Abstract
Marked elevations of B-type natriuretic peptide (BNP) are not generally seen in patients with heart failure and preserved ejection fraction (HFpEF). The objective of this study was to examine the clinical and laboratory characteristics of a large cohort of patients with HFpEF and markedly elevated BNP. A retrospective examination of 421 inpatients at a university hospital admitted with a diagnosis of HFpEF was performed. Clinical and echocardiographic data in 4 groups of patients with levels of BNP ≤ 100 pg/mL, 100–400 pg/mL, 400–1,000 pg/mL and > 1,000 pg/mL were compared. Patients with HFpEF and BNP > 1,000 pg/mL (28% of the population) were characterized by impaired renal function and greater use of anti-hypertensive medications. A subset of these patients with BNP > 1,000 pg/mL had normal renal function (21%) and were significantly older, more frequently female, and tended to have lower ejection fractions. Conversely, patients with HFpEF and BNP ≤ 100 pg/mL were younger and had preserved renal function. BNP was inversely related to the likelihood of subsequent admission for heart failure, but not to myocardial infarction or death. In conclusion: BNP > 1,000 pg/mL is seen in almost 1/3 of patients hospitalized with HFpEF. This elevation of BNP often reflects impaired renal function, but can also be seen in patients with preserved renal function but relatively impaired systolic function.
Keywords: B-type natriuretic peptide, diastolic heart failure, chronic kidney disease
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is becoming a more common diagnosis as the prevalence of patients with hypertension, diabetes, chronic kidney disease (CKD) and advancing age increases. HFpEF is now the cause of clinical heart failure in approximately 50% of patients, is a frequent cause of hospitalization, and is associated with significant morbidity and mortality[1]. The diagnosis of HFpEF depends on the clinical diagnosis of heart failure in the setting of preserved ejection fraction, usually with an ejection fraction > 45%[1]. In addition to clinical assessment, the severity of heart failure is often assessed by measuring B-type natriuretic peptide (BNP), a peptide hormone released by cardiomyocytes in response to increased wall stress[2]. In contrast to heart failure with impaired systolic function (EF ≤ 45%) where serum levels of BNP are often > 1,000 pg/mL in patients with severe dysfunction[3], the levels of BNP in HFpEF tend to be lower, with mean values in the literature reported in the range of 400–500 pg/mL[2]. However, BNP values > 1,000 can now be seen in HFpEF[4] and may denote a worse prognosis. In order to characterize and identify these patients, the purpose of this study was to examine the clinical and echocardiographic characteristics of patients with markedly elevated BNP.
PATIENTS AND METHODS
Patients and patient selection
This study was approved by the Institutional Review Board of the University of California Davis.
Patients admitted to the University of California Davis Medical Center between 7/1/2010 and 6/30/2011 with a diagnosis of HFpEF (ICD9 code 428) were examined. The diagnosis of HFpEF was made by the hospital attending physician and was based on clinical signs and symptoms of heart failure with imaging evidence of preserved left ventricular ejection fraction (>45%) by echocardiography. Demographic, clinical, and echocardiographic data were obtained from chart review of the electronic medical record and the echocardiographic data base. The characteristics of the patients are shown in Table 1. A total of 421 patients met entry criteria, and were analyzed based on the maximum BNP values obtained during the index hospitalization. BNP was measured using an immunoenzymatic assay (AlereInc, Waltham MA). Concurrent echocardiography data were obtained including left ventricular ejection fraction, chamber dimensions, left ventricular mass and E/A ratio. These measurements were derived from the echocardiographic images using American Society of Echocardiography standards[5]. Cardiovascular outcomes (death, myocardial infarction, and rehospitalization for heart failure) were recorded for a mean of 2.7 years.
