Abstract
Objective
Epithelial cell adhesion molecule (EpCAM) has experienced a renaissance lately as a binding site for targeted therapy as well as a prognostic marker in epithelial malignancies. Aim of this study was to study EpCAM as a potential prognostic marker in epithelial ovarian cancer (EOC).
Methods
EpCAM expression was assessed by immunohistochemistry on paraffin-embedded primary EOC-tissue samples. EpCAM overexpression was defined as an expression of EpCAM of 76% to 100%. Tissue samples and clinical data were systematically collected within the international and multicenter "Tumorbank Ovarian Cancer" network.
Results
Seventy-four patients, diagnosed with EOC between 1994 and 2009, were included in the study (median age, 56 years; range, 31 to 86 years). The majority of the patients (81.1%) presented with an advanced stage International Federation of Gynecology and Obstetrics (FIGO) III/IV disease. Histology was of the serous type in 41 patients (55.4%), endometrioid in 19 (25.6%), and mucinous in 14 (19%). EpCAM was overexpressed in 87.7%. Serous tumors overexpressed EpCAM significantly more often than mucinous tumors (87.8% vs. 78.6%, p=0.045); while no significant difference was noted between the other histological subgroups. EpCAM overexpression was significantly associated with a better progression free survival and higher response rates to platinum based chemotherapy (p=0.040 and p=0.048, respectively). EpCAM was identified as an independent prognostic marker for overall survival (p=0.022).
Conclusion
Our data indicate a significant association of EpCAM overexpression with a more favorable survival in EOC-patients. Serous cancers showed a significant EpCAM overexpression compared to mucinous types. Larger multicenter analyses are warranted to confirm these findings.
Keywords: Epithelial cell adhesion molecule, Ovarian neoplasms, Survival
INTRODUCTION
Epithelial cell adhesion molecule (EpCAM) is a transmembraneous glycoprotein expressed on epithelial cells. Normally it is expressed in the intercellular space connecting epithelial cells, but also on the surface of various epithelial tumor cells [1]. In tumors, EpCAM is usually not limited to the intercellular space, but expressed in a chaotic pattern over the entire surface of the tumor cell [2]. While EpCAM is one of the oldest cancer antigens, discovered as early as in 1979, it has only recently been used as a binding agent for targeted therapy in the oncologic treatment setting [3]. Efforts by different study groups have since then resulted in the development and even European Medicines Agency-approval of EpCAM-specific antibodies such as catumaxomab for treatment [4,5]. Furthermore, EpCAM might not only be used as a target for treatment but also as specific biomarker for histopathology evaluation as well as for prognosis [6,7].
While EpCAM can be found on nearly all epithelial tumor cells, the level of EpCAM-expression may significantly differ between the various histological types [7]. There is a growing evidence that these different expression levels might be of significant prognostic value [8,9,10]. A clear prognostic importance of EpCAM-expression has been observed for breast cancer, carcinomas of the gall bladder and carcinoma of the kidney and esophagus [8,10,11,12].
Epithelial ovarian cancer (EOC), one of the leading causes of cancer-related mortality in women, was found to exhibit a high level of EpCAM-expression in the majority of cases. However, only few studies have been performed to identify the prognostic value of EpCAM in EOC. Since EpCAM was found to promote carcinogenesis and invasiveness, the purpose of this study was to evaluate if EpCAM expression might be a prognostic marker in EOC. Furthermore, the level of EpCAM expression in EOC and a potential impact of EpCAM expression on chemotherapy response were our secondary aims.
MATERIALS AND METHODS
Tissue samples of EOC-patients were collected during primary cytoreductive surgery after informed consent according to standard operation procedures for the Tumor Bank Ovarian Cancer project. Out of this tumor bank 74 patients were randomly selected for this analysis within a 15 year period (1994 to 2009). Twenty normal ovaries gained from other gynecological surgeries due to benign reasons and colon cancer specimens with known EpCAM overexpression were used as controls. Ethical approval was obtained by the Ethical Committee of the Charité University Medicine Berlin. All patients were chemotherapy naive at the time of the tissue collection.
Immediately after collection, the tissue was embedded in paraffin blocks and later examined by two experts of the ovarian cancer team at the Pathology Department Charité Campus Mitte. Clinical-surgical and follow-up data were collected from the validated documentation system (Intraoperative-Mapping-of-Ovarian-Cancer [IMO]), specifically developed for ovarian neoplasms with a particular focus on the description of the tumor pattern, maximal tumor burden, postoperative tumor residuals (0, <0.5, <1, <2, and >2 cm) and the amount of intraoperative ascites (none, </>500 mL). IMO represents a detailed surgical and histopathological documentation system developed to obtain a better and more objective description of the ovarian tumor spread within the abdominal cavity and to define more precisely the histopathological features of the malignancy [13]. Within the Tumor Bank Ovarian Cancer project (http://www.toc-network.de), tumor tissue, ascites, serum, and blood were collected from each EOC-patient.
