Abstract
An essential component of allogeneic and autologous hematopoietic cell transplantation (HCT) is the conditioning regimen administered before the hematopoietic cell infusion. Early regimens relied on dose intensity, assuming that high-dose chemoradiotherapy would eliminate malignant disease and reinfusion of the graft would then restore hematopoiesis. However, as the contribution of graft-versus-tumor effects to the success of allogeneic HCT was recognized over time, in an effort to exploit these, many investigators lowered the dose of radiation and chemotherapeutic agents in the preparative regimen. This resulted in a major paradigm shift, and consequently, the pool of eligible patients underwent a remarkable expansion. In this article, we provide a review of the definition of high-dose, reduced-intensity, and nonmyeloablative conditioning regimens, the most commonly used agents and combinations, and the evolution of some early regimens. We also provide a brief review of the toxicities associated with these regimens.
Introduction
Hematopoietic cell transplantation (HCT) is a potentially curative therapeutic approach for a variety of malignant and nonmalignant hematopoietic diseases. When HCT is performed in patients with malignant disorders, preparative or conditioning regimens are administered as part of the procedure to achieve 2 goals: provide sufficient immunoablation to prevent graft rejection and reduce the tumor burden. Traditionally, these goals have been achieved by using otherwise supralethal doses of total body irradiation (TBI) and chemotherapeutic agents with nonoverlapping toxicities. However, as it was recognized that immunologic reactions of donor cells against malignant host cells (ie, graft-versus-tumor [GVT] effects) substantially contributed to the effectiveness of HCT, reduced-intensity and nonmyeloablative conditioning regimens have been developed, making HCT applicable to older and medically infirm patients.
Definitions
The intensity of conditioning regimens can vary substantially, and when selecting the optimal conditioning regimen for any given patient, disease-related factors such as diagnosis and remission status, as well as patient-related factors including age, donor availability, and presence of comorbid conditions, need to be considered. In rare situations, such as children with severe combined immunodeficiency1 or patients with severe aplastic anemia who have syngeneic donors, HCT can be performed without the administration of a preparative regimen.
Although full consensus has not been reached within the HCT community, conditioning regimens have been classified as high-dose (myeloablative), reduced-intensity, and nonmyeloablative, following the Reduced-Intensity Conditioning Regimen Workshop, convened by the Center for International Blood and Marrow Transplant Research (CIBMTR) during the Bone Marrow Transplantation Tandem Meeting in 2006.2 During this meeting, 56 HCT professionals representing 44 institutions from 9 countries agreed on criteria (previously known as the Champlin criteria) to define the general characteristics of a reduced-intensity conditioning (RIC) regimen (Table 1). Based on these criteria, myeloablative, or “high-dose” regimens, consisting of alkylating agents (single or multiple) with or without TBI, are expected to ablate marrow hematopoiesis, not allowing autologous hematologic recovery. In contrast, nonmyeloablative regimens, although causing minimal cytopenias, do not require stem cell support.3 Regimens that do not fit the definition for myeloablative or nonmyeloablative conditioning are classified as RIC regimens: they result in potentially prolonged cytopenias, and they require hematopoietic stem cell support. What differentiates RIC regimens from myeloablative regimens is that the dose of alkylating agents or TBI is generally reduced by ≥30%. It is important to recognize that the intensity of regimens classified as reduced-intensity by these criteria can vary substantially and represents a continuum. Examples of reduced-intensity and nonmyeloablative regimens are shown in Table 2.
Table 1.
Acceptance of the Champlin criteria as the characteristics defining a RIC regimen at the RIC Regimen Workshop convened by the CIBMTR
| Criterion | Percentage of HCT professionals that strongly agree or agree |
|---|---|
| Results in reversible myelosuppression (usually within 28 d) when given without stem cell support | 67 |
| Results in mixed chimerism in a proportion of patients at the time of first assessment | 71 |
| Associated with a low rate of nonhematologic toxicity | 71 |
Adapted from Giralt et al.104
Table 2.
Examples of RIC and nonmyeloablative regimens according to commonly used agents and combinations
| RIC regimens | Nonmyeloblative regimens |
|---|---|
| TBI ≤500 cGy as a single fraction or ≤800 cGy if fractionated | FLU + CY + ATG |
| Total BU ≤9 mg/kg | FLU + AraC + Ida |
| Total MEL <140 mg/m2 | Cladribine + AraC |
| Thiotepa <10 mg/kg | Total lymphoid irradiation + ATG |
| TBI ≤2 Gy ± purine analog |
High-dose conditioning regimens
TBI-based regimens
For patients with hematologic malignancies undergoing autologous and allogeneic HCT, high-dose TBI has been widely used as part of the conditioning regimen due to its immunosuppressive properties, its effectiveness against most leukemias and lymphomas, and its ability to penetrate to sanctuary sites. The majority of regimens combined 12- to 16-Gy TBI, usually fractionated, with other chemotherapeutic agents, most commonly, cyclophosphamide, based on its antineoplastic and immunomodulatory properties.4-8 In general, higher doses of TBI, although reducing the relapse risk, resulted in increased, often fatal gastrointestinal, hepatic, and pulmonary toxicities, secondary malignancies, and impaired growth and development in children. In a randomized trial, patients with acute myeloid leukemia (AML) in first remission receiving 15.75 Gy had decreased relapse rates compared with patients receiving 12 Gy; however, this effect was offset by an increase in transplant-related deaths, resulting in similar survival.6,9 In addition to the delivered dose, other factors, such as dose rate, fractionation, interval between fractions, and the source of radiation (cobalt-60 vs linear accelerator) could also impact both the antineoplastic and toxic effects of TBI. Fractionation resulted in decreased organ toxicity but also sustained antineoplastic effects, due to a higher proportion of intact repair mechanisms retained in normal tissues as opposed to leukemic cells.10 Hyperfractionation (multiple fractions per day) with lung shielding resulted in a decreased incidence of interstitial pneumonitis of 4%, down from 50% observed with single-fraction TBI without lung shielding.11,12 The majority of TBI schedules in use today are either fractionated or hyperfractionated.
In addition to cyclophosphamide, various agents, such as cytarabine (AraC),13 etoposide,14 melphalan,15 and busulfan,16 have been combined with high-dose TBI as conditioning regimens; however, due to the lack of randomized trials, there is currently no evidence suggesting that any of these combinations are superior to cyclophosphamide and high-dose TBI.
The administration of high-dose TBI is associated with immediate and delayed toxicities, although it is not always possible to distinguish which component of the conditioning regimen is responsible for any given toxicity. Nausea, vomiting, transient acute parotiditis, xerostomia, mucositis, and diarrhea are commonly observed acute complications. Interstitial pneumonitis, idiopathic pulmonary fibrosis, and reduced lung pulmonary function can also be related to high-dose TBI. In addition, renal damage can occur and can be delayed (up to ∼2 years) after high-dose TBI.17 The occurrence of sinusoidal obstruction syndrome (SOS; formerly known as veno-occlusive disease of the liver) is more common in chemotherapy-based regimens (see below). Long-term side effects of high-dose TBI include infertility, cataract formation,18 hyperthyroidism and thyroiditis, and secondary malignancies19; however, the association of the latter is likely overestimated and is currently debated.20,21
High-dose chemotherapy-based regimens
To avoid short- and long-term toxicities associated with high-dose TBI, especially in patients who received previous radiation therapy, high-dose chemotherapy-based regimens have been developed both in the autologous and allogeneic settings where TBI is replaced with additional chemotherapeutic agents. Alkylating agents remain the mainstay of such regimens, due to favorable toxicity profile (marrow toxicity as dose-limiting toxicity) and their effect on nondividing tumor cells. Nonhematologic toxicity has limited the use of other agents, such as anthracyclines and taxanes, in this setting.