Table 1. Patient characteristics by BNP category [mean (SD)].
| BNP ≤ 100(n = 54) | BNP > 100≤ 400(n = 110) | BNP > 400≤ 1,000(n = 140) | BNP > 1,000(n = 117) | P-value | |
| Age (years) | 61.4 (12.5) | 69.5 (14) | 69.5 (14.6) | 68 (16.2) | 0.005F |
| Gender | |||||
| Female | 27 (50%) | 63 (57.3%) | 85 (60.7%) | 69 (59%) | 0.561C |
| Male | 27 (50%) | 47 (42.7%) | 55 (39.3%) | 48 (41%) | |
| Creatinine (mg/dL) | 1.5 (1.1) | 1.5 (1.2) | 1.9 (2.1) | 2.7 (2.4) | <0.001L |
| BUN (g/dL) | 26.7 (21.8) | 26.5 (19) | 29.2 (19.4) | 33.9 (21.8) | 0.004L |
| Glucose (mg/dL) | 144.7 (68.5) | 139.7 (55.6) | 139.3 (66.9) | 144.2 (69.9) | 0.908L |
| Hematocrit (%) | 37.5 (5.6) | 35.2 (5.6) | 33.8 (6.6) | 33.6 (6.7) | 0.001F |
| Hemoglobin (g/dL) | 12.4 (1.9) | 11.6 (1.9) | 11.2 (2.2) | 11.1 (2.2) | 0.001F |
| Troponin (ug/L) | 0 (0) | 0 (0.1) | 0.1 (0.6) | 0.2 (0.9) | <0.001L |
| CKD IV or V | 7 (13%) | 19 (17%) | 37 (26%) | 50 (43%) | <0.001c |
| Dialysis | 0 | 1 (1.9%) | 8 (5.7%) | 21 (17.5%) | < 0.001c |
| eGFR (mL/minute/m2) | 68.5 (31.6) | 61.8 (33.1) | 54.1 (29.8) | 40.4 (27.2) | <0.001L |
| Echocardiographic measures | |||||
| LVEF (%) | 59.8 (6.6) | 60.3 (7.9) | 61.1 (6.7) | 58.2 (8) | 0.139F |
| RA (mm) | 41.5 (9.3) | 41.4 (8.7) | 42.1 (9.6) | 42.9 (10.8) | 0.767F |
| LA (mm) | 43.5 (7.5) | 42.3 (7.6) | 42.7 (7.9) | 44.7 (8.4) | 0.146F |
| AO (mm) | 32 (4.3) | 32.7 (5.1) | 31.5 (6.5) | 32.2 (5.5) | 0.506F |
| RVD (mm) | 37.3 (10.2) | 38.9 (9.9) | 39.1 (8.7) | 37.8 (12.2) | 0.672F |
| LVD (mm) | 47.8 (7.7) | 47.8 (6.9) | 48.4 (8.5) | 46.7 (8.2) | 0.529F |
| LVS (mm) | 31.8 (8.2) | 31.7 (7.2) | 32.5 (9.3) | 31 (8.8) | 0.676F |
| Fractional shortening | 34 (9) | 33.9 (10.2) | 33.6 (10.6) | 35.3 (12.6) | 0.725F |
| IVS (mm) | 12.4 (2.9) | 12.5 (3.2) | 11.9 (2.8) | 12.4 (2.7) | 0.456L |
| EA ratio | 1.2 (0.5) | 1.1 (0.6) | 1.1 (0.5) | 1.4 (0.7) | 0.083L |
| LV mass (gm) | 277 (105.7) | 280.5 (111.2) | 266.4 (102.5) | 270.9 (107) | 0.808L |
| Medication use | |||||
| ACE inhibitor | 30 (55.6%) | 74 (67.3%) | 95 (67.9%) | 74 (63.2%) | 0.389C |
| ARB | 13 (24.1%) | 26 (23.6%) | 30 (21.4%) | 30 (25.6%) | |
| Aspirin | 40 (74.1%) | 84 (76.4%) | 113 (80.7%) | 88 (75.2%) | 0.662C |
| Beta blocker | 33 (61.1%) | 74 (67.3%) | 103 (73.6%) | 80 (68.4%) | 0.376C |
| CCB (DHP) | 21 (38.9%) | 51 (46.4%) | 71 (50.7%) | 69 (59%) | 0.071C |
| CCB (non-DHP) | 17 (31.5%) | 28 (25.5%) | 39 (27.9%) | 28 (23.9%) | 0.736C |
| Clonidine | 9 (16.7%) | 17 (15.5%) | 24 (17.1%) | 31 (26.5%) | 0.133C |
| Diabetes medication | 27 (50%) | 65 (59.1%) | 85 (60.7%) | 61 (52.1%) | 0.370C |
| Digoxin | 12 (22.2%) | 20 (18.2%) | 30 (21.4%) | 18 (15.4%) | 0.585C |
| Hydralazine | 18 (33.3%) | 46 (41.8%) | 58 (41.4%) | 62 (53%) | 0.075C |
