Abstract
Recently, a new imaging method was proposed by Reichenbach et al (Radiology 1997;204:272–277) to image small cerebral venous vessels specifically. This method, referred to as high-resolution blood oxygen level-dependent venography (HRBV), relies on the susceptibility difference between the veins and the brain parenchyma. The resulting phase difference between the vessels and the brain parenchyma leads to signal losses over and above the usual T2* effect. At 1.5 T, a rather long TE (roughly 40 msec) is required for this cancellation to become significant, leading to enhanced susceptibility artifacts and a long data acquisition time. In this study, we examine the utility of incorporating a clinically available T1 reducing contrast agent, Omniscan (Sanofi Winthrop Pharmaceuticals, NY, NY), with the HRBV imaging approach to reduce susceptibility artifacts and imaging time while maintaining the visibility of cerebral veins. Using a double-dose injection of Omniscan, we were able to reduce TE from 40 to 25 msec. This led to a decrease in TR from 57 to 42 msec, allowing a 26% reduction in data acquisition time while maintaining the visibility of cerebral venous vessels and reducing susceptibility artifacts.
Index terms: BOLD, venogram, contrast agent
Recently, a novel method was proposed to image cerebral veins (1). This method relies on the difference in susceptibility between the venous blood and the adjacent brain parenchyma. Using a high-resolution, three-dimensional T2*-weighted gradientecho sequence, Reichenbach et al (1) demonstrated that a good overall representation of cerebral venous structures can be obtained in normal volunteers. We refer to this high-resolution blood oxygen level-dependent (BOLD) venographic imaging technique as HRBV. More recently, Shaibani et al (2) utilized this technique in patients with occult malformations including cavernous hemangiomas (cavernomas), venous angiomas, and capillary telangiectasias. They reported a higher sensitivity in depicting the locations and extent of occult malformations using HRBV imaging when compared with the commonly used pre- and post-contrast T1-weighted images, T2-weighted images, FLAIR images or conventional time-of-flight MR angiographic images. Their findings underscore the potential clinical utility of HRBV imaging.
However, two major limiting factors have prevented HRBV from being accepted in daily clinical practice. First, a three-dimensional (3D) sequence with a long TE is required to allow the phase of venous blood and the adjacent brain tissue to become opposed phase so that signal cancellation will occur. It is both the phase development and signal cancellation that leads to the visualization of the veins. A TE of roughly 40 msec is normally used, assuming that the cerebral venous blood oxygen saturation is about 55% (3). This leads to a long data acquisition time, making the method more susceptible to patient motion. Second, when a long TE is used, the image quality is compromised by a reduction of the signal-to-noise ratio (SNR) and an increased sensitivity to susceptibility artifacts from air/tissue interfaces. In this paper, we will demonstrate that a shorter TE and shorter TR can be used following administration of a T1-reducing contrast agent to obtain HRBV images with comparable visualization of cerebral veins to those non-contrast-enhanced scans acquired with a longer TE.
THEORY
Assume a voxel can be viewed as a two-compartment model in which the blood and the brain parenchyma occupy volume fractions of λ and 1 − λ, respectively. In the case of an artery, which contains fully oxygenated blood, the signal intensity of this voxel is simply the linear sum of the signal from each compartment. However, venous blood has a different susceptibility than arterial blood. When the voxel is occupied in part by a vein, the signal intensity of the voxel will be a function of TE, λ, the angle (θ) between the blood vessel and the static magnetic field, the hematocrit, and the fractional blood oxygen saturation (Y) (4–6). Assuming that the venous blood and the brain parenchyma have the same spin density and T2* (7), the signal intensity can be written as
| (1) |
where ρ is spin density, and fb and fp contain the imaging parameter dependence of the blood and parenchyma, respectively. Furthermore, it has been suggested that the frequency changes caused by the presence of deoxyhemoglobin molecules in the intravascular compartment can be written as (6)
| (2) |
where γ is gyromagnetic ratio and Δχ, which has been shown to be 0.18 ppm (8), is the susceptibility difference between fully deoxygenated and oxygenated blood. Subsequently, the phase shift, ϕ(TE, Y) induced by the presence of deoxyhemoglobin molecules is 2πδf(θ)TE. Therefore, for a hematocrit of 0.45 and a field strength of 1.5 T, the deoxyhemoglobin-induced phase shift in the intravascular compartment can be approximated as (6)
| (3) |
For future discussion, we rewrite Eq. [1]as
| (4) |
where we have set
| (5) |
This two-compartment model only takes into account the intravascular effects of the BOLD phenomena and it is certain that more signal cancellation occurs from the extravascular effects (please see the discussion section for more details).