Busulfan is an alkylating agent with profound toxic effect on nondividing marrow cells including early myeloid precursors. It is also active in a variety of malignancies, including chronic myeloid leukemia (CML) and other myeloproliferative disorders, AML, lymphomas, acute lymphocytic leukemia (ALL), and multiple myeloma. However, busulfan cannot be administered as a single agent as HCT conditioning due to its limited toxicity to mature lymphocytes. Another major limitation to its use in the setting of HCT was its availability solely in oral form, resulting in substantial variability in plasma levels between patients,22 which in turn contributed to variable pharmacokinetics, toxicity, and response in patients receiving the same weight-based or body surface area–based dose. In general, although low steady-state plasma busulfan levels were found to be associated with graft rejection,23 there was also a direct association observed between high plasma busulfan levels and regimen-related toxicity, such as SOS.22,24 In addition, steady-state plasma busulfan levels <918 ng/mL were also correlated with an increased risk of relapse in chronic phase CML patients undergoing HLA-matched related and unrelated donor HCT.25 Based on these observations, and the fact that steady-state busulfan plasma concentrations can be achieved rapidly and can be predicted from first-dose kinetics, the strategy of targeted steady-state busulfan dosing emerged and has been used successfully in the setting of high-dose conditioning regimens.26 When intravenous (IV) busulfan became available, pharmacokinetic data demonstrated less individual variation, and retrospective comparisons indicated less liver toxicity associated with the IV form. A regimen consisting of high-dose busulfan (16 mg/kg total dose) and cyclophosphamide (200 mg/kg total dose) was developed,27 modified (total cyclophosphamide dose was decreased to 120 mg/kg),28 and has been widely used since in patients undergoing autologous and allogeneic HCT.
In more recently developed regimens, to further reduce regimen-related toxicity, cyclophosphamide was replaced with fludarabine, a nucleoside analog with considerable immunosuppressive properties that also has a synergizing effect with alkylators by inhibiting DNA repair. The combination of busulfan and fludarabine was found to have more favorable toxicity profile in patients with myeloid leukemias,29,30 although, in a more recent randomized trial including 126 patients with leukemia and myelodysplastic syndrome (MDS), overall, relapse-free, and event-free survivals appeared inferior to those achieved with busulfan and cyclophosphamide conditioning.31
Another alkylating agent, melphalan (140 mg/m2), has also been successfully combined with busulfan (16 mg/kg) in the setting of both autologous and allogeneic HCT with encouraging results in small, single institution studies.32,33 Combinations of busulfan, thiotepa, and cyclophosphamide, as well as busulfan, cyclophosphamide, and etoposide, have also been used for both autologous and allogeneic HCT.34-36 In addition, melphalan is often combined with carmustine (BCNU), etoposide, and AraC (BEAM), a regimen used in patients with non-Hodgkin’s lymphoma (NHL) undergoing autologous HCT.37 This, and similar non–radiation-containing regimens (eg, cyclophosphamide, BCNU, VP16 [etoposide] [CBV]38 and BCNU, etoposide, AraC, cyclophosphamide [BEAC]39) are especially useful to replace TBI-based regimens in lymphoma patients who have already received dose-limiting irradiation. Common toxicities associated with high-dose BCNU, especially at doses exceeding 450 mg/m2, include pulmonary toxicity (often fatal pulmonary fibrosis) and, less frequently, SOS, hemorrhagic cystitis, and nephrotoxicity. These combinations are sufficiently immunosuppressive and have been explored in the allogeneic setting40
Melphalan has been used as a single agent or in combination with TBI as conditioning for patients with multiple myeloma undergoing autologous HCT; however, a randomized trial (single-agent melphalan at 200 mg/m2 vs melphalan 140 mg/m2 with 8-Gy TBI) showed a survival advantage for single-agent melphalan.15
In more recent studies, treosulfan, a water-soluble prodrug of a bifunctional alkylating agent approved in Europe for the treatment of advanced ovarian carcinoma, has been explored in combination with fludarabine and other agents as conditioning for autologous and allogeneic HCT. The dose-limiting toxicity was mucositis, diarrhea, and dermatitis in clinical trials involving autologous stem cell rescue.41 The combination of treosulfan and fludarabine with or without low-dose TBI was associated with high engraftment rates, substantially decreased regimen-related toxicity and transplant-related mortality, and improved survival in patients with myeloid malignancies undergoing allogeneic HCT.42,43 Further clinical trials are needed to define the role of treosulfan-based regimens in the autologous and allogeneic HCT setting.
High-dose chemoradiotherapy vs high-dose chemotherapy
A limited number of studies have been performed to compare high-dose TBI and chemotherapy-based regimens. In patients with chronic phase CML, a cyclophosphamide (120 mg/kg) and high-dose TBI (10-12 Gy)-based regimen appeared equivalent to the combination of busulfan (16 mg/kg) and cyclophosphamide (120 mg/kg) in 2 prospective randomized studies44,45; however, both of these early trials used oral busulfan without individual dose adjustments based on plasma busulfan levels. Another study comparing the 2 regimens, also using fixed-dose oral busulfan without dose adjustments in patients with AML, showed improved disease-free and overall survival, lower relapse rates, and lower transplant-related mortality associated with the TBI-based conditioning regimen.46 Long-term follow-up of these and another study showed that the combination of busulfan/cyclophosphamide and cyclophosphamide/TBI provided similar long-term survival in patients with CML, whereas in patients with AML, a nonsignificant 10% lower survival rate was observed after busulfan/cyclophosphamide.47 Similarly, a retrospective, registry-based study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation showed that outcomes using IV busulfan/cyclophosphamide were not statistically different from those after cyclophosphamide/TBI.48 A retrospective analysis conducted by the CIBMTR compared outcomes of allogeneic HCT in patients with AML in first remission receiving cyclophosphamide/TBI (n = 200) vs busulfan/cyclophosphamide (n = 381)-based conditioning and showed similar transplant-related mortality and relapse-free and overall survival, whereas the relapse risk was lower in patients receiving TBI-based conditioning.49 The question was revisited recently by the CIBMTR in a retrospective analysis based on a more recent dataset including 1230 patients with AML in first remission who underwen cyclophosphamide/TBI-based vs busulfan/cyclophosphamide-based conditioning followed by allogeneic HCT.50 In this analysis, patients receiving IV busulfan were included and analyzed separately from those receiving oral busulfan. It is unclear in this registry-based dataset to what extent busulfan dose adjustments were performed based on pharmacokinetic data; however, multivariate analysis showed statistically significantly less nonrelapse mortality (NRM) and better overall and leukemia-free survival in patients receiving IV, but not oral, busulfan compared with TBI. Finally, a recent, prospective cohort study conducted through the CIBMTR showed that, compared with TBI, IV busulfan resulted in superior survival with no increased risk of relapse or treatment-related mortality in patients with myeloid malignancies, supporting the use of high-dose IV busulfan vs TBI in these patients.51
Reduced intensity and nonmyeloablative conditioning regimens
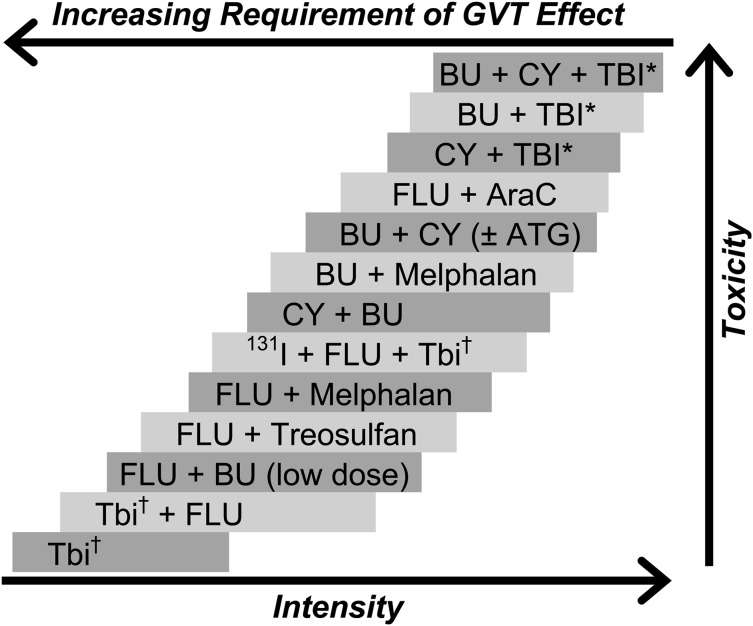
Observations from the late 1970s and early1980s suggesting that patients who developed acute or chronic graft-versus-host disease (GVHD) after allogeneic HCT had improved relapse-free survival led to the recognition that allogeneic hematopoietic cells not only rescued patients from the hematologic toxicity of high-dose conditioning regimens but, through immunologic GVT effects, also contributed to the cure of malignant diseases.52-54 These findings were further supported by lower relapse rates associated with the use of unmodified grafts compared with autologous,55 syngeneic,56 or T cell-depleted grafts.57 The hypothesis that GVT effects are capable of eradicating malignant disorders led to the development of reduced-intensity and nonmyeloablative regimens that made allogeneic HCT an accessible therapeutic option for older and medically infirm patients who previously were not considered candidates for high-dose conditioning. Although there is a considerable range in the intensity of these regimens, a uniform pattern has been the replacement of cytotoxic components of the conditioning regimen with less toxic but immunosuppressive agents to enable engraftment. Figure 1 outlines the spectrum of commonly used regimens in relation to their estimated immunosuppressive and myelosuppressive potency. As in the setting of HLA-matched allografts, both host-versus-graft and graft-versus-host reactions are primarily mediated by T-lymphocytes; therefore, presumably, modulation of host-versus-graft reactions would also contribute to the prevention of GVHD.