| Hydrochlorothiazide | 12 (22.2%) | 50 (45.5%) | 65 (46.4%) | 41 (35%) | 0.007C |
| Nitrate | 10 (18.5%) | 28 (25.5%) | 36 (25.7%) | 32 (27.4%) | 0.662C |
| Loop diuretic | 40 (74.1%) | 87 (79.1%) | 105 (75%) | 85 (72.6%) | 0.719C |
| Spironolactone | 10 (18.5%) | 28 (25.5%) | 33 (23.6%) | 14 (12%) | 0.048C |
| Statin | 32 (59.3%) | 71 (64.5%) | 93 (66.4%) | 78 (66.7%) | 0.787C |
| Medical history | |||||
| Alcohol abuse | 3 (5.6%) | 5 (4.5%) | 3 (2.1%) | 3 (2.6%) | 0.522C |
| Tobacco abuse | 10 (18.5%) | 14 (12.7%) | 11 (7.9%) | 10 (8.5%) | 0.096C |
| Hypertension | 31 (57.4%) | 73 (66.4%) | 96 (68.6%) | 67 (57.3%) | 0.521C |
| Ischemic heart disease | 10 (18.5%) | 33 (30%) | 45 (32.1%) | 38 (32.5%) | 0.290C |
| Diabetes | 16 (29.6%) | 50 (45.5%) | 65 (46.4%) | 45 (38.5%) | 0.302C |
| Hyperlipidemia | 21 (38.9%) | 58 (52.7%) | 80 (57.1%) | 52 (44.4%) | 0.182C |
| Cerebrovascular disease | 0 | 0 | 4 (2.9%) | 1 (0.9%) | 0.153C |
F = P-value for global test of any differences between BNP categories from ANOVA F-test. L = P-value from ANOVA F-test, data log transformed prior to analysis. C = P-value from chi-square test. K = P-value from Kruskal-Wallis test. LVEF: left ventricular ejection fraction; RA: right atrium; LA: left atrium; AO: aorta; RVD: right ventricle; LVD: left ventricle diastole; LVS: left ventricle systole; IVS: interventricular septum; DHP: dihydropyridine.
Data analysis
Patients were grouped based on BNP levels (≤ 100, > 100 and ≤ 400, > 400 and ≤ 1,000, and > 1,000 pg/mL). Continuous patient characteristics were compared between BNP groups using ANOVA, with the data log transformed where a histogram indicated data skewness. For patient characteristics with a significant ANOVA F-test for the global test of any differences among BNP categories, pairwise comparisons between groups were conducted using the Tukey-Kramer method. Frequencies for categorical patient characteristics were compared between groups using chi-square tests. In the case of a significant chi-square test, adjusted residuals[6] were examined to determine the nature of the differences between groups. The Kruskal-Wallis test was used to compare median CKD stage between groups. This analysis was repeated in patients with BNP > 1000, comparing patients with and without a history of CKD (eGFR> 60 mL/min/m2 as determined by the MDRD equation[7]). A multiple linear regression model was used to estimate the joint effects of selected patient characteristics on BNP levels. Variables selected for inclusion in the model were those that differed significantly between BNP groups in univariate analysis. Data are presented as mean +/− standard deviation (SD). A P value ≤ 0.05 was used to reject the null hypothesis.
