Abstract
Objective
Research has suggested that greater psychophysiological reactivity to stress increases risk of dementia and that those with the Type A behavior pattern (TABP) are predisposed to elevated stress reactivity and cardiovascular disease (CVD), but no study has evaluated the associations amongst TABP, CVD and dementia prospectively. Hence, the present study aimed to investigate dementia risk in relation to TABP and CVD.
Methods
A population-based cohort of 1,069 persons with a baseline mean age of 64.81 years from the Swedish Twin Registry was followed consecutively for 23 years. Based on self-reported items, TABP was measured using 6 scales: Ambition, Stress, Hard-driving, Neuroticism, Cynicism, and Paranoia. CVD was self-reported and dementia was diagnosed adhering to DSMIII- R or DSM-IV criteria.
Results
TABP was generally not associated with dementia risk. However, significant interaction effects of stress, paranoia, and cynicism with CVD on dementia risk were observed. That is, for those with CVD, high scores on stress, paranoia, and cynicism were associated with increased risk of dementia (hazard ratio [HR]=1.43, 95% confidence interval [CI]=0.95–2.15; HR=1.39, 95% CI=0.83–2.33; HR=1.25, 95% CI=0.76–2.06, respectively), whereas for those who did not have CVD, high scores on these measures appeared to be protective (HR=0.76, 95% CI=0.50–1.14; HR=0.55, 95% CI=0.34–0.89; HR=0.50, 95% CI=0.29–0.84, respectively).
Conclusion
Some features of TABP confer an increased risk for dementia in those with CVD, whereas those without CVD are protected. When evaluating the risk of dementia, CVD and personality traits should be taken into consideration.
Keywords: Dementia, Personality, Type A Behavior Pattern, Cardiovascular disease, Longitudinal studies
While public health efforts have helped increase longevity over the last century, the incidence and prevalence of dementia have consequently increased as old age is the strongest known risk factor for dementia (Fratiglioni, De Ronchi, & Aguero-Torres, 1999). Dementia, a chronic and debilitating disorder in which cognitive processes such as memory, cognition, language, judgment and behavior are affected to the extent that normal daily functioning is disrupted, imposes significant economic and emotional burdens on society (Knopman, 2011). The most common type of dementia is Alzheimer’s disease (AD); the second most common subtype is ischemic vascular disease (VaD), however, neuropathologic investigations suggest there are often multiple forms of pathology in the same patient (Vinters et al., 2000). The fact that some older adults develop dementia and some do not indicates that dementia is not a normal process of aging (Whalley, 2002). Thus, there is a need to pinpoint risk factors associated with dementia and to understand whether risk factors act in an additive or interactive manner. Here, dementia risk is prospectively examined in relation to the Type A behavior pattern (TABP) and cardiovascular disease (CVD).
It is generally agreed that TABP is a multi-dimensional personality construct belonging to individuals with poor behavioral coping mechanisms (Lichtenstein, Pedersen, Plomin, de Faire, & McClearn, 1989). Although the classification of TABP varies somewhat across studies, research has nevertheless characterized TABP as being comprised of excessive drive, free-floating hostility, neuroticism, high ambition and general impatience (Rosenman et al., 1964; Smith, O'Keeffe, & Allred, 1989; Williams et al., 1980). It has been remarked that individuals who carry a combination of the above mentioned traits differ from non-Type A persons not only in personality and self-evaluative processes, but also in their physiological reaction to perceived stressful situations (Dembroski, MacDougall, Shields, Petitto, & Lushene, 1978; Rosenman et al., 1964). A major focus has been on Type A personality in relation to CVD, a group of disorders of the heart and blood vessels including events such as heart attack and stroke. Despite the controversy surrounding TABP as a predictor of CVD, considerable research supports an association between the two, (Lichtenstein et al., 1989; Miller, Turner, Tindale, Posavac, & Dugoni, 1991; Smith & MacKenzie, 2006; Williams et al., 1980), particularly when individual facets of TABP are assessed (Smith, Glazer, Ruiz, & Gallo, 2004). The mechanism for this relationship is often suggested to be hyper cardiovascular reactivity to stress via neuro-endocrine pathways. The behavioral responses in TABP could be activating the hypothalamic-pituitaryadrenal (HPA) axis, the primary system activated during a stress response (Rosmond & Bjorntorp, 2000). Prolonged activation of the HPA axis could, in turn, produce an above average amount of glucocorticoid hormones such as cortisol, which could then lead to cardiovascular problems or exacerbate cardiovascular conditions in patients with coronary heart disease (Steptoe & Kivimaki, 2012).
Increasing evidence supports that CVD is associated with an elevated risk of dementia, including VaD as well as AD (Eriksson, Bennet, Gatz, Dickman, & Pedersen, 2010; Qiu et al., 2006; Rosano & Newman, 2006). Moreover, risk factors for vascular disease appear to be linked to both VaD and AD, and having cerebrovascular disease in the presence of AD likely results in an additive effect with respect to impairment (Breteler, 2000). While an extensive amount of research has assessed the relationship between Type A personality traits and CVD, and more recently CVD and dementia, few studies have examined the link between TABP and dementia risk, and to an even lesser extent, the associations amongst TABP, CVD, and dementia in one model. Assuming there is an association between TABP and dementia, one might imagine such an association as being either mediated or moderated by CVD.
In initial tests of the hypothesis that Type A behavior influences risk of dementia, two case-control studies that investigated premorbid behavior characteristics among persons with dementia did not report an association (Amaducci et al., 1986; Motomura et al., 1998). Another case-control study observed that individuals with mild cognitive impairment, a state often thought to be a precursor to dementia, were characteristically more hostile than healthy matched controls (Clement, Belleville, Belanger, & Chasse, 2009). Two population-based longitudinal studies have explored the relationship between midlife stress and dementia 30 to 35 years later. Crowe et al. concluded that self-reported reactivity to stress was a predictor of elevated dementia risk (Crowe, Andel, Pedersen, & Gatz, 2007), while the other study found a relationship between self-reported frequent psychological stress and subsequent dementia risk (Johansson et al., 2010). Studies on proneness to psychological distress, measured as high neuroticism, have found an association with AD (Wilson et al., 2003) and cognitive decline (Crowe, Andel, Pedersen, Fratiglioni, & Gatz, 2006). Similarly, higher neuroticism evaluated before dementia onset has been reported to be associated with AD neuropathology (Duberstein et al., 2011; Terracciano et al., 2013). Conversely, one prospective study with a 6 year follow-up did not observe neuroticism alone to be associated with higher dementia risk, but amongst people with an inactive or socially isolated lifestyle, low neuroticism was shown to be protective (Wang et al., 2009). In sum, the literature concerning the relationship between Type A-related traits and dementia is suggestive, but scarce and sometimes contradictory. Further, many previous studies have adopted case-control designs or had relatively short follow-up periods, thus limiting the ability to differentiate baseline personality from dementia-affected personality.