MATERIALS AND METHODS
All protocols described below were approved by the Human Studies Committee at the School of Medicine, Washington University and an informed consent was signed and obtained from all volunteers. Eight normal healthy volunteers were studied. The subjects were divided into two groups depending on the dose of the contrast agent received. Two subjects received a dose of 0.1 mmol/kg, and six subjects received a dose of 0.2 mmol/kg of contrast agent. In the latter group, three subjects were imaged with the multi-echo scan and the other three were imaged with a single echo sequence. The two subjects who received a single dose of contrast agent were imaged with the single echo sequence. The contrast agent Omniscan (SanofiWinthrop Pharmaceuticals, NY, NY) was used for this study.
In addition to a conventional scout sequence, two other sequences were used: a 3D multi-echo sequence and a single-echo, high-resolution, 3D, T2*-weighted, gradient-echo sequence. The multi-echo sequence was used to assess the quality of HRBV as a function of TE pre- and post-contrast so that an optimal TE could be determined (n = 3). The time interval between two echoes was made as short as possible at the expense of resolution. The imaging parameters were as follows: TR 100 msec; flip angle 25°; field of view (FOV) 256 × 256 mm2 with a matrix size of 256 × 256; and 32 partitions with a slice thickness of 2 mm. In total, eight echoes were collected ranging from TE 6.3 msec to TE 84.7 msec with an increment of 11.2 msec. Velocity compensation was applied along both the slice-select and frequency-encoding directions to minimize signal dephasing caused by flowing spins. This sequence was used to acquire images pre-contrast and repeated 3 times post-contrast so that the effects of clearance of the contrast agent could be investigated.
After determining the optimal TE, the 3D T2*-weighted, single-echo, low-bandwidth, high-resolution gradient-echo sequence was used (n = 3). Velocity compensation along all three axes was applied to minimize flow artifacts. The bandwidth in these sequences is 2.5 times smaller than that in the multi-echo sequence and should yield a 1.6 fold better SNR in the images. Three sets of images with different TEs (25, 30, and 40 msec), different TRs (42, 47, and 57 msec), and different flip angles (13°, 15°, and 20°) were acquired pre- and post-contrast, respectively. The corresponding total data acquisition times for the three TRs used were 9.73, 8.02, and 7.19 minutes, respectively. The images with a TE of 40 msec were acquired first, followed by TE 30 msec and TE 25 msec pre-contrast. This temporal order was reversed immediately after the injection of contrast so that images with a TE of 25 msec have the maximum effects from the contrast agent. In all cases, an FOV of 160 × 256 mm2 with a matrix size of 160 × 512 and 64 partitions with 2mmthickness were used.
Data Analysis
Since paramagnetic structures have a higher resonance frequency, the phase of small veins parallel to the main field is negative relative to the brain parenchyma and cerebrospinal fluid (CSF). As described in Reichenbach et al (1), a phase mask was created by setting all phase values between 0 and +π to unity and by normalizing the phase values from 0 to −π to values ranging linearly from 1 to 0, respectively. Subsequently, the magnitude images were multiplied four times by the phase mask to augment the visibility of the venous structures. Finally, a minimum intensity projection (MIP) technique over every adjacent four slices was used to display the processed data.
