Abstract
Objective
Water-tight closure of the dura in extracranial-intracranial (EC-IC) bypass is impossible because the superficial temporal artery (STA) must run through the dural defect. Consequently, subdural hygroma and subcutaneous cerebrospinal fluid (CSF) collection frequently occur postoperatively. To reduce these complications, we prospectively performed suturing of the arachnoid membrane after STA-middle cerebral artery (STA-MCA) and evaluated the clinical usefulness.
Materials and Methods
Between Mar. 2005 and Oct. 2010, extracranial-intracranial arterial bypass (EIAB) with/without encephalo-myo-synangiosis was performed in 88 cases (male : female = 53 : 35). As a control group, 51 patients (57 sides) underwent conventional bypass surgery without closure of the arachnoid membrane. Postoperative computed tomography (CT) scan was performed twice in three days and seven days later, respectively, for evaluation of the presence of subdural fluid collection and other mass lesions.
Results
The surgical result was excellent, with no newly developing ischemic event until recent follow-up. The additional time needed for arachnoid suture was five to ten minutes, when three to eight sutures were required. Post-operative subdural fluid collection was not seen on follow-up computed tomography scans in all patients.
Conclusion
Arachnoid suturing is simple, safe, and effective for prevention of subdural fluid collection in EC-IC bypass surgery, especially the vulnerable ischemic hemisphere.
Keywords: Arachnoid suture, Extracranial-intracranial bypass surgery, Subdural hygroma, Mass effect
INTRODUCTION
Subdural hygromas, which are subdural collections of fluid such as cerebrospinal fluid (CSF), can occur under variable intracranial conditions, such as infection, congenital malformation, overdrainage of the shunt system, craniofacial disproportions, post-spinal anesthesia and tearing of arachnoid membrane due to head trauma, and intracranial procedure/operation.10) This lesion can cause headache and/or ischemic symptoms because of increased intracranial pressure (ICP) and compression of brain parenchyma and/or vascular structure such as vein of Labbe.2) This symptomatic subdural hygroma can be managed by surgical drainage (craniotomy, shunt and/or burr hole drainage) and spinal blood patch.10)
Tearing of arachnoid membrane, which is one step in extracranial-intracranial artery bypass (EIAB) for direct revascularization, causes continuous leakage of CSF into subdural space (Fig. 1) and has the potential of symptomatic subdural hygroma and hematoma.8) In this paper, two cases which manifested as cerebral ischemia due to subdural fluid collection after EIAB were reported and a new surgical technique was introduced for prevention of this complication.
Fig. 1.
Cerebrospinal fluid (CSF) leakage after tearing of arachnoid membrane for confirming recipient artery.
MATERIALS AND METHODS
Between Mar. 2005 and Oct. 2010, EIAB with/without encephalo-myo-synangiosis was performed in 88 cases (male : female = 53 : 35). Mean age of patients was 52.5 years old (13 - 77 years old). The indications for superficial temporal artery-middle cerebral artery (STA-MCA) bypass surgery were hemodynamic compromise due to atherosclerotic stenoocclusive cerebrovascular disease in 65 cases and moyamoya disease in 23 cases. EIAB with suturing for subarachnoid membrane was performed in 31 cases between Sep. 2005 and Jun. 2006. By contrast, EIAB without suturing was performed during the other period in 57 cases.
Postoperative computed tomography scan was performed at three, seven days for evaluation of the presence of subdural fluid collection and other mass lesions such as epidural hematoma. All patients underwent surgery performed by a single surgeon at one institute.
RESULTS
The diagnosis was determined by ischemic or hemorrhagic event. EMS was performed with EIAB in 15 cases, and clipping for the unruptured aneurysm was performed with bypass surgery in two cases. There was no intraoperative problem such as leakage or anestheologic problem. The intact flow of the bypass site was checked by trans-cranial Doppler (TCD).