Taken together, dementia has been studied in relation to CVD and to aspects of TABP, but there remains a gap in the literature concerning the associations amongst TABP, CVD, and dementia. Hence, this study aims to explore whether TABP is a risk factor for dementia, and to examine whether CVD mediates or moderates such a relationship using baseline Type A personality measures and dementia diagnoses obtained up to 23 years later in a population-based sample.
Methods and Materials
Participants
The participants of the longitudinal Swedish Adoption/Twin Study of Aging (SATSA) were drawn from the population-based Swedish Twin Registry (STR). The sampling procedures and selection criteria for participants have been described in detail previously (Finkel & Pedersen, 2004; Pedersen et al., 1991). In short, SATSA consists of two longitudinal components: a questionnaire assessment and an in-person-testing (IPT) phase. In 1984, the first wave of questionnaires (Q1) that consisted of two parts was mailed out to all participants that were alive from the baseline sample. The second part (Q1 Blue) was mailed out only to those responding to the first part (Q1 Red). Subsequent questionnaires were sent out in 3-year intervals. Questionnaire items from both parts of Q1 that were of relevance to the present study included questions about personality and health. Starting in 1986, a subsample of SATSA twins (n=645) aged 50 and above participated in the first IPT assessment. All previous IPT participants as well as twins who had participated in Q1 and had turned 50 years of age since the last IPT were invited to subsequent IPTs at rolling 3-year intervals between 1986 and 2007. The IPT sessions were comprised of an interview, cognitive testing, and a health examination performed by registered nurses in a healthcare setting or in the participant’s own home. The present study used data from the first questionnaire and dementia diagnoses from IPT1 through IPT7 as well as from three Swedish population health registers that will be described in greater detail below.
Of the 2,018 participants who responded to Q1 Red (71% response rate), 1,736 participants responded to Q1 Blue (86% response rate). Those who were aged 50 years and above (n = 1,509) were selected for the current analyses so that the sample was representative of the population at risk for dementia. As dementia is slowly progressive, those who developed the condition within 5 years after baseline (n = 63) were excluded to reduce the risk of including persons with dementia-related personality changes. Participants were also excluded if they were diagnosed with dementia prior to or at baseline Q1 (n = 24), if they died within 5 years after baseline (n = 177), or if they had missing information (n = 176). The exclusion of those who died shortly after baseline was to avoid including persons experiencing changes in personality that are associated with terminal decline. Thus, a total of 1,069 persons (M = 64.81 years, SD = 8.25) at baseline were followed up until diagnosis of dementia, death, or the end of the study period.
SATSA has been approved by the Ethics Committee at Karolinska Institutet. All participants received a letter that described the purpose, content and duration of the SATSA study and were assured confidentiality and anonymity as part of the informed consent process. Participants were informed that their involvement in the study was voluntary and that they were free to withdraw from the study at any point in time.
Measurement of the Type A Behavior Pattern
TABP was assessed with six scales: Ambition, Stress, Hard-driving, Neuroticism, Cynicism and Paranoia. The ambition, stress, and hard-driving scales used items derived from the Framingham Type A scale (Haynes, Feineib, Levine, Scotch, & Kannel, 1978) and the Bortner scale (Bortner, 1969), both of which have been widely used as measures of TABP and tested in relation to coronary heart disease (Smith et al., 1989). Originally a total of 16 items were used; however, five of these items were not used to measure Type A in this study because the work-related elements of these items would have only allowed them to be applicable to those who were working at the time the questionnaire was distributed. Therefore, the scales were based on 11 items; five from the Framingham scale and six based on the Bortner scale. The neuroticism scale was from a short version of the Eysenck Personality Inventory (Floderus, 1974). The paranoid and cynicism scales consisted of a total of 19 items from the Cook and Medley Hostility scale (Costa, Zonderman, McCrae, & Williams, 1986), which have been validated and found to be correlated with measures of hostility and agreeableness from the NEO personality inventory (Barefoot, Dodge, Peterson, Dahlstrom, & Williams, 1989). Paranoia and cynicism items were included in Q1Blue while all other items were included in Q1 Red.
All Type A measures mentioned above were derived by summing the scores for items that corresponded with the particular Type A trait. A high score signified a high degree of the Type A trait while a low score signified the opposite. All items except for neuroticism items used a 5-point Likert scale response format (1=strongly agree, 2=agree, 3=neither agree nor disagree, 4=disagree, 5=strongly disagree). Neuroticism was based on the sum of 9 yes-no items, in which 0 represented low neuroticism and 9 represented high neuroticism. Cronbach’s alphas for the scales (with the exception of the single item stress scale) were: ambition (4 items; .56), harddriving (5 items; .54), neuroticism (9 items; .74), paranoia (10 items; .69), and cynicism (9 items; .74). Details concerning the items of each scale can be found in the online Appendix.
Dementia Diagnoses
Participants were continuously screened for dementia throughout the study. To be considered as a suspected case, at least one of the following criteria had to be met: a score lower than 24 on the Mini-Mental State Examination (MMSE), a decline of 3 points or more on the MMSE since the participant’s last visit, low scores on the cognitive tests, a history of dementia documented in their medical records, being suspected of having dementia by the research nurses, and/or being reported of having cognitive problems by a proxy (Gatz et al., 1997). Further, those who were non-responders at any point were contacted and asked to respond to a telephone-screening interview that incorporated a 10-item Mental Status Questionnaire, other brief cognitive measures, and questions concerning health and daily functioning (Gatz, Reynolds, Finkel, Pedersen, & Walters, 2010).
Suspected cases were diagnosed during a consensus conference based on all available information from the IPT visit, which included cognitive testing and nurses’ impressions, as well as proxy interviews, and reviews of medical records. Through IPT 4, participants suspected of cognitive impairment were invited for a complete clinical work-up that included a full somatic and neurological evaluation as well as a neuropsychological examination by a physician. This information was also considered during the consensus conference (Gatz et al., 1997). From IPT 5 and onward, the dementia diagnoses were based upon information derived from the aforementioned IPT visits and from information gathered from the longitudinal follow-up of various aspects, particularly cognitive data (Dahl et al., 2010; Gatz et al., 2010). All participants were diagnosed according to the edition of the Diagnostic and Statistical Manual of Mental Disorders current at that time (either DSM-III-R or DSM-IV) (American Psychiatric Association, 1987, 1994). A complete differential diagnosis of dementia was assigned based on standard criteria for each disease entity. Participants diagnosed with dementia were given a differential diagnosis of AD, VaD, mixed dementia, other specific dementias such as Parkinson’s disease dementia, dementia secondary to other conditions, or dementia not otherwise specified.
