Abstract
Objective
This study sought to evaluate platelet volume indices (mean platelet volume [MPV], platelet distribution width [PDW], and platelet large cell ratio [P-LCR]) in varicocele patients, and compare it with platelet volume parameters in healthy controls.
Methods
This cross-sectional study involved 2 groups: group 1 included 51 varicocele subjects and group 2 consisted of 50 healthy control subjects of similar ages. Peripheral venous blood samples were collected with ethylenediaminetetraacetic acid-K2 anticoagulant between 8:30 AM and 10 AM following an overnight fast. Platelet volume parameters (MPV, PDW, and P-LCR) were measured in both groups within 2 hours of sampling.
Results
The mean PDW, MPV, and P-LCR were 13.9±2.5%, 10.1±1.3 fL, and 27.3±7.8% in varicocele patients, respectively, and were 12.6±2.4%, 9.3±1.1 fL, and 21.9±6.4% in the control group, respectively. The mean PDW, MPV, and P-LCR were significantly higher in the varicocele group than the control group.
Conclusion
The results of the present study suggest that vascular components may play an important role in the pathophysiology of varicocele; therefore, there is a great need for prospective studies to confirm this relationship.
Keywords: Blood platelets, Mean platelet volume, Varicoceles, Vascular diseases
Introduction
Varicocele, which is defined as an abnormal venous dilatation and tortuosity of the pampiniform plexus, is regarded as the most common surgically correctable vascular cause of infertility in men [1,2]. Some evidence has shown an association between systemic varicosity and varicocele. There is also a positive association between varicocele and coronary artery ectasia. This may bring the existence of common mechanisms to mind like general vascular disorders [3,4].
On the other hand, it has been shown that the platelet size reflects platelet activity and can be assessed by platelet volume indices (PVI) [5,6]. PVI include the mean platelet volume (MPV), platelet distribution width (PDW), and platelet large cell ratio (P-LCR). The MPV is a measurement of the average size of platelets found in circulation in the blood. The MPV does not reflect the variation in platelet size observed microscopically. The PDW is computed as the coefficient of variation of the average volume of the platelet population. A high PDW indicates that the platelets are more variable in volume than normal [7,8]. The P-LCR indicates the percentage of large platelets with a volume >12 fL among the total of all platelets [9].
Previous studies have demonstrated that large platelets are metabolically and enzymatically more active than small platelets [10]. Platelet volume indices, particularly the MPV, have been demonstrated to increase with vascular disorders [11,12,13]. Based on our knowledge, there is one study evaluating the relationship between PVI and varicocele. This study found the MPV to be an important index of the PVI in varicocele patients [14], but in some situations, abnormal thrombopoiesis is associated with an increase in the PDW or P-LCR, despite the maintenance of a normal MPV [7]. Therefore, the assessment of the PDW and P-LCR with MPV can be valuable in the determination of a possible relationship between the PVI and varicocele.
The aim of this study was to assess the association between varicocele and the PVI as an indicator of platelet activity. This association may partly demonstrate the pathophysiology of varicocele.
Methods
This cross-sectional study was performed in Imam Reza Educational Hospital, Mashhad University of Medical Sciences, Mashhad, Iran in 2013. The study was approved by the Ethics Committee of Vice-Chancellor of Research of the university and all participants gave their informed consent. The study involved 2 groups: group 1 included 51 adult varicocele subjects (mean age, 25.8±5.4) who complained of scrotal pain or infertility and group 2 consisted of 50 healthy adult control subjects (mean age, 25.3±4.8).
Diagnoses of varicocele were made by physical examination by 2 urologists. If there was a disagreement over the diagnosis, scrotal Doppler ultrasound was performed to confirm the diagnosis. At room temperature, the 2 urologists performed an inspection and palpation of each patient's varicose veins both before and after the Valsalva maneuver. The physical examination was performed while the patient was standing. Based on some studies, the grading of varicoceles by physical examination has the greatest value and has some effects on fertility and response to treatment [15]. The varicocele was categorized based on the Dubin grading system [16] as follows: grade III, visible varicose veins; grade II, varicose veins that can be palpated without the Valsalva maneuver but cannot be seen; or grade I, varicose veins that can be palpated only after the Valsalva maneuver. In addition, the sperm parameters of the patients were analyzed.
