Abstract
Purpose
The aim of this study was to determine whether school-aged children with Kawasaki disease (KD) have an increased risk for early atherosclerosis.
Methods
The study included 98 children. The children were divided into the following groups: group A (n=19), KD with coronary arterial lesions that persisted or regressed; group B (n=49), KD without coronary arterial lesions; and group C (n=30), healthy children. Anthropometric variables and the levels of biochemical markers, including total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A, apolipoprotein B, homocysteine, high-sensitivity C-reactive protein (hs-CRP), and brachial artery stiffness using pulse wave velocity were compared among the three groups.
Results
There were no significant differences in blood pressure and body index among the three groups. Additionally, there was no sex-specific difference. Moreover, the levels of triglyceride, HDL-C, apolipoprotein A, and hs-CRP did not differ among the three groups. However, the levels of total cholesterol (P=0.018), LDL-C (P=0.0003), and apolipoprotein B (P=0.029) were significantly higher in group A than in group C. Further, the level of homocysteine and the aortic pulse wave velocity were significantly higher in groups A and B than in group C (P=0.0001).
Conclusion
School-aged children after KD have high lipid profiles and arterial stiffness indicating an increased risk for early atherosclerosis.
Keywords: Risk factors of cardiovascular diseases, Atherosclerosis, Mucocutaneous lymph node syndrome
Introduction
Long-term cardiovascular complications of persistent coronary arterial lesion secondary to Kawasaki disease (KD) are well documented. The previous long-term studies have reported that patients with normal coronary artery or transient dilation during the acute phase of illness generally have the same cardiovascular risks as the general population during the transition into adulthood1,2). However, concerns have been raised regarding the possibility of increased predisposition to premature atherosclerosis in children with KD in their transition into adulthood3,4). This may be due to ongoing functional and structural abnormalities of affected vessels, abnormal profile of known risk factors for atherosclerosis and the presence of a state of chronic inflammation4,5,6,7). Data on the cardiovascular risk profile long after resolution of the acute phase of KD remain limited and controversial4,5). Noninvasive assessment of systemic arteries has shown inconsistent and uncertain relationships with the degree of coronary artery involvement8,9,10).
We aimed to assess whether or not school-aged children after KD have increased risk factors suggestive of early atherosclerosis.
Materials and methods
1. Subjects
This study was conducted with three groups based on the patients' status and the presence or absence of coronary complications: patients with KD with persistent or regressed coronary artery lesions (CALs) (group A), patients with KD without CALs (group B), and age- and sex-matched healthy controls (group C). The patients with KD were diagnosed at or referred to the Chungnam National University Hospital in Daejeon, Korea. The subjects were recruited during the study period from January 2006 to July 2011. Healthy age-matched subjects (6-12 years of age) were recruited as control subjects in this study, and they were patients who previously discharged from our clinic with a diagnosis of functional murmur. Exclusion criteria for both KD and control subjects included a history of a condition that might affect vascular function such as rheumatologic disease, diabetes or other chronic medical conditions.
2. Methods
As general characteristics for all patients were taken, body weight and height were measured, and body mass index was calculated accordingly. Before taking blood pressure and cardiovascular assessments, all patients rested for at least 15 minutes. Blood pressure was measured twice in the right arm with automatic sphygmomanometer (Dinamap, Critikon Inc., Tampa, FL, USA), with subjects in the seated position, and the average of the two readings was taken. From the medical records, the following patient data were retrieved: interval from disease onset to time of study, coronary complication, cardiac symptoms, and medications taken at the time of study. Coronary artery aneurysms were documented by serial two-dimensional echocardiography.
Venous blood was withdrawn for measurement of nonfasting total cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A (Apo-A), apolipoprotein B (Apo-B), high-sensitivity C-reactive protein (hs-CRP), and homocysteine levels. All of the aforementioned measurements were obtained by standard laboratory methods.