RESULTS
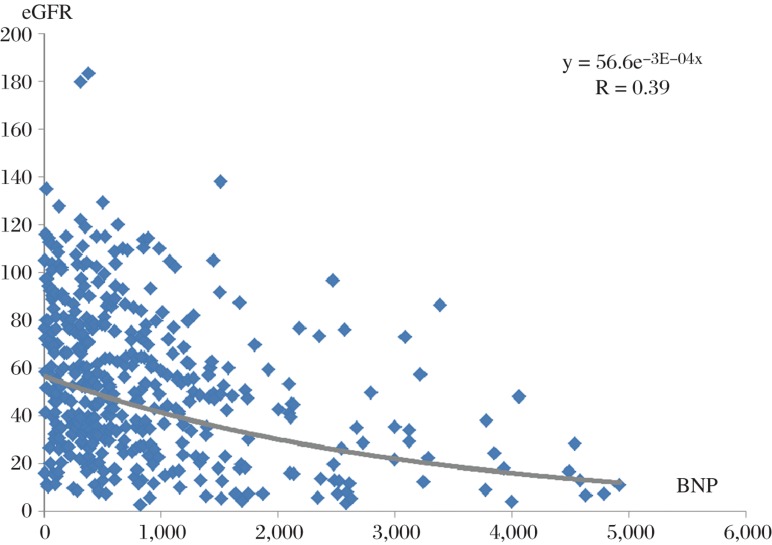
Patient characteristics are shown in Table 1. Patients with a BNP > 1,000 pg/mL comprised 28% of inpatients with a clinical diagnosis of HFpEF. Patients with BNP > 1,000 pg/mL, compared to those with a BNP ≤ 100 pg/mL, were characterized by a significantly higher age, higher levels of serum creatinine and BUN, lower eGFR, a higher prevalence of CKD IV or V and use of dialysis, lower hemoglobin, higher peak levels of troponin, and greater use of anti-hypertensive medications such as thiazides and spironolactone. However, when compared to all other patients, only eGFR and troponin was significantly different in the patients with BNP > 1,000 pg/mL. When BNP was plotted as a function of eGFR, there was a significant relationship, r = 0.39 (Fig. 1). Importantly, variables that were not significantly different between BNP groups included left ventricular size, ejection fraction, or wall thickness, as well as sex, diagnosis of diabetes or ischemic heart disease, and use of beta-blockers.
Fig. 1. Relationship of BNP (pg/mL) and renal function (eGFR, mg/minute/m2) for the entire cohort of patients.
The relationship between these 2 variables was statistically significant, r = 0.39, P ≤ 0.05. Correlation coefficient derived using log transformation of the data.
In order to examine the effect of renal dysfunction and reduced clearance on the serum level of BNP, the characteristics of patients with BNP > 1,000 pg/mL but without CKD (defined as eGFR> 60 mL/minute/m2) were examined and compared to those patients with CKD (Table 2). In comparison to patients with CKD, the patients with a BNP > 1,000 pg/mL but without CKD, comprising 21% of patients, had marginally lower mean values of BNP (1,677 +/− 608 vs 2,101 +/− 1,063 pg/mL), P = 0.06, and a lower use of anti-hypertensive medications such as calcium channel blockers, clonidine, and hydralazine. Echo parameters also differed significantly between these two groups, with patients without CKD having a lower E/A ratio (1.0 +/− 0.6 vs. 1.4 +/− 0.7), but a greater LV mass (335.9 +/− 138.9 vs. 273.8 +/− 106.5 gms, P < 0.05). There was a trend toward lower ejection fractions in those patients without CKD (LVEF 55.4 +/− 9.3 vs. 59.0 +/− 7.7, P = 0.08).
Table 2. Patient characteristics by CKD - patients with BNP > 1,000 (pg/mL).