All patients underwent a primary maximum tumor debulking with maximal surgical effort aiming at optimal tumor residuals, including en bloc resection techniques; most received pelvic and paraaortic lymph node dissection, peritonectomy, and upper abdominal procedures. Tumor residuals were documented after each surgery.
1. Immunohistochemistry
The expression of EpCAM was analyzed by immunohistochemistry using the avidin-biotin complex method. The paraffin embedded tissue was cut in 4 µm sections and mounted onto adhesive-coated glass slides. Before EpCAM staining, hematoxylin and eosin-stained slides were prepared for each case to identify representative tumor regions. After deparaffinization and rehydration, the slides were pretreated in a microwave oven in citrate buffer. To block endogenous peroxidase the slides were placed in 10% hydrogen peroxide for 30 minutes. Unspecific binding was blocked by incubation with 1% bovine serum albimune in Tris-buffered saline for 30 minutes. The primary murine monoclonal antibody HO-3 (clone HO-3-19) targeting EpCAM was applied at a dilution of 1 : 100 and then the slides were stored in a moist chamber at 8℃ over night. On the following day, the slides were incubated with a biotinylated horse anti-mouse secondary antibody in a dilution of 1:200 in a moist chamber for 40 minutes. The ABC complex was applied on the tissue in a moist chamber for 40 minutes. The diaminobenzidine solution was then incubated on the slides for 6 minutes. Finally, the slides were counterstained with Mayer's hematoxylin and dehydrated before mounting. Between incubations the slides were washed with water or with Tris-buffered saline. Colon cancer tissues and the tissue of benign ovaries were used as positive and negative controls.
2. Evaluation of EpCAM expression
EpCAM expression was defined as a specific membranous staining of the tumor cells. EpCAM expression was evaluated by a proportion score and an intensity score. The proportion score was to evaluate the amount of tumor cells positively stained; four different groups were defined: 0% to 10% as no expression; 11% to 50% as low expression; 51% to 75% as moderate expression; and 76% to 100% as overexpression. To evaluate the intensity three different groups of intensity were defined: + as a weak, ++ as a moderate, and +++ as a strong staining intensity. This approach was based on Bellone et al. [14]. All slides were independently evaluated by two experts without knowing the clinical outcome, using light-microscopy.
3. Statistical analysis
PASW ver. 19 (IBM Co., Armonk, NY, USA) was used for the statistical analyses. The chi-square test and Kendall's tau-b test were applied to correlate the EpCAM expression with the clinical data. Estimates of median survival and 95% confidence interval (CI) were calculated using the Kaplan-Meier method. Log-rank tests were used for univariate statistical comparisons. Adjusted hazard ratios (HRs) were estimated with the Cox proportional hazards model. Crude and adjusted odds ratios (ORs) for residual tumor and platinum sensitivity were obtained using logistic regression analysis.
RESULTS
Seventy-four patients with primary EOC were included in the study. Median age at diagnosis was 56 years (range, 31 to 86 years). The majority of the patients (81.1%) presented with an advanced-stage International Federation of Gynecology and Obstetrics (FIGO) III/IV disease; 48.6% had positive lymph nodes; and 12.2% distant metastases. Distant metastases were found as pleural carcinosis (three patients), in the lungs (one patient), liver parenchyma (three patients), as well as axillary and inguinal lymph nodes (two patients). Detailed histopathological characteristics are presented in Table 1. During a median follow-up time of 39.7 months (range, 2.1 to 173 months) 46 of the evaluated patients (62.2%) had a relapse, and 44 patients (59.5%) have died. A complete macroscopic tumor clearance could be achieved in 52 patients (70.3%). In 11 patients (14.9%) tumor residuals were ≤1 cm and in 10 patients (13.5%) >1 cm. The majority of the patients (87.8%) received adjuvant chemotherapy. A combination of carboplatinum and paclitaxel was administered to 70.3% of the patients. The 4.1% of the patients received single-agent carboplatinum while 1.4% received single-agent paclitaxel. The 12.3% of the patients were treated with other platinum-based regimens.
Table 1.
