda A, Nurko S, et al. Accuracy of Pain Recall in Children. J Pediatr Gastroenterol Nutr. 2012;55(3):288–91. doi: 10.1097/MPG.0b013e31824cf08a. [DOI] [PubMed] [Google Scholar]
- 42.Engsbro AL, Simren M, Bytzer P. The Rome II and Rome III criteria identify the same subtype-populations in irritable bowel syndrome: agreement depends on the method used for symptom report. Neurogastroenterol Motil. 2012;24(7):604–11. e266. doi: 10.1111/j.1365-2982.2012.01908.x. [DOI] [PubMed] [Google Scholar]
- 43.Weinland SR, Morris CB, Hu Y, Leserman J, Bangdiwala SI, Drossman DA. Characterization of episodes of irritable bowel syndrome using ecological momentary assessment. Am J Gastroenterol. 2011;106(10):1813–20. doi: 10.1038/ajg.2011.170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