For those who were lost to IPT follow-up or for those who had been screened but not diagnosed with dementia at least one year before the register’s date of diagnosis, dementia status and age of dementia ascertainment were pulled from the Cause of Death Register (CDR) and the inpatient and outpatient records of the National Patient Register (NPR) to classify dementia status.1 If there was discordance between the sources, the consensus diagnoses were considered the gold standard.
CVD measures
CVD was self-reported in the baseline questionnaire. One was classified as having CVD if any of the following cardiovascular conditions were present: hypertension without use of antihypertensive medication, angina pectoris, myocardial infarction, phlebitis, circulation problems in the limbs, claudication, thrombosis, stroke, tachycardia, having a heart valve problem, and having a heart operation. Persons with hypertension taking antihypertensive medication were not considered as having CVD. Based upon literature suggesting self-reported stroke as being underreported (Englert et al., 2010), national health register diagnoses of hemorrhagic stroke and ischemic stroke that occurred before baseline were extracted from the National Patient Register and the Cause of Death Register as a supplement to the self-reported data on CVD.2 An additional CVD variable categorized into three groups in terms of the life threatening severity of the cardiovascular conditions as rated previously by a five-member panel of physicians was created (Gold, Malmberg, McClearn, Pedersen, & Berg, 2002).
Covariates
Covariates known to be associated with risk of dementia include sex, education, and Apolipoprotein E (ApoE) status. Education, which was based on self-reports, was defined as the highest achieved level and was categorized into two categories: elementary education and education beyond elementary school. ApoE ε4 is the most well-established risk genotype associated with dementia, and because it is also associated with CVD, it is relevant to include ApoE status as a covariate. ApoE status was categorized as carrier and non-carrier of the ε4 allele and was obtained from blood samples collected during the IPT 3 assessment. Hence, information on ApoE status was only available for 500 participants.
Statistical Analysis
All analyses were performed in STATA version 12.0. One-sample analysis of variance tests (ANOVA) and chi-squared tests were performed to examine differences between sample participants and respondent drop-outs. Cronbach’s alpha was calculated to test the internal consistency of the Type A scales. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CI) to evaluate the association between TABP and all-cause dementia as well as CVD and dementia. Age was used as the time scale in Cox regression, and as such, there was no need to model for the effect of age as it was absorbed into the estimate of the baseline hazard.
To correct for the clustering effect resulting from including related individuals, i.e. twins, a robust sandwich estimator was utilized during regression analyses. A robust standard error signifies the variance of a sample containing random and independent observations as opposed to a sample composed of twin pairs in which there is a clustering effect resulting from the correlated characteristics between twin members.
The associations between the Type A traits and the risk of dementia were assessed using continuous and dichotomous median-split variables. First, crude analyses were performed to examine the associations between each TABP subscale and the risk for all-cause dementia. In the next model, a mediation model, age, sex, education, and CVD were controlled for. Logistic regression was performed to examine whether there was a significant association between TABP and CVD. To investigate the role of CVD as a moderator in the relationship between TABP and dementia, TABP was analyzed as the main effect and then stratified by CVD status. Interaction terms were used in Cox analyses to compute strata-specific estimates.
In secondary analyses that were based on a subsample of 500 participants, ApoE ε4 status was added to the above multivariate adjusted model including CVD in order to assess whether the presence or absence of ApoE ε4 alleles mediated the association between TABP and dementia. An interaction of TABP and ApoE status on dementia risk was also tested.
Results
Of the 1,069 participants, 190 persons (18%) developed dementia during a median of 19.06 years. The mean age of dementia ascertainment was 83.05 years (SD = 6.22), and the range was between 63 and 97 years. The incidence rate of all-cause dementia was 10.2 cases per 1000 person-years (95% CI = 0.009–0.012). The most common cause of dementia was AD (53%), followed by VaD (23%). The participants included those who had complete information on sex, age, CVD status and the Type A measures from Q1 Red while respondent drop-outs included those who had missing information for some of these measures. Results from an ANOVA test indicated that respondent drop-outs were significantly older (M = 69.52, SD = 8.98) than participants (M = 64.81, SD = 8.25, respectively), F(1, 1,269) = 53.19, p < .001). Compared to participants, respondent drop-outs were also less educated and had a higher proportion of CVD, X 2(1, N = 1,269) = 19.47, p < .001; X2(1, N = 1,269) = 4.73, p = .03, respectively. Since fewer participants responded to the Q1 Blue questionnaire, all analyses with paranoia or cynicism were based on a sample of 836 persons of which 132 were cases of dementia.
TABP and Dementia
Participants who were later diagnosed with dementia generally scored slightly lower on TABP scales, i.e. being less Type A, than those who remained non-demented. The only significant difference in scores was observed for the ambition scale (M = 15.80, SD = 2.76 for demented; M = 16.23, SD = 2.46 for non-demented; F (1, 1,069) = 4.57, p = .03).
In Cox regression analyses, the Type A measures were first analyzed in their continuous forms in relation to dementia risk (Table 1). A higher score on ambition, which was based on items such as, “I am ambitious,” was associated with a lower risk of dementia, even when controlling for sex, education, and CVD. Other TABP measures were not significantly associated with dementia. Comparable results were observed based on analyses using median-dichotomized Type A measures (results not shown).
Table 1.
Analyses of the effect of continuous Type A variables on predicting dementia (N = 1,069; n = 190 cases)
| Crude | Adjustedb | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Stress | 1.03 | 0.92–1.15 | .61 | 1.02 | 0.92–1.13 | .73 |
| Hard-driving | 0.97 | 0.93–1.02 | .22 | 0.98 | 0.94–1.02 | .26 |
| Ambition | 0.94 | 0.89–0.98 | .02 | 0.93 | 0.88–0.98 | .01 |
| Neuroticism | 0.99 | 0.93–1.05 | .80 | 0.98 | 0.92–1.04 | .55 |
| Paranoiaa | 0.99 | 0.96–1.02 | .49 | 0.99 | 0.96–1.02 | .39 |
| Cynicisma | 0.98 | 0.95–1.01 | .21 | 0.98 | 0.95–1.01 | .16 |
Note. HR = hazard ratio; CI = confidence interval.