Patients' characteristics (n=74)
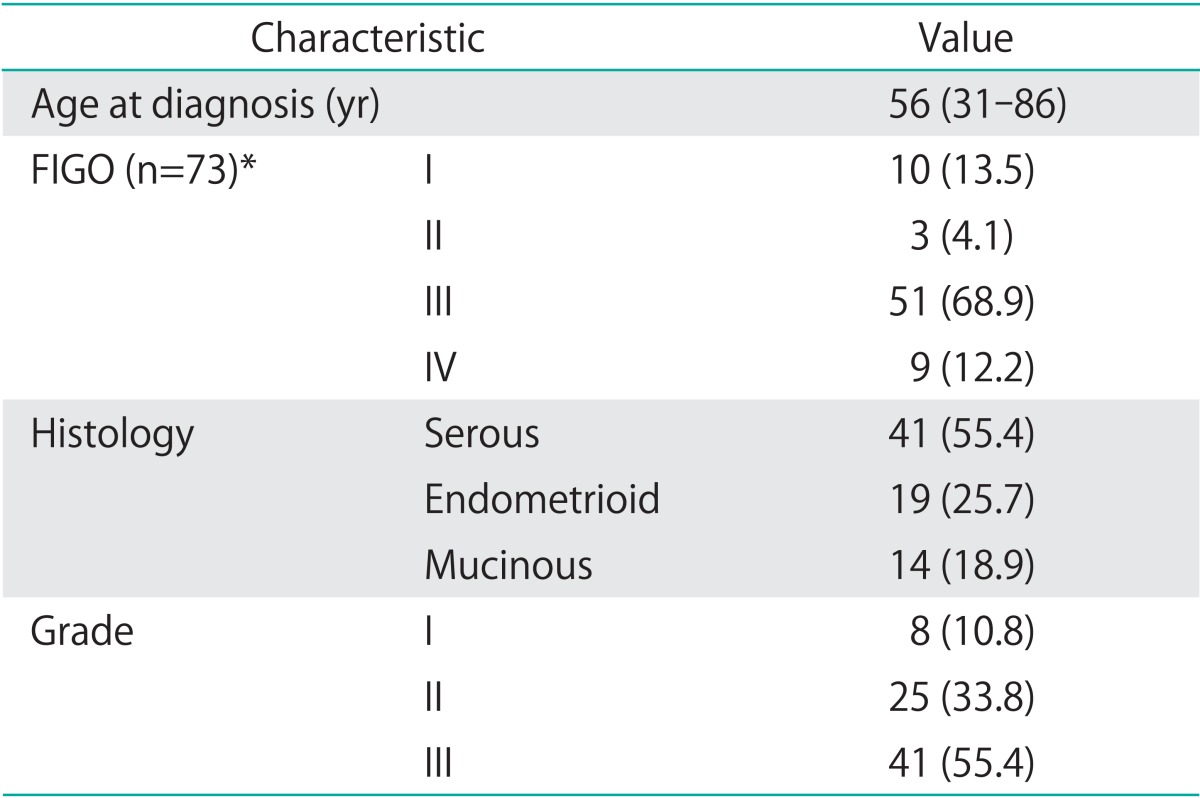
Values are presented as median (range) of number (%).
*International Federation of Gynecology and Obstetrics (FIGO) stage was not assessable in one patient.
1. EpCAM expression according to histology
The patients were divided in three subgroups according to histology. The most relevant differences between the various histological subgroups are as follows: serous tumors and endometrioid tumors were significantly more often poorly differentiated compared to mucinous tumors (65.9% vs. 16.7%, p<0.001; 63.2% vs. 16.7%, p=0.001, respectively). Patients with serous tumors had significantly higher rates of positive lymph node status than patients with mucinous or endometrioid tumors (61.0% vs. 21.4% and 42.1%, respectively; p=0.009 and p=0.218, respectively).
Advanced stage disease FIGO III/IV was significantly more frequently associated with serous than mucinous (p=0.001) or endometrioid (p=0.028) histology. Interestingly, the mucinous subtype regardless of tumor stage seemed to have a trend for a better survival than patients with nonmucinous histology (p=0.084).
As mentioned above, EpCAM overexpression was defined as 76% to 100% of the cells being positively stained with an anti-EpCAM antibody. EpCAM was overexpressed in a total of 65 patients (87.8%). All normal ovaries that were stained as controls showed strong expression of EpCAM on the surface epithelium. Overexpression was found in 36 serous (87.8%), 18 endometrioid (94.7%), and 11 mucinous tumors (78.6%). In serous tumors EpCAM was significantly higher expressed than in mucinous tumors (p=0.045). There was no significant correlation between EpCAM expression and the other histological subtypes.
EpCAM did not correlate with FIGO stage, grading, tumor stage, lymph node metastasis, distant metastasis, or tumor residuals. Examples of immunohistochemistry staining can be seen in Fig. 1.
Fig. 1.
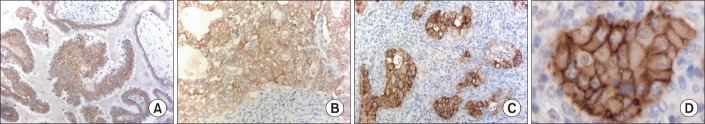
Immunohistochemistry staining for epithelial cell adhesion molecule (EpCAM). (A) Mucinous ovarian cancer; bar 100 µm. (B) Endometrioid ovarian cancer; bar 50 µm. (C) Serous ovarian cancer; bar 100 µm. (D) Demonstration of the membranous staining of EpCAM, example extracted from panel C; bar 20 µm.
2. EpCAM expression and survival
Estimated median overall survival was 47.6 months (95% CI, 38.1 to 57.1 months) and median progression free survival (PFS) 18.0 months (95% CI, 14.7 to 21.3 months).