| No CKD(n = 24) | CKD(n = 93) | P-Value | |
| Age (years) | 70.2 (13.5) | 67.7 (16.3) | 0.493L |
| Gender | |||
| Female | 13 (54.2%) | 54 (58.1%) | 0.731C |
| Male | 11 (45.8%) | 39 (41.9%) | |
| BNP | 1677 (608) | 2101 (1063) | 0.064L |
| eGFR | 81.9 (17.6) | 29.9 (17.5) | <0.001L |
| Creatinine | 0.9 (0.16) | 3.2 (2.5) | <0.001L |
| BUN | 15.5 (6.7) | 38.8 (22.6) | <0.001L |
| Glucose | 139.8 (46.8) | 147.7 (79) | 0.857L |
| Hematocrit | 35.4 (5) | 32.7 (7.3) | 0.043F |
| Hemoglobin | 11.7 (1.8) | 10.7 (2.4) | 0.025F |
| Troponin | 0.4 (1.5) | 0.1 (0.2) | 0.697L |
| Echocardiographic measures | |||
| LVEF | 55.4 (9.3) | 59.0 (7.7) | 0.077F |
| RA | 43.5 (12.3) | 42.6 (10.2) | 0.712F |
| LA | 45.2 (8.9) | 44.3 (8.3) | 0.613F |
| AO | 32.8 (4.9) | 31.9 (5.8) | 0.484F |
| RVD | 38.4 (12.1) | 37.2 (12.3) | 0.666F |
| LVD | 45.9 (8.7) | 47.2 (8) | 0.504F |
| LVS | 28.9 (9.4) | 32.2 (8.4) | 0.088F |
| Fractional shortening | 38.2 (11.1) | 33.7 (13) | 0.110F |
| IVS | 12.8 (2.7) | 12.3 (2.7) | 0.383L |
| EA ratio | 1.0 (0.6) | 1.4 (0.7) | 0.011L |
| LV mass | 335.8 (138.9) | 273.8 (106.5) | 0.018L |
F = P-value for global test of any differences between BNP categories from ANOVA F-test. L = P-value from ANOVA F-test, data log transformed prior to analysis. C = P-value from chi-square test. K = P-value from Kruskal-Wallis test.
Abbreviations and units same as Table 1.
Patients with a clinical diagnosis of HFpEF but normal BNP (≤ 100 pg/mL) comprised 13% of the study population and were characterized by younger age (61.4 +/− 12.5 years) and preserved renal function (eGFR 68.8 +/− 31.9 mL/minute/m2), although 13 of 54 (24%) patients in this group had creatinine levels greater than the upper limit of normal (1.28 gm/dL), with values as high as 5.87 mg/dL.
The level of BNP in the index hospitalization did not predict subsequent death or hospitalization for a myocardial infarction. However, BNP levels were lower in patients subsequently hospitalized for heart failure during the follow-up period (631.2 +/− 647.7 vs 907.2 +/− 956.8 pg/mL, P = 0.04).
DISCUSSION
The role of B-type natriuretic peptide in heart failure
B-type natriuretic peptide (BNP) is a peptide hormone released in response to increased ventricular wall stress, and is often used to diagnose heart failure due to systolic dysfunction (HFrEF)[7]. In patients with heart failure with preserved ejection fraction (HFpEF), elevations in BNP have been reported to correlate with left ventricular end-diastolic wall stress[8], age, and abnormal diastolic function (assessed by left atrial volume index), and inversely with LV ejection fraction[9]. Median values of BNP in patients with HFpEF are generally lower than those with reduced ejection fraction, with median values of 413[10] and 445 pg/mL[2] previously being reported in these patients. Thus, BNP values > 1,000 pg/mL are unusual and may identify a different cohort of patients at different risk for further cardiovascular events[4].
Clinical predictors of BNP in HFpEF
In the current study, the prevalence of BNP > 1,000 pg/mL was 28% (117/421) and BNP > 2,000 pg/mL was 11% (45/421). Patients with a BNP > 1,000 pg/mL, when compared to those with a normal BNP (≤ 100 pg/mL), were characterized by significantly higher age, worse renal function, anemia, elevated troponins, and use of anti-hypertensive agents. Important variables that were not associated with a markedly elevated BNP included LV mass, dimensions and ejection fraction, as well as gender, a diagnosis of diabetes, or history of ischemic heart disease.
Marked elevations of BNP are frequently seen in systolic heart failure and generally reflect increased wall stress due to left ventricular enlargement without compensatory wall thickening[11]. In our cohort, patients with BNP > 1,000 pg/mL did not have evidence of increased wall stress, as their left ventricular end diastolic dimensions, wall thickness and left ventricular mass were similar to the other groups. These findings parallel those of Niizuma et al.[11], who found no correlation between BNP concentrations and left ventricular end-diastolic wall stress in patients with HFpEF.