Based on N = 836 and 132 cases.
Adjusted for sex, education and cardiovascular disease with robust standard error. There was no need to adjust for age since age was used as the time scale in Cox regression.
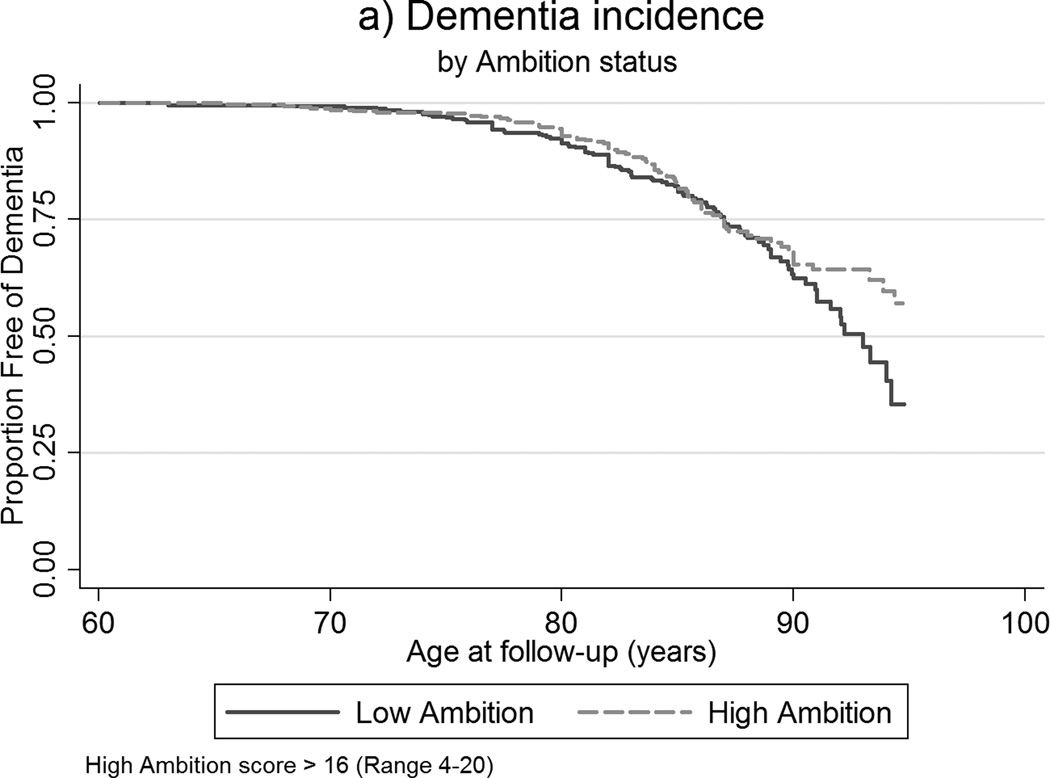
The difference in dementia incidence between low and high ambition levels is illustrated in Figure 1, where over half of those scoring high on ambition remained free of dementia whereas only approximately 30% of those with low ambition scores did not develop dementia.
Figure 1.
Kaplan-Meier survival curves of age of dementia incidence by ambition status.
TABP and CVD
A robust binary logistic model adjusted for age, sex and education was used to calculate the odds of having CVD for each of the six TABP measures. Stress, neuroticism, paranoia and cynicism were found to be significantly associated with an increased odds of CVD (OR = 1.22, 95% CI = 1.10–1.34; OR = 1.15, 95% CI = 1.09–1.22; OR = 1.04, 95% CI = 1.02–1.07; OR = 1.04, 95% CI = 1.02–1.07). Ambition and hard-driving were not associated with CVD.
CVD and Dementia
Logistic regression analysis confirmed that CVD was associated with an increased risk of dementia, even after adjusting for age, sex, and education (HR = 1.34, 95% CI = 1.01–1.77).
TABP, CVD, and Dementia
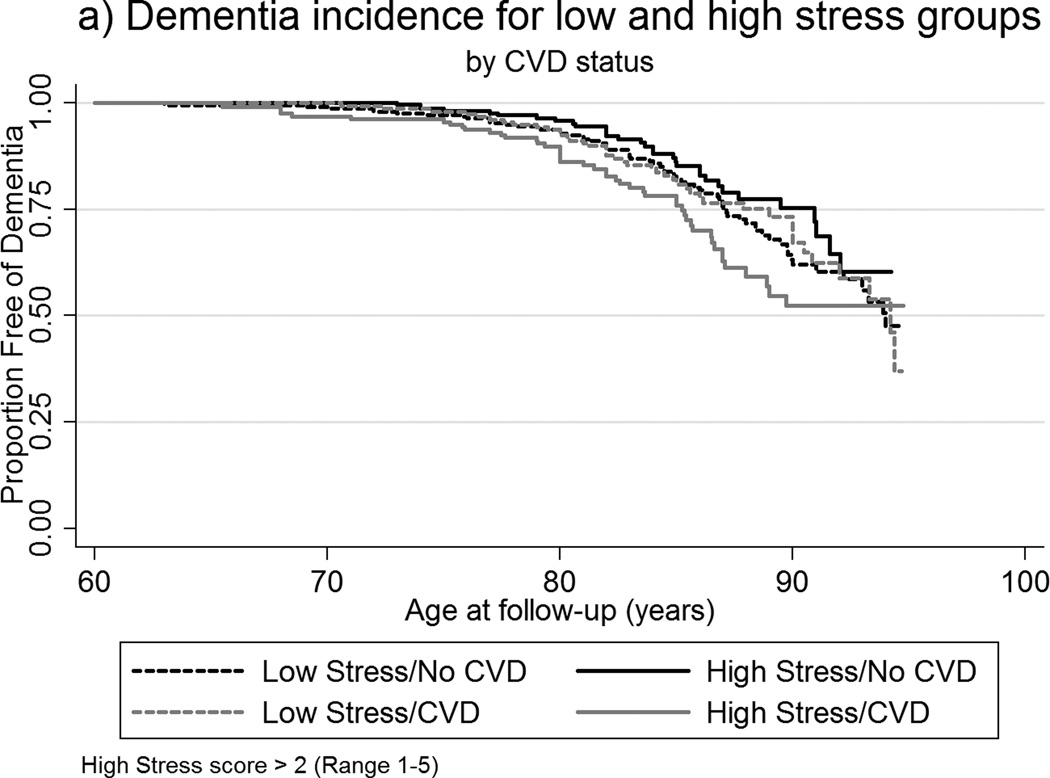
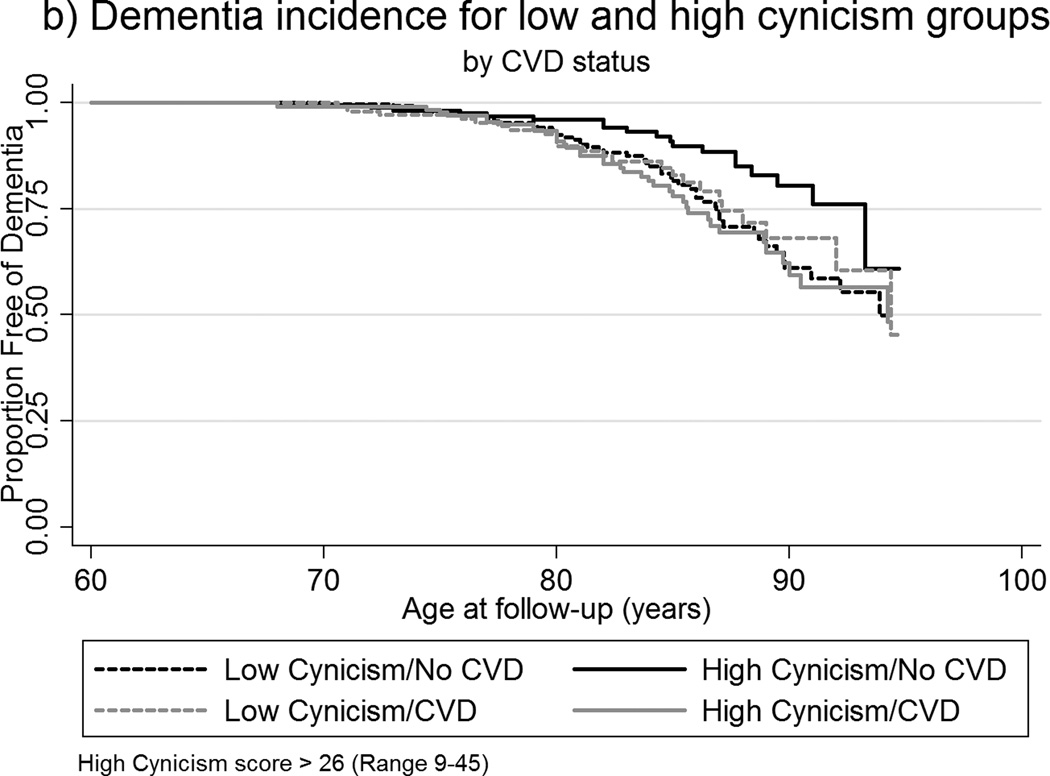
To investigate a moderation of the relationship of TABP and dementia by CVD status, Cox regression analyses using interaction terms were performed (Table 2). Interaction terms for CVD and median-dichotomized measures of stress, paranoia, and cynicism were significant. In those with CVD, high scores on stress, paranoia, and cynicism were associated with increased risk of dementia, whereas for those who did not have CVD, high scores on these measures appeared to be protective. An illustration of the interaction is depicted in the Kaplan Meier survival curves shown in Figures 2a–2b. There were no significant interaction effects for ambition, hard-driving, and neuroticism.
Table 2.
Analyses of the effect of dichotomized Type A measures on predicting dementia y CVD status (N = 1,069)
| No CVD (n = 656) |
CVD (n = 413) |
||||
|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI |
p value of interaction term |
|
| High vs. Low Stress | 0.76 | 0.50–1.14 | 1.43 | 0.95–2.15 | .03 |
| High vs. Low Hard-driving | 0.77 | 0.52–1.14 | 1.26 | 0.84–1.90 | .09 |
| High vs. Low Ambition | 0.62 | 0.41–0.92 | 0.94 | 0.62–1.42 | .15 |
| High vs. Low Neuroticism | 0.83 | 0.56–1.23 | 1.04 | 0.69–1.58 | .44 |
| High vs. Low Paranoiaa | 0.55 | 0.34–0.89 | 1.39 | 0.83–2.33 | .01 |
| High vs. Low Cynicisma | 0.50 | 0.29–0.84 | 1.25 | 0.76–2.06 | .01 |
