Heart failure (HF) is a leading cause of mortality and morbidity in the industrialized world and imposes a substantial burden on the public health. In the United States, HF is the primary cause of death for more than 60,000 people annually and a contributing factor in over 282,000 cases.1 Despite guideline-recommended therapy for patients with HF and reduced ejection fraction,1 the 1-year mortality in patients with New York Heart Association functional class III to IV HF on maximal medical therapy is 35% to 40%.2 Based on recent estimates, an estimated 5.1 million adult Americans have HF, and projections show that by the year 2030 the prevalence of HF in the United States will increase by 25%.2 By any metric, HF imposes a major public health and financial burden on society. The lack of new disease-modifying pharmacological therapy for HF over the past two decades further amplifies these concerns.
Importance of Hospitalization for Acute HF Syndrome (AHFS)
Hospitalization for AHFS is a significant predictor of increased mortality, recurrent hospitalization, increased resource consumption, worsened functional status, and worsened quality of life.3 Even after excluding patients with shock, several recent studies indicate that the rate of the composite endpoint of death or re-hospitalization at 60-days post discharge is consistently >30% among patients hospitalized for AHFS.4-6 Though studies have identified some patient characteristics affecting the risk of this composite endpoint, no widely accepted risk prediction model has emerged to date.7
Failure of Short-Term Interventions
Previous large-scale studies have examined numerous interventions for preventing post-hospitalization death or re-hospitalization. Although some in-hospital treatments for AHFS have favorably affected in-hospital metrics, such as the rate of decongestion,8, 9 or dyspnea scores,10 nearly all have failed to impact post-hospitalization mortality and/or readmission. Included among these failed interventions are intravenous milrinone,11 hemodynamically-guided therapy,12 tolvaptan,13 levosimendan,14 rolofylline,5 nesiritide,15, 16 and ultrafiltration.17 Currently, the lone exception is recombinant relaxin-2 therapy with the RELAX-AHF trial reporting reduced 180-day mortality but no improvement in the rates of cardiovascular death or rehospitalization.10 Thus, while AHFS hospitalization identifies increased risk, it appears that sustained interventions during the vulnerable early months after a HF hospitalization will be required to affect the high rates of re-hospitalization and mortality in this population.
Why cardiac metabolism as a therapeutic target for HF
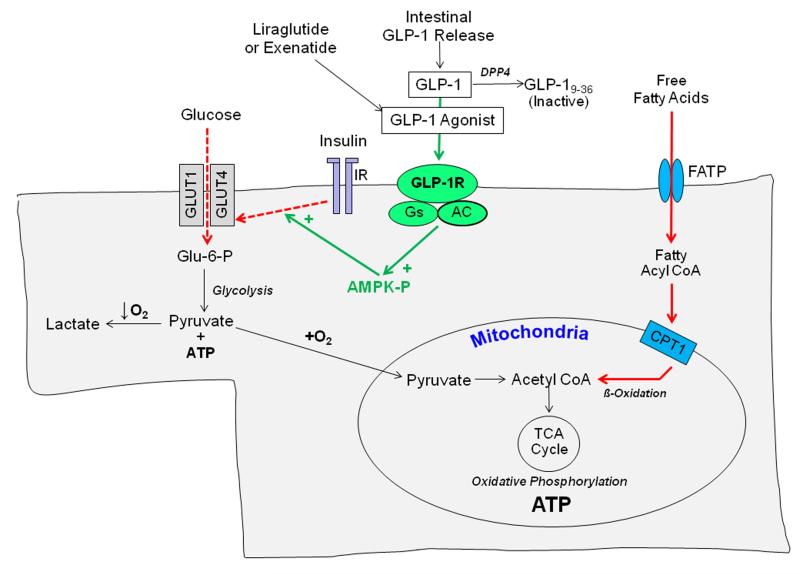
Even in the absence of acute coronary insufficiency, an increasing body of literature supports the concept of the failing heart as an energy-starved organ.18, 19 The heart consumes more energy per gram than any other organ, and myocardial metabolic demands are further increased in the setting of HF by pathological hypertrophy, increased wall stress, neurohormonal stimulation and vasoconstriction. As illustrated in the Figure, cardiac myocytes can use a variety of substrates to generate ATP. Under normal circumstances, fatty acids constitute the predominant fuel for ATP generation by cardiac myocytes.20 In pathologic cardiac hypertrophy, changes in transcription factors such as PPARα and co-factors such as PGC-1α reduce fatty acid oxidation by downregulating genes involved in fatty acid transport19 and utilization21, 22 resulting in an increased reliance on glucose metabolism. However, as HF continues to progress, the myocardium becomes progressively insulin-resistant, which limits uptake of vitally needed glucose by the plasma membrane transporters GLUT1 and GLUT4.23 The net result of these metabolic perturbations is a substantial impairment in both fatty acid and glucose metabolism such that the heart becomes substrate constrained. Importantly, myocardial insulin resistance is observed in both diabetics and nondiabetics.24, 25 Because no currently used HF therapy directly targets these fundamental metabolic derangements, there is an opportunity to develop metabolic modulators as a new class of HF therapeutics.
Figure. Cardiac Myocyte Substrate Utilization in Heart Failure.
