Abstract
Background and Objective:
Periodontal disease has been linked with an increased risk of various systemic diseases. A plausible biologic explanation for this link includes the opportunity for oral pathogens to translocate to the circulation as a result of breakdown in integrity of the oral epithelium. This study refined a methodology used to detect endotoxin activity in the serum of subjects with indolent periodontal infections.
Material and Methods:
The QCL® Kinetic Chromogenic Assay (Cambrex) is a kinetic measure of endotoxin activity. Sera from 211 pregnant women with periodontitis enrolled in the Obstetrics and Periodontal Therapy Trial were used to develop the assay further and to evaluate the detection of endotoxin activity that might accompany a low-level bacteremia in chronic periodontitis.
Results:
We optimized the system to increase the sensitivity and reproducibility of the assay. The refined system was able to detect endotoxin activity in serum at > 0.0125 EU/mL. At baseline (13–16 wk of gestation), 35.5% of the women were positive for endotoxin activity (1.62 ± 2.21; range: 0.38–15 EU/mL).
Conclusion:
This report describes a sensitive measure of endotoxin activity in serum. The procedure allowed us to document levels of this microbial virulence factor in serum of individuals with indolent infections such as periodontal disease.
Keywords: periodontitis, endotoxin, serum, pregnancy, indolent infection
Endotoxin classically describes the biologic activity of the lipopolysac-charide structure comprising the outer membrane of gram-negative bacteria (1). Lipopolysaccharide, through engagement of lipopolysaccharide-binding protein (2,3) in serum and CD14 antigen on various cell types, particularly monocytes/macrophages, activates host cells at very low concentrations (3–5). In addition, Toll-like receptor-2 and -4, as cell-surface pattern-recognition receptors, bind lipo-polysaccharide as a ligand to initiate a cascade of multiple intracellular signaling pathways (4,6–8). These interactions between lipopolysaccharide and host cells result in up-regulation of the transcription of numerous genes, leading to the elevated production of an array of cytokines, chemokines and lipid mediators that contribute to both acute and chronic inflammatory responses to control the infection (3,9). Systemic bacteremia with gram-negative bacteria, resulting in a septic infection, delivers lipopolysaccharide to the circulation. Circulating endotoxin in the vasculature triggers the release of cytokines and acute-phase reactants, contributing to disseminated intravascular coagulation pathways leading to multiple organ system failure (10–12).
In contrast to overt septic infections, various human diseases are expressed as chronic infections. These are usually localized to specific tissues or organs and, through induction of a chronic inflammatory response, can lead to loss of function of the tissues/organs. Moreover, the chronic local inflammation can release factors that may elicit systemic inflammatory responses (13,14). Periodontitis is a disease with localized tissue destruction, reflecting a chronic immunoinflammatory lesion (15–17) initiated by oral infection with a complex microbial ecology in sub-gingival biofilms (18–20). The tissue destruction undermines the integrity of the epithelial barrier in the periodontium and allows bacteria from the biofilms to translocate into the circulation (21–23). This can occur as a result of simple toothbrushing, but clearly the severity and extent of periodontitis accentuates the ability for bacteria to enter the bloodstream (24– 26). However, in contrast to overt septic infections, the bacteremia being seeded from the oral cavity is generally at low levels and relatively transient for individual episodes (25–28). Nevertheless, the chronicity of the disease and infection creates an indolent challenge to the systemic circulation that has been proposed to be a contributing facet of systemic health changes that accompany periodontitis (24,29–34).
The primordial nature of the necessity for both vertebrates and invertebrates to protect against bacterial infections leading to endotoxicity was recognized by the identification of phagocytic cells in these species that detect and react to lipopolysaccharide/endotoxin (35–37). The ability of endotoxin to activate these phagocytic cells led to the development of a sensitive assay for this toxic contaminant using amoebocytes from the horseshoe crab, Limulus polyphemus (38). The original assay documented coagulation of the amoebocytes and has been used to determine endotoxin contamination of medical and biological reagents, as well as to estimate the endotoxin levels in biological fluids during septic infections (38,39). These early methods have been adapted to more quantitative colorimetric methods and kinetic assessments to enhance sensitivity of the assays (39,40). However, these techniques have not been effectively used to estimate the endotoxin levels in the sera of patients with indolent infections and low levels of serum endotoxin, such as those with periodontitis. This report describes a modification of a commercial assay for endotoxin that substantially increased the sensitivity of the detection of this toxin in serum and demonstrates its utility in documenting endotoxin levels in the sera of patients with periodontitis.