RESULTS
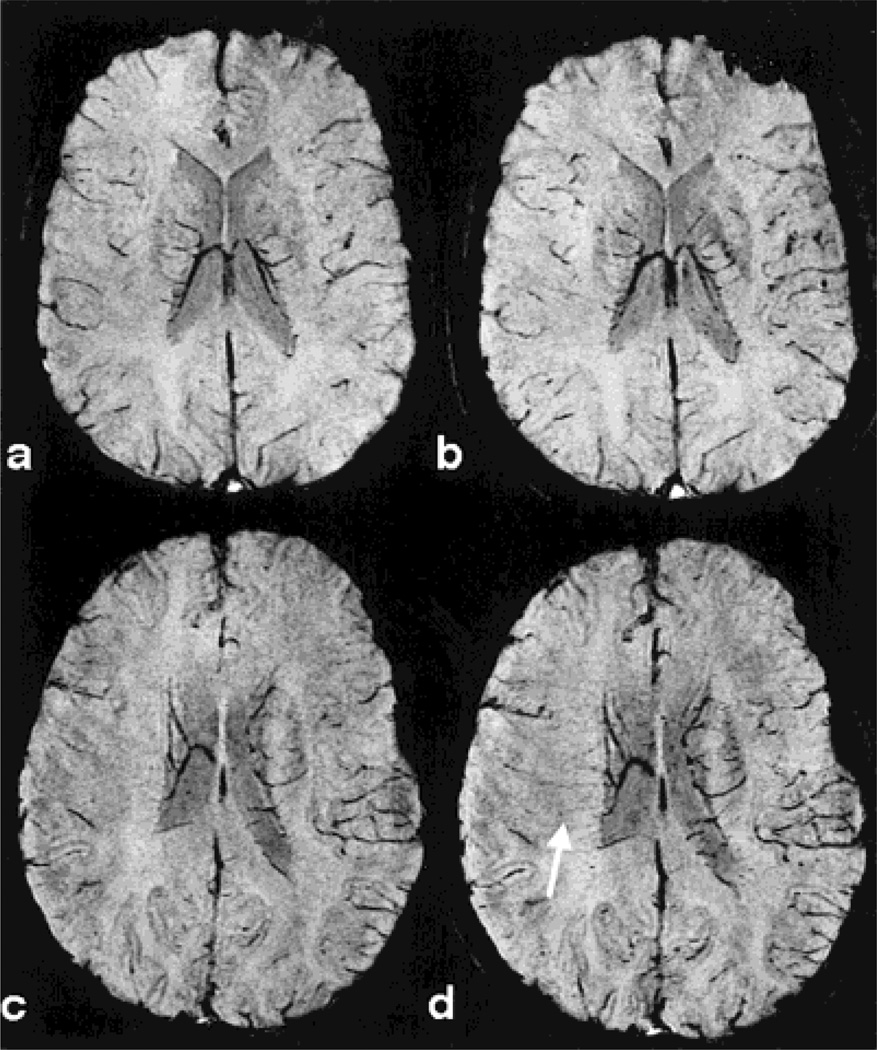
In all cases, an improvement in the conspicuity of venous vessels was observed following administration of contrast agent compared with the pre-contrast images. Furthermore, this effect was dose-dependent with double dose contrast providing superior venous visualization compared to single dose contrast. Figure 1 shows the comparison of HRBV between a single dose (Fig. 1b) and a double dose of contrast agent (Fig. 1d) from two separate volunteers. The high-resolution, single-echo, 3D sequence with a TE of 40msec was used to acquire images pre-contrast (Fig. 1a,c) and post-contrast (Fig. 1b,d), respectively. Only a slight improvement in vascular visualization is exhibited when comparing a pre- (Fig. 1a) with post-contrast (Fig. 1b) single dose study while a more noticeable improvement in vascular visualization post-contrast is observed when a double dose of contrast was used. This is most striking for those deep white matter veins (arrow), which are not seen in both pre-contrast and single dose post-contrast HRBV images but become visible after administration of a double dose of contrast (Fig. 1d).
Figure 1.
A comparison of images from two volunteers pre-and post-contrast. The first volunteer received a single dose of contrast agent. There are some improvements in venous vessel visualization from pre-contrast (a) to post-contrast (b). The second volunteer, on the other hand, received a double dose of contrast agent. A substantial improvement in the venous vessel visualization (arrow) is observed from pre-contrast (c) to post-contrast (d). All images were acquired with a TE of 40 msec.