Occlusion or rupture of the bypass artery did not occur in any cases. In two cases, postoperative brain CT showed epidural hematoma. Surgical management was required in one case. In two cases, new cerebral infarction was detected on the site, which was unrelated to preoperative ischemic event. None of the cases required surgical intervention, however, deterioration of neurology was observed. Subdural hygroma was detected on postoperative brain CT in 30 cases. Among them, 24 cases (80%) belonged to the group without suturing for subarachnoid membrane. Three cases (10%) showed symptoms related to mass effect of subdural fluid collection such as hemiparesis or deterioration of mental status. Among three cases, surgical management was required in two cases. All patients recovered to preoperative status.
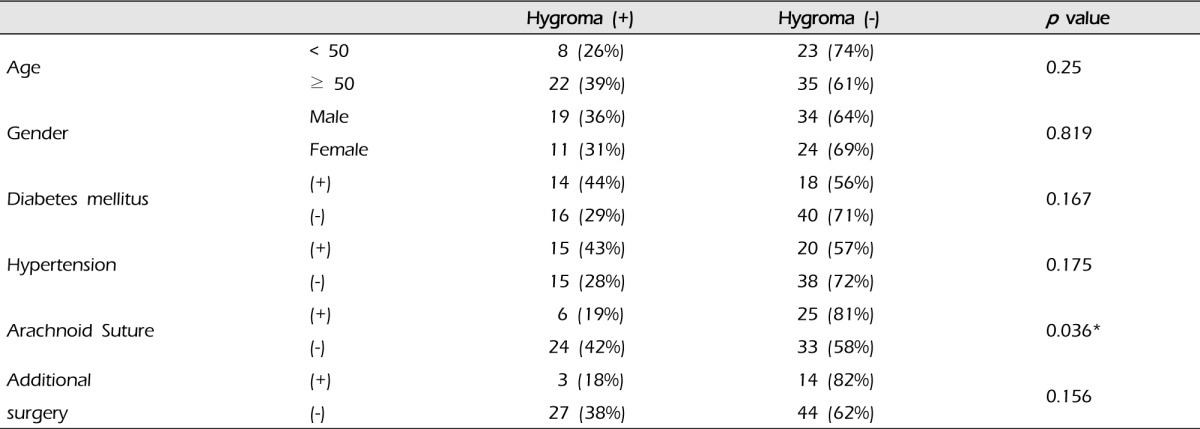
The relationship of postoperative subdural hygroma with several factors was evaluated: age, gender, diabetes milletus, hypertension, and additional surgical intervention except EIAB and intraoperative arachnoid suture (Table 1). The group with arachnoid suture (31 cases) had six (19%) subdural hygromas compared with 24 cases (38%) in the group without that (57 cases) (p = 0.036). In addition, all subdural hygromas that caused neurological symptoms occurred in the group without arachnoid suture. Additional surgical intervention such as EMS did not increase the risk of subdural hygroma (p = 0.156), and the others did not show statistical significance (Table 1).
Table 1.
Relationships between subdural hygroma and several factors
CASE REPORTS
Illustrative cases
Case I
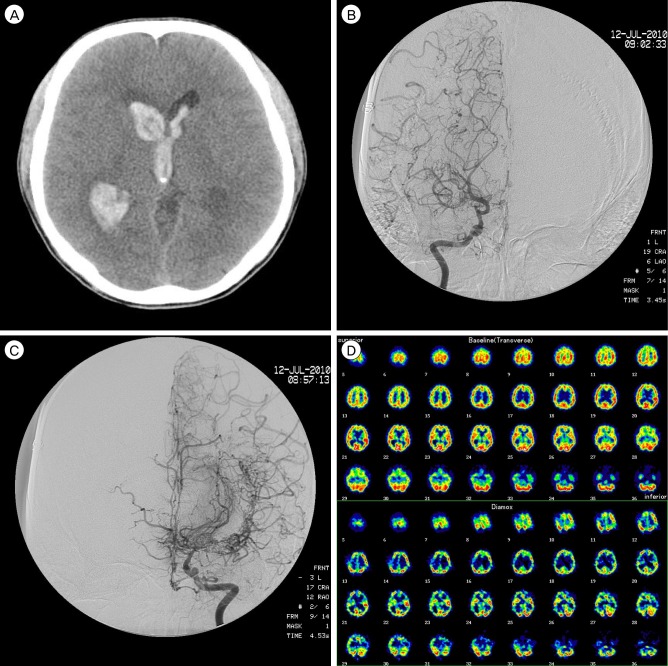
A 63-year-old woman, who had taken antihypertensive medications for seven years, complained of intermittent motor aphagia with right hemiparesis (Grade IV/III) for one month. On admission at a local hospital, brain CT showed a low attenuated lesion on the left caudate nucleus and basal ganglion, and brain magnetic resonance image (MRI) showed a high signal on a T2 weight image (T2WI) and a low signal on a T1 weight image (T1WI), suspicious chronic cerebral infarction (Fig. 2A). For evaluation of this lesion, neck CT angiography (CTA) was performed and showed left internal carotid artery (ICA) stenosis, which was shown on ultrasonography with color Doppler to the neck. Brain single proton emission computed tomography (SPECT) showed the perfusion defect on the area of both ICAs, especially left (Fig. 2B). Digital subtraction angiography (DSA) showed the obstruction of the left ICA, luminal irregularity of the right ICA and right vertebral artery (VA) (Fig. 2C).