The following patients were excluded from the study: subjects with a history of coronary artery disease, hypertension, hyperlipidemia, peripheral vascular disease, diabetes mellitus, testicular tumor, hydrocele, undescended testis, inguinal hernia, epididymo-orchitis, inguinal and scrotal surgeries, splenectomy, thrombotic thrombocytopenic purpura (TTP), idiopathic thrombocytopenic purpura (ITP), myeloproliferative disorders, leukemia, Bernard-Soulier syndrome, epinephrine injection within the previous one month, thrombocytopenia (less than 150×103/µL), or thrombocytosis (more than 450×103/µL).
Peripheral venous blood samples were collected with ethylenediaminetetraacetic acid (EDTA-K2) anticoagulant between 8:30 AM and 10 AM following an overnight fast. To avoid platelet swelling and reliably measure the PVI in the EDTA, the laboratory analysis should be done within 2 hours of venipuncture [17,18]. Therefore, whole blood samples were collected in EDTA-containing tubes and the platelet indices were measured within 60 minutes after sampling. The total blood count, including hemoglobin (Hb), white blood cells (WBC), platelets (Plt), and platelet volume parameters were measured in both groups. An automated blood cell counter was used for these measurements (Sysmex K21N analyser, Kobe, Japan).
The Kolmogorov-Smirnov test was used to test the normality of distribution. For normally distributed values, the differences between groups were evaluated by an independent sample t-test and analysis of variance. The Mann-Whitney U test was used to assess the difference between non-parametric values. Statistical analyses were performed using the SPSS ver. 16 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered to be statistically significant.
Results
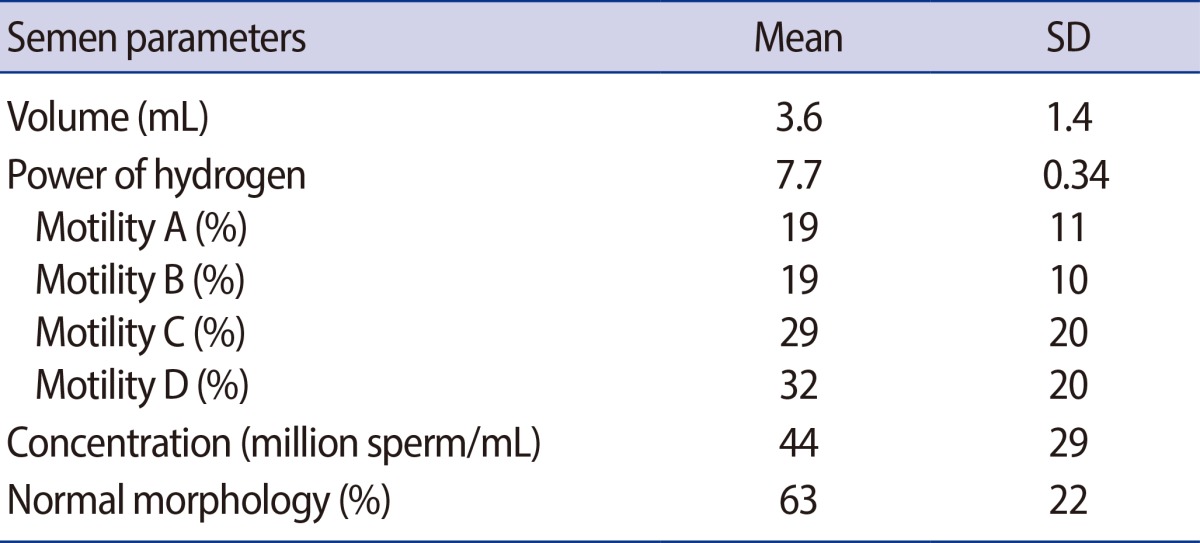
The mean age (±SD) and age range of patients were 25.8 (±5.4) years and 18 to 40 years, and for the control group, 25.3 (±4.8) years and 18-37 years, respectively. Based on the physical examination, 37 patients (72.5%) had left-side varicocele, 5 (9.8%) had right-side varicocele, and bilateral varicocele was seen in 9 cases (17.6%). The most common grade of varicocele was grade III in 21 patients (41.2%). Nineteen patients (37.2%) had grade II and 11 cases (21.6%) had grade I varicocele. The seminal parameters of group 1 are outlined in Table 1.
Table 1.