Note. HR = hazard ratio; CI = confidence interval; CVD = cardiovascular disease. All above analyses were adjusted for sex and education with robust standard error. There was no need to adjust for age since age was used as the time scale in Cox regression.
Based on N = 836; No CVD (n = 515); CVD (n = 321).
Figure 2.
a–b. Kaplan-Meier survival curves of age of dementia incidence by: (a) CVD status among low and high stress groups; (b) CVD status among low and high cynicism groups.
In a secondary analysis, ApoE ε4 allele status was, as expected, a significant risk factor for dementia. Carriers of the ε4 allele were more than twice as likely to develop dementia compared to non-carriers, even when age and sex were taken into consideration (HR = 2.29, 95% CI = 1.57–3.34). Upon performing a Cox regression analysis of the subsample with ApoE status (n = 500), the effect of each of the Type A measures on predicting dementia while controlling for ApoE status as well as sex, education, and CVD remained unchanged (results not shown). There were no significant interactions between ApoE status and the TABP measures on dementia risk.
Additional sensitivity analyses were performed. First, point estimates generated from testing a CVD variable categorized into three levels based on life threatening severity with risk of dementia were similar to those based on analyses using the dichotomous (presence or absence) CVD variable. Of those classified as having CVD, 48 (12%) persons had somewhat life threatening CVD and 365 (88%) persons had very life threatening CVD. The hazard ratio for dementia associated with somewhat life threatening CVD was 1.44 (95% CI = 0.79–2.61), and 1.32 (95% CI = 0.98–1.77) for very life threatening CVD. Although the associations were not significant, the point estimates were similar to that based on the analysis of the binary CVD variable (HR = 1.34). In another sensitivity analysis, the CVD association with VaD (HR = 1.88, 95% CI = 1.04–3.40) was assessed. Although not significantly different from that for all dementias, the somewhat elevated estimate suggests that the risk of dementia is in part driven by the association of CVD and VaD.
Discussion
In this prospective study with a 23-year follow-up time, the association between TABP and dementia was assessed. There was evidence for interactions with CVD, whereby high scores on TABP measures—namely stress, paranoia, and cynicism—conferred an increased dementia risk in those with CVD, but a protective effect in those without CVD. Ambition was found to have a protective effect on dementia, but there was little evidence that the other TABP measures influenced dementia risk.