Fig. 2.
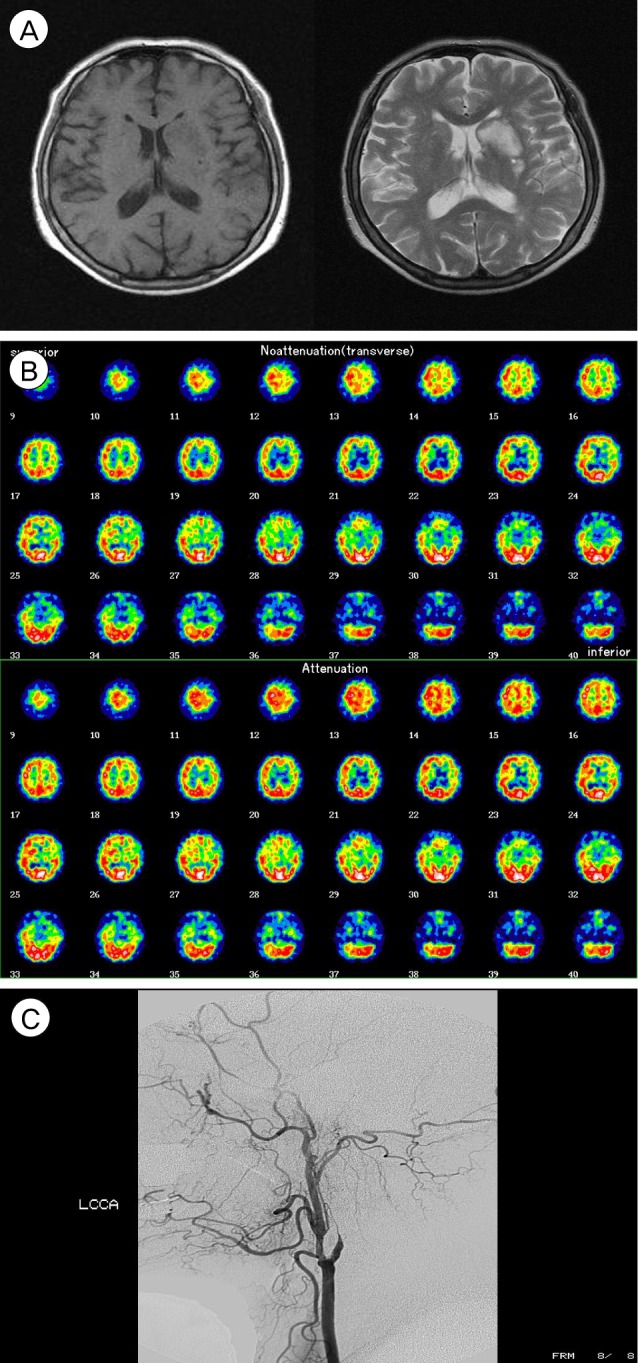
(A) Brain magnetic resonance imaging (MRI) shows a high signal on a T2 weight image (T2WI) and a low signal on T1WI. (B) Brain single proton emission computed tomography (SPECT) shows perfusion defect on the left internal carotid artery (ICA) territories, especially in the left frontal lobe. (C) Digital subtraction angiography (DSA) shows the obstruction of the left ICA and non-visualization of the left ICA branches such as the anterior cerebral artery and middle cerebral artery (MCA).