Material and methods
Patient population
The patients included 211 women recruited at the University of Kentucky College of Dentistry into the 'Obstetrics and Periodontal Therapy' clinical trial (31). This was a multicenter randomized clinical trial carried out to evaluate the effects of periodontal therapy during the second trimester of pregnancy on the incidence of preterm and/or low-birthweight deliveries. Women at least 16 years of age were enrolled between 13 and 16 wk 6 days of gestation and were randomly assigned to receive scaling and root planing plus monthly tooth polishing before 21 wk of gestation (test group) followed by monthly periodontal maintenance or scaling and root planing after delivery (control group). Women had to have 20 natural teeth, with bleeding on probing on at least 35% of all tooth sites and four or more teeth with pockets of ≥ 4 mm and attachment loss ≥ 2 mm. Women were ineligible if they had multiple fetuses, required antibiotic prophylaxis prior to dental treatment, had a medical condition that precluded elective dental treatment, had extensive tooth decay, or were likely to have fewer than 20 remaining teeth after treatment of tooth decay, abscesses, or other non-periodontal pathoses. Serum was obtained from blood samples taken at baseline, which was after randomization and entry into the study and prior to any therapy. An additional serum sample was obtained at 29–32 wk of gestation, which followed periodontal therapy in the treatment group (n = 106).
Endotoxin level
The QCL® Kinetic Chromogenic Assay (Cambrex, East Rutherford, NJ, USA) is a kinetic measure of endotoxin activity. Escherichia coli 055:B5 endotoxin (BioWhittaker, Walkersville, MD, USA) was used as a standard for the assay. All reagents, Eppendorf pipette tips (Fisher Scientific, Pittsburgh, PA, USA) and 96-well Costar flat-bottom plates (Fisher Scientific) were pyrogen free. All reagents were brought to room temperature (25°C) before starting the assay, which was performed on a dry heat block. A stock standard of endotoxin was prepared at 50 EU/mL (reconstituted with endotoxin activity-free water). This standard was vigorously vortexed for ≥ 10min and a standard curve was created by dilution 1:20 in normal serum and endotoxin activity-free water (range: 10–0.025 EU/mL). All experimental samples were diluted 1:20 with endotoxin activity-free water. The sample blank was normal human serum diluted 1:20. We also included in each assay an internal control that was an aliquot of 1:20 normal human serum spiked with 5 EU/mL of the endotoxin activity standard. We determined that this normal serum lacked detectable endotoxin activity with the increased sensitivity of this method. In addition, when spiked with endotoxin activity and analyzed, the estimated endotoxin activity levels were within 5% of the amount of the spike in the sample. All samples, standards, blank and control were heated to 85°C for 15 min, then 100 lL of standards and samples were loaded into the plates and incubated at 37°C for 10 min. Immediately before the assay, limulus amoebocyte lysate lysate was reconstituted with 5.2 mL/vial of endotoxin activity-free water. The colorimetric changes reflecting endotoxin levels were measured using a Biotech Elx808cse Microplate Reader with WINK QCL version 3 software (Canton, OH, USA).
Statistical analysis
A Friedman nonparametric analysis of variance for dependent samples was used to determine differences in endotoxin activity levels in baseline serum samples vs. serum samples taken at 29–32 wk of gestation. A Bland & Altman plot (41) (Analyse-It Software Ltd., Leeds, UK) was used to estimate the level of agreement between independent assessments of endotoxin activity in a set of samples. diffierences in categories of endotoxin activity detected in the sera were evaluated between baseline samples and those taken at 29–32 wk of activity using a Cochran Mantel–Haenszel test (JMP 7.0; SAS, Cary, NC, USA). The relationship between endotoxin activity category and clinical presentation was developed by stratifying the patients into tertiles based upon either the mean bleeding (e.g. inflammation) or the mean attachment loss (e.g. disease severity) for each patient. This enabled the use of a chi-square test to estimate the relationship between endotoxin activity and clinical parameters of periodontitis.
Results
Initially we determined the reproducibility of the modified assay using the human serum spiked with known concentrations of endotoxin activity compared with the endotoxin activity standard prepared in distilled H2O as normally prescribed in the commercial kit (Fig. 1). The results demonstrated significant inhibition of endotoxin activity values with both 1:10 and 1:20 dilutions of serum. Additionally, simply adding human serum albumin at 0.1% to the endotoxin activity/DH2O standard decreased the assay dynamics (data not shown). Clearly, the inhibition was dependent upon the amount of serum present (1:10 vs. 1:20). Thus, the assay was modified to be performed with a standard curve generated by adding known amounts of endotoxin activity to a serum control, because a standard curve using DH2O would significantly underestimate the endotoxin activity levels. Figure 2 demonstrates the characteristics of multiple runs of the endotoxin activity standard in human serum, and describes a reproducible standard curve. Also, the minimal detectable dose of the assay performed in serum was calculated to be < 0.0125 EU/mL. Figure 3 depicts a Precision Plot of the characteristics of an internal serum standard that was included in all runs. This internal standard was a 1:20 dilution of a single serum sample spiked with 5 EU/mL of the E. coli endotoxin activity, just prior to each assay. The results demonstrate a mean of all the runs of 5.108 EU/mL, with a standard deviation of 1.239 and a coefficient of variation of 24.8%.