Median overall survival of patients with EpCAM overexpression (76% to 100%) was 49.4 months (95% CI, 24.552 to 74.248) as compared to 14.2 months for patients with an EpCAM expression ≤50% (95% CI, 11.159 to 17.241) and 43.1 months in patients with an EpCAM expression of 51% to 75% (95% CI, 23.176 to 63.024), showing a significant association of EpCAM expression and overall survival (p=0.015) (Table 2, Fig. 2). Compared to patients with a weak or no EpCAM expression (≤50%) patients with an EpCAM overexpression (76% to 100%) had a significantly better prognosis (p=0.008). In a multivariate analysis including age, residual tumor after surgery, FIGO, grading, platinum response, and histology EpCAM was shown to be an independent prognostic marker for survival (p=0.022) (Table 3). EpCAM expression had no significant effect on PFS in Kaplan-Meier analysis (Table 4, Fig. 3). However, in a multivariate analysis after adjusting for age, histology, FIGO, grading and residual tumor after surgery it was seen that patients with an EpCAM overexpression have a significantly longer PFS than patients with an EpCAM expression ≤50% (p=0.040; HR, 0.168; 95% CI, 0.031 to 0.924) (Table 5).
Table 2.
EpCAM expression correlated to overall survival (Kaplan-Meier)
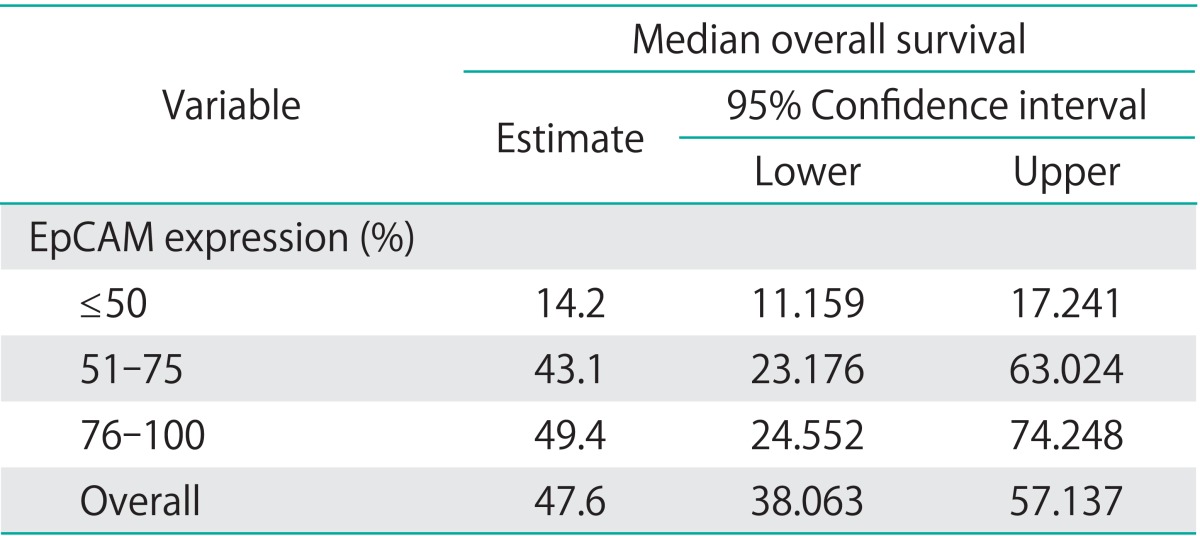
EpCAM, epithelial cell adhesion molecule.
p=0.015
Fig. 2.
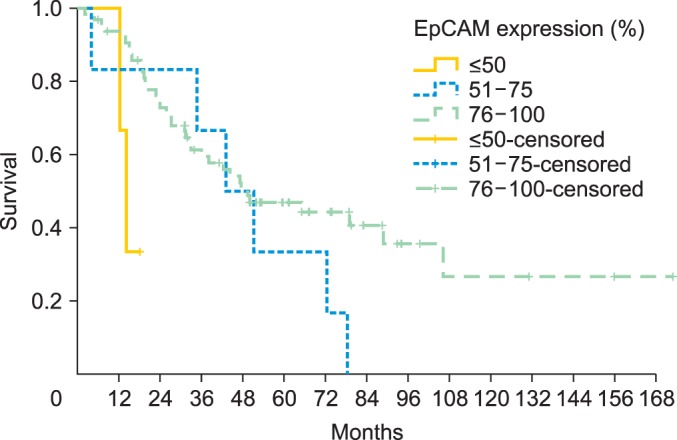
Overall survival illustrated for patients with different epithelial cell adhesion molecule (EpCAM) expression rates (n=74).
Table 3.