Seminal parameters of varicocele patients
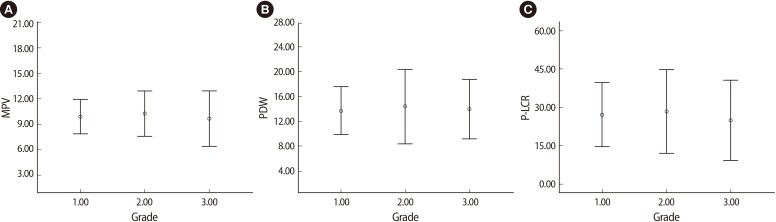
The hematologic parameters of the patients with varicocele and the control group are shown in Table 2. Although the mean Hb, WBC, and Plt levels were similar in both groups, the mean PDW, MPV, and P-LCR were significantly higher in the varicocele group than the control group. There were no significant correlations among the PDW, MPV, and P-LCR and varicocele grade (Figure 1).
Table 2.
Comparison of hematologic parameters between varicocele and control groups
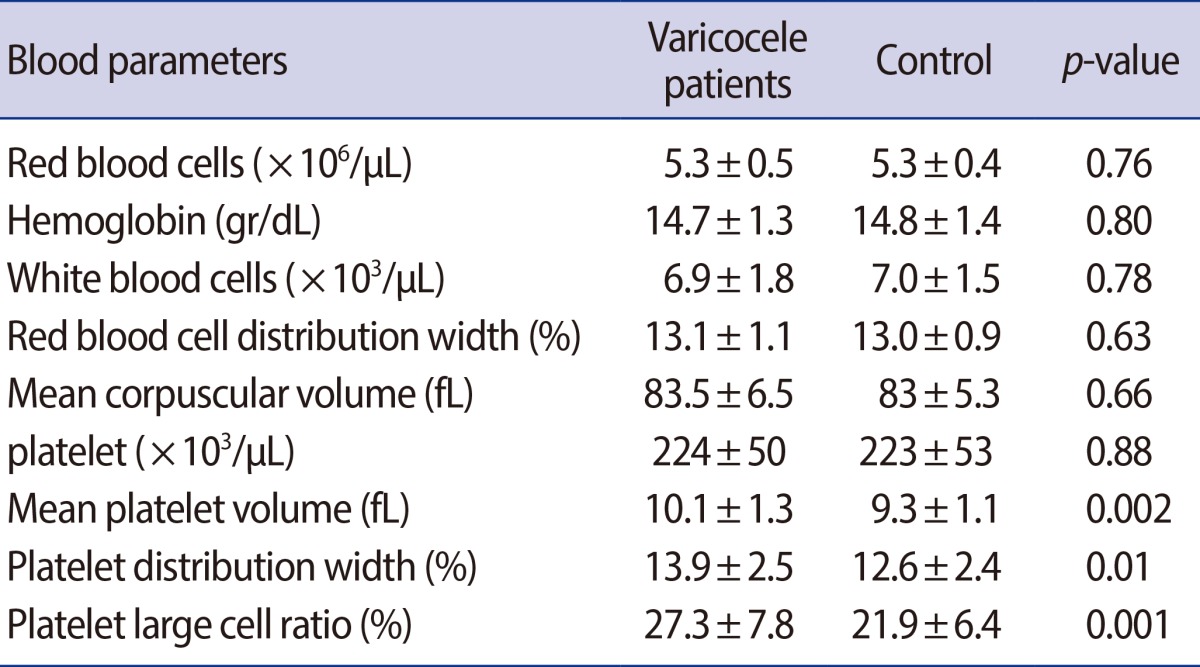
Values are presented as mean±SD.
Figure 1.
There were no significant correlations between the varicocele grade and (A) mean platelet volume (MPV; p-value=0.43, (B) platelet distribution width (PDW; p-value=0.74), or (C) platelet large cell ratio (P-LCR; p-value=0.41).
Discussion
Several theories have been developed to explain the causes of varicocele, but there is not yet a unifying theory. There are three theories on varicocele pathophysiology. First, the left internal spermatic vein drains perpendicularly into the left renal vein; this perpendicular insertion results in an increase in hydrostatic pressure in the pampiniform venous plexus, causing left side varicocele. Second, malfunction or absence of the venous valves results in tortuosity of veins. Third, the "nutcracker effect" occurs when the testicular vein is compressed between the aorta and superior mesenteric artery [16,19].