In contrast to patients with marked elevations of BNP, a recent study identified patients with a clinical diagnosis of HFpEF and normal BNP ≤ 100 pg/mL in 29% of outpatients[12]. Those patients were characterized as younger women with obesity, but less frequently with CKD. In our study of inpatients with a BNP ≤ 100 pg/mL and a clinical diagnosis of HFpEF (n = 54, prevalence of 13%), these patients, in comparison to those with a BNP > 1,000 pg/mL, were younger, had a lower prevalence of CKD IV (8%) and CKD V (5%), and less frequent use of anti-hypertensive medications. In contrast to the prior study, there was no difference in gender distribution. While the previously reported greater prevalence of normal BNP in outpatients likely represents the less severe disease found in the outpatient populations compared to those hospitalized with HFpEF, these two studies indicate that there are common factors, primarily younger age and a low prevalence of significant CKD, in patients with HFpEF and BNP ≤ 100 pg/mL.
The role of CKD
Elevated BNP in patients with chronic kidney disease may reflect the extent of cardiac dysfunction or, alternatively, altered metabolism of BNP in renal failure. While the relationship of BNP to left ventricular end-diastolic wall stress is significant in patients with end-stage renal disease and systolic dysfunction, that relationship was not found by Nizuma et al. in patients with preserved systolic function[2]. In that study, BNP was significantly elevated in those patients with end-stage renal disease (GFR < 15 mL/minute/1.73 m2), 93% of whom were on hemodialysis. However, BNP was not significantly elevated in patients with HFpEF who had CKD I–IV compared to those with normal renal function, suggesting that markedly elevated BNP is seen primarily in those with CKD V, generally on dialysis.
The clearance of BNP is two-fold. BNP binds to natriuretic peptide receptor C which is responsible for its clearance via receptor -mediated endocytosis and lysosomal degradation[13], with subsequent removal of the peptide by the kidneys. BNP is also enzymatically degraded to an inactive form by neutral endopeptidase[14]. Thus, both reduced renal function and reduced activity of neutral endopeptidase can result in higher serum levels of BNP.
In the current study, there was a statistically significant inverse relationship with BNP and eGFR (Fig. 1). Furthermore, 17.5% of patients with a BNP > 1,000 ng/mL had a history of ESRD or were on hemodialysis, compared to 0, 0.9, and 5.7% in patients with BNP ≤ 100, 100–400, and 400–1,000, respectively. Thus, these data confirm the importance of severely impaired renal function in resultant levels of BNP > 1,000 pg/mL.
Approximately one-fifth (21%) of the patients with markedly elevated BNP > 1,000 pg/mL had normal renal function (defined as eGFR> 60 mL/minute/m2). Thus, while renal dysfunction contributes to elevated serum levels of BNP, it is not a requirement. Those patients without CKD appeared to be different than those with CKD, tending to have lower mean BNP values, and a lower prevalence of anti-hypertensive medications. There was a suggestion of lower systolic function in this cohort. While there were no differences between these groups in LV mass, these data suggest those patients with elevated BNP but without CKD may represent an elderly population with a lower burden of chronic hypertensive and renal disease, yet have elevated wall stress due to mildly impaired systolic function in addition to diastolic dysfunction[15]. Although speculative, another possible explanation for their markedly elevated BNP in the absence of CKD is reduced activity of neutral endopeptidase, as seen in patients with aging[16].
The prognostic implications of markedly elevated BNP were paradoxical, as BNP > 100 pg/mL did not predict either subsequent death or myocardial infarction, yet patients with a relatively lower BNP during index hospitalization had a higher rate of subsequent hospitalization for heart failure. This may reflect the higher LV mass in patients with preserved renal function yet BNP > 1,000 pg/mL (Table 2). While this latter observation needs further study, these data suggest that, in contrast to systolic heart failure (HFrEF), the level of BNP in HFpEF does not seem to provide prognostic information regarding death or MI[3].