Fig. 1.
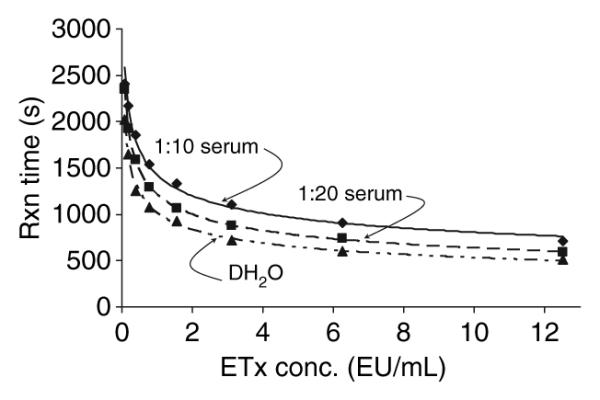
Dose–response curves for endotoxin activity reactions with Escherichia coli lipopolysaccharide. The standard method is the curve of lipopolysaccharide diluted in DH2O. Comparisons were made with identical concentrations of lipopolysaccharide diluted 1:10 and 1:20 in normal human serum containing no detectable endotoxin activity. The curves were derived from triplicate determinations for each condition. Etx, endotoxin activity; Rxn, reaction.
Fig. 2.
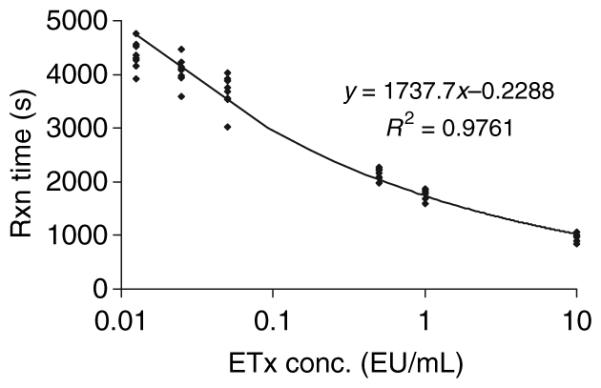
Characteristics of the dose–response curve of Escherichia coli lipopolysaccharide diluted 1:20 in normal human serum with no detectable endotoxin activity. Each point denotes an independent assay with duplicate determinations. The calculated minimal detectable dose was < 0.0125 EU/mL. The regression equation was derived from a power curve fit to the logarithm of the endotoxin activity concentrations. The percentage coefficient of variation for the assay ranged from 4.7 to 8.6% across the range of endotoxin activity concentrations in this curve. Etx, endotoxin activity; Rxn, reaction.
Fig. 3.
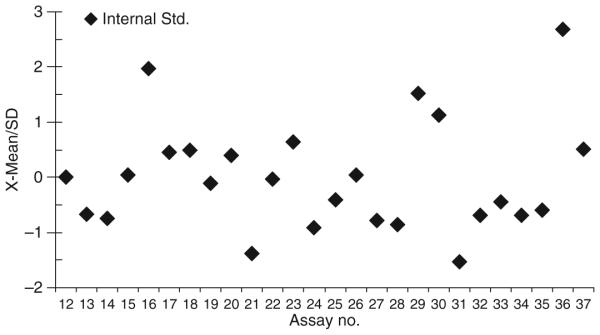
Consistency of the internal serum control (normal human serum spiked with 5.0 EU/mL). Each point denotes an independent assay and represents duplicate determinations in each assay. Mean values, and one and two standard deviations, for the group of assays are included.
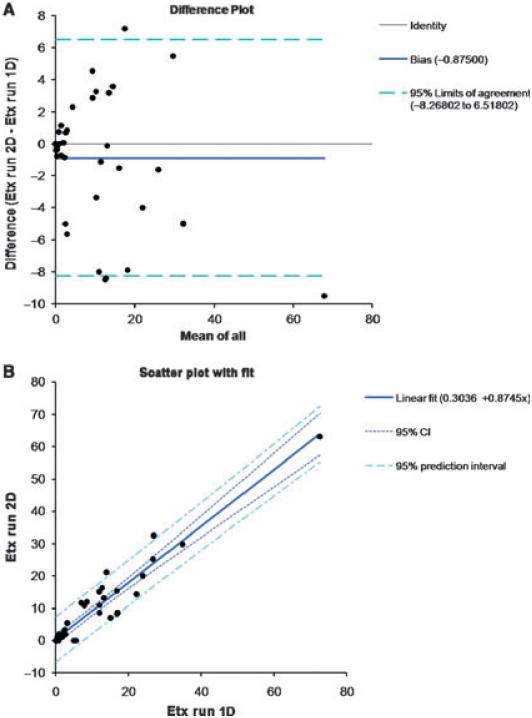
Analysis of 43 samples that in an initial endotoxin activity analysis demonstrated levels of endotoxin below detectable levels to > 50 EU/mL, was performed to assess the agreement of levels of endotoxin activity in independent assays (Fig. 4). The results (Fig. 4A) demonstrated a correlation of the absolute difference vs. the average difference of 0.78. Upper and lower 95% confidence intervals were plotted and show that 3/43 samples fell outside this range. Additionally, the scatter plot (Fig. 4B) provided a demonstration of the linear regression of the two runs and showed a highly significant (p < 0.001) correlation with an adjusted R2 of 0.89. While the overall precision of the assay across the range of endotoxin activity concentrations was acceptable, we observed variations in the coefficient of repeatability, ranging from 0.88 to 10.98 over the 2-log range of concentrations. This estimation suggested that a categorical description of the endotoxin activity levels in the patients would probably provide a more accurate representation for comparing serum endotoxin activity challenge and changes rather than using exact endotoxin activity measures. Thus, we developed five categories of endotoxin activity levels in serum: 1, > 0.0125 to < 1; 3, 1 to < 5; 4, 5 to < 10; 1 EU/mL; 3, 1 to < 5 EU/mL; 4, 5 to < 10 EU/mL; and 5, > 10 EU/mL.