Multivariate analysis (Cox regression) for EpCAM and overall survival
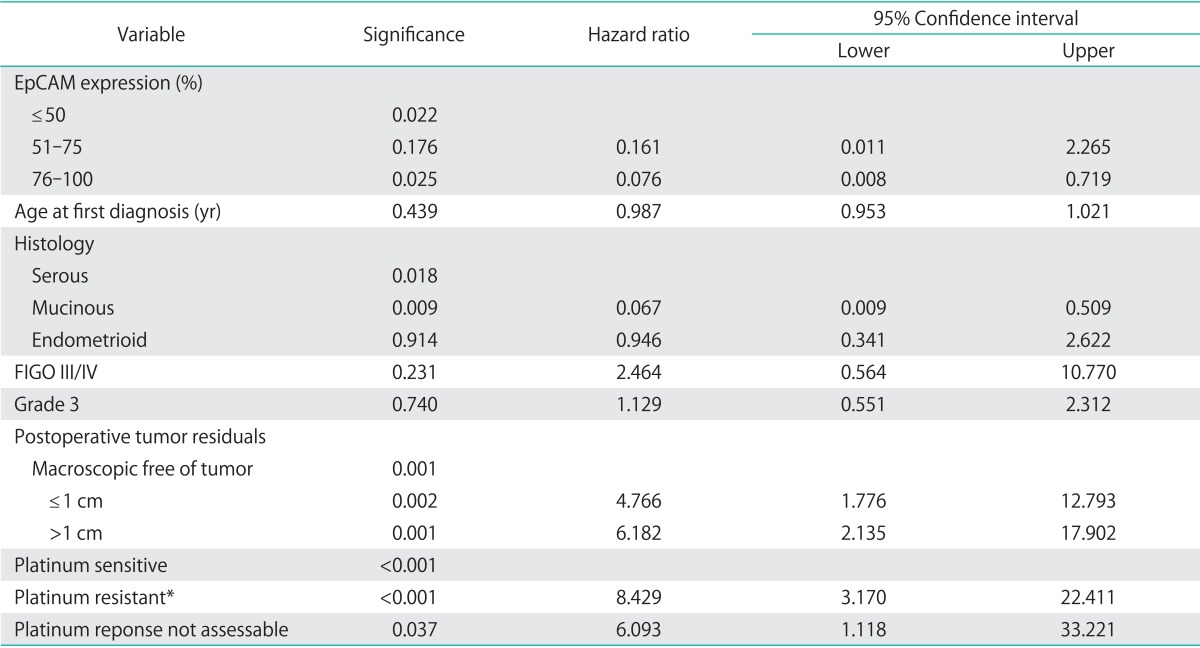
EpCAM, epithelial cell adhesion molecule; FIGO, International Federation of Gynecology and Obstetrics.
*Platinum resistant was defined as recurrence within 6 months after completion of the last cycle of platinum-based chemotherapy.
Table 4.
EpCAM expression correlated to progression free survival (Kaplan-Meier)
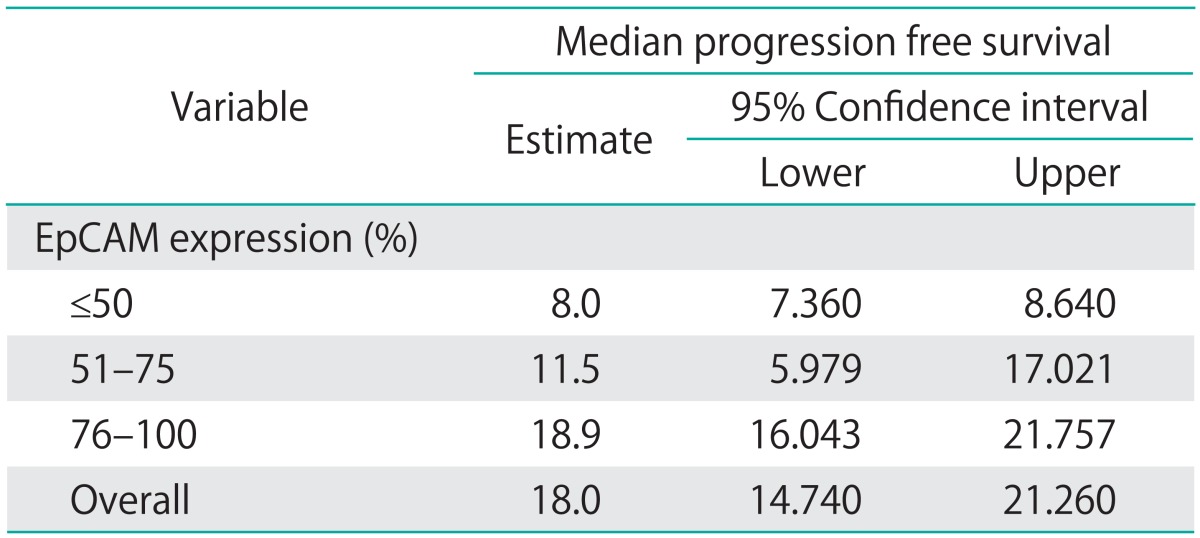
EpCAM, epithelial cell adhesion molecule.
p=0.144.
Fig. 3.