Limitations
A strength of this study was the large number of patients included in a study of HFpEF. However, as a retrospective study from one institution, this data set was limited to observing differences in patients characterized by a clinical diagnosis of HFpEF in the inpatient setting. Thus, these data may not directly apply to outpatients. The diagnosis was established by the treating physician and, as a clinical diagnosis, it is possible that criteria for the diagnosis were not consistent across patients. Also, there are some data that were not obtained from the studies. For example, left atrial volume index was not consistently measured and was therefore not included in the echocardiographic parameters[9].
In conclusion, marked elevations of BNP (> 1,000 pg/mL) are common in patients with a clinical diagnosis of HFpEF, comprising 28% of the patients in this sample, and primarily characterized by severe CKD and hypertension. CKD is not obligatory, as approximately one-fifth of patients with BNP > 1,000 pg/mL had normal renal function; these patients tended to be older with lower ejection fractions. In contrast to systolic heart failure, markedly elevated BNP does not confer a negative prognosis, perhaps because of confounding disease processes.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through grant #UL1 TR000002
References
- 1.Wood P, Piran S, Liu PP. Diastolic heart failure: progress, treatment challenges, and prevention. Can J Cardiol. 2011;27:302–10. doi: 10.1016/j.cjca.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Niizuma S, Iwanaga Y, Yahata T, Tamaki Y, Goto Y, Nakahama H, et al. Impact of left ventricular end-diastolic wall stress on plasma B-type natriuretic peptide in heart failure with chronic kidney disease and end-stage renal disease. Clin Chem. 2009;55:1347–53. doi: 10.1373/clinchem.2008.121236. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K, Tsutamoto T, Wada A, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1587–93. doi: 10.1016/s0735-1097(00)00912-8. [DOI] [PubMed] [Google Scholar]
- 4.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;14:1498–506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 5.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Agresti A. An introduction to categorical data analysis. New York, N.Y.: John Wiley & Sons; 2007. [Google Scholar]
- 7.O'Meara E, Chong KS, Gardner RS, Jardine AG, Neilly JB, McDonagh TA. The Modification of Diet in Renal Disease (MDRD) equations provide valid estimations of glomerular filtration rates in patients with advanced heart failure. Eur J Heart Fail. 2006;8:63–7. doi: 10.1016/j.ejheart.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Baggish AL, Lloyd-Jones DM, Blatt J, Richards AM, Lainchbury J, O'Donoghue M, et al. A clinical and biochemical score for mortality prediction in patients with acute dyspnoea: derivation, validation and incorporation into a bedside programme. Heart. 2008;94:1032–7. doi: 10.1136/hrt.2007.128132. [DOI] [PubMed] [Google Scholar]
- 9.Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, et al. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742–8. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Jaubert MP, Armero S, Bonello L, Nicoud A, Sbragia P, Paganelli F, et al. Predictors of B-type natriuretic peptide and left atrial volume index in patients with preserved left ventricular systolic function: an echocardiographic-catheterization study. Arch Cardiovasc Dis. 2010;103:3–9. doi: 10.1016/j.acvd.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P, et al. Bedside B-Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol. 2003;41:2010–7. doi: 10.1016/s0735-1097(03)00405-4. [DOI] [PubMed] [Google Scholar]
- 12.Iwanaga Y, Kihara Y, Niizuma S, Noguchi T, Nonogi H, Kita T, et al. BNP in overweight and obese patients with heart failure: an analysis based on the BNP-LV diastolic wall stress relationship. J Card Fail. 2007;13:663–7. doi: 10.1016/j.cardfail.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, et al. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic Peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–6. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–68. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Vanderheyden M, Bartunek J, Goethals M. Brain and other natriuretic peptides: molecular aspects. Eur J Heart Fail. 2004;6:261–8. doi: 10.1016/j.ejheart.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Morris DA, Boldt LH, Eichstädt H, Ozcelik C, Haverkamp W. Myocardial systolic and diastolic performance derived by 2-dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ Heart Fail. 2012;5:610–20. doi: 10.1161/CIRCHEARTFAILURE.112.966564. [DOI] [PubMed] [Google Scholar]
- 17.Reckelhoff JF, Baylis C. Proximal tubular metalloprotease activity is decreased in the senescent rat kidney. Life Sci. 1992;50:959–63. doi: 10.1016/0024-3205(92)90174-n. [DOI] [PubMed] [Google Scholar]