Figure 1.
Selected conditioning regimens of different dose intensities. AraC, cytosine arabinoside; ATG, antithymocyte globulin (or thymoglobulin); 131I, anti-CD45 antibody conjugated to 131I. BU, busulfan; CY, cyclophosphamide; Flu, fludarabine (various dosing schedules). *High-dose TBI (800-1320 cGy). †Low-dose TBI (200-400 cGy). Reproduced from Deeg and Sandmaier.106
In general, reduced-intensity and nonmyeloablative allogeneic HCT results in a varying degree of initial mixed donor-host chimerism,58 the degree of which can differ in distinct cellular subsets, depending on the intensity of the actual conditioning regimen, the HLA disparity between donor and host, graft composition, and other factors. Complete donor chimerism develops rapidly after reduced-intensity regimens that use more myelosuppressive drug combinations; in contrast, the achievement of full donor engraftment can take months when less intense, nonmyeloablative conditioning regimens are used. Of note, particularly in the nonmyeloablative setting, higher numbers of grafted T cells, CD14+ cells, natural killer cells, and CD34+ cells were associated with higher levels of donor T-cell chimerism, and high T-cell monocyte and CD34+ cell numbers reduced the risk of graft rejection.59
Although mixed chimerism may be sufficient to correct the phenotype of certain congenital nonmalignant disorders, full donor chimerism may be necessary to exert GVT effects to control malignant diseases. It is important to note that the sensitivity of different hematologic malignancies to GVT effects can vary substantially, a phenomenon not entirely understood. The efficacy of GVT effects can be also impacted by the tumor burden and the proliferation rate of the malignancy. This was demonstrated in a study including 834 consecutive patients undergoing a minimally toxic regimen of 2-Gy TBI with or without fludarabine, where the antitumor effect of each regimen relied almost exclusively on GVT effects, allowing estimation of the extent of these effects.60 Relapse rates per patient year at risk were calculated for 29 different diseases and disease stages. Patients with lymphoid malignancies in complete remission (CLL, ALL in first complete remission, NHL at any stage except for aggressive lymphomas not in remission, multiple myeloma) experienced the lowest relapse rates, whereas patients with advanced myeloid malignances and aggressive lymphomas not in remission were at the highest risk for relapse after nonmyeloablative conditioning, suggesting that additional interventions, such as pretransplant cytoreductive therapy or post-transplant maintenance therapy, may be necessary to achieve disease control in the latter group. The emergence of novel therapies targeting mutations or expression of specific antigens through antibodies or adoptive immunotherapy could provide viable, personalized options for maintenance therapy following reduced-intensity allogeneic transplants in an effort to reduce post-HCT relapse. Examples include ongoing trials testing the peritransplant use of monoclonal antibodies (eg, rituximab), cytotoxic T-lymphocytes (eg, transduced with WT-1-specific T-cell receptor), tyrosine-kinase inhibitors (such as imatinib,61 dasatinib, and nilotinib for Philadelphia-positive ALL and AC-220 for FMS-like tyrosine kinase 3–internal tandem duplication–positive AML), and others.
Commonly used RIC regimens
Early studies reported by the MD Anderson Cancer Center used purine nucleoside analog-based regimens followed by allogeneic HCT for the treatment of hematologic malignancies.62,63 In subsequent reports, also from the MD Anderson group, purine analogs (fludarabine or cladribine) were combined with different doses of melphalan (180, 140, and eventually 100 mg/m2) in patients with high-risk AML and MDS undergoing allogeneic HCT.64,65 Of note, these studies showed efficacy of this regimen in patients not in complete remission but with high NRM rates, resulting in 40% and 23% 2-year survival in patients with active disease and circulating blasts, respectively, at the time of transplant. An update of outcomes, however, reported high rates of 4-year overall and progression-free survival (71% and 68%, respectively) in 36 patients in complete remission at the time of HCT.
Slavin et al reported a regimen incorporating fludarabine, oral busulfan (8 mg/kg), and anti-thymocyte globulin (ATG) in 26 younger patients with both hematologic malignancies and genetic disorders undergoing HCT from HLA-identical siblings.66 All patients achieved partial or complete donor chimerism. With the exception of 2 patients, all patients developed absolute neutropenia, and a total of 13 patients developed some form of SOS (4 moderate or severe), suggesting that this regimen was still relatively intense. This regimen also showed activity in patients with heavily pretreated malignant lymphoma, all of whom had rapid engraftment,67 and was evaluated using HLA-matched unrelated donors as well.68,69
A more intensified, sequential regimen of cytoreduction with fludarabine, cytarabine, and amsacrine, followed by 3 days of rest, and then 4-Gy TBI, ATG, and cyclophosphamide (80-120 mg/kg; the fludarabine, AraC, amsacrine regimen), also incorporating early donor lymphocyte infusion, was developed in Germany and showed promising results for high-risk AML and MDS patients, including patients with primary induction failure and adverse risk cytogenetics.70 Subsequent studies implied that replacing TBI with busulfan in this regimen may further improve outcomes.71,72
In separate studies, several groups of investigators described the incorporation of clofarabine, a rationally designed, second-generation purine nucleoside analog with intrinsic antileukemic activity, into RIC regimens for allogeneic HCT, in combination with an alkylating agent such as busulfan or with TBI.73,74 These single institution studies, albeit small, showed that conditioning regimens incorporating clofarabine in various combinations (mostly in combination with an alkylating agent) and followed by allogeneic HCT were well tolerated and safe. The replacement of fludarabine with clofarabine in busulfan-containing preparative regimens did not seem to impact engraftment.