Two months from the onset of symptoms, the operation was performed. Direct anastomaosis between STA and angular branch of distal MCA was performed uneventfully for the obstruction of the left ICA.
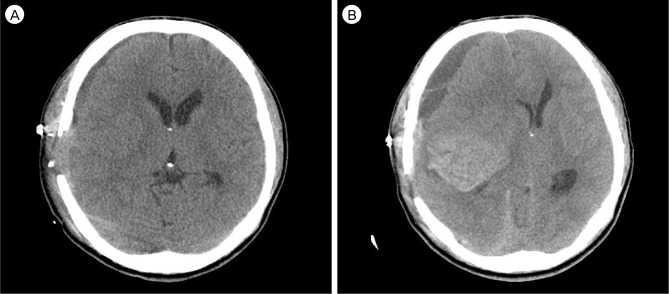
The neurological examination showed no change compared with that of the preoperative state. On the fourth postoperative day (POD), she complained of sudden motor aphagia and deteriorated right hemiparesis (Grade IV/II). Brain CTA showed subdural hygroma on the left fronto-temporal area with midline deviation (approximately 8 mm) and intact bypass artery (Fig. 3A). Emergency trephination with one burr-hole for subdural hygroma was performed under local anesthesia. The yellowish fluid was drained through the silicon catheter used for trephination. After drainage through the trephination tip for three days, her neurological status recovered to its preoperative state (Grade IV/III) and the silicon catheter for trephination was removed after checking the brain CT, which showed a decrease of subdural hygroma and correction of midline deviation (Fig. 3B).
Fig. 3.
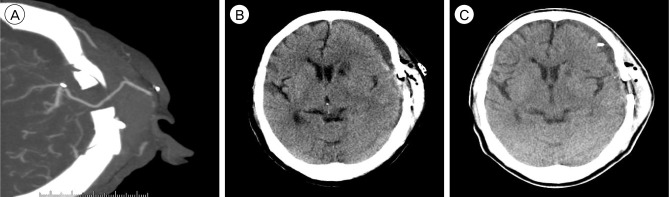
Brain computed tomography angiography (CTA) shows an intact bypass artery (A) and subdural fluid collection (B). (C) Brain CT after trephination shows greater decrease of subdural fluid collection.
Case II
A 35-year-old, right-handed male without significant past medical history presented with confused mentation and headache. Prior to this, he was asymptomatic, and had normal developmental milestones. Initial unenhanced brain CT showed all ventricular intraventricular hemorrhage (Fig. 4A), therefore, extraventricular drainage was performed immediately. He showed gradual improvement, and, after two months, was completely asymptomatic.
Fig. 4.
(A) Brain CT shows all ventricular intraventricular hemorrhage. (B) Right ICA angiography shows occlusion at the supraclinoid portion with prominent collateral arteries. (C) Left ICA angiography also shows MCA occlusion. (D) Resting hypoperfusion and decreased vascular reserve in both MCA territories are observed in SPECT.
DSA was performed. Right ICA angiography showed an occlusion at the supraclinoid portion with prominent collateral arteries (Fig. 4B). Left ICA angiography also showed middle cerebral artery (MCA) occlusion (Fig. 4C). Resting hypoperfusion and decreased vascular reserve in both MCA territories were observed in SPECT (Fig. 4D). The patient was diagnosed with moyamoya disease. Right-side superficial temporal artery MCA anastomosis was performed.
After surgery, the neurological status was the same with preoperative status. Brain CT showed subdural hematoma on the right frontotemporoparietal region. However, five days later, the patient presented with sudden comatose mentation. He had isocoria with dilation of both pupils and showed evidence of decorticate posturing to painful stimulation. CT showed a newly developed acute intracerebral hemorrhage in the right temporal lobe and increased subdural hygroma in the right frontal area (Fig. 5B).
Fig. 5.
(A) Brain CT shows subdural hematoma on the right frontotemporoparietal region. (B) Newly developed acute intracerebral hemorrhage in the right temporal lobe and increased subdural hygroma in the right frontal area.