The present study found that the PVI (MPV, PDW, and P-LCR) are higher in varicocele patients than in normal healthy controls. The PVI, particularly MPV, has been accepted as a parameter of platelet activity [20]. Several studies have reported the presence of larger platelets to be a probable risk factor for developing vascular disorders such as myocardial infarction, myocardial angina, coronary artery atherosclerosis, and stroke [21,22,23].
This study detected higher PVI, particularly MPV values, in patients with varicocele. Furthermore, Yetkin et al. [4] showed that patients with coronary artery ectasia have an increased prevalence of varicocele. Another study reported positive correlations between the presence of peripheral varicose veins and varicocele [3]. Based on this evidence and the findings of the present study, we postulated that platelet activation may play an important role in the pathophysiologic basis of varicocele. This theory carries two important implications. First, platelet activation in varicocele may be due to future vascular disorders like cardiovascular disease. There is a great need for methodologically rigorous prospective studies that assess the incidence of vascular disease in varicocele patients. Second, to the best of our knowledge, there is one study evaluating the relationship between MPV and varicocele. In this study, Bozkurt et al. [14] reported that an increase in the MPV is dependent on the severity of varicocele, and the increase in the varicocele grade is associated with a higher MPV in varicocele patients. Therefore, the underlying mechanism of increased PVI in varicocele patients is unclear, and we believe that the body of literature requires other studies to confirm this relationship and provide further insights into this issue.
The PVI can be affected by many factors, such as a history of coronary artery disease, hypertension, hyperlipidemia, peripheral vascular disease, diabetes mellitus, splenectomy, thrombotic thrombocytopenic purpura, idiopathic thrombocytopenic purpura, myeloproliferative disorders, leukemia, thrombocytopenia, and thrombocytosis [6,7,10]. As noted earlier in the method section, we excluded patients with these conditions to avoid generating misleading results.
Another factor that can affect the measurement of MPV is the delay between blood sampling and laboratory analyses. Maximal changes in the MPV occur after the first 2 hours after sampling [16]. As a result, variation in intervals between venipuncture and laboratory analyses can lead to unreliable data. To reliably measure the MPV in the present study, all laboratory analyses were done within 2 hours of blood sampling.
There are 3 limitations that must be acknowledged and addressed regarding our study. First, the relatively small sample size of this study affected the study power. Second, the design of the present study is cross-sectional and thus cannot confirm a causal relationship. Third, we did not evaluate other markers of platelet activation such as beta-thromboglobulin and platelet factor IV.
In conclusion, this study showed a higher PVI in patients with varicocele. We postulated that platelet activation may play an important role in the pathophysiologic basis of varicocele, but the present study is cross-sectional and cannot confirm causality. Therefore, there is a great need for prospective research to confirm this relationship.
Acknowledgments
The authors would like to thank Dr. Shirin Irani for her invaluable comments on manuscript preparation.
Footnotes
The present study was the result of a doctoral thesis supported financially by the Vice President for Research, Mashhad University of Medical Sciences (grant number: 910059).
No potential conflict of interest relevant to this article was reported.
References
- 1.Jarow JP. Effects of varicocele on male fertility. Hum Reprod Update. 2001;7:59–64. doi: 10.1093/humupd/7.1.59. [DOI] [PubMed] [Google Scholar]
- 2.Redmon JB, Carey P, Pryor JL. Varicocele: the most common cause of male factor infertility? Hum Reprod Update. 2002;8:53–58. doi: 10.1093/humupd/8.1.53. [DOI] [PubMed] [Google Scholar]
- 3.Kilic S, Aksoy Y, Sincer I, Oguz F, Erdil N, Yetkin E. Cardiovascular evaluation of young patients with varicocele. Fertil Steril. 2007;88:369–373. doi: 10.1016/j.fertnstert.2006.11.119. [DOI] [PubMed] [Google Scholar]