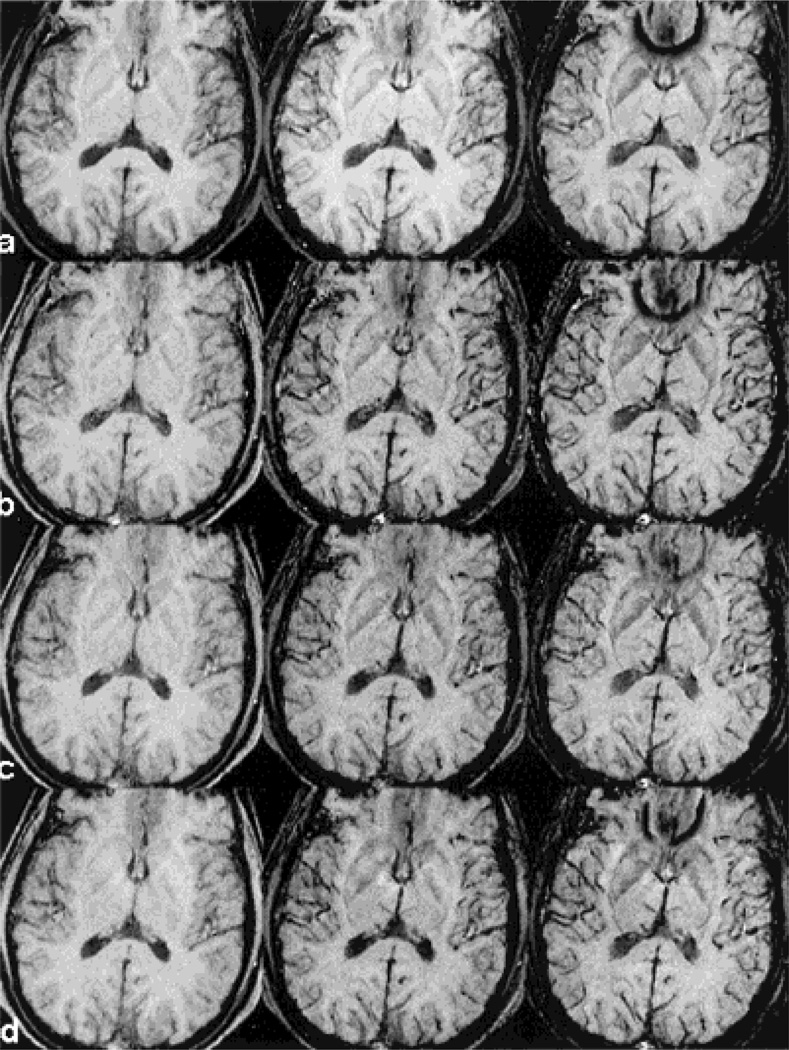
A subset of the multi-echo images used to determine the appropriate choice of echo time post-contrast is shown in Fig. 2. The shorter echo images (TE 17.5 msec) show little signal loss from veins. The signal from blood becomes brighter with the injection of a contrast agent at this short echo time because it remains in phase with the brain parenchyma. At a TE of 28.7 msec, on the other hand, there are already substantial improvements in venous visualization. Although the venous visibility post-contrast continues to improve at 40 msec, the images acquired at a TE of 28.7 msec exhibit sufficient venous information to make it the echo time of choice to reduce susceptibility artifacts and imaging time. From the perspective of temporal variation, images in rows b–d in Fig. 2 demonstrate that there is little loss of venous visualization during the first 30 minutes post-contrast. This indicates that the clearance of the contrast agent has minimal effects on vascular visualization during the time window covered in this study.
Figure 2.
HRBV images from a subset of the multi-echo sequence are shown to demonstrate the signal cancellation for three different TEs (17.5, 28.7, and 39.9 msec for images from left to right in each row) pre-contrast (row a) and three time points post-contrast (rows b–d). Images in row b were collected immediately after the injection of contrast agent, row c 10 minutes later, and row d 20minutes later. There is little change in venous vessel visualization during the first 30 minutes after the injection of contrast agent.