Fig. 4.
Reproducibility of the independent assessment of endotoxin activity in sera containing elevated levels of endotoxin activity. Forty-three sera with initial endotoxin activity measurements ranging from below the minimal detectable dose to > 50 EU/mL were re-analyzed in an independent assay in triplicate. (A) Bland & Altman plot of the differences in values plotted against the average difference with a 95% confidence interval for the assays. (B) Scatter plot of the 43 individual values and linear regression demonstrating the correlation. 1D, one-dimensional; 2D, two-dimensional; CI, confidence interval; Etx, endotoxin activity.
Figure 5 shows the distribution of endotoxin activity levels in the serum of pregnant women at baseline and at 29–32 wk of gestation. The figure demonstrates that the majority of the population at either visit had negligible endotoxin activity levels in serum; however, a distinct subset of the patients showed detectable levels of this bacterial marker in the blood. The distribution of endotoxin activity categories in serum from pregnant women at baseline and at 29–32 wk of gestation showed no difference in endotoxin activity between baseline samples and those taken at 29–32 wk of gestation across the entire population (Fig. 6). The distribution of subjects in endotoxin activity categories at baseline and at 29–32 wk of gestation (in the treatment group) is provided in Fig. 7. The results show that in both the treatment and control patients, a higher frequency of subjects had negative endotoxin activity (category 1) at 29–32 wk of gestation (70.4%) compared with baseline (64.0%).
Fig. 5.

Cumulative distribution of endotoxin activity levels in serum from the patients at baseline (Visit B) and 29–32 wk (Visit 5). Each point denotes the endotoxin activity level in an individual patient sample.
Fig. 6.
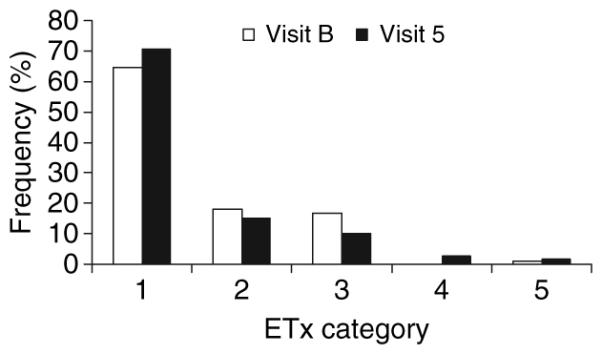
Distribution of samples in endotoxin activity categories comparing base-line (Visit B) with 29–32 wk (Visit 5) samples from the same population. The bars denote the frequency of samples at each visit in the various categories: 1, < 0.0125; 2, > 0.0125 to < 1; 3, 1 to < 5; 4, 5 to < 10; and 5, > 10 EU/mL. Etx, endotoxin activity.
Fig. 7.

Distribution of samples in endotoxin activity categories comparing baseline (B) with 29–32 wk (Visit 5-V) for the control (C) and treated (T) subsets of the patients. The bars denote the frequency of sera from the patients demonstrating various levels of endotoxin activity. Etx, endotoxin activity.
The relationship of endotoxin activity category to baseline clinical presentation of the pregnant women is presented in Fig. 8. The results do not support any difference in baseline clinical parameters irrespective of endotoxin activity in serum.
Fig. 8.