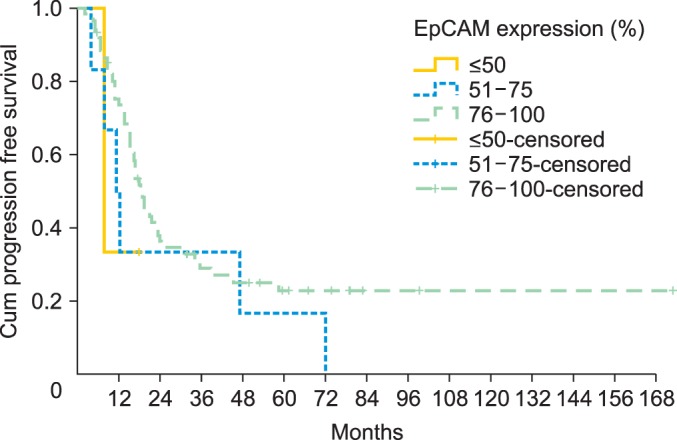
Progression free survival illustrated for patients with different epithelial cell adhesion molecule (EpCAM) expression rates (n=74).
Table 5.
Multivariate analyses (Cox regression) for EpCAM and progression free survival
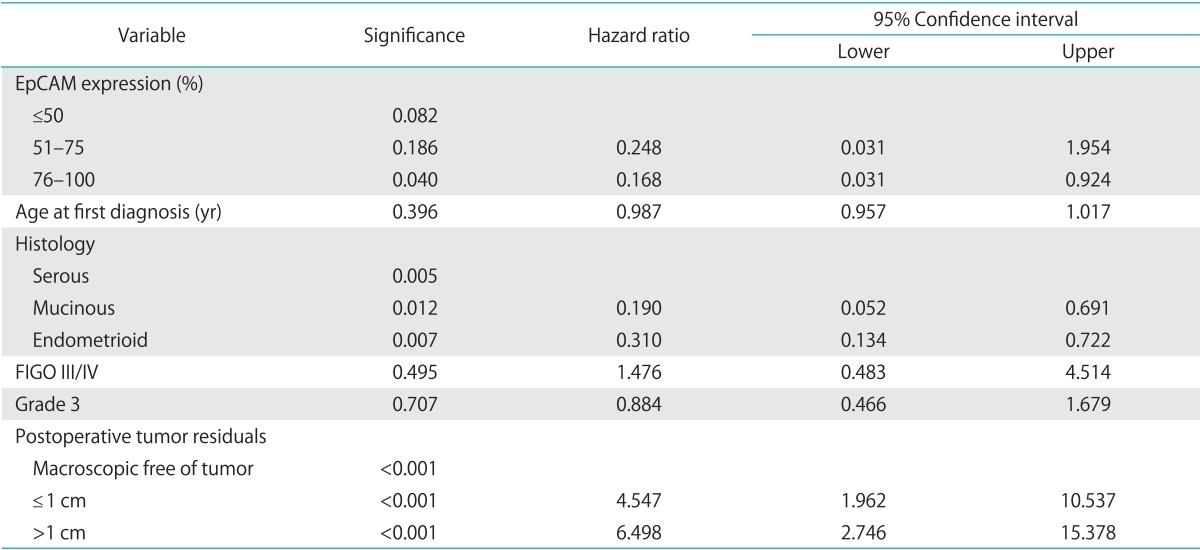
EpCAM, epithelial cell adhesion molecule; FIGO, International Federation of Gynecology and Obstetrics.
Moreover, EpCAM overexpression was associated with a significant higher rate of response to platinum compared to those patients with an EpCAM expression ≤75% (OR, 7.004; 95% CI, 1.017 to 48.248; p=0.048). There was neither a significant correlation of EpCAM expression and tumor residuals in univariate analyses (p=0.713) nor in multivariate analyses (p=0.578).
DISCUSSION
In the present analysis we could identify for the first time a significant association of EpCAM overexpression with a more favorable overall survival and higher response to platinum-based chemotherapy in EOC patients. Furthermore, we could show that serous and endometrioid tumors appeared to present a significantly higher rate of EpCAM overexpressing cells as compared to mucinous tumors.
The role and the clinical implication of EpCAM in carcinogenesis remain unclear. On the one hand, there is data suggesting a positive role of EpCAM in carcinogenesis in translational as well as in clinical research. In vitro analyses might explain the beneficial properties of EpCAM by its function as cell-cell-adhesion molecule due to anticipation of tumor cell distribution [6]. Clinical studies also reported a distinct and significant positive impact of EpCAM overexpression on overall survival for renal cell, gastric, and esophageal carcinoma [8,12,15]. Patients in our collective with EpCAM overexpression had an overall survival of 49.4 months while patients with EpCAM expression ≤50% survived only 14.2 months respectively. The translational and clinical findings mentioned above could be seen as support of this data.
On the other hand, the role of EpCAM is discussed controversially as there are also reports that suggest that EpCAM-related mechanisms can promote tumor growth. Munz et al. [16] reported that EpCAM can upregulate c-myc and induce cell proliferation. Moreover, in vitro data also propose a role of EpCAM in immunosuppression and tumor cell proliferation [17,18]. Clinical studies that report EpCAM overexpression as a negative prognostic marker in breast cancer and carcinoma of the gallbladder, support these in vitro findings [9,10,11]. The fact, that EpCAM was reported as a positive prognostic marker in one tumor entity, while being linked to a negative prognosis in another, might suggest that the role of EpCAM in carcinogenesis is closely tied to the tumor biology of the different entities.