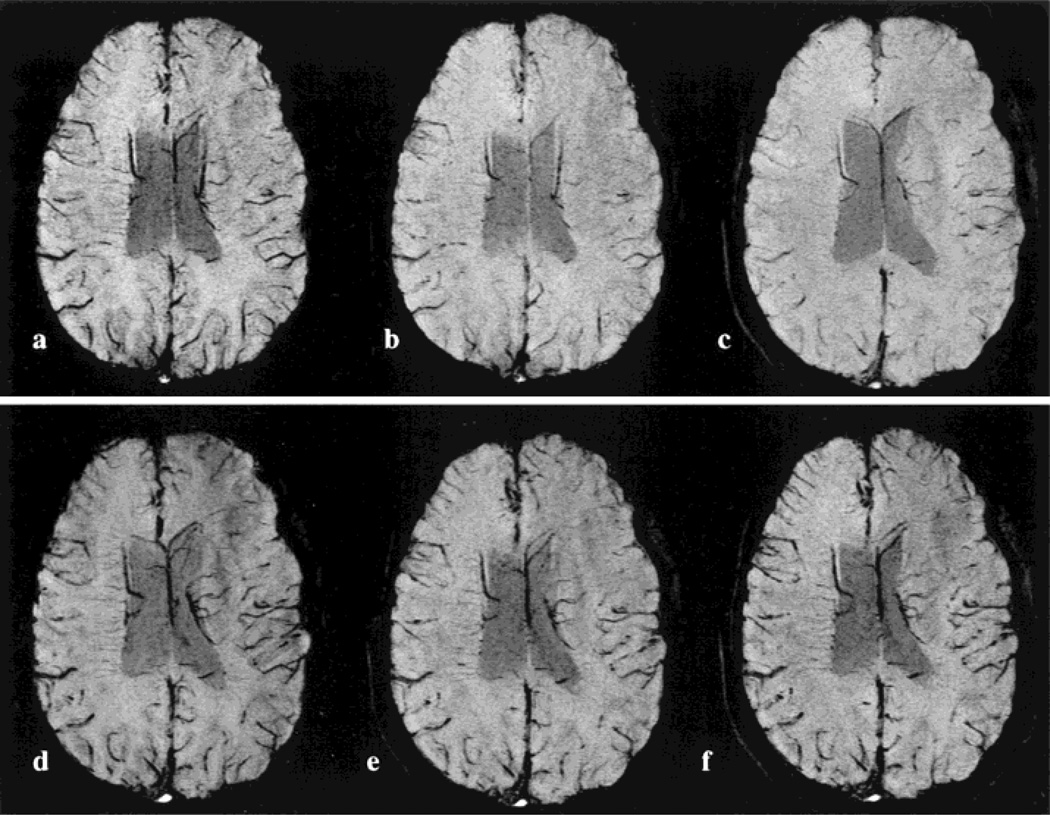
An example of the HRBV mIP images for the lower bandwidth, single-echo acquisitions is given in Fig. 3. Here, TE 40 msec (Fig. 3a), TE 30 msec (Fig. 3b), and TE 25 msec (Fig. 3c) pre-contrast images are compared with the corresponding mIP images obtained after the injection of contrast agent (Fig. 3d–f). The image acquired with TE 40 msec shows greater conspicuity of the veins than both the TE 25 and 30 msec pre-contrast images. However, when results obtained with the same TE are compared before and after administration of contrast, a dramatic improvement in venous visualization is observed post-contrast for all TEs. More importantly, the vascular conspicuity obtained with the TE 25 msec post-contrast image is comparable to the pre-contrast TE 40 msec images. The susceptibility artifacts around the sinuses are also considerably reduced.
Figure 3.
A comparison of HRBV with different TEs pre- (a–c) and post-contrast (d–f) with the single-echo 3D sequence. The TE values are 40, 30, and 25 msec for images from left to right in each row.
DISCUSSION
It has been recognized that deoxyhemoglobin can be used as an endogenous contrast agent and that changes in its concentration within blood vessels induce signal intensity changes in T2*-weighted images, the so-called BOLD contrast (9–11). Since the discovery of the BOLD effect (9–11), numerous papers have reported its utility in functional brain mapping where changes in the deoxyhemoglobin concentration in response to external sensory inputs and/or cognitive paradigms are used to reveal brain activation (11,12). BOLD has also been proved to be of particular value in the understanding of pathophysiological conditions in association with the alterations of cerebral hemodynamics and oxygen metabolism by measuring the changes of R2* (1/T2*) in the brain parenchyma before and after physiological manipulations (13–19). These applications underscore the potential clinical utility of the BOLD effect.
One common feature of these applications of BOLD imaging is that the change of R2* either in the blood and/or the brain parenchyma causes MR signal alteration in T2*-weighted images. However, an additional component of the BOLD effect, the induced phase shift with respect to the adjacent brain parenchyma by deoxygenated venous blood is exploited in this study to visualize venous structures (6). An excellent representation of cerebral venous anatomy is obtained with this approach, as demonstrated in the Results section. However, to improve the quality and clinical utility of HRBV imaging further, the long TE required needs to be shortened. Therefore, the main goals of this study were to reduce TE so that susceptibility-induced artifacts at air/tissue interfaces could be minimized and to reduce TR so that shorter acquisition times could be achieved. To accomplish these goals, a T1 shortening contrast agent was used in conjunction with the HRBV technique. The basic concept behind HRBV imaging is a cancellation of venous signal with background tissue. This cancellation will be small if the blood signal fraction is small. By using a T1 reducing contrast agent, the blood signal fraction should increase for a given echo time, and the cancellation phenomena could, therefore, be enhanced. The details of these concepts are addressed next.