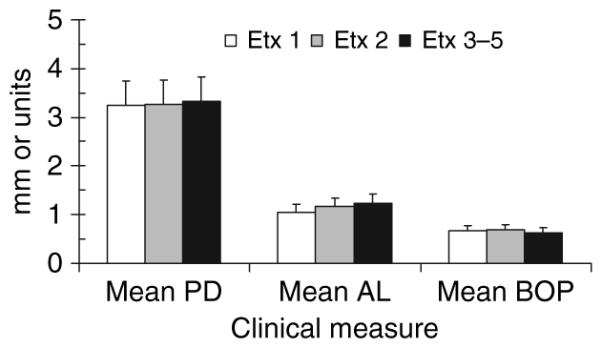
Relationship between clinical oral parameters and serum endotoxin activity levels at baseline. The bars denote the mean values of the mouth pocket depths (PD) or attachment loss (AL) and the mean bleeding index [bleeding on probing (BOP)] of the mouth for the subjects stratified into endotoxin activity categories (see Fig. 6 legend). The vertical brackets enclose one standard deviation. Etx, endotoxin activity.
Discussion
Mucosal bacterial infections that invade or translocate across epithelial barriers into the systemic circulation with hematogenous spread of the infection to distant tissues/organs, remains a significant human health problem. As reported by the National Center for Health Statistics of the CDC, annual deaths from infectious disease rank third in the USA, with deaths from septicemia the 10th leadingcause of death (http://www.cdc.gov/nchs/fastats/deaths.htm). However, various chronic infections without mortal consequences can contribute to the increasing recognition of the impact of chronic diseases on the population. These types of chronic infections can result in a constant low-level challenge to the host, resulting in the potential for a cumulative alteration in the function of cells, tissues, or organs that eventually would demonstrate notable disease symptoms. Periodontal disease represents this type of indolent infection, in which localized collateral damage of oral tissues as a result of chronic host–bacterial interactions can result in microbial challenge to the vascular system and distant tissues (16,25).
Substantial evidence demonstrates that bacteria from the oral cavity can enter the bloodstream. The gingival tissues are structurally composed of an extensive capillary network that not only can deliver cells and biomolecules from the vascular system, but also enables the translocation of oral bacteria and/or their products into the local draining lymph nodes and the systemic circulation. Normal oral health procedures, including toothbrushing, have been shown to contribute to a transient bacteremia with oral microorganisms (25,26). This process is enhanced in cases where the gingival tissue is more fragile as a result of inflammation and is exacerbated in periodontitis as a result of breaks in the integrity of the gingival epithelial barrier (42). The results of this translocation have been described in detail and may cause subacute bacterial endocarditis, primarily resulting from oral streptococcal species (25,43,44), and infection foci in various tissues including liver, brain and joints (45–50) from oral bacteria. More recent studies have suggested that the chronic local infection in periodontitis provides a chronic microbial challenge to the systemic circulation that can enable oral bacteria to colonize vascular tissues (51), placental/amniotic tissues and the fetus (52), and alter pancreatic functions (30). Alternatively, this chronic systemic challenge has been noted to increase circulating levels of pro-inflammatory cytokines and acute-phase proteins, reflecting a stress on host systems and contributing to altered functions of critical tissues or organs (15,53).
The oral cavity has been estimated to harbor over 700 species of micro-organisms and demonstrates variations in the colonization patterns of the range of niches in the oral cavity (18), differences in the total and relative contributions of individual species to healthy compared with disease biofilms (19) and variable arrays of potential virulence factors produced by individual species (54–56). In fact, certain oral species have been demonstrated to have an enhanced capacity to invade host epithelial and endothelial cells (57–59), which would be expected to play a role in their capacity to translocate into the systemic circulation. However, in any given patient who harbors the potential for oral colonization by 75–100 different species, it is a challenge to document the frequency and magnitude of bacterial translocation across a population or within individual patients over time. Various molecular genetic techniques have been employed to address these questions. The use of species-specific primers is somewhat limited by the range of species that could be translocating in an individual subject, while the use of 'universal' prokaryotic primers are not uniform in their effectiveness for the broad range of oral bacteria (60,61). Because the principal bacteria that have been associated with pathogenic biofilms in periodontitis are gram-negative, the availability of a sensitive system for detection of endotoxin could provide a broad evaluation of microbial challenge to the systemic circulation that occurs in periodontitis. Moreover, available data supports that 'endotoxin assays' will also detect the lipoteichoic acid of gram-positive bacteria (62–64). Thus, this report describes our modification of a commercial kinetic endotoxin activity assay system to estimate the microbial challenge to the systemic circulation.
The findings demonstrated that we could detect endotoxin activity in serum resulting from an indolent infection, such as periodontitis, with high sensitivity and reproducibility. This required the inclusion of a standard endotoxin activity curve prepared in serum because the standard protocol in DH2O significantly underestimated the quantity and frequency of endotoxin activity in the experimental samples. We also determined that inclusion of a freshly spiked serum sample in every assay provided an internal control of the between-assay reproducibility. As we characterized the details of the technology it became clear that attempting to provide absolute quantities of endotoxin activity at low levels in the serum was not particularly robust. However, stratifying the endotoxin activity levels into categories with a range of increasing amounts provided an accurate estimation of microbial presence in the serum samples. The system was then used to assess its utility in determining levels and changes in endotoxin activity in a cohort of pregnant women with periodontitis, and relating these levels to treatment and oral clinical presentation.