- 4.Yetkin E, Kilic S, Acikgoz N, Ergin H, Aksoy Y, Sincer I, et al. Increased prevalence of varicocele in patients with coronary artery ectasia. Coron Artery Dis. 2005;16:261–264. doi: 10.1097/00019501-200508000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Park Y, Schoene N, Harris W. Mean platelet volume as an indicator of platelet activation: methodological issues. Platelets. 2002;13:301–306. doi: 10.1080/095371002220148332. [DOI] [PubMed] [Google Scholar]
- 6.Leader A, Pereg D, Lishner M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med. 2012;44:805–816. doi: 10.3109/07853890.2011.653391. [DOI] [PubMed] [Google Scholar]
- 7.Jackson SR, Carter JM. Platelet volume: laboratory measurement and clinical application. Blood Rev. 1993;7:104–113. doi: 10.1016/s0268-960x(05)80020-7. [DOI] [PubMed] [Google Scholar]
- 8.Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14:28–32. [PMC free article] [PubMed] [Google Scholar]
- 9.Hong H, Xiao W, Maitta RW. Steady increment of immature platelet fraction is suppressed by irradiation in single-donor platelet components during storage. PLoS One. 2014;9:e85465. doi: 10.1371/journal.pone.0085465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nena E, Papanas N, Steiropoulos P, Zikidou P, Zarogoulidis P, Pita E, et al. Mean Platelet Volume and Platelet Distribution Width in non-diabetic subjects with obstructive sleep apnoea syndrome: new indices of severity? Platelets. 2012;23:447–454. doi: 10.3109/09537104.2011.632031. [DOI] [PubMed] [Google Scholar]
- 11.Corash L, Tan H, Gralnick HR. Heterogeneity of human whole blood platelet subpopulations. I. Relationship between buoyant density, cell volume, and ultrastructure. Blood. 1977;49:71–87. [PubMed] [Google Scholar]
- 12.Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol. 1982;50:509–519. doi: 10.1111/j.1365-2141.1982.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 13.Khandekar MM, Khurana AS, Deshmukh SD, Kakrani AL, Katdare AD, Inamdar AK. Platelet volume indices in patients with coronary artery disease and acute myocardial infarction: an Indian scenario. J Clin Pathol. 2006;59:146–149. doi: 10.1136/jcp.2004.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozkurt Y, Soylemez H, Sancaktutar AA, Islamoglu Y, Kar A, Penbegul N, et al. Relationship between mean platelet volume and varicocele: a preliminary study. Urology. 2012;79:1048–1051. doi: 10.1016/j.urology.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Will MA, Swain J, Fode M, Sonksen J, Christman GM, Ohl D. The great debate: varicocele treatment and impact on fertility. Fertil Steril. 2011;95:841–852. doi: 10.1016/j.fertnstert.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masson P, Brannigan RE. The varicocele. Urol Clin North Am. 2014;41:129–144. doi: 10.1016/j.ucl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Shakiba B. Mean platelet volume after renal transplant: time is important. Exp Clin Transplant. 2013;11:296. doi: 10.6002/ect.2013.0050. [DOI] [PubMed] [Google Scholar]
- 18.Lance MD, van Oerle R, Henskens YM, Marcus MA. Do we need time adjusted mean platelet volume measurements? Lab Hematol. 2010;16:28–31. doi: 10.1532/LH96.10011. [DOI] [PubMed] [Google Scholar]
- 19.Naughton CK, Nangia AK, Agarwal A. Varicocele and male infertility: part ii. pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2001;7:473–481. doi: 10.1093/humupd/7.5.473. [DOI] [PubMed] [Google Scholar]
- 20.De Luca G, Santagostino M, Secco GG, Cassetti E, Giuliani L, Franchi E, et al. Mean platelet volume and the extent of coronary artery disease: results from a large prospective study. Atherosclerosis. 2009;206:292–297. doi: 10.1016/j.atherosclerosis.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Bath P, Algert C, Chapman N, Neal B PROGRESS Collaborative Group. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 2004;35:622–626. doi: 10.1161/01.STR.0000116105.26237.EC. [DOI] [PubMed] [Google Scholar]
- 22.Yasar AS, Bilen E, Yuksel IO, Arslantas U, Karakas F, Kirbas O, et al. Association between admission mean platelet volume and coronary patency after thrombolytic therapy for acute myocardial infarction. Turk Kardiyol Dern Ars. 2010;38:85–89. [PubMed] [Google Scholar]
- 23.Pikija S, Cvetko D, Hajduk M, Trkulja V. Higher mean platelet volume determined shortly after the symptom onset in acute ischemic stroke patients is associated with a larger infarct volume on CT brain scans and with worse clinical outcome. Clin Neurol Neurosurg. 2009;111:568–573. doi: 10.1016/j.clineuro.2009.04.002. [DOI] [PubMed] [Google Scholar]