Echocardiography was performed using a Vivid7 (GE health-care Korea, Seoul, Korea) and the transducer with a frequency of 5 and 7 MHz. The coronary artery segments measurement included the internal lumen diameters of the left main coronary artery, left anterior descending artery, and right coronary artery. The coronary artery dimensions were normalized for body surface area as z scores and calculated using regression equations. Patients with any coronary artery segment with a z score ≥2.5 were considered to have CALs.
Stiffness of brachio-radial artery was assessed by measuring the pulse wave velocity (PWV) of the subjects in the supine position. PWV is related to the square root of elastic modulus, according to the Moens-Korteweg equation. Hence, the stiffer the artery, the faster the PWV. Previously validated photoplethysmographic technique was used to measure the transit time required for the pulse to travel at the wrist11). The transit time was determined from the time delay between the foot of the corresponding brachial and radial pulse wave. The average of three readings was taken. PWV was calculated by dividing the distance between the two points by transit time. PWV was measured by ocillometric method (Colin Co., Komki, Japan), and we calculated the mean value of both arms.
3. Data analysis
Data are described as frequencies and the mean±standard deviation. To compare the differences in variables among the three groups, one-way analysis of variance (ANOVA) was performed. When values of P<0.05 were yielded by ANOVA, multiple comparisons between two groups by the unpaired t test were performed. A value of P<0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA).
Results
A total of 98 subjects were studied. Out of 19 patients of group A, 8 patients had persistent CAL, whereas 11 patients had regressed CAL. In total 68 patients with KD, all except 6 patients had received intravenous immunoglobulin therapy during the acute phase of the illness while only 8 patients were maintained on long-term aspirin therapy. The group A was studied for 68.95±31 months after KD, whereas group B was studied for 52.29±26.51 months after KD. None were symptomatic and none required coronary artery interventions. The demographic and hemodynamic profiles are summarized in Table 1. There were no significant differences in gender, age, duration of follow-up, blood pressure and body mass index among the three groups.
Table 1.
Demographic and hemodynamic profiles of the three groups
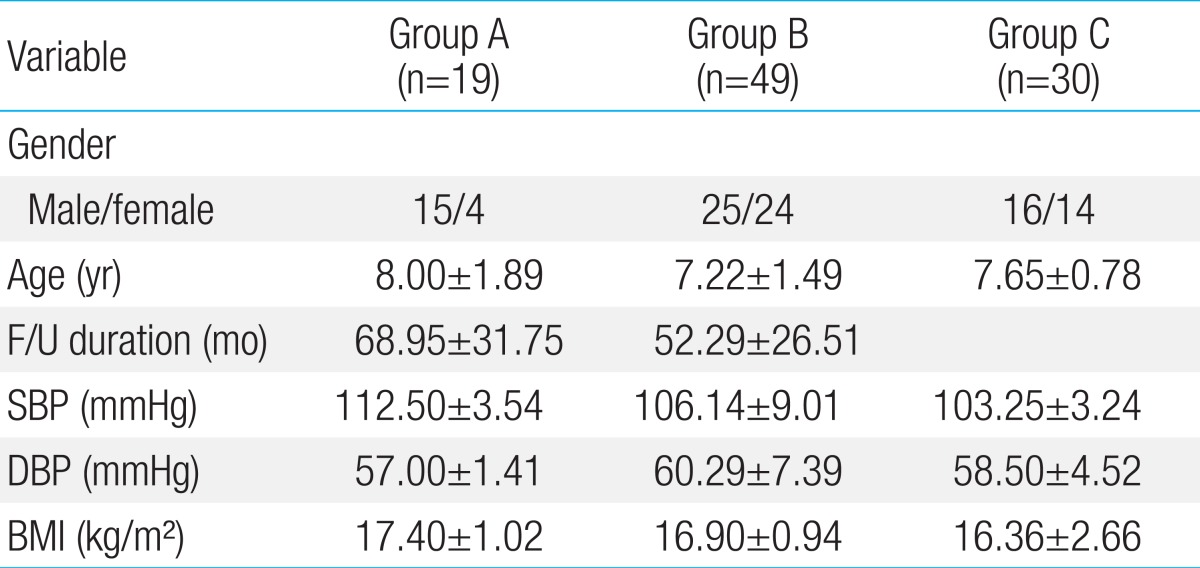
Values are presented as mean±standard deviation.