The results showed that approximately 30–35% of the women in this study had endotoxin activity detectable in baseline serum samples, expressing levels from 10–2000-fold above the minimal detectable dose level of the assay. While generally it appeared that the frequency of endotoxin activity-positive serum samples were higher at baseline compared with those at 29–32 wk of gestation, we could not demonstrate an effect of the periodontal therapy on this frequency. Similarly, there were no statistical relationships between baseline oral clinical presentation (i.e. pocket depth, attachment loss, bleeding on probing) and the distribution of endotoxin activity-positive sera; however, there did appear to be a positive trend with both pocket depth and attachment loss being related to the frequency of increasing endotoxin activity levels (i.e. endotoxin activity categories 3–5).
In conclusion, this report describes a methodology for detection of endotoxin activity in serum from patients who could be expected to demonstrate some level of indolent systemic challenge (i.e. periodontitis). The results demonstrated that only a subset of the subjects had systemic endotoxin activity, which could highlight a more 'at-risk' group for complications of chronic periodontal infections. This technology can be readily expanded to a larger population to evaluate: (i) changes in endotoxin activity with therapy; (ii) relationship to clinical presentation; (iii) relationship to systemic inflammatory/immune responses; and (iv) association with adverse systemic outcomes.
Acknowledgements
This work was supported by U.S.P.H.S. grant U01 DE014338 from the National Institute for Dental and Craniofacial Research. We thank Dr Jim Hodges and Ms Helen Voelker, University of Minnesota, for data management support in this project.
References
- 1.Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J Dent Res. 2005;84:584–595. doi: 10.1177/154405910508400702. [DOI] [PubMed] [Google Scholar]
- 2.Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006;8:946–952. doi: 10.1016/j.micinf.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11:225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 4.Moreno C, Merino J, Ramirez N, Echeverria A, Pastor F, Sanchez-Ibarrola A. Lipopolysaccharide needs soluble CD14 to interact with TLR4 in human monocytes depleted of membrane CD14. Microbes Infect. 2004;6:990–995. doi: 10.1016/j.micinf.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 is an acute-phase protein. J Immunol. 2004;172:4470–4479. doi: 10.4049/jimmunol.172.7.4470. [DOI] [PubMed] [Google Scholar]
- 6.Stoll LL, Denning GM, Weintraub NL. Endotoxin, TLR4 signaling and vascular inflammation: potential therapeutic targets in cardiovascular disease. Curr Pharm Des. 2006;12:4229–4245. doi: 10.2174/138161206778743501. [DOI] [PubMed] [Google Scholar]
- 7.Ulevitch RJ. Toll gates for pathogen selection. Nature. 1999;401:755–756. doi: 10.1038/44490. [DOI] [PubMed] [Google Scholar]
- 8.Hajishengallis G, Sharma A, Russell MW, Genco RJ. Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis. Ann Periodontol. 2002;7:72–78. doi: 10.1902/annals.2002.7.1.72. [DOI] [PubMed] [Google Scholar]
- 9.Madianos PN, Bobetsis YA, Kinane DF. Generation of inflammatory stimuli: how bacteria set up inflammatory responses in the gingiva. J Clin Periodontol. 2005;32(suppl 6):57–71. doi: 10.1111/j.1600-051X.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 10.Aue G, Carroll N, Kressel BR, Hardi R, Horne MK. 3rd. Disseminated intravascular coagulation in an ambulatory young woman. J Lab Clin Med. 2005;146:192–196. doi: 10.1016/j.lab.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Jellema WT, Veerman DP, De Winter RJ, et al. In vivo interaction of endotoxin and recombinant bactericidal/permeability-increasing protein (rBPI23): hemodynamic effects in a human endotoxemia model. J Lab Clin Med. 2002;140:228–235. doi: 10.1067/mlc.2002.127170. [DOI] [PubMed] [Google Scholar]
- 12.Brandtzaeg P, Kierulf P, Gaustad P, et al. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- 13.Damare SM, Wells S, Offenbacher S. Eicosanoids in periodontal diseases:potential for systemic involvement. Adv Exp Med Biol. 1997;433:23–35. doi: 10.1007/978-1-4899-1810-9_5. [DOI] [PubMed] [Google Scholar]
- 14.Sekut L, Menius JA, Jr, Brackeen MF, Connolly KM. Evaluation of the significance of elevated levels of systemic and localized tumor necrosis factor in different animal models of inflammation. J Lab Clin Med. 1994;124:813–820. [PubMed] [Google Scholar]