Commonly used nonmyeloablative regimens
A low-dose, 2-Gy TBI-based preparative regimen was developed at the Fred Hutchinson Cancer Research Center, which was feasible for delivery in the outpatient setting.75 To prevent graft rejection and increase pretransplantation host T-cell immunosuppression, fludarabine at 90 mg/m2 was added to the 2-Gy TBI. Peritransplant immunosuppression consisted of cyclosporine and mycophenolate mofetil. A more recent publication summarized outcomes in 1092 patients with HLA-matched related (n = 611) and HLA-matched unrelated (n = 481) donors undergoing conditioning with 2-Gy TBI with or without fludarabine (90 mg/m2).76 As with most reduced-intensity conditioning regimens, the leading cause of treatment failure was relapse, with a 5-year relapse-related mortality rate of 34.5%. Most relapses occurred early while the immune system was compromised. The 5-year NRM rate was 24%; most NRM occurred in patients with a previous history of GVHD.
A nonmyeloablative regimen containing fludarabine (90 mg/m2) and cyclophosphamide (2250 mg/m2) in conjunction with peritransplant rituximab was developed at the MD Anderson Cancer Center and was shown to induce sustained long-term clinical and molecular remission in patients with relapsed, chemosensitive follicular lymphoma.77 Of note, this regimen has been used successfully in patients with other CD20+ B-cell NHL histologies and was most effective in patients with chemosensitive disease.78
A different regimen has been developed by the Stanford group, based on murine studies, to facilitate the presence of natural killer/T cells that suppress GVHD but retain GVT effects.79,80 This regimen combines total lymphoid irradiation (8-12 Gy) delivered over 11 days and ATG (1.25 mg/kg) administered over 5 days. This approach resulted in low rates of acute GVHD in initial reports (2 of 37 patients), with evident antitumor activity and promising survival (71% and 77% in patients with lymphoid malignancies and acute leukemia, respectively, at a median follow up of 482 days).81
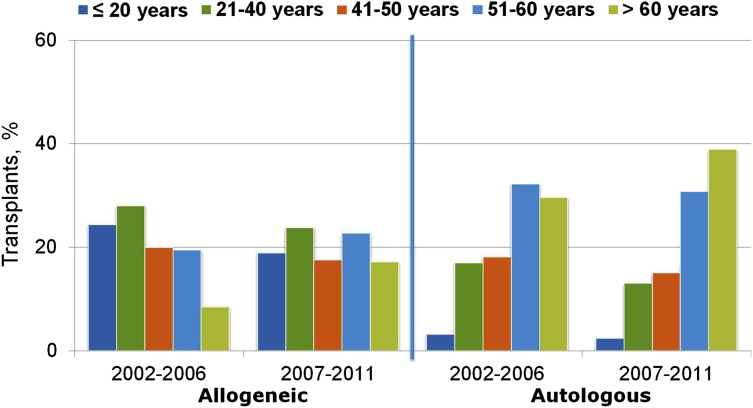
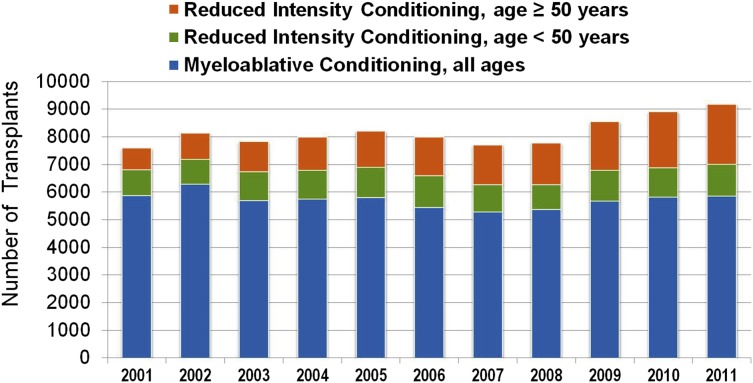
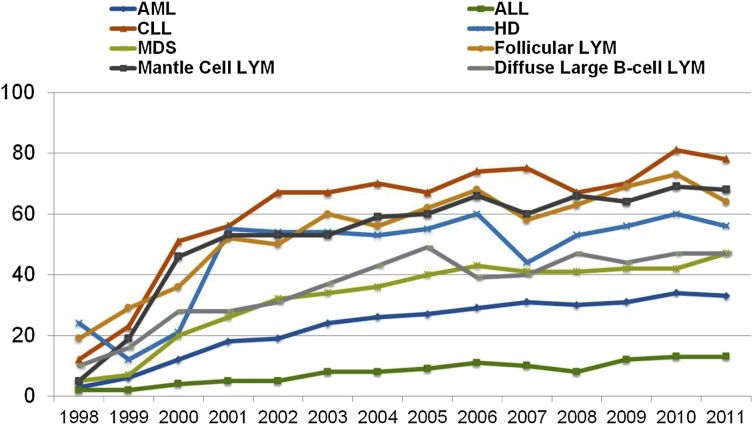
Although the above reduced-intensity and nonmyeloablative regimens have been used successfully by different centers, at present they have not been compared with each other in prospective randomized trials. Nevertheless, the adoption of less toxic conditioning regimens has expanded the number of patients eligible to undergo allogeneic HCT to include patients who previously had been excluded due to age or comorbidities. The number of transplants performed in patients >50 years of age increased more than in any other age group between 2001 and 2011 (Figures 2 and 3), and currently, patients in their mid- to late 70s can be considered for allogeneic HCT. Not surprisingly, the proportion of reduced-intensity transplants has increased in all disease categories (Figure 4), with higher proportions in diseases associated with older or more heavily treated patients.
Figure 2.
Trends in transplant by type and recipient age, 2002 to 2006 and 2007 to 2011, CIBMTR data. Transplants are for AML, ALL, NHL, Hodgkin’s lymphoma, and multiple myeloma. Figure originally presented as slide 6 of Pasquini and Wang.107
Figure 3.
Allogeneic transplants registered with the CIBMTR, 2001 to 2011, by conditioning regimen intensity and age. CIBMTR data. Figure originally presented as slide 21 of Pasquini and Wang.107
Figure 4.
Percentage of RIC allo-HCTs, registered with CIBMTR, 1998 to 2011, by year of transplant and disease. CIBMTR data. Figure originally presented as slide 23 of Pasquini and Wang.107
Radioimmunotherapy-based regimens
The observation that the majority of leukemias and lymphomas were exquisitely radiosensitive and that higher doses of external beam TBI substantially reduced the risk of relapse in early studies of allogeneic HCT but resulted in increased toxicity due to radiation-induced damage to normal organs6,9 led to the hypothesis that targeted radiotherapy using radiolabeled monoclonal antibodies would be superior to external beam TBI in achieving disease control without imposing excessive toxicity. These approaches aim to deliver higher doses of radiation to the tumor site while relatively sparing normal organs from exposure to TBI. To achieve a favorable biodistribution of a radiolabeled monoclonal antibody, an ideal antigen would be expressed homogeneously on the tumor cell surface and would lack expression on normal cells. Lacking such an antigen, methods to target lineage-specific hematopoietic antigens, such as CD20, CD33, and CD45, have been successfully developed in the autologous and allogeneic HCT setting.82-85
Anti-CD20 radioimmunoconjugates
NHL cells are highly sensitive to radiation and stably express lineage-specific antigens, making them ideal targets for radioimmunotherapy. Two anti-CD20 radioimmunoconjugates, 131I-tositumomab (Bexxar) and 90Y-ibritumomab (Zevalin), have been approved by the US Food and Drug Administration for the treatment of relapsed, refractory, or transformed follicular lymphoma.