DISCUSSION
Cerebral ischemia with structural abnormality such as moyamoya disease, occlusion of MCA or ICA can be managed with the operation for cerebral revascularization. EIAB, which was introduced by Yasargil, is known as the standard surgical procedure with indirect pial synangiosis such as encephalo-myo-synangiosis (EMS).3) Despite the favorable outcome of revascularization surgery, there are several complications after EIAB skin necrosis around the donor artery, infection, perioperative cerebral infarction, headache, nausea, seizure, newly developed cerebral infarction or neurological deficit, postoperative intracranial hemorrhage, and subdural fluid collection.1),3),4)
Postoperative neurological deficit was reported at the rate of 1.9 to 8.8% of cases in adults with moyamoya disease,7) however, it was reported in 4 to 20% of patients who underwent EIAB procedures.4) According to Kuroda et al.,5) some studies in the literature have reported that postoperative neurological deficit can be caused by an unknown mechanism.4) Ohue et al.7) suggested that the postoperative neurological deficit can be caused by hyperfusion rather than ischemic changes using image study such as brain SPECT and MRI. And, in some cases, some factors other than hyperfusion may be related to the postoperative neurological deficit.7) The incidence of chronic subdural hematoma (CSDH) after the operation was reported as 4.8% in patients with stroke and 6.7% in patients with moyamoya disease.1) Andoh et al. reported that some cases have a high risk for CSDH in patients with brain atrophy, postoperative air in the subdural space and subdural effusion.1) Subdural hygroma caused by tearing of the arachnoid membrane has a potential risk for compression of the brain like swollen temporal muscle in EMS reported by Fujimura et al.3) In addition, theoretically, the mass effect can cause mechanical tearing of the suture site between donor and recipient artery due to tension caused by thickness of collected fluid. Favorable outcomes were reported for postoperative CSDH, except for one death.6) Therefore, discussion about prevention of postoperative subdural fluid collection is very important and worthwhile.
In general, if postoperative neurological deficit appears, the operator will consider of cerebral ischemia or hyperperfusion during acute stage after surgery.3) These complications were reported in many literature studies and prevented with several introduced methods such as the strict control of blood pressure to normotension or slight hypotension against cerebral hyperperfusion or medications of antiplatelet therapy against embolic ischemic events.4),7),9) However, discussion for prevention of postoperative CSDH is rare.
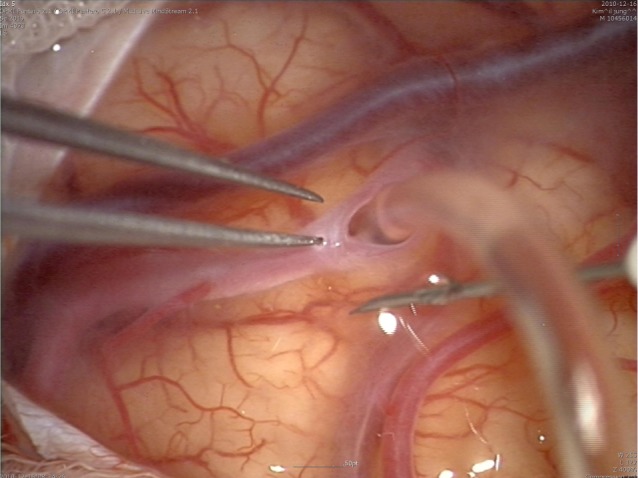
To prevent this complication, we introduce suturing of arachnoid membrane (Fig. 6). This procedure can be performed with suturing between torn arachnoid membrane for finding of recipient artery with three to eight times suturing using 10-0 nylon. Closure can prevent leakage of CSF into subdural space. In addition, the procedure can prevent kinking of a bypass artery with anchoring of a bypass artery with sutured arachnoid membrane.
Fig. 6.