- 15.Kinane DF, Bartold PM. Clinical relevance of the host responses of periodontitis. Periodontol 2000. 2007;43:278–293. doi: 10.1111/j.1600-0757.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 16.Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005;49:491–516. doi: 10.1016/j.cden.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 19.Haffajee AD, Socransky SS. Introduction to microbial aspects of periodontal biofilm communities, development and treatment. Periodontol 2000. 2006;42:7–12. doi: 10.1111/j.1600-0757.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 20.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 21.Page RC. The pathobiology of periodontal diseases may affect systemic diseases: inversion of a paradigm. Ann Periodontol. 1998;3:108–120. doi: 10.1902/annals.1998.3.1.108. [DOI] [PubMed] [Google Scholar]
- 22.Desvarieux M, Demmer RT, Rundek T, et al. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Circulation. 2005;111:576–582. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pussinen PJ, Vilkuna-Rautiainen T, Alfthan G, et al. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arterioscler Thromb Vasc Biol. 2004;24:2174–2180. doi: 10.1161/01.ATV.0000145979.82184.9f. [DOI] [PubMed] [Google Scholar]
- 24.Mattila KJ, Pussinen PJ, Paju S. Dental infections and cardiovascular diseases: a review. J Periodontol. 2005;76:2085–2088. doi: 10.1902/jop.2005.76.11-S.2085. [DOI] [PubMed] [Google Scholar]
- 25.Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B. Bacteraemia following periodontal procedures. J Clin Periodontol. 2005;32:708–713. doi: 10.1111/j.1600-051X.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 26.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33:401–407. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 27.Takai S, Kuriyama T, Yanagisawa M, Nakagawa K, Karasawa T. Incidence and bacteriology of bacteremia associated with various oral and maxillofacial surgical procedures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:292–298. doi: 10.1016/j.tripleo.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547–558. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak KF, Taylor GW, Dawson DR, Ferguson JE, 2nd, Novak MJ. Periodontitis and gestational diabetes mellitus: exploring the link in NHANES III. J Public Health Dent. 2006;66:163–168. doi: 10.1111/j.1752-7325.2006.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 30.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 31.Michalowicz BS, Hodges JS, DiAngelis AJ, et al. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355:1885–1894. doi: 10.1056/NEJMoa062249. [DOI] [PubMed] [Google Scholar]
- 32.Bobetsis YA, Barros SP, Offenbacher S. Exploring the relationship between periodontal disease and pregnancy complications. J Am Dent Assoc. 2006;137(suppl 2):7S–13S. doi: 10.14219/jada.archive.2006.0403. [DOI] [PubMed] [Google Scholar]
- 33.Meurman JH, Sanz M, Janket SJ. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med. 2004;15:403–413. doi: 10.1177/154411130401500606. [DOI] [PubMed] [Google Scholar]
- 34.Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. 2003;8:38–53. doi: 10.1902/annals.2003.8.1.38. [DOI] [PubMed] [Google Scholar]
- 35.Wiens M, Korzhev M, Krasko, et al. Innate immune defense of the sponge suberites domuncula against bacteria involves a MyD88-dependent signaling pathway. Induction of a perforin-like molecule. J Biol Chem. 2005;280:27949–27959. doi: 10.1074/jbc.M504049200. [DOI] [PubMed] [Google Scholar]
- 36.de Eguileor M, Grimaldi A, Tettamanti G, Valvassori R, Cooper EL, Lanzavecchia G. Lipopolysaccharide-dependent induction of leech leukocytes that cross-react with vertebrate cellular differentiation markers. Tissue Cell. 2000;32:437–445. doi: 10.1054/tice.2000.0132. [DOI] [PubMed] [Google Scholar]
- 37.Weiss CA, 3rd, Wasiluk KR, Kellogg TA, Dunn DL. Bactericidal and endotoxin neutralizing activity of a peptide derived from limulus antilipopolysaccharide factor. Surgery. 2000;128:339–344. doi: 10.1067/msy.2000.108061. [DOI] [PubMed] [Google Scholar]
- 38.Naqvi SB, Mirza T, Sheikh D, Abbas T. Application of limulus amebocyte lysate (LAL) test for detecting endotoxin (pyrogen) in large volume parenterals. Pak J Pharm Sci. 2004;17:89–94. [PubMed] [Google Scholar]
- 39.Dehus O, Hartung T, Hermann C. Endotoxin evaluation of eleven lipopoly-saccharides by whole blood assay does not always correlate with Limulus amebocyte lysate assay. J Endotoxin Res. 2006;12:171–180. doi: 10.1179/096805106X102156. [DOI] [PubMed] [Google Scholar]
- 40.Ong KG, Leland JM, Zeng K, Barrett G, Zourob M, Grimes CA. A rapid highly-sensitive endotoxin detection system. Biosens Bioelectron. 2006;21:2270–2274. doi: 10.1016/j.bios.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 42.Vitkov L, Krautgartner WD, Hannig M. Surface morphology of pocket epithelium. Ultrastruct Pathol. 2005;29:121–127. doi: 10.1080/01913120590916832. [DOI] [PubMed] [Google Scholar]