High-dose 131I-tositumomab was explored as conditioning, followed by autologous stem cell rescue, in heavily pretreated B-cell NHL patients >60 years of age, with encouraging results.86 The Nebraska group has explored the combination of conventional doses of 131I-tositumomab and high-dose BEAM conditioning followed by autologous HCT in patients with chemotherapy-refractory or multiply-relapsed B-cell NHL.87 Encouraging results have been reported with conditioning regimens combining 90Y-ibritumomab and high-dose BEAM followed by autologous HCT.88,89 Limited data exist on the use of anti-CD20-based radioimmunotherapy as part of conditioning therapy followed by allogeneic HCT.90
Anti-CD45 radioimmunoconjugates
The CD45 antigen (common leukocyte antigen) is expressed on the surface of virtually all hematopoietic cells, except mature red cells and platelets. In addition, CD45 expression has been detected in 85% to 90% of AML and ALL, and the antigen does not internalize after antibody binding. In a phase 1 study, patients with advanced acute leukemia or MDS were treated with escalating doses of 131I-labeled CD45 antibody, in combination with high-dose cyclophosphamide and TBI conditioning, followed by autologous or allogeneic HCT.91 This antibody was eventually explored in combination with high-dose targeted busulfan and cyclophosphamide conditioning,92 and, more recently, in combination with fludarabine and 2-Gy TBI in elderly patients with advanced AML or MDS93 with promising results, especially in patients with relapsed or refractory leukemias who would not have been eligible for high-dose conditioning. Conjugation of anti-CD45 antibody with alternative radioisotopes including 90Y is currently explored in clinical trials.
Anti-T cell antibody-containing regimens
Antibodies targeting T lymphocytes, such as ATG and alemtuzumab, are commonly incorporated into high-dose and RIC regimens to decrease the incidence of graft rejection and to prevent GVHD. In general, ATG does not exert its immunomodulatory effects exclusively through in vivo host and donor T-lymphocyte depletion. Other mechanisms contribute, such as the modulation of cell surface molecules mediating interactions of lymphocytes and the endothelium, the modulation and depletion of antigen-presenting cells, and the induction of regulatory T lymphocytes.94,95 Consequently, the efficacy of ATG in preventing graft rejection and GVHD is affected by several factors including the dose administered, its source and formulation (rabbit vs horse; thymoglobulin, ATG-Fresenius vs Atgam), the timing of administration, the degree of HLA disparity between host and donor, the graft source (marrow vs granulocyte colony-stimulating factor-mobilized peripheral blood stem cells [PBSCs]), and the intensity of the conditioning regimen used, all of which complicates any attempt to compare the various single institution phase 2 studies currently being conducted.
Several prospective randomized trials compared the efficacy of ATG in preventing GVHD in the setting of high-dose conditioning regimens, the discussion of which is beyond the scope of this review, with a primary focus on conditioning regimens. A meta-analysis of 7 such randomized trials concluded that, although administration of horse or rabbit ATG resulted in a significant reduction in the risk of grades III and IV acute GVHD, this did not translate into improved NRM or overall survival.96
In contrast, to our knowledge, there have been no prospective phase 3 randomized trials reported to date that compare in vivo T-cell depletion with T cell-replete allografts in the context of RIC regimens. As discussed, the success of these regimens relies more heavily on immune-mediated GVT effects; therefore, it is critical to understand the impact that in vivo T-cell depletion may have on the control of malignant disorders vs nonmalignant conditions in the RIC setting. Phase 2 trials from single institutions have showed promising results,97 and there is emerging evidence that incremental changes in the dose can have substantial impact on outcomes, eg, reduction of thymoglobulin dose from 7.5 to 6 mg/kg resulted in sustained control of GVHD but reduced NRM in patients undergoing HCT with RIC from unrelated donors.98
Observations of a large retrospective analysis from the CIBMTR seem to caution the merits of in vivo T-cell depletion in conjunction with RIC regimens followed by allogeneic HCT. In this study, in vivo T-cell depletion with ATG or alemtuzumab resulted in increased relapse incidence and decreased disease-free survival in a cohort of 1676 patients with hematologic malignancies receiving chemotherapy-based RIC regimens, followed by related or unrelated donor HCT, using marrow or PBSCs as a graft source.99 A more recent, but also retrospective, registry-based analysis from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation focused on a more homogeneous patient cohort and included 1250 patients with AML in first complete remission undergoing HLA-identical sibling PBSC transplantation with chemotherapy-based RIC regimens.100 In this study, in vivo T-cell depletion was successful in preventing acute and chronic GVHD, but was associated with similar relapse risk, NRM, and disease-free and overall survivals. In this study it was also observed that, among patients receiving busulfan-based RIC, the use of ATG at a dose of <6 mg/kg did not result in an increased risk of relapse, whereas with ATG doses ≥6 mg/kg, there was a nonsignificant trend of higher relapse risk.
In summary, although in vivo T-cell depletion with ATG may reduce GVHD in patients undergoing RIC followed by allogeneic HCT, the dose and timing of ATG can substantially impact outcomes, cautioning against the routine use in this context. Future prospective randomized trials evaluating this approach should aim to use low-intermediate doses of ATG to define the potential benefit of integrating ATG into RIC or nonmyeloablative regimens.
To decrease GVHD rates, several groups have incorporated alemtuzumab into the conditioning regimen as another approach to achieve in vivo T-cell depletion.101,102 These studies showed that, although inclusion of alemtuzumab in the preparative regimen was associated with durable engraftment and a low incidence of GVHD, this did not necessarily translate into a survival benefit when retrospectively compared with similar regimens not containing alemtuzumab. Concerns regarding infections and relapse have limited its more widespread use in the transplant setting.
Choice of conditioning regimens
As standard regimens have not been established for the various forms of HCT, clinical practice varies substantially among institutions. Most regimens have been evaluated in phase 1 or 2 trials only, and randomized phase 3 trials are lacking in the field. As patient selection and local patterns of supportive care have a great impact on outcomes, it is difficult to compare the relative efficacy of different regimens based on reports of phase 2 trials. An ongoing, phase 3 multicenter trial by the Blood and Marrow Transplant Clinical Trials Network addresses this problem by comparing outcomes after allogeneic HCT in patients with AML or MDS receiving high-dose vs RIC regimens. This trial was designed to assess the impact of regimen intensity on outcomes after allogeneic stem cell transplantation for MDS or AML, based on the hypothesis that reducing the intensity of the conditioning regimen would decrease treatment-related mortality without increasing relapse, thereby improving overall survival. Of note, the trial was halted permanently by the National Heart, Lung and Blood Institute Data and Safety Monitoring Board in April 2014, as there appeared to be a benefit of the high-dose conditioning over the RIC regimen for patients who met the eligibility criteria (age ≤ 65 years, AML or MDS with <5% marrow blasts, and an HCT-specific comorbidity index score of ≤4).
When evaluating a patient for allogeneic HCT, the diagnosis, disease status, donor availability (ie, HLA disparity, predicting the risk of rejection), graft source, and patient-related factors such as the presence or absence of comorbid conditions should all influence the choice of conditioning regimen.
In recent, mostly retrospective reports, patient age did not show statistically significant association with overall survival, NRM, or relapse.103 The intrinsic selection bias in these studies needs to be recognized. Nevertheless, the data show that age alone should not be considered a contraindication to allogeneic HCT. Older patients and those with preexisting comorbid conditions should be evaluated for allogeneic HCT with RIC or nonmyeloablative regimens, carefully assessing risks and benefits.
Disease-associated factors should also be considered, such as diagnosis and remission status. As discussed above, higher relapse rates have been observed with RIC and nonmyeloablative regimens, and the sensitivity of different hematologic malignancies to GVT effects can vary substantially. Patients at high risk for post-HCT relapse with RIC and nonmyeloablative regimens (eg, advanced myeloid malignances and aggressive lymphomas not in remission) should be considered for more intense regimens, and if not eligible, additional interventions can be used, such as pretransplant cytoreductive therapy or post-transplant maintenance therapy, preferably in the context of a clinical trial. As remission status at the time of HCT is an important prognostic factor that predicts risk of relapse in patients with both myeloid and lymphoid malignancies, allogeneic HCT should be pursued at early disease stages, possibly as soon as complete remission is achieved in patients with high-risk diseases.
In conclusion, the development of reduced-intensity regimens made allogeneic HCT accessible to older and medically infirm patients. The role of dose intensity and reduced-intensity regimens is yet to be defined in younger populations; however, it is possible that with the evolution and optimization of peritransplant interventions and post-transplant maintenance therapies, this approach will provide a platform for the treatment of younger patients as well.