Theoretical Considerations
The current understanding of the BOLD effect indicates that there are intravascular and extravascular sources of signal dependence around veins. The former leads to a phase dependence of venous blood as described in Eq. [3] and a signal dependence as described in Eq. [1]. The extravascular effects, on the other hand, lead to a local reduction in T2* of the surrounding tissue, which also depends on the vessel orientation. In this study, the phase changes of blood and the resulting cancellation of the venous signal with the surrounding brain parenchyma are used to complement the T2* signal loss. For a blood vessel with Y = 0.55, a hematocrit = 0.45, and that is parallel to the magnetic field, a −π phase shift with respect to the surrounding brain parenchyma can be obtained with a TE of about 45 msec. In this study, an echo time of 40 msec was used to avoid aliasing. The resulting signal cancellation is still excellent and is consistent with the findings of Reichenbach et al (1).
To understand the venous cancellation further, consider the case where θ = 0° and the venous blood exhibits a −π phase shift relative to the brain parenchyma. The signal of a voxel is then proportional to the absolute value of 1 − λ − λ′ where λ is the volume fraction of blood and λ′ is defined in Eq. [3] as the signal fraction of blood. Two different approaches can be utilized to improve venous conspicuity with HRBV. First, with a reduction of voxel size, λ will be increased with respect to the brain parenchyma volume fraction, leading to more signal cancellation and better vascular visualization. This improved cancellation is seen when comparing the smaller signal loss in Fig. 2 where voxel size is 1 × 1 ×2mm3 with larger signal loss of Fig. 1 or 3 where voxel size is 0.5 × 1 × 2 mm3. This improved vascular visibility highlights the importance of using high-resolution images to maximize the effects of signal cancellation between venous blood and the adjacent brain parenchyma.
Second, when a T1 shortening contrast agent is used, λ′ will increase with respect to 1 − λ resulting in a better delineation of veins compared with non-contrast enhanced HRBV. This improvement in vascular conspicuity should be further enhanced with a higher dose of contrast agent as long as λ + λ′ remains less than 0.5. In good agreement with these predictions, our findings demonstrate that imaging of the venous vasculature is facilitated by the use of a contrast agent for all TEs and the improvements are best seen with a double dose of contrast agent (Fig. 1). More importantly, there is a comparable venous visualization between pre-contrast TE 40 msec and post-contrast TE 25 msec, leading to the conclusion that a shorter TE of 25 msec can be used to obtain HRBV images. The superior cancellation may result from two effects of the contrast agent. In addition to the expected increase in λ′ due to a reduction of T1 of blood, an extra phase shift is also anticipated after the injection of the paramagnetic contrast agent. This extra phase shift is roughly 50° with a double dose of contrast agent for TE 25 msec changing the phase from 112.5° pre-contrast to 162.5° post-contrast. This brings the phase of blood close to opposition with that of the surrounding brain parenchyma at this relatively short TE.
Dependence
As discussed in the Materials and Methods section, when a negative phase change occurs, the multiplication of the phase mask with the magnitude images highlights the venous structures. However, Eq. [2] suggests that at angles greater than 54.7°, the phase will become positive. In this case, the value of the phase mask would be unity for these vessels and it would be ineffective in augmenting the conspicuity of veins.
Despite these theoretical predictions, even for those vessels with θ greater than 54.7°, delineation of veins was further improved by application of the phase mask. Specifically, deep white matter veins, which are nearly perpendicular to the static magnetic field and are not clearly visible in the originally collected images, are still enhanced after the application of the phase mask (arrow, Fig. 1). This discrepancy may be explained by considering the extravascular effects that could induce an extra signal loss in addition to that due to intravascular effects. Quantitative modeling of vessel geometry would be required to predict fully both intravascular and extravascular contributions to the phase of the vessel. This is beyond the scope of this paper.