- 43.Meurman JH, Hamalainen P. Oral health and morbidity – implications of oral infections on the elderly. Gerodontology. 2006;23:3–16. doi: 10.1111/j.1741-2358.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 44.Rose LF, Mealey B, Minsk L, Cohen DW. Oral care for patients with cardiovascular disease and stroke. J Am Dent Assoc. 2002;133(suppl):37S–44S. doi: 10.14219/jada.archive.2002.0378. [DOI] [PubMed] [Google Scholar]
- 45.Roy S, Ellenbogen JM. Seizures, frontal lobe mass, and remote history of periodontal abscess. Arch Pathol Lab Med. 2005;129:805–806. doi: 10.5858/2005-129-805-SFLMAR. [DOI] [PubMed] [Google Scholar]
- 46.Marques da Silva R, Caugant DA, Josefsen R, Tronstad L, Olsen I. Characterization of Streptococcus constellatus strains recovered from a brain abscess and periodontal pockets in an immuno-compromised patient. J Periodontol. 2004;75:1720–1723. doi: 10.1902/jop.2004.75.12.1720. [DOI] [PubMed] [Google Scholar]
- 47.Wagner KW, Schon R, Schumacher M, Schmelzeisen R, Schulze D. Case report: brain and liver abscesses caused by oral infection with Streptococcus intermedius. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e21–e23. doi: 10.1016/j.tripleo.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Gendron R, Grenier D, Maheu-Robert L. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2000;2:897–906. doi: 10.1016/s1286-4579(00)00391-9. [DOI] [PubMed] [Google Scholar]
- 49.Papaioannides D, Boniatsi L, Korantzo-poulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15:77–79. doi: 10.1159/000089391. [DOI] [PubMed] [Google Scholar]
- 50.Bartz H, Nonnenmacher C, Bollmann C, et al. Micromonas (peptostreptococcus) micros: unusual case of prosthetic joint infection associated with dental procedures. Int J Med Microbiol. 2005;294:465–470. doi: 10.1016/j.ijmm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Offenbacher S, Elter JR, Lin D, Beck JD. Evidence for periodontitis as a tertiary vascular infection. J Int Acad Periodontol. 2005;7:39–48. [PubMed] [Google Scholar]
- 52.Kostadinov S, Pinar H. Amniotic fluid infection syndrome and neonatal mortality caused by Eikenella corrodens. Pediatr Dev Pathol. 2005;8:489–492. doi: 10.1007/s10024-005-0010-2. [DOI] [PubMed] [Google Scholar]
- 53.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76:2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 54.Fine DH, Kaplan JB, Kachlany SC, Schreiner HC. How we got attached to Actinobacillus actinomycetemcomitans: a model for infectious diseases. Periodontol 2000. 2006;42:114–157. doi: 10.1111/j.1600-0757.2006.00189.x. [DOI] [PubMed] [Google Scholar]
- 55.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 56.Holt SC, Kesavalu L, Walker S, Genco CA. Virulence factors of Porphyromonas gingivalis. Periodontol 2000. 1999;20:168–238. doi: 10.1111/j.1600-0757.1999.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 57.Eick S, Reissmann A, Rodel J, Schmidt KH, Pfister W. Porphyromonas gingivalis survives within KB cells and modulates inflammatory response. Oral Microbiol Immunol. 2006;21:231–237. doi: 10.1111/j.1399-302X.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 58.Vitkov L, Krautgartner WD, Hannig M. Bacterial internalization in periodontitis. Oral Microbiol Immunol. 2005;20:317–321. doi: 10.1111/j.1399-302X.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 59.Roth GA, Moser B, Huang SJ, et al. Infection with a periodontal pathogen induces procoagulant effects in human aortic endothelial cells. J Thromb Haemost. 2006;10:2256–2261. doi: 10.1111/j.1538-7836.2006.02128.x. [DOI] [PubMed] [Google Scholar]
- 60.Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43:3944–3955. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirakodu SS, Govindaswami G, Novak MJ, Ebersole JL, Novak KF. Optimizing qPCR for the quantification of periodontal pathogesn in a complex plaque biofilm. Open Dent J. 2008;2:49–55. doi: 10.2174/1874210600802010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schroder NW, Schumann RR. Non-LPS targets and actions of LPS binding protein (LBP) J Endotoxin Res. 2005;11:237–242. doi: 10.1179/096805105X37420. [DOI] [PubMed] [Google Scholar]
- 63.Draing C, Pfitzenmaier M, Zummo S, et al. Comparison of lipoteichoic acid from different serotypes of Streptococcus pneumoniae. J Biol Chem. 2006;281:33849–33859. doi: 10.1074/jbc.M602676200. [DOI] [PubMed] [Google Scholar]
- 64.Morath S, Geyer A, Spreitzer I, Hermann C, Hartung T. Structural decomposition and heterogeneity of commercial lipoteichoic acid preparations. Infect Immun. 2002;70:938–944. doi: 10.1128/iai.70.2.938-944.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]