Acknowledgments
The authors thank Helen Crawford and Bonnie Larson for help in manuscript preparation and Jeannine McCune for helpful comments on the manuscript.
Research funding was provided by grants CA018029, CA078902, and CA015704 from the National Insitututes of Health, National Cancer Institute.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers.
Authorship
Contribution: B.G. and B.M.S. drafted and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brenda M. Sandmaier, 1100 Fairview Ave N. D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: bsandmai@fhcrc.org.
References
- 1.Grunebaum E, Mazzolari E, Porta F, et al. Bone marrow transplantation for severe combined immune deficiency. JAMA. 2006;295(5):508–518. doi: 10.1001/jama.295.5.508. [DOI] [PubMed] [Google Scholar]
- 2.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas ED, Clift RA, Hersman J, et al. Marrow transplantation for acute nonlymphoblastic leukemic in first remission using fractionated or single-dose irradiation. Int J Radiat Oncol Biol Phys. 1982;8(5):817–821. doi: 10.1016/0360-3016(82)90083-9. [DOI] [PubMed] [Google Scholar]
- 5.Brochstein JA, Kernan NA, Groshen S, et al. Allogeneic bone marrow transplantation after hyperfractionated total-body irradiation and cyclophosphamide in children with acute leukemia. N Engl J Med. 1987;317(26):1618–1624. doi: 10.1056/NEJM198712243172602. [DOI] [PubMed] [Google Scholar]
- 6.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76(9):1867–1871. [PubMed] [Google Scholar]
- 7.Petersen FB, Deeg HJ, Buckner CD, et al. Marrow transplantation following escalating doses of fractionated total body irradiation and cyclophosphamide—a phase I trial. Int J Radiat Oncol Biol Phys. 1992;23(5):1027–1032. doi: 10.1016/0360-3016(92)90909-2. [DOI] [PubMed] [Google Scholar]
- 8.Demirer T, Petersen FB, Appelbaum FR, et al. Allogeneic marrow transplantation following cyclophosphamide and escalating doses of hyperfractionated total body irradiation in patients with advanced lymphoid malignancies: a Phase I/II trial. Int J Radiat Oncol Biol Phys. 1995;32(4):1103–1109. doi: 10.1016/0360-3016(95)00115-f. [DOI] [PubMed] [Google Scholar]
- 9.Clift RA, Buckner CD, Appelbaum FR, Sullivan KM, Storb R, Thomas ED. Long-term follow-Up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. [Letter] Blood. 1998;92(4):1455–1456. [PubMed] [Google Scholar]
- 10.Peters LJ, Withers HR, Cundiff JH, Dicke KA. Radiobiological considerations in the use of total-body irradiation for bone-marrow transplantation. Radiology. 1979;131(1):243–247. doi: 10.1148/131.1.243. [DOI] [PubMed] [Google Scholar]
- 11.Shank B, Hopfan S, Kim JH, et al. Hyperfractionated total body irradiation for bone marrow transplantation: I. Early results in leukemia patients. Int J Radiat Oncol Biol Phys. 1981;7(8):1109–1115. doi: 10.1016/0360-3016(81)90170-x. [DOI] [PubMed] [Google Scholar]
- 12.Shank B, O’Reilly RJ, Cunningham I, et al. Total body irradiation for bone marrow transplantation: the Memorial Sloan Kettering Cancer Center experience. Radiother Oncol. 1990;18(Suppl 1):68–81. doi: 10.1016/0167-8140(90)90180-5. [DOI] [PubMed] [Google Scholar]
- 13.Riddell S, Appelbaum FR, Buckner CD, et al. High-dose cytarabine and total body irradiation with or without cyclophosphamide as a preparative regimen for marrow transplantation for acute leukemia. J Clin Oncol. 1988;6(4):576–582. doi: 10.1200/JCO.1988.6.4.576. [DOI] [PubMed] [Google Scholar]
- 14.Horning SJ, Chao NJ, Negrin RS, et al. The Stanford experience with high-dose etoposide cytoreductive regimens and autologous bone marrow transplantation in Hodgkin’s disease and non-Hodgkin’s lymphoma: preliminary data. Ann Oncol. 1991;2(Suppl 1):47–50. doi: 10.1093/annonc/2.suppl_1.47. [DOI] [PubMed] [Google Scholar]
- 15.Moreau P, Facon T, Attal M, et al. Intergroupe Francophone du Myélome. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood. 2002;99(3):731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 16.Scott B, Deeg HJ, Storer B, et al. Targeted busulfan and cyclophosphamide as compared to busulfan and TBI as preparative regimens for transplantation in patients with advanced MDS or transformation to AML. Leuk Lymphoma. 2004;45(12):2409–2417. doi: 10.1080/10428190412331283206. [DOI] [PubMed] [Google Scholar]
- 17.Ozsahin M, Pène F, Touboul E, et al. Total-body irradiation before bone marrow transplantation. Results of two randomized instantaneous dose rates in 157 patients. Cancer. 1992;69(11):2853–2865. doi: 10.1002/1097-0142(19920601)69:11<2853::aid-cncr2820691135>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.van Kempen-Harteveld ML, Struikmans H, Kal HB, et al. Cataract-free interval and severity of cataract after total body irradiation and bone marrow transplantation: influence of treatment parameters. Int J Radiat Oncol Biol Phys. 2000;48(3):807–815. doi: 10.1016/s0360-3016(00)00669-6. [DOI] [PubMed] [Google Scholar]
- 19.Socié G, Curtis RE, Deeg HJ, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18(2):348–357. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia S, Ramsay NK, Steinbuch M, et al. Malignant neoplasms following bone marrow transplantation. Blood. 1996;87(9):3633–3639. [PubMed] [Google Scholar]
- 21.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19(2):464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 22.Grochow LB. Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol. 1993;20(4 Suppl 4):18–25. [PubMed] [Google Scholar]
- 23.Slattery JT, Sanders JE, Buckner CD, et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. [Erratum appears in Bone Marow Transplantation 1996 Oct; 18(4):829] Bone Marrow Transplant. 1995;16(1):31–42. [PubMed] [Google Scholar]
- 24.Grochow LB, Jones RJ, Brundrett RB, et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989;25(1):55–61. doi: 10.1007/BF00694339. [DOI] [PubMed] [Google Scholar]
- 25.Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89(8):3055–3060. [PubMed] [Google Scholar]
- 26.Radich JP, Gooley T, Bensinger W, et al. HLA-matched related hematopoietic cell transplantation for chronic-phase CML using a targeted busulfan and cyclophosphamide preparative regimen. Blood. 2003;102(1):31–35. doi: 10.1182/blood-2002-08-2619. [DOI] [PubMed] [Google Scholar]
- 27.Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309(22):1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 28.Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood. 1987;70(5):1382–1388. [PubMed] [Google Scholar]
- 29.Bornhäuser M, Storer B, Slattery JT, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102(3):820–826. doi: 10.1182/blood-2002-11-3567. [DOI] [PubMed] [Google Scholar]
- 30.Iravani M, Evazi MR, Mousavi SA, et al. Fludarabine and busulfan as a myeloablative conditioning regimen for allogeneic stem cell transplantation in high- and standard-risk leukemic patients. Bone Marrow Transplant. 2007;40(2):105–110. doi: 10.1038/sj.bmt.1705685. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Joo YD, Kim H, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013;31(6):701–709. doi: 10.1200/JCO.2011.40.2362. [DOI] [PubMed] [Google Scholar]
- 32.Alegre A, Lamana M, Arranz R, et al. Busulfan and melphalan as conditioning regimen for autologous peripheral blood stem cell transplantation in multiple myeloma. Br J Haematol. 1995;91(2):380–386. doi: 10.1111/j.1365-2141.1995.tb05307.x. [DOI] [PubMed] [Google Scholar]
- 33.Vey N, De Prijck B, Faucher C, et al. A pilot study of busulfan and melphalan as preparatory regimen prior to allogeneic bone marrow transplantation in refractory or relapsed hematological malignancies. Bone Marrow Transplant. 1996;18(3):495–499. [PubMed] [Google Scholar]