Group A, patients with Kawasaki disease (KD) with persistent or regressed coronary artery lesions (CALs); group B, patients with KD without CALs; group C, age- and sex-matched healthy controls; F/U, follow-up period after diagnosis of KD; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index.
The lipid profile of cohort is summarized in Table 2. The levels of total cholesterol, LDL-C, and Apo-B were significantly higher in group A subjects than those of group C subjects. However, these differences among KD groups were not significant. The levels of Apo-A, HDL-C, and triglyceride were not significantly different among the three groups.
Table 2.
Lipid profiles of the three groups
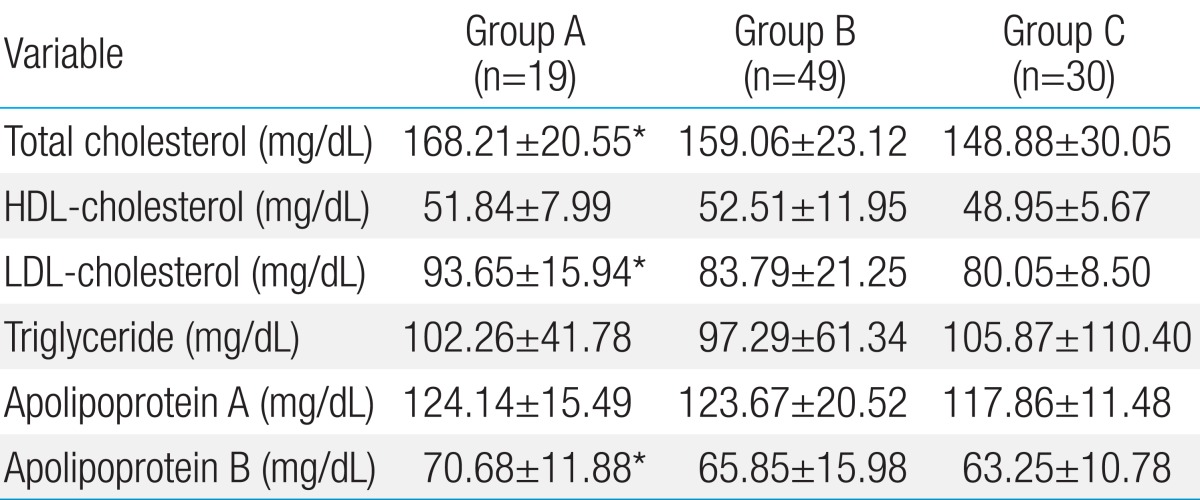
Values are presented as mean±standard deviation.
Group A, patients with Kawasaki disease (KD) with persistent or regressed coronary artery lesions (CALs); group B, patients with KD without CALs; group C, age- and sex-matched healthy controls; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
*P<0.05 versus group C.