The findings regarding the relationship between TABP and CVD as well as CVD and dementia are in agreement with the literature. Psychological stress has been shown to be associated with CVD (Rosengren et al., 2004), and CVD has been linked to cognitive impairment and dementia (Stampfer, 2006). Thus, one might expect an association between TABP and dementia that is mediated by CVD. However, CVD did not mediate the relationship, but instead appeared to be a moderator. Persons with CVD may be more sensitive to the perturbations of the HPA axis by stress, which may lead to greater risk of dementia. For those without CVD, having a certain amount of stress may help maintain homeostasis and, hence, may be protective against dementia. It is possible that the observed protective effect of cynicism and paranoia could be attributed to these individuals having some genetic predisposition that allows them to better withstand the effects of aging, similar to the hardy survivor effect.
While having TABP is a risk factor for CVD, there are ways of modifying behavior such as engaging in behavioral therapy or reducing psychosocial factors that influence stress reactivity (Linden, Phillips, & Leclerc, 2007). Taken together with the results confirming CVD as being associated with higher risk of dementia and the findings of the present study, these interventions may hold relevance for reducing dementia risk among those with CVD who have greater stress, cynicism, and paranoia.
The only Type A measure that was observed to be significantly related to dementia regardless of CVD status was ambition. Ambition was not associated with CVD, and the protective effect of ambition for dementia risk remained even when CVD and education were controlled for. This suggests that being ambitious has beneficial effects on dementia risk that go beyond education attainment. Thus, a byproduct of having the trait of ambition could be related to being more active in the cognitive, physical and social sense. Ambition was also significantly correlated to the trait of Openness to Experience (results not shown), which has been associated with higher cognitive performance (Sharp, Reynolds, Pedersen, & Gatz, 2010). Further, higher ambition as a protective factor for dementia is supported by the theory that optimizing cognitive function decreases risk of dementia (La Rue, 2010).
Among the TABP measures, neuroticism was the most well-researched in relation to dementia risk (Crowe et al., 2006; Duberstein et al., 2011; Terracciano et al., 2013; Wang et al., 2009; Wilson et al., 2003). In the present study, neuroticism was not found to be a significant predictor of dementia, which is in accordance with the study by Wang et al., but in contrast to other studies assessing dementia risk in relation to neuroticism. It is possible that previous studies with relatively shorter follow-up periods could not distinguish direction of causation, and elevated neuroticism might have been a preclinical sign of dementia. While Wang et al. did not find neuroticism to be independently related to dementia, low neuroticism was found to be associated with reduced dementia risk amongst those with inactive or socially isolated lifestyles (Wang et al., 2009). Crowe and colleagues found greater neuroticism to be associated with increased risk of cognitive impairment (Crowe et al., 2006), a state that sometimes, though not necessarily, precedes dementia. Although the study had a follow-up time of 25 years, the measure of cognitive impairment was cross-sectional. As such, their study did not allow for elapsed time to onset of cognitive impairment to be taken into account. Thus, the relationship between neuroticism and dementia remains unclear.
Strengths and Limitations
There are several strengths of the present study, notably the prospective design, the median follow-up time of 19 years, and the use of a population-based sample. Previous studies indicate that personality change precedes early symptoms of dementia (Robins Wahlin & Byrne, 2011). Thus, there is a risk that persons with undetected dementia may not be able to provide reliable responses with regards to personality, or that personality might have changed as part of the dementia process. The long follow-up time in this study allows for convincing arguments that personality may be a predictor rather than a reflection of clinical symptoms of dementia. Access to data on age at dementia ascertainment allowed use of Cox modeling to examine the relative risk of incident dementia over time rather than at a particular time point. The sample size was sufficiently large for capturing the effects of TABP on dementia risk. Further, the risk of reverse causality is low due to the use of stringent exclusion criteria during the selection process of study participants.
In addition to the study’s strengths, there are also limitations. Self-reports of TABP may be less robust predictors compared to interviewer-based assessments (Miller et al., 1991). If so, this would drive findings toward the null. Nevertheless, the TABP measures were based on items from validated scales for Type A behavior (Lichtenstein et al., 1989; Smith et al., 1989). Reliabilities for the scales were generally acceptable based on Cronbach’s alpha statistics. There are few generally acceptable definitions of CVD for research purposes. Epidemiologic studies customarily employ dichotomous measures rather than continuous measures, as there are no obvious ways in which to quantify dose-response indicators of severity of disease. The use of a dichotomous CVD variable could result in information loss; however, a sensitivity analysis with a CVD variable categorized into three levels according to life threatening severity gave similar estimates as that based on the dichotomous CVD variable. Dementia status was not evaluated by a physician from IPT 5 and forward as was previously done in prior IPTs. However, in later evaluations of dementia, all data from previous IPTs including an extensive cognitive test battery, medical records as well as the research nurses’ evaluation of cognitive status were available. The availability of longitudinal cognitive data made it possible to identify drops in cognitive performance. Further, nurse evaluations have been shown to have high sensitivity (0.80) and specificity (0.98) (Dahl, Berg, & Nilsson, 2007). There may have been possible selection bias as the participants had a younger mean age compared to that of respondent dropouts. However, as the proportion of dementia cases was not significantly different between participants and respondent drop-outs, such bias is likely to be minimal. When related individuals, such as twins, are studied, one must always evaluate whether the sample is representative of the general population for the measures of interest. There is substantial evidence that elderly Scandinavian twins are generalizable to a population of singletons of comparable age (Simmons et al., 1997), and that dementia prevalence (Gatz et al., 2005), all cause mortality and cardiovascular mortality do not differ significantly between twins and the general population (Christensen et al., 2001). The standard errors were corrected for including related individuals.
Conclusion
This study has shown that there are interactions between CVD with stress, paranoia, and cynicism on the risk of dementia, wherein high scorers of these traits with CVD were at greater risk for dementia while high scorers without CVD were protected. Individuals at risk for CVD might consider behavioral management therapy not only to reduce the risk of developing future cardiovascular conditions, but also to decrease the risk of dementia in later life. As this is the only prospective cohort study with a long follow-up period that has investigated the relationship of TABP, CVD, and incident dementia, intervention studies are called for to explore whether reducing stress levels in CVD patients will decrease dementia risk or delay onset of dementia.
Supplementary Material
Footnotes
The following ICD codes were used to identify dementia diagnoses: 290.0, 290.1, 290.4, 290.8, 290.9, 294.1, 331.0, 331.1, 331.2, 331.9 (ICD-9); F00, F01, F02, F03, F05.1, G30, G31.1, G31.8A (ICD-10).