- 34.Dimopoulos MA, Alexanian R, Przepiorka D, et al. Thiotepa, busulfan, and cyclophosphamide: a new preparative regimen for autologous marrow or blood stem cell transplantation in high-risk multiple myeloma. Blood. 1993;82(8):2324–2328. [PubMed] [Google Scholar]
- 35.Przepiorka D, Nath R, Ippoliti C, et al. A phase I-II study of high-dose thiotepa, busulfan and cyclophosphamide as a preparative regimen for autologous transplantation for malignant lymphoma. Leuk Lymphoma. 1995;17(5-6):427–433. doi: 10.3109/10428199509056853. [DOI] [PubMed] [Google Scholar]
- 36.Jones RJ, Santos GW. New conditioning regimens for high risk marrow transplants. Bone Marrow Transplant. 1989;4(Suppl 4):15–17. [PubMed] [Google Scholar]
- 37.Mills W, Chopra R, McMillan A, Pearce R, Linch DC, Goldstone AH. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin’s lymphoma. J Clin Oncol. 1995;13(3):588–595. doi: 10.1200/JCO.1995.13.3.588. [DOI] [PubMed] [Google Scholar]
- 38.Weaver CH, Appelbaum FR, Petersen FB, et al. High-dose cyclophosphamide, carmustine, and etoposide followed by autologous bone marrow transplantation in patients with lymphoid malignancies who have received dose-limiting radiation therapy. J Clin Oncol. 1993;11(7):1329–1335. doi: 10.1200/JCO.1993.11.7.1329. [DOI] [PubMed] [Google Scholar]
- 39.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 40.Przepiorka D, van Besien K, Khouri I, et al. Carmustine, etoposide, cytarabine and melphalan as a preparative regimen for allogeneic transplantation for high-risk malignant lymphoma. Ann Oncol. 1999;10(5):527–532. doi: 10.1093/oxfordjournals.annonc.a010369. [DOI] [PubMed] [Google Scholar]
- 41.Scheulen ME, Hilger RA, Oberhoff C, et al. Clinical phase I dose escalation and pharmacokinetic study of high-dose chemotherapy with treosulfan and autologous peripheral blood stem cell transplantation in patients with advanced malignancies. Clin Cancer Res. 2000;6(11):4209–4216. [PubMed] [Google Scholar]
- 42.Nemecek ER, Guthrie KA, Sorror ML, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2011;17(3):341–350. doi: 10.1016/j.bbmt.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gyurkocza B, Gutman J, Nemecek ER, et al. Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20(4):549–555. doi: 10.1016/j.bbmt.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clift RA, Buckner CD, Thomas ED, et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994;84(6):2036–2043. [PubMed] [Google Scholar]
- 45.Devergie A, Blaise D, Attal M, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: a report from the French Society of Bone Marrow Graft (SFGM). Blood. 1995;85(8):2263–2268. [PubMed] [Google Scholar]
- 46.Blaise D, Maraninchi D, Archimbaud E, et al. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-Cytoxan versus Cytoxan-total body irradiation as preparative regimen: a report from the Group d’Etudes de la Greffe de Moelle Osseuse. Blood. 1992;79(10):2578–2582. [PubMed] [Google Scholar]
- 47.Socié G, Clift RA, Blaise D, et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. Blood. 2001;98(13):3569–3574. doi: 10.1182/blood.v98.13.3569. [DOI] [PubMed] [Google Scholar]
- 48.Nagler A, Rocha V, Labopin M, et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen—a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31(28):3549–3556. doi: 10.1200/JCO.2013.48.8114. [DOI] [PubMed] [Google Scholar]
- 49.Litzow MR, Pérez WS, Klein JP, et al. Comparison of outcome following allogeneic bone marrow transplantation with cyclophosphamide-total body irradiation versus busulphan-cyclophosphamide conditioning regimens for acute myelogenous leukaemia in first remission. Br J Haematol. 2002;119(4):1115–1124. doi: 10.1046/j.1365-2141.2002.03973.x. [DOI] [PubMed] [Google Scholar]
- 50.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122(24):3863–3870. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bredeson C, LeRademacher J, Kato K, et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood. 2013;122(24):3871–3878. doi: 10.1182/blood-2013-08-519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300(19):1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 53.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED and the Seattle Marrow Transplant Team. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304(25):1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan KM, Weiden PL, Storb R, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73(6):1720–1728. [PubMed] [Google Scholar]
- 55.Gorin NC, Labopin M, Fouillard L, et al. Retrospective evaluation of autologous bone marrow transplantation vs allogeneic bone marrow transplantation from an HLA identical related donor in acute myelocytic leukemia. A study of the European Cooperative Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 1996;18(1):111–117. [PubMed] [Google Scholar]
- 56.Fefer A, Sullivan KM, Weiden P, et al. Graft versus leukemia effect in man: the relapse rate of acute leukemia is lower after allogeneic than after syngeneic marrow transplantation. Prog Clin Biol Res. 1987;244:401–408. [PubMed] [Google Scholar]
- 57.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. [PubMed] [Google Scholar]
- 58.Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94(9):3234–3241. [PubMed] [Google Scholar]
- 59.Baron F, Maris MB, Storer BE, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005;19(5):822–828. doi: 10.1038/sj.leu.2403718. [DOI] [PubMed] [Google Scholar]
- 60.Kahl C, Storer BE, Sandmaier BM, et al. Relapse risk in patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110(7):2744–2748. doi: 10.1182/blood-2007-03-078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ram R, Storb R, Sandmaier BM, et al. Non-myeloablative conditioning with allogeneic hematopoietic cell transplantation for the treatment of high-risk acute lymphoblastic leukemia. Haematologica. 2011;96(8):1113–1120. doi: 10.3324/haematol.2011.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89(12):4531–4536. [PubMed] [Google Scholar]
- 63.Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97(3):631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 64.Oran B, Giralt S, Saliba R, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol Blood Marrow Transplant. 2007;13(4):454–462. doi: 10.1016/j.bbmt.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Popat U, de Lima MJ, Saliba RM, et al. Long-term outcome of reduced-intensity allogeneic hematopoietic SCT in patients with AML in CR. Bone Marrow Transplant. 2012;47(2):212–216. doi: 10.1038/bmt.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91(3):756–763. [PubMed] [Google Scholar]
- 67.Nagler A, Slavin S, Varadi G, Naparstek E, Samuel S, Or R. Allogeneic peripheral blood stem cell transplantation using a fludarabine-based low intensity conditioning regimen for malignant lymphoma. Bone Marrow Transplant. 2000;25(10):1021–1028. doi: 10.1038/sj.bmt.1702392. [DOI] [PubMed] [Google Scholar]
- 68.Nagler A, Aker M, Or R, et al. Low-intensity conditioning is sufficient to ensure engraftment in matched unrelated bone marrow transplantation. Exp Hematol. 2001;29(3):362–370. doi: 10.1016/s0301-472x(00)00655-x. [DOI] [PubMed] [Google Scholar]
- 69.Bornhäuser M, Thiede C, Platzbecker U, et al. Dose-reduced conditioning and allogeneic hematopoietic stem cell transplantation from unrelated donors in 42 patients. Clin Cancer Res. 2001;7(8):2254–2262. [PubMed] [Google Scholar]
- 70.Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23(24):5675–5687. doi: 10.1200/JCO.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 71.Detrait MY, Chevallier P, Sobh M, et al. Outcome of high-risk and refractory AML/MDS patients receiving a FLAMSA sequential chemotherapy regimen followed by reduced-intensity conditioning (RIC) and allogeneic hematopoietic stem cell transplantation (allo-HSCT) [abstract]. Blood. 2011;118(21):1957.