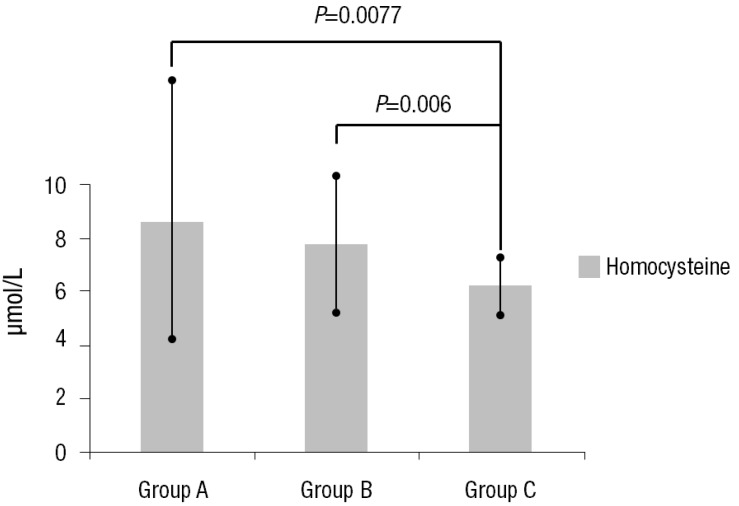
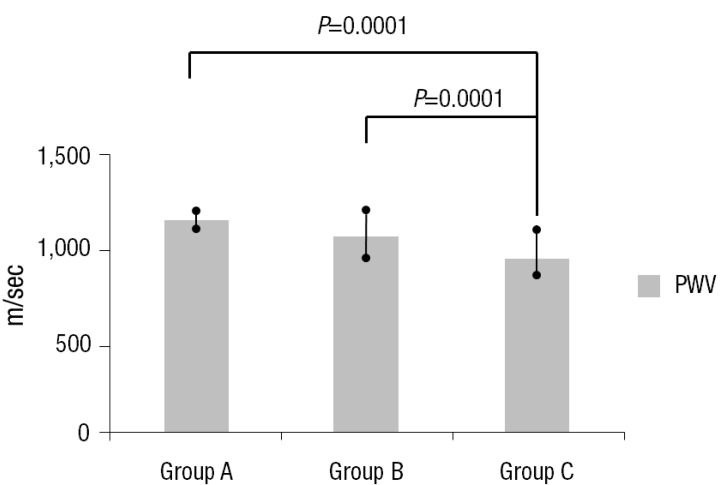
The serum homocysteine levels in groups A, B, and C subjects were 8.67±4.61 µmol/L, 7.85±3.08 µmol/L, and 6.20±1.15 µmol/L respectively. The differences among the groups were significant (Table 3) (Fig. 1). However, the differences in the level of hs-CRP were not significant (Table 3). The PWV of group A, B, and C subjects were 1,181.50±7.78 m/sec, 1,076.86±164.10 m/sec, and 966.71±88.70 m/sec respectively. PWVs of KD subjects (groups A and B) were higher than those of healthy subjects (group C) (Table 3) (Fig. 2).
Table 3.
Serum markers and arterial stiffness for atherosclerosis in the three groups
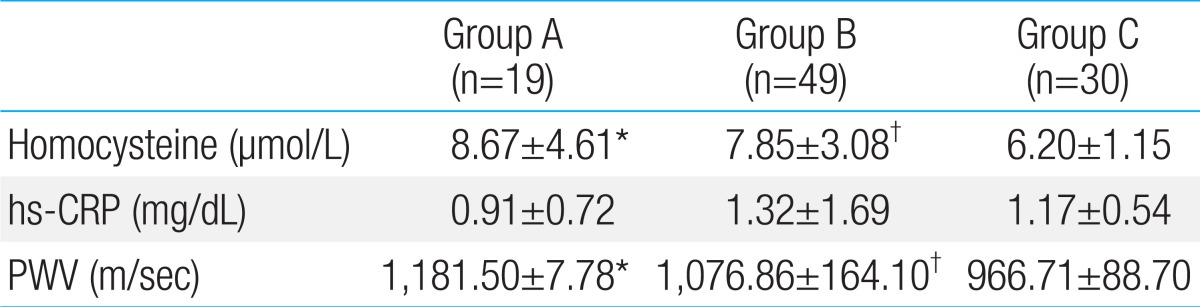
Values are presented as mean±standard deviation.
Group A, patients with Kawasaki disease (KD) with persistent or regressed coronary artery lesions (CALs); group B, patients with KD without CALs; group C, age- and sex-matched healthy controls; hs-CRP, high sensitivity C-reactive protein; PWV, pulse wave velocity.
*P<0.05 versus group C. †P<0.05 versus group C.
Fig. 1.
Homocysteine level in the three groups. Values are presented as mean±standard deviation. Group A, patients with Kawasaki disease (KD) with persistent or regressed coronary artery lesions (CALs); group B, patients with KD without CALs; group C, age- and sex-matched healthy controls.
Fig. 2.
Pulse wave velocity in the three groups. Values are presented as mean±standard deviation. Group A, patients with Kawasaki disease (KD) with persistent or regressed coronary artery lesions (CALs); group B, patients with KD without CALs; group C, age- and sex-matched healthy controls; PWV, pulse wave velocity.