Only few data exist on EpCAM expression in ovarian cancer. To our knowledge, only two studies have analysed the prognostic significance of EpCAM overexpression in EOC [19,20]. Spizzo et al. [19] found a significant correlation of EpCAM overexpression with a worse survival in patients with EOC after primary cytoreduction, while Heinzelmann-Schwarz et al. [20] could not identify a significant impact of overexpression on survival. These findings are contrary to our results. A possible explanation could be heterogeneity clinical and histopathological characteristics of the patients. A comparison between Spizzo's collective of 199 patients and ours, showed a difference in terms of tumor stage (68% and 81.1% with FIGO stages III/IV in the collective of Spizzo compared to ours respectively) and grading (45.7% grade 3 in the study by Spizzo et al. [19] compared to 55.4% in our study), indicating that our collective was composed of more patients with advanced stages and unfavourable prognosis. Also our collective contained more patients with serous histology (40.7% and 55.4% with serous histology in the Spizzo's study and ours, respectively). In Spizzo's study it was not looked at tumor residuals and chemotherapy response. Furthermore, we also found a higher frequency of EpCAM overexpression in our collective than in the study by Spizzo et al. [19] (87.8 % vs. 68.8 %). Despite these diverse characteristics of the patient collectives, overall survival was found to be 46 months in both Spizzo's analysis and our analysis [19].
In our study, EpCAM overexpression did significantly impact overall survival in both Kaplan-Meier and Cox regression analyses. Regarding PFS, indeed, there was a significant impact of EpCAM overexpression in Cox regression analysis. This might explain the effect of overexpression on response to platinum based chemotherapy, which is, linked to PFS. Since histology had a strong influence on PFS, univariate survival analysis with the Kaplan-Meier method was not able to demonstrate a significant association of EpCAM overexpression with a longer PFS. The multivariate Cox regression model, however, that included histology in the analysis was therefore capable to show a significant impact of EpCAM overexpression on PFS despite the relatively small collective.
Another interesting finding of our analysis was the fact that mucinous histology was linked to better overall survival. This result was surprising since the mucinous subtype is mostly described to have a negative impact on prognosis. The trend for a better survival of mucinous tumors could be explained by the tumor characteristics of our mucinous tumors. As described in the results section, these tumors have a less-advanced tumor stage, a rarer lymph node involvement and a lower rate of G3 tumors. These differences are coincidental and can be drawn back to selection bias in our randomly selected sample. Based on the limited number of patients, our results have to be interpreted with caution. Nevertheless, the role of EpCAM expression in mucinous ovarian cancers is unclear and is warranted to be examined.
One of the major limitation of this study is the restricted amount of patients (n=74), making this study an exploratory analysis. The low rate of tumors without EpCAM overexpression in our collective (n=9) exposes our analyses to the risk of selection bias since the tumor samples, that were evaluated, were randomly chosen. To overcome this limitation and to minimize selection bias, a larger trial is warranted after this initial exploratory analysis.
In the light of new evolving targeted therapies such as catumaxomab and adecatumumab that use EpCAM as the main binding structure, it is necessary to further understand the role of EpCAM in ovarian cancer [4,21]. The high rate of expression noted in our analysis supports the rationale for anti-EpCAM treatment in this tumor entity.
In conclusion, our results indicate that EpCAM overexpression might be a prognostic marker in EOC, as well as a predictive factor for response to platinum-based chemotherapy.
Footnotes
Parts of the results have been chosen for abstract publication only related to the ASCO 2012: Woopen H, Pietzner K, Richter R, Fotopoulou C, Joens T, Braicu I, et al. Prognostic value of epithelial cell adhesion molecule (EpCAM) in patients with primary epithelial ovarian cancer (abstract). J Clin Oncol 2012;30(Suppl):e15531.
Dr. Horst Lindhofer is CEO of TRION Research GmbH. All remaining authors have declared no conflicts of interest.