- 72.Kröger N, Zabelina T, Wolschke C, et al. Induction chemotherapy followed immediately by busulfan-based reduced conditioning and allografting in elderly patients with advanced MDS or sAML. [abstract] Blood. 2009;114(22):3387. [Google Scholar]
- 73.Chevallier P, Labopin M, Buchholz S, et al. Clofarabine-containing conditioning regimen for allo-SCT in AML/ALL patients: a survey from the Acute Leukemia Working Party of EBMT. Eur J Haematol. 2012;89(3):214–219. doi: 10.1111/j.1600-0609.2012.01822.x. [DOI] [PubMed] [Google Scholar]
- 74.van Besien K, Stock W, Rich E, et al. Phase I-II study of clofarabine-melphalan-alemtuzumab conditioning for allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(6):913–921. doi: 10.1016/j.bbmt.2011.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 76.Storb R, Gyurkocza B, Storer BE, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31(12):1530–1538. doi: 10.1200/JCO.2012.45.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111(12):5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sauter CS, Barker JN, Lechner L, et al. A phase II study of a nonmyeloablative allogeneic stem cell transplant with peritransplant rituximab in patients with B cell lymphoid malignancies: favorably durable event-free survival in chemosensitive patients. Biol Blood Marrow Transplant. 2014;20(3):354–360. doi: 10.1016/j.bbmt.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lan F, Zeng D, Higuchi M, Huie P, Higgins JP, Strober S. Predominance of NK1.1+TCR alpha beta+ or DX5+TCR alpha beta+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: “natural suppressor” cells. J Immunol. 2001;167(4):2087–2096. doi: 10.4049/jimmunol.167.4.2087. [DOI] [PubMed] [Google Scholar]
- 80.Pillai AB, George TI, Dutt S, Strober S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood. 2009;113(18):4458–4467. doi: 10.1182/blood-2008-06-165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lowsky R, Takahashi T, Liu YP, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353(13):1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 82.Appelbaum FR, Matthews DC, Eary JF, et al. The use of radiolabeled anti-CD33 antibody to augment marrow irradiation prior to marrow transplantation for acute myelogenous leukemia. Transplantation. 1992;54(5):829–833. doi: 10.1097/00007890-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 83.Press OW, Eary JF, Appelbaum FR, et al. Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support. N Engl J Med. 1993;329(17):1219–1224. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 84.Jurcic JG, Caron PC, Nikula TK, et al. Radiolabeled anti-CD33 monoclonal antibody M195 for myeloid leukemias. Cancer Res. 1995;55(23 Suppl):5908s–5910s. [PubMed] [Google Scholar]
- 85.Gopal AK, Gooley TA, Maloney DG, et al. High-dose radioimmunotherapy versus conventional high-dose therapy and autologous hematopoietic stem cell transplantation for relapsed follicular non-Hodgkin lymphoma: a multivariable cohort analysis. Blood. 2003;102(7):2351–2357. doi: 10.1182/blood-2003-02-0622. [DOI] [PubMed] [Google Scholar]
- 86.Gopal AK, Rajendran JG, Gooley TA, et al. High-dose [131I] tositumomab (anti-CD20) radioimmunotherapy and autologous hematopoietic stem cell transplantation for adults greater than or equal to 60 years of age with relapsed or refractory B-cell lymphoma. J Clin Oncol. 2007;25(11):1396–1402. doi: 10.1200/JCO.2006.09.1215. [DOI] [PubMed] [Google Scholar]
- 87.Vose JM, Bierman PJ, Enke C, et al. Phase I trial of iodine-131 tositumomab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(3):461–467. doi: 10.1200/JCO.2005.05.117. [DOI] [PubMed] [Google Scholar]
- 88.Krishnan A, Nademanee A, Fung HC, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(1):90–95. doi: 10.1200/JCO.2007.11.9248. [DOI] [PubMed] [Google Scholar]
- 89.Shimoni A, Zwas ST, Oksman Y, et al. Yttrium-90-ibritumomab tiuxetan (Zevalin) combined with high-dose BEAM chemotherapy and autologous stem cell transplantation for chemo-refractory aggressive non-Hodgkin’s lymphoma. Exp Hematol. 2007;35(4):534–540. doi: 10.1016/j.exphem.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 90.Shimoni A, Hardan I, Shem-Tov N, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20(2):322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 91.Matthews DC, Appelbaum FR, Eary JF, et al. Phase I study of (131)I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94(4):1237–1247. [PubMed] [Google Scholar]
- 92.Pagel JM, Appelbaum FR, Eary JF, et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107(5):2184–2191. doi: 10.1182/blood-2005-06-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pagel JM, Gooley TA, Rajendran J, et al. Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2009;114(27):5444–5453. doi: 10.1182/blood-2009-03-213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111(7):3675–3683. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haidinger M, Geyeregger R, Poglitsch M, et al. Antithymocyte globulin impairs T-cell/antigen-presenting cell interaction: disruption of immunological synapse and conjugate formation. Transplantation. 2007;84(1):117–121. doi: 10.1097/01.tp.0000266677.45428.80. [DOI] [PubMed] [Google Scholar]
- 96.Kumar A, Mhaskar AR, Reljic T, et al. Antithymocyte globulin for acute-graft-versus-host-disease prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation: a systematic review. [Review] Leukemia. 2012;26(4):582–588. doi: 10.1038/leu.2011.349. [DOI] [PubMed] [Google Scholar]
- 97.Devillier R, Fürst S, El-Cheikh J, et al. Antithymocyte globulin in reduced-intensity conditioning regimen allows a high disease-free survival exempt of long-term chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(3):370–374. doi: 10.1016/j.bbmt.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 98.Hamadani M, Blum W, Phillips G, et al. Improved nonrelapse mortality and infection rate with lower dose of antithymocyte globulin in patients undergoing reduced-intensity conditioning allogeneic transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2009;15(11):1422–1430. doi: 10.1016/j.bbmt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baron F, Labopin M, Blaise D, et al. Impact of in vivo T-cell depletion on outcome of AML patients in first CR given peripheral blood stem cells and reduced-intensity conditioning allo-SCT from a HLA-identical sibling donor: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(3):389–396. doi: 10.1038/bmt.2013.204. [DOI] [PubMed] [Google Scholar]
- 101.Kottaridis PD, Milligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96(7):2419–2425. [PubMed] [Google Scholar]
- 102.Pérez-Simón JA, Kottaridis PD, Martino R, et al. Spanish and United Kingdom Collaborative Groups for Nonmyeloablative Transplantation. Nonmyeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders. Blood. 2002;100(9):3121–3127. doi: 10.1182/blood-2002-03-0701. [DOI] [PubMed] [Google Scholar]
- 103.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306(17):1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giralt S, Ballen K, Rizzo D, et al. doi: 10.1016/j.bbmt.2008.12.497. Reduced-Intensity Conditioning Regimen Workshop: Defining the dose spectrum. Report of a workshop convened by the Center for International Blood and Marrow Transplant Research Biology of Blood and Marrow Transplantation, Vol. 15. issue 3. Elsevier Science; March 2009:367-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bacigalupo A, Ballen K, Rizzo D, et al. doi: 10.1016/j.bbmt.2009.07.004. Defining the intensity of conditioning regimens, working definitions. Biology of Blood and Marrow Transplantation, Vol. 15, issue 12. Elsevier Science; December 2009:1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deeg HJ, Sandmaier BM. Who is fit for allogeneic transplantation? Blood. 2010;116(23):4762–4770. doi: 10.1182/blood-2010-07-259358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides 2013. http://www.cibmtr.org. Accessed May 16, 2014.