ICD codes that were used to detect cases of hemorrhagic stroke are as follows: 331 (ICD-7), 432-434 (ICD-8), and 432-434 (ICD-9). ICD codes used to identify ischemic stroke events included: 332 (ICD-7), 431 (ICD-8), and 431-432 (ICD-9).
Contributor Information
Kathleen Bokenberger, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm.
Nancy L. Pedersen, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm Department of Psychology, University of Southern California, Los Angeles
Margaret Gatz, Department of Psychology, University of Southern California, Los Angeles Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm.
Anna K. Dahl, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm School of Health Sciences, Jönköping University, Jönköping
References
- Amaducci LA, Fratiglioni L, Rocca WA, Fieschi C, Livrea P, Pedone D, et al. Risk factors for clinically diagnosed Alzheimer's disease: a case-control study of an Italian population. Neurology. 1986;36(7):922–931. doi: 10.1212/wnl.36.7.922. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, editor. Diagnostic and statistical manual of mental disorders: DSM-III-R. 4th ed. Washington, D.C.: 1987. text rev. ed. [Google Scholar]
- American Psychiatric Association, editor. Diagnostic and statistical manual of mental disorders: DSM-IV. 4th ed. ed. Washington, D.C.: 1994. [Google Scholar]
- Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB., Jr The Cook-Medley hostility scale: item content and ability to predict survival. Psychosomatic medicine. 1989;51(1):46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Bortner RW. A short rating scale as a potential measure of pattern A behavior. Journal of Chronic Diseases. 1969;22(2):87–91. doi: 10.1016/0021-9681(69)90061-7. [DOI] [PubMed] [Google Scholar]
- Breteler MM. Vascular involvement in cognitive decline and dementia. Epidemiologic evidence from the Rotterdam Study and the Rotterdam Scan Study. Annals of the New York Academy of Sciences. 2000;903:457–465. doi: 10.1111/j.1749-6632.2000.tb06399.x. [DOI] [PubMed] [Google Scholar]
- Christensen K, Wienke A, Skytthe A, Holm NV, Vaupel JW, Yashin AI. Cardiovascular mortality in twins and the fetal origins hypothesis. Twin research : The Official Journal of the International Society for Twin Studies. 2001;4(5):344–349. doi: 10.1375/1369052012506. [DOI] [PubMed] [Google Scholar]
- Clement F, Belleville S, Belanger S, Chasse V. Personality and psychological health in persons with mild cognitive impairment. Canadian Journal on Aging. 2009;28(2):147–156. doi: 10.1017/S0714980809090126. [DOI] [PubMed] [Google Scholar]
- Costa PT, Zonderman AB, McCrae RR, Williams RB. Cynicism and paranoid alienation in the Cook and Medley HO Scale. Psychosomatic Medicine. 1986;48(3–4):283–285. doi: 10.1097/00006842-198603000-00014. Retrieved from http://www.psychosomaticmedicine.org. [DOI] [PubMed] [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Fratiglioni L, Gatz M. Personality and risk of cognitive impairment 25 years later. Psychology and Aging. 2006;21(3):573–580. doi: 10.1037/0882-7974.21.3.573. [DOI] [PubMed] [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Gatz M. Do Work-related Stress and Reactivity to Stress Predict Dementia More Than 30 Years Later? Alzheimer Disease and Associated Disorders. 2007;21(3):205–209. doi: 10.1097/WAD.0b013e31811ec10a. [DOI] [PubMed] [Google Scholar]
- Dahl A, Berg S, Nilsson SE. Identification of dementia in epidemiological research: a study on the usefulness of various data sources. Aging Clinical and Experimental Research. 2007;19(5):381–389. doi: 10.1007/BF03324718. [DOI] [PubMed] [Google Scholar]
- Dahl A, Hassing LB, Fransson E, Berg S, Gatz M, Reynolds CA, Pedersen NL. Being overweight in midlife is associated with lower cognitive ability and steeper cognitive decline in late life. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2010;65(1):57–62. doi: 10.1093/gerona/glp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembroski TM, MacDougall JM, Shields JL, Petitto J, Lushene R. Components of the type A coronary-prone behavior pattern and cardiovascular responses to psychomotor performance challenge. Journal of Behavioral Medicine. 1978;1(2):159–176. doi: 10.1007/BF00846637. [DOI] [PubMed] [Google Scholar]
- Duberstein PR, Chapman BP, Tindle HA, Sink KM, Bamonti P, Robbins J, Franks P. Personality and risk for Alzheimer's disease in adults 72 years of age and older: a 6-year follow-up. Psychology and Aging. 2011;26(2):351–362. doi: 10.1037/a0021377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert H, Muller-Nordhorn J, Seewald S, Sonntag F, Voller H, Meyer-Sabellek W, Willich SN. Is patient self-report an adequate tool for monitoring cardiovascular conditions in patients with hypercholesterolemia? Journal of Public Health (Oxford, England) 2010;32(3):387–394. doi: 10.1093/pubmed/fdq013. [DOI] [PubMed] [Google Scholar]
- Eriksson UK, Bennet AM, Gatz M, Dickman PW, Pedersen NL. Nonstroke cardiovascular disease and risk of Alzheimer disease and dementia. Alzheimer Disease and Associated Disorders. 2010;24(3):213–219. doi: 10.1097/WAD.0b013e3181d1b99b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL. Processing Speed and Longitudinal Trajectories of Change for Cognitive Abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology, and Cognition. 2004;11(2–3):325–345. [Google Scholar]
- Floderus B. Psycho-social factors in relation to coronary heart disease and associated risk factors. Tryckindustri, Solna: Tr.; 1974. [Google Scholar]
- Fratiglioni L, De Ronchi D, Aguero-Torres H. Worldwide prevalence and incidence of dementia. Drugs and Aging. 1999;15(5):365–375. doi: 10.2165/00002512-199915050-00004. [DOI] [PubMed] [Google Scholar]
- Gatz M, Fratiglioni L, Johansson B, Berg S, Mortimer JA, Reynolds CA, Pedersen NL. Complete ascertainment of dementia in the Swedish Twin Registry: the HARMONY study. Neurobiology of Aging. 2005;26(4):439–447. doi: 10.1016/j.neurobiolaging.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, Ahlbom A. Heritability for Alzheimer's disease: the study of dementia in Swedish twins. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 1997;52(2):M117–M125. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Finkel D, Pedersen NL, Walters E. Dementia in Swedish twins: predicting incident cases. Behavioral Genetics. 2010;40(6):768–775. doi: 10.1007/s10519-010-9407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold CH, Malmberg B, McClearn GE, Pedersen NL, Berg S. Gender and health: a study of older unlike-sex twins. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2002;57(3):S168–S176. doi: 10.1093/geronb/57.3.s168. [DOI] [PubMed] [Google Scholar]