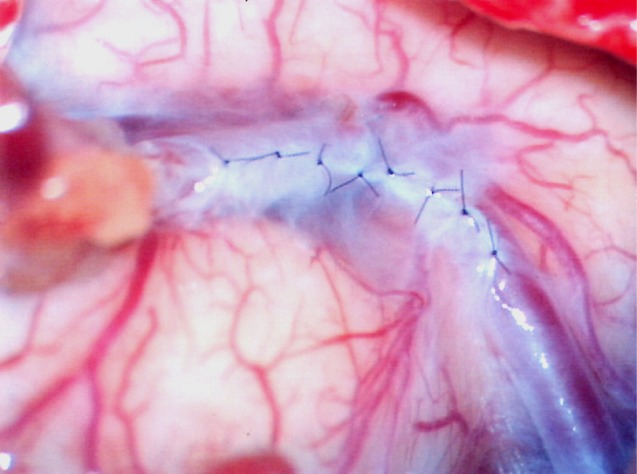
Intraoperative finding of arachnoid suturing three times using 10-0 nylon.
CONCLUSION
Results of our study showed that suturing of arachnoid membrane during EIAB is simple, safe, and effective for prevention of subdural fluid collection postoperatively and can be easily performed within 10 minutes by an experienced bypass surgeon. Therefore, we suggest that this intraoperative procedure is worthy of wide use.
References
- 1.Andoh T, Sakai N, Yamada H, Yano H, Hirayama H, Imao Y, et al. Chronic subdural hematoma following bypass surgery-report of three cases. Neurol Med Chir (Tokyo) 1992 Aug;32(9):684–689. doi: 10.2176/nmc.32.684. [DOI] [PubMed] [Google Scholar]
- 2.Das K, Murali R, Lindstrom CJ, Couldwell WT. Symptomatic subdural hygroma and temporal lobe edema after translabyrinthine removal of acoustic neuroma. Skull Base. 2001 May;11(2):137–142. doi: 10.1055/s-2001-14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujimura M, Kaneta T, Shimizu H, Tominaga T. Cerebral ischemia owing to compression of the brain by swollen temporal muscle used for encephalo-myo-synangiosis in moyamoya disease. Neurosurg Rev. 2009 Apr;32(2):245–249. doi: 10.1007/s10143-009-0184-6. discussion 249. [DOI] [PubMed] [Google Scholar]
- 4.Furuya K, Kawahara N, Morita A, Momose T, Aoki S, Kirino T. Focal hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease. Case report. J Neurosurg. 2004 Jan;100(1):128–132. doi: 10.3171/jns.2004.100.1.0128. [DOI] [PubMed] [Google Scholar]
- 5.Kuroda S, Kamiyama H, Abe H, Asaoka K, Mitsumori K. Temporary neurological deterioration caused by hyperperfusion after extracranial-intracranial bypass-case report and study of cerebral hemodynamics. Neurol Med Chir (Tokyo) 1994 Jan;34(1):15–19. doi: 10.2176/nmc.34.15. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa Y, Shimoyama M, Kashiwaba T, Suzuki Y, Gotoh S, Miyasaka K, et al. [Reconstructive operation of Moyamoya disease and its problems] No Shinkei Geka. 1981;9(3):305–314. Japanese. [PubMed] [Google Scholar]
- 7.Ohue S, Kumon Y, Kohno K, Watanabe H, Iwata S, Ohnishi T. Postoperative temporary neurological deficits in adults with moyamoya disease. Surg Neurol. 2008 Mar;69(3):281–286. doi: 10.1016/j.surneu.2007.01.047. discussion 286-7. [DOI] [PubMed] [Google Scholar]
- 8.Sonobe M, Takahashi S, Kubota Y, Shirane R. [Chronic subdural hematoma developing after EMS for moyamoya disease] No Shinkei Geka. 1982 Aug;10(8):857–859. Japanese. [PubMed] [Google Scholar]
- 9.Uno M, Nakajima N, Nishi K, Shinno K, Nagahiro S. Hyperperfusion syndrome after extracranial-intracranial bypass in a patient with moyamoya disease-case report. Neurol Med Chir (Tokyo) 1998 Jul;38(7):420–424. doi: 10.2176/nmc.38.420. [DOI] [PubMed] [Google Scholar]
- 10.Verdu MT, Martinez-Lage JF, Alonso B, Sanchez-Ortega JL, Garcia-Candel A. Non-surgical management of intracranial subdural hematoma complicating spinal anesthesia. Neurocirugia (Astur) 2007 Feb;18(1):40–43. [PubMed] [Google Scholar]