Discussion
The objective of this study was to assess whether or not school-aged children after KD have increased risk factors for early atherosclerosis. Our cohort study of 68 school-aged children demonstrated that some cardiovascular risk factors for early atherosclerosis such as total cholesterol, LDL-C and Apo-B of lipid profiles, homocysteine and PWV are significantly higher in KD subjects than healthy subjects. However, there were no differences in hs-CRP, HDL-C, and Apo-A.
Several studies have recently suggested a greater long-term incidence of traditional cardiovascular risk factors in KD patients. Plasma lipid profiles, such as triglycerides, total cholesterol, LDL-C, and HDL-C levels, can be measured as predictors of atherosclerosis, and are associated with people who have coronary artery diseases12). According to Newburger et al.13), KD patients have decreased HDL-C levels after acute illness of KD. A recent study has shown that the potential effect of inflammation on coronary risk in patients with coronary dysfunction is correlated with HDL-C level, which suggests that HDL-C may be useful for the prevention of future cardiovascular risk in KD patients14). In their follow-up of KD patients at a mean of 7 years after KD, Cheung et al.5) demonstrated that patients with CAL had reduced not only HDL-C, but also Apo-A and all risk factors for cardiovascular disease. Yet, inconsistent correlation between lipid profile and the degree of early atherosclerosis have been reported in adolescent and young adult after KD6,8,9,10,15). McCrindle et al.10) showed that KD patients with 10 to 20 years of age had lower Apo-A levels than those in the healthy control subjects in 2007. Gupta-Malhotra et al.9) showed that levels of cholesterol and Apo-B, the protein in LDL-C and a proven risk factor for cardiovascular disease, are significantly elevated in patients with KD. In the findings of present study performed at 4-6 years after acute illness of KD, the levels of total cholesterol, LDL-C, and Apo-B were significantly higher in patients with persistent or regressed CAL than those of healthy control subjects. However, these differences among KD groups were not significant. Our findings suggest that the severity of vasculitis in the acute phase is not only partially reflected by development of CAL, but also related to late lipid abnormalities. The present studies disagree with those of Cheung et al.5) and McCrindle et al.10) Other lipid profile of LDL-C and apo-B was significantly increased instead of HDL-C and Apo-A. Potential reasons for the differences in study results in this subgroup of KD patients include differences in peripheral vascular health related to race and ethnicity or methological issues. Inhibition of lipoprotein lipase may decrease Apo-A levels by increasing LDL-C levels and therefore by increasing its catabolism16). Increased LDL-C levels have been described in adults with chronic inflammation due to rheumatoid arthritis17). Indeed, there is increasing evidence for unabated low-grade vasculitis after the end of the acute phase of KD. In order to know the true significance of these inconsistent findings among the recent studies, a study large enough to control for other variables that may influence lipid levels, such sex, age, body mass index and diet, is suggested.
Serum hs-CRP and serum amyloid-A have recently been regarded as reliable clinical markers for the prediction of coronary events, independent of other known chronic renal failure6). Also an elevated homocysteine level has been documented to be an independent risk factor for atherosclerosis in adults. Linnebank et al.18) showed that homocysteine levels and polymorphisms involved in homocysteine metabolism also have no significant causal effects on early atherosclerosis, and homocysteine levels are confounded with other stroke risk factors although it remains uncertain what these factors are. Our study showed that homocysteine level is significantly higher in KD groups than that of healthy children, but the levels of hs-CRP were not significantly different between KD and healthy subjects. It might suggest that homocysteine may be considered as the biomarker of early atherosclerosis even though validation is needed in finding relationship between the atherosclerosis and school-aged children after KD.