References
- 1.Ogura E, Senzaki H, Yoshizawa K, Hioki K, Tsubura A. Immunohistochemical localization of epithelial glycoprotein EGP-2 and carcinoembryonic antigen in normal colonic mucosa and colorectal tumors. Anticancer Res. 1998;18(5B):3669–3675. [PubMed] [Google Scholar]
- 2.Xie X, Wang CY, Cao YX, Wang W, Zhuang R, Chen LH, et al. Expression pattern of epithelial cell adhesion molecule on normal and malignant colon tissues. World J Gastroenterol. 2005;11:344–347. doi: 10.3748/wjg.v11.i3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 4.Sebastian M, Passlick B, Friccius-Quecke H, Jager M, Lindhofer H, Kanniess F, et al. Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM x anti-CD3): a phase I study. Cancer Immunol Immunother. 2007;56:1637–1644. doi: 10.1007/s00262-007-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Gun BT, Melchers LJ, Ruiters MH, de Leij LF, McLaughlin PM, Rots MG. EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis. 2010;31:1913–1921. doi: 10.1093/carcin/bgq187. [DOI] [PubMed] [Google Scholar]
- 7.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura H, Kato H, Faried A, Sohda M, Nakajima M, Fukai Y, et al. Prognostic significance of EpCAM expression in human esophageal cancer. Int J Oncol. 2007;30:171–179. [PubMed] [Google Scholar]
- 9.Spizzo G, Went P, Dirnhofer S, Obrist P, Simon R, Spichtin H, et al. High Ep-CAM expression is associated with poor prognosis in node-positive breast cancer. Breast Cancer Res Treat. 2004;86:207–213. doi: 10.1023/B:BREA.0000036787.59816.01. [DOI] [PubMed] [Google Scholar]
- 10.Varga M, Obrist P, Schneeberger S, Muhlmann G, Felgel-Farnholz C, Fong D, et al. Overexpression of epithelial cell adhesion molecule antigen in gallbladder carcinoma is an independent marker for poor survival. Clin Cancer Res. 2004;10:3131–3136. doi: 10.1158/1078-0432.ccr-03-0528. [DOI] [PubMed] [Google Scholar]
- 11.Gastl G, Spizzo G, Obrist P, Dunser M, Mikuz G. Ep-CAM overexpression in breast cancer as a predictor of survival. Lancet. 2000;356:1981–1982. doi: 10.1016/S0140-6736(00)03312-2. [DOI] [PubMed] [Google Scholar]
- 12.Seligson DB, Pantuck AJ, Liu X, Huang Y, Horvath S, Bui MH, et al. Epithelial cell adhesion molecule (KSA) expression: pathobiology and its role as an independent predictor of survival in renal cell carcinoma. Clin Cancer Res. 2004;10:2659–2669. doi: 10.1158/1078-0432.ccr-1132-03. [DOI] [PubMed] [Google Scholar]
- 13.Sehouli J, Konsgen D, Mustea A, Oskay-Ozcelik G, Katsares I, Weidemann H, et al. "IMO": intraoperative mapping of ovarian cancer. Zentralbl Gynakol. 2003;125:129–135. doi: 10.1055/s-2003-41864. [DOI] [PubMed] [Google Scholar]
- 14.Bellone S, Siegel ER, Cocco E, Cargnelutti M, Silasi DA, Azodi M, et al. Overexpression of epithelial cell adhesion molecule in primary, metastatic, and recurrent/chemotherapy-resistant epithelial ovarian cancer: implications for epithelial cell adhesion molecule-specific immunotherapy. Int J Gynecol Cancer. 2009;19:860–866. doi: 10.1111/IGC.0b013e3181a8331f. [DOI] [PubMed] [Google Scholar]
- 15.Songun I, Litvinov SV, van de Velde CJ, Pals ST, Hermans J, van Krieken JH. Loss of Ep-CAM (CO17-1A) expression predicts survival in patients with gastric cancer. Br J Cancer. 2005;92:1767–1772. doi: 10.1038/sj.bjc.6602519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munz M, Kieu C, Mack B, Schmitt B, Zeidler R, Gires O. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene. 2004;23:5748–5758. doi: 10.1038/sj.onc.1207610. [DOI] [PubMed] [Google Scholar]
- 17.Osta WA, Chen Y, Mikhitarian K, Mitas M, Salem M, Hannun YA, et al. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64:5818–5824. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- 18.Gutzmer R, Li W, Sutterwala S, Lemos MP, Elizalde JI, Urtishak SL, et al. A tumor-associated glycoprotein that blocks MHC class II-dependent antigen presentation by dendritic cells. J Immunol. 2004;173:1023–1032. doi: 10.4049/jimmunol.173.2.1023. [DOI] [PubMed] [Google Scholar]
- 19.Spizzo G, Went P, Dirnhofer S, Obrist P, Moch H, Baeuerle PA, et al. Overexpression of epithelial cell adhesion molecule (Ep-CAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancer. Gynecol Oncol. 2006;103:483–488. doi: 10.1016/j.ygyno.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry J, Scolyer RA, Davies MJ, et al. Overexpression of the cell adhesion molecules DDR1, Claudin 3, and Ep-CAM in metaplastic ovarian epithelium and ovarian cancer. Clin Cancer Res. 2004;10:4427–4436. doi: 10.1158/1078-0432.CCR-04-0073. [DOI] [PubMed] [Google Scholar]
- 21.Naundorf S, Preithner S, Mayer P, Lippold S, Wolf A, Hanakam F, et al. In vitro and in vivo activity of MT201, a fully human monoclonal antibody for pancarcinoma treatment. Int J Cancer. 2002;100:101–110. doi: 10.1002/ijc.10443. [DOI] [PubMed] [Google Scholar]