- Haynes SG, Feineib M, Levine S, Scotch N, Kannel WB. The relationship of psychosocial factors to coronary heart disease in the Framingham Study. American Journal of Epidemiology. 1978;107(5):384–402. doi: 10.1093/oxfordjournals.aje.a112557. [DOI] [PubMed] [Google Scholar]
- Johansson L, Guo X, Waern M, Ostling S, Gustafson D, Bengtsson C, Skoog I. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain: A Journal of Neurology. 2010;133(Pt 8):2217–2224. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- Knopman D. Cecil Medicine. 24 ed. Philadelphia, Pa: Saunders Elsevier; 2011. Alzheimer’s disease and other dementias. [Google Scholar]
- La Rue A. Healthy brain aging: role of cognitive reserve, cognitive stimulation, and cognitive exercises. Clinics in Geriatric Medicine. 2010;26(1):99–111. doi: 10.1016/j.cger.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Pedersen NL, Plomin R, de Faire U, McClearn GE. Type A behavior pattern, related personality traits and self-reported coronary heart disease. Personality and Individual Differences. 1989;10(4):419–426. [Google Scholar]
- Linden W, Phillips MJ, Leclerc J. Psychological treatment of cardiac patients: a meta-analysis. European Heart Journal. 2007;28(24):2972–2984. doi: 10.1093/eurheartj/ehm504. [DOI] [PubMed] [Google Scholar]
- Miller TQ, Turner CW, Tindale RS, Posavac EJ, Dugoni BL. Reasons for the trend toward null findings in research on Type A behavior. Psychological Bulletin. 1991;110(3):469–485. doi: 10.1037/0033-2909.110.3.469. [DOI] [PubMed] [Google Scholar]
- Motomura N, Ohkubo F, Tomota Y, Akagi H, Asano A, Seo T. Premorbid psychosocial behavior in demented patients. The International Journal of Neuroscience. 1998;95(3–4):167–172. doi: 10.3109/00207459809003338. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, McClearn GE, Plomin R, Nesselroade JR, Berg S, DeFaire U. The Swedish Adoption Twin Study of Aging: an update. Acta Geneticae Medicae et Gemellologiae. 1991;40(1):7–20. doi: 10.1017/s0001566000006681. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and alzheimer disease: A population-based cohort study. Archives of Internal Medicine. 2006;166(9):1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- Robins Wahlin TB, Byrne GJ. Personality changes in Alzheimer's disease: a systematic review. International Journal of Geriatric Psychiatry. 2011;26(10):1019–1029. doi: 10.1002/gps.2655. [DOI] [PubMed] [Google Scholar]
- Rosano C, Newman AB. Cardiovascular disease and risk of Alzheimer's disease. Neurological Research. 2006;28(6):612–620. doi: 10.1179/016164106X130407. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- Rosenman RH, Friedman M, Straus R, Wurm M, Kositchek R, Hahn W, Werthessen NT. A predictive study of coronary heart disease. JAMA : The Journal of the American Medical Association. 1964;189:15–22. doi: 10.1001/jama.1964.03070010021004. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. Journal of Internal Medicine. 2000;247(2):188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Sharp ES, Reynolds CA, Pedersen NL, Gatz M. Cognitive engagement and cognitive aging: is openness protective? Psychology and Aging. 2010;25(1):60–73. doi: 10.1037/a0018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons SF, Johansson B, Zarit SH, Ljungquist B, Plomin R, Mcclearn GE. Selection Bias in Samples of Older Twins?: A Comparison between Octogenarian Twins and Singletons in Sweden. Journal of Aging and Health. 1997;9(4):553–567. doi: 10.1177/089826439700900407. [DOI] [PubMed] [Google Scholar]
- Smith TW, Glazer K, Ruiz JM, Gallo LC. Hostility, anger, aggressiveness, and coronary heart disease: an interpersonal perspective on personality, emotion, and health. Journal of Personality. 2004;72(6):1217–1270. doi: 10.1111/j.1467-6494.2004.00296.x. [DOI] [PubMed] [Google Scholar]
- Smith TW, MacKenzie J. Personality and risk of physical illness. Annual Review of Clinical Psychology. 2006;2:435–467. doi: 10.1146/annurev.clinpsy.2.022305.095257. [DOI] [PubMed] [Google Scholar]
- Smith TW, O'Keeffe JL, Allred KD. Neuroticism, symptom reports, and type A behavior: interpretive cautions for the Framingham Scale. Journal of Behavioral Medicine. 1989;12(1):1–11. doi: 10.1007/BF00844745. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ. Cardiovascular disease and Alzheimer's disease: common links. Journal of Internal Medicine. 2006;260(3):211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nature reviews. Cardiology. 2012;9(6):360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- Terracciano A, Iacono D, O'Brien RJ, Troncoso JC, An Y, Sutin AR, Resnick SM. Personality and resilience to Alzheimer's disease neuropathology: a prospective autopsy study. Neurobiology of Aging. 2013;34(4):1045–1050. doi: 10.1016/j.neurobiolaging.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-X, Karp A, Herlitz A, Crowe M, Kåreholt I, Winblad B, Fratiglioni L. Personality and lifestyle in relation to dementia incidence. Neurology. 2009;72(3):253–259. doi: 10.1212/01.wnl.0000339485.39246.87. [DOI] [PubMed] [Google Scholar]
- Whalley LJ. Brain ageing and dementia: what makes the difference? The British Journal of Psychiatry: The Journal of Mental Science. 2002;181:369–371. doi: 10.1192/bjp.181.5.369. [DOI] [PubMed] [Google Scholar]
- Williams RB, Jr, Haney TL, Lee KL, Kong YH, Blumenthal JA, Whalen RE. Type A behavior, hostility, and coronary atherosclerosis. Psychosomatic Medicine. 1980;42(6):539–549. doi: 10.1097/00006842-198011000-00002. Retrieved from http://www.psychosomaticmedicine.org. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer's disease. Neurology. 2003;61(11):1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Ellis WG, Zarow C, Zaias BW, Jagust WJ, Mack WJ, Chui HC. Neuropathologic substrates of ischemic vascular dementia. Journal of Neuropathology and Experimental Neurology. 2000;59(11):931–945. doi: 10.1093/jnen/59.11.931. Retrieved from http://ovidsp.tx.ovid.com/ [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.