Many studies of cardiovascular risk factors for premature atherosclerosis have recently reported in adult with arterial stiffness, carotid arterial intima-media thickening and significant decreased % flow mediated dilation (FMD). However, noninvasive assessment of vessels has shown inconsistent abnormalities. FMD and intima media thickness (IMT) were not associated with PWV independently of cardiovascular risk factors in young adults19). PWV reflects a different aspect of vascular damage than FMD or IMT. The PWV is simple to measure and is a powerful marker for the risk of atherosclerosis in adults8,9,10). Patients with a history of KD have a higher PWV than patients in control group. Moreover, there is no significant difference in the PWV between the KD patients with or without CALs5). However, in other study PWV does not differ significantly between KD patients with normal-appearing coronary arteries and healthy controls9). Noto et al.20) reported that KD patients with persistent CALs, compared with control patients, had the carotid arterial wall with a higher IMT and lower dispensability. Decreased %FMD reflects endothelial cell dysfunction. Ishikawa and Iwashima21) showed that the children with KD already had arterial endothelial dysfunction within 5 years since the onset of illness. The present study shows that PWV is higher in school-aged KD subjects than healthy subjects regardless of CAL. The reason for difference in study results is not clear, but methological or genetic issues may be involved. Further comparison study between the inconsistent abnormalities of known cardiovascular risk factors for atherosclerosis reported in previous study and those in present study is suggested. It is still unclear about a long-term prognosis of KD regardless of CAL. Therefore, counseling on life style factors affecting cardiovascular health including dyslipidemia, hypertension, smoking, and obesity, is an important aspect of long-term management in all patients with a previous history of KD. Both American Heart Association22) and the Japanese23) guidelines recommend healthy life style counseling and cardiovascular risk assessment through primary health care for all KD patients every 5 years as minimal follow-up regardless of CAL.
Study limitations include the small number of patients investigated. As we present a retrospective study, a prospective assessment of the cardiovascular risk factors for early atherosclerosis during different phases of KD is warranted. Also, we evaluated the patients in whom complete KD was diagnosed and excluded patients with incomplete or atypical KD.
In conclusion, the cardiovascular risk factors for early atherosclerosis, including lipid profiles and arterial stiffness, are observed even in school-aged children with a history of KD. The healthy life style counseling and cardiovascular risk assessment for all KD patients are warranted regardless of CAL.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease: a 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 2.Gersony WM. The adult after Kawasaki disease the risks for late coronary events. J Am Coll Cardiol. 2009;54:1921–1923. doi: 10.1016/j.jacc.2009.06.057. [DOI] [PubMed] [Google Scholar]
- 3.Burns JC, Shike H, Gordon JB, Malhotra A, Schoenwetter M, Kawasaki T. Sequelae of Kawasaki disease in adolescents and young adults. J Am Coll Cardiol. 1996;28:253–257. doi: 10.1016/0735-1097(96)00099-x. [DOI] [PubMed] [Google Scholar]
- 4.Silva AA, Maeno Y, Hashmi A, Smallhorn JF, Silverman ED, McCrindle BW. Cardiovascular risk factors after Kawasaki disease: a case-control study. J Pediatr. 2001;138:400–405. doi: 10.1067/mpd.2001.111430. [DOI] [PubMed] [Google Scholar]
- 5.Cheung YF, Yung TC, Tam SC, Ho MH, Chau AK. Novel and traditional cardiovascular risk factors in children after Kawasaki disease: implications for premature atherosclerosis. J Am Coll Cardiol. 2004;43:120–124. doi: 10.1016/j.jacc.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 6.Mitani Y, Sawada H, Hayakawa H, Aoki K, Ohashi H, Matsumura M, et al. Elevated levels of high-sensitivity C-reactive protein and serum amyloid-A late after Kawasaki disease: association between inflammation and late coronary sequelae in Kawasaki disease. Circulation. 2005;111:38–43. doi: 10.1161/01.CIR.0000151311.38708.29. [DOI] [PubMed] [Google Scholar]