Choices of TE
To determine the shortest TE that yields sufficient venous visualization post-contrast in a single scan, a multi-echo 3D gradient-echo sequence was utilized to obtain HRBV images pre- and post-contrast. This is necessary initially because of the changing concentration of the contrast agent as a function of time. Although it is ideal to use similar imaging parameters, spatial resolution and sequence design between the multi-echo and the single-echo sequences, in order to keep interecho spacing short, resolution was reduced to 1 mm in the read direction, velocity compensation along the phase-encoding direction was not applied, and the bandwidth was increased for the multi-echo sequence. These modifications could potentially lead to a reduction in blood volume fraction in a given voxel, misregistration artifacts for fast flowing spins, and a reduction in SNR, respectively, for the multi-echo sequence. Despite these drawbacks, Fig. 2 demonstrated that the echo time could be reduced to at least 28.7 msec post-contrast while maintaining good vascular contrast. Conversely, when the single-echo sequence was utilized, these drawbacks were removed, making it possible to investigate even shorter echo times. Furthermore, since the effects of contrast agent on HRBV persisted for up to 30 minutes, as shown in Fig. 2, we were able to vary the echo times in the single-echo sequence from 25 to 30 to 40 msec in a single setting. As shown in Fig. 3, a dramatic improvement in vascular visualization was obtained when comparing images obtained pre- and post-contrast even for a TE as short as 25 msec. Although further optimization may be possible, we believe that this is a reasonable practical choice of TE for clinical applications.
Technical Aspects
Not all imaging parameters remained the same as the echo time changed. Specifically, the flip angle was reduced as the TR became shorter in our study. This modification is justified because CSF can also be used as a source for signal cancellation with blood in addition to the signal from brain parenchyma. Small venous vessels located in cortical sulci are often surrounded by CSF instead of brain parenchyma. Therefore, it is desirable to maintain high signal from CSF in order to maximize the effects of signal cancellation. Apart from using a long TE to maintain high signal from CSF, more spin density-weighted images can be obtained by using a small flip angle so that relatively high signal from CSF is preserved. Conversely, in order to appreciate fully the effects of the contrast agent in reducing T1 of blood and, hence, to improve the visualization of the venous vasculature in HRBV, a large flip angle should be used after the injection of a contrast agent. Since the T1 of CSF is rather long (on the order of 4 seconds), this would suppress the signal of CSF, resulting in a paradoxical effect in the sulci where the loss of signal from CSF would degrade visualization of venous vessels. For this reason, the flip angle is reduced from 20° to 13° as both TR and TE are reduced from 57 and 40 msec to 42 and 25 msec, respectively. Although 13° appears low, it is, in fact, on the order of the Ernst angle for the blood (roughly 15°) pre-contrast, which leads to a substantial T1 suppression of signal from blood. However, postcontrast, the Ernst angle increases to roughly 30° and little T1 suppression occurs at 13° leading to the expected increase in signal from vessels.
Since TE is the main determinant for TR, reduction of TE from 40 to 25 msec allows for a reduction of TR from 57 to 42 msec. This results in a 26% decrease in total data acquisition time (from 9.73 to 7.19 minutes). In addition, the reduction in TE minimizes the susceptibility artifacts induced by air-tissue interfaces, as shown in Fig. 2. The former should greatly improve the applicability of HRBV in a clinical setting and make the method less subject to patient motion while the latter should extend the anatomical coverage of HRBV.
CONCLUSIONS
High-resolution MR BOLD venographic imaging is a technique to augment the visibility of deoxygenated blood. It cannot, at this point, be used to quantify vessel size or oxygen saturation, but rather manifests as a clinically useful tool in revealing venous vasculature. It has been shown to be of particular value in diagnosing venous vascular malformations. It may also have potential applications in several other disease entities associated with the central nervous system such as stroke and tumors, in which changes in the pathophysiology may be reflected in the HRBV images. This paper demonstrates that HRBV image quality can be improved by using a T1 reducing contrast agent with shorter echo times than originally proposed. These, in turn, make it possible to scan patients more quickly, thereby improving its utility in a clinical environment.
ACKNOWLEDGMENTS
K. Vo is the recipient of an RSNA seed grant.
Contract grant sponsor: NIH; Contract grant number: NS35147; Contract grant sponsor: RSNA; Contract grant sponsor: Siemens Medical Systems.