- 7.Iemura M, Ishii M, Sugimura T, Akagi T, Kato H. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart. 2000;83:307–311. doi: 10.1136/heart.83.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalla Pozza R, Bechtold S, Urschel S, Kozlik-Feldmann R, Netz H. Subclinical atherosclerosis, but normal autonomic function after Kawasaki disease. J Pediatr. 2007;151:239–243. doi: 10.1016/j.jpeds.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 9.Gupta-Malhotra M, Gruber D, Abraham SS, Roman MJ, Zabriskie JB, Hudgins LC, et al. Atherosclerosis in survivors of Kawasaki disease. J Pediatr. 2009;155:572–577. doi: 10.1016/j.jpeds.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 10.McCrindle BW, McIntyre S, Kim C, Lin T, Adeli K. Are patients after Kawasaki disease at increased risk for accelerated atherosclerosis? J Pediatr. 2007;151:244–248. doi: 10.1016/j.jpeds.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 11.Cheung YF, Taylor MJ, Fisk NM, Redington AN, Gardiner HM. Fetal origins of reduced arterial distensibility in the donor twin in twin-twin transfusion syndrome. Lancet. 2000;355:1157–1158. doi: 10.1016/s0140-6736(00)02068-7. [DOI] [PubMed] [Google Scholar]
- 12.Cabana VG, Gidding SS, Getz GS, Chapman J, Shulman ST. Serum amyloid A and high density lipoprotein participate in the acute phase response of Kawasaki disease. Pediatr Res. 1997;42:651–655. doi: 10.1203/00006450-199711000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Newburger JW, Burns JC, Beiser AS, Loscalzo J. Altered lipid profile after Kawasaki syndrome. Circulation. 1991;84:625–631. doi: 10.1161/01.cir.84.2.625. [DOI] [PubMed] [Google Scholar]
- 14.Nishtar S, Wierzbicki AS, Lumb PJ, Lambert-Hammill M, Turner CN, Crook MA, et al. Waist-hip ratio and low HDL predict the risk of coronary artery disease in Pakistanis. Curr Med Res Opin. 2004;20:55–62. doi: 10.1185/030079903125002595. [DOI] [PubMed] [Google Scholar]
- 15.Ou CY, Tseng YF, Lee CL, Chiou YH, Hsieh KS. Significant relationship between serum high-sensitivity C-reactive protein, high-density lipoprotein cholesterol levels and children with Kawasaki disease and coronary artery lesions. J Formos Med Assoc. 2009;108:719–724. doi: 10.1016/S0929-6646(09)60395-8. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg IJ, Blaner WS, Vanni TM, Moukides M, Ramakrishnan R. Role of lipoprotein lipase in the regulation of high density lipoprotein apolipoprotein metabolism: studies in normal and lipoprotein lipase-inhibited monkeys. J Clin Invest. 1990;86:463–473. doi: 10.1172/JCI114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakatos J, Harsagyi A. Serum total, HDL, LDL cholesterol, and triglyceride levels in patients with rheumatoid arthritis. Clin Biochem. 1988;21:93–96. doi: 10.1016/s0009-9120(88)80094-8. [DOI] [PubMed] [Google Scholar]
- 18.Linnebank M, Moskau S, Farmand S, Fliessbach K, Kolsch H, Bos M, et al. Homocysteine and carotid intima-media thickness in a german population: lack of clinical relevance. Stroke. 2006;37:2840–2842. doi: 10.1161/01.STR.0000244764.02851.d3. [DOI] [PubMed] [Google Scholar]
- 19.Koivistoinen T, Virtanen M, Hutri-Kahonen N, Lehtimaki T, Jula A, Juonala M, et al. Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: the Cardiovascular Risk in Young Finns Study and the Health 2000 Survey. Atherosclerosis. 2012;220:387–393. doi: 10.1016/j.atherosclerosis.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Noto N, Okada T, Yamasuge M, Taniguchi K, Karasawa K, Ayusawa M, et al. Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesions. Pediatrics. 2001;107:1095–1099. doi: 10.1542/peds.107.5.1095. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa T, Iwashima S. Endothelial dysfunction in children within 5 years after onset of Kawasaki disease. J Pediatr. 2013;163:1117–1121. doi: 10.1016/j.jpeds.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 22.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 23.JCS Joint Working Group. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2008): digest version. Circ J. 2010;74:1989–2020. doi: 10.1253/circj.cj-10-74-0903. [DOI] [PubMed] [Google Scholar]