REFERENCES
- 1.Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204:272–277. doi: 10.1148/radiology.204.1.9205259. [DOI] [PubMed] [Google Scholar]
- 2.Shaibani A, Yoon M, Haacke EM, Lin W, Lee BCP. Evaluation of angiographically occult vascular malformation using a new high-resolution technique for MR venography; Proceedings of the 36th Annual Meeting of the American Society of Neuroradiology; 1998. [Google Scholar]
- 3.Haacke EM, Lai S, Reichenbach JR, et al. In vivo measurement of blood oxygen saturation using magnetic resonance imaging: a direct validation of the blood oxygen level-dependent concept in functional brain imaging. Hum Brain Mapping. 1997;5:341–346. doi: 10.1002/(SICI)1097-0193(1997)5:5<341::AID-HBM2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Yablonskiy DA, Haacke EM. Theory and NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med. 1994;32:749–763. doi: 10.1002/mrm.1910320610. [DOI] [PubMed] [Google Scholar]
- 5.Kennan RP, Gao JH, Zhong J, Gore JC. A General model of microcirculatory blood flow effects in gradient sensitized MRI. Med Phys. 1994;21:539–545. doi: 10.1118/1.597170. [DOI] [PubMed] [Google Scholar]
- 6.Haacke EM, Lai S, Yablonskiy DA, Lin W. In vivo validation of the BOLD mechanism: a review of signal changes in gradient echo functional MRI in the presence of flow. Int J Imaging System Tech. 1995;6:153–163. [Google Scholar]
- 7.Li D, Wang Y, Waight D. Blood oxygen saturation assessment in vivo using T2* estimation. Magn Reson Med. 1998;39:685–690. doi: 10.1002/mrm.1910390503. [DOI] [PubMed] [Google Scholar]
- 8.Weisskoff RM, Kiihne S. MRI susceptometry: image-based measurement of absolute susceptibility of MR contrast agents and human blood. Magn Reson Med. 1992;24:375–383. doi: 10.1002/mrm.1910240219. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image in rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa S, Lee TM. Magnetic resonance imaging of blood vessels at high-fields. Magn Reson Med. 1990;16:9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Menon RS, Tank DW, et al. Functional brain mapping by blood oxygenation level dependent contrast magnetic resonance imaging. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwong K, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner R, Le Bihan D, Moonen CTW, Despres D, Frank J. Echoplanar time course MRI of cat brain oxygenation changes. Magn Reson Med. 1991;22:159–166. doi: 10.1002/mrm.1910220117. [DOI] [PubMed] [Google Scholar]
- 14.Prielmeier F, Nagatomo Y, Frahm J. Cerebral blood oxygenation in rat brain during hypoxic hypoxia: quantitative MRI of effective transverse relaxation rates. Magn Reson Med. 1994;31:678–681. doi: 10.1002/mrm.1910310615. [DOI] [PubMed] [Google Scholar]
- 15.Jezzard P, Heineman F, Taylor J, et al. Comparison of EPI gradientecho contrast changes in cat brain caused by respiratory challenges with direct simultaneous evaluation of cerebral oxygenation via a cranial window. NMR Biomed. 1994;7:35–44. doi: 10.1002/nbm.1940070107. [DOI] [PubMed] [Google Scholar]
- 16.Lin W, Paczynski RP, Celik A, et al. Experimental hypoxemic hypoxia: changes in R2* of brain parenchyma accurately reflect the combined effects of changes in arterial and cerebral venous oxygen saturation. Magn Reson Med. 1998;39:474–481. doi: 10.1002/mrm.1910390318. [DOI] [PubMed] [Google Scholar]
- 17.Hoppel BE, Weisskoff RM, Thulborn KR, et al. Measurement of regional blood oxygenation and cerebral hemodynamics. Magn Reson Med. 1993;30:715–723. doi: 10.1002/mrm.1910300609. [DOI] [PubMed] [Google Scholar]
- 18.Kwong KK, Wanke I, Donahue KM, Davis TL. EPI imaging of global increase of brainMR signal with breath-hold preceded by breathing O2. Magn Reson Med. 1995;33:448–452. doi: 10.1002/mrm.1910330322. [DOI] [PubMed] [Google Scholar]
- 19.De Crespigny AJ, Wendland MF, Derugin N, Kozniewska E, Moseley ME. Real-time observation of transient focal ischemia and hyperemia in cat brain. Magn Reson Med. 1992;27:391–397. doi: 10.1002/mrm.1910270220. [DOI] [PubMed] [Google Scholar]