Under non-ischemic conditions, cardiac myocytes readily use both fatty acids and glucose to efficiently generate ATP via oxidative phosphorylation. Fatty acids are the predominant substrate under normal conditions. In chronically overloaded hearts, altered expression of key metabolic genes reduces fatty acid uptake and metabolism (solid red arrows). As heart failure progresses, cardiac myocyte insulin resistance develops and leads to reductions in glucose uptake via the transporters GLUT1 and GLUT4 (dotted red arrows). Together, these changes impair the uptake and utilization of two chief substrates for ATP generation via oxidative phosphorylation. Either naturally occurring GLP-1 or degradation-resistant GLP-1 agonists reduce insulin resistance and increase cardiac myocyte glucose uptake via signaling through the GLP-1R receptor that induces phosphorylation (activation) of AMP-activated protein kinase (AMPK), as highlighted by green arrows.
Abbreviations: GLP-1 – glucagon-like peptide; FATP – fatty acid transport protein; CPT1 -carnitine palmitoyltransferase 1; IR – insulin receptor; DPP IV – dipeptidyl-peptidase 4; Glu-6-P – Glucose-6-phosphate; AC – adenylate cyclase; AMPK-P – activated AMPK.
GLP-1 is a metabolic modulator with therapeutic potential in HF
Glucagon-like peptide-1 (GLP-1) is a naturally occurring incretin peptide released from intestinal L cells that enhances cellular glucose uptake by stimulating insulin secretion and by enhancing insulin sensitivity in target tissues.26 In normal physiology, endogenous GLP-1 is implicated in the control of appetite and satiety.27 Administration of exogenous GLP-1 as a continuous infusion in patients with type 2 diabetes causes an impressive increase in insulin sensitivity in both skeletal muscle and adipose tissue, with substantial improvements in insulin-mediated glucose uptake.28 Importantly, the insulin stimulating actions of GLP-1 cease at glucose levels below 4 mM (72 mg/dl) which mitigates the risk of hypoglycemia.28, 29 GLP-1 also promotes increased glucose uptake in target tissues via mechanisms independent of increases in circulating insulin.30 Receptors for GLP-1 have been identified in rodent31 and human32 myocardium, thereby identifying myocardium as a direct target for GLP-1 action. As illustrated in the Figure, recent studies indicate that GLP-1 receptor binding activates adenylate cyclase and phosphorylates (activates) the cAMP-dependent protein kinase (AMPK).33 In turn, activated AMPK enhances insulin-dependent signaling, increased glucose and other cardioprotective actions. Recognizing that mitochondrial dysfunction contributes to defects in substrate utilization in HF,34 it is relevant that GLP-1 agonists improve mitochondrial function in vitro35 and reduce mitochondrial oxidative stress and damage within the hearts of rodents with type 2 diabetes mellitus.36
These observations support GLP-1 as a potential pharmacologic modulator to enhance myocardial glucose metabolism. In contrast to recombinant GLP-1 that requires continuous infusion due to rapid hydrolysis in vivo, degradation-resistant GLP-1 analogues permit intermittent subcutaneous administration with dosing intervals ranging from 12-hours to one week. Two GLP-1 agonists, including exenatide and liraglutide, are currently FDA approved for use in diabetes mellitus and offer interesting cardiovascular effects that may potentially be important in HF. Exenatide is available in both twice daily and once weekly formulations and liraglutide is administered once daily. More generally, though small molecules and devices have dominated HF therapeutics, protein-based therapies administered by SQ injection expand the potential for pathophysiologic modulation.
Acute Effects of GLP-1 in HF
In both preclinical studies employing animal models and clinical investigations, short-term GLP-1 infusions have demonstrated salutary cardiac effects. In conscious, chronically instrumented dogs, GLP-1 (1.5 pmol/kg/min) attenuated myocardial stunning after brief periods of myocardial ischemia with regional wall motion and isovolumic left ventricular relaxation recovering significantly earlier in treated vs. control animals (6 vs. 24 hours). GLP-1 induced equivalent protective effects in rat models of transient37 and sustained38 coronary occlusion. In dogs with pacing-induced cardiomyopathy, a 48-hour infusion of GLP-1 increased stroke volume, LV dP/dt and LVEF while decreasing LVEDP and SVR. Identical dosing of GLP-1 produced no changes in normal control normal dogs,39 demonstrating that GLP-1 specifically targets pathological processes. Likewise, in 11 patients with severe left ventricular dysfunction following acute myocardial infarction and reperfusion, subjects treated with a 3-day GLP-1 infusion had greater increase in LVEF (from 29±2 % to 39±3%, p<0.01) compared with historical controls (28±2% to 29±2%) and GLP-1 treated patients had a shorter length of stay (6 vs. 10 days, p<0.02).40 In a randomized, double-blind, crossover clinical trial in 20 patients with a low LVEF (<35%) and NYHA III-IV HF symptoms,40 Nathanson et al. reported that the GLP-1 agonist exenatide significantly increased cardiac index and decreased pulmonary wedge pressure at 3 and 6 hours after the infusion had begun while placebo infusion induced no changes in these variables.41 In contrast, Halbirk et al. did not observe change in LVEF or cardiac index during a 48-hour infusion of recombinant GLP-1 in nondiabetic patients with ischemic heart disease and NYHA II-III symptoms of HF.42 The predominance of data indicates that GLP-1 is neither a positive nor negative inotrope.
Chronic GLP-1 Therapy in HF
Data supporting a therapeutic benefit for GLP-1 therapy in patients with chronic HF are derived from small pilot studies and retrospective analyses. In 12 patients with chronic severe HF (NYHA III-IV), a continuous, five-week infusion of recombinant GLP-1 was associated with significant improvements in LVEF, Minnesota QOL score, 6 minute walk distance, and exercise VO2max, while historical controls had no changes in these parameters.43 The favorable effects of GLP-1 therapy were similar in magnitude in diabetics and in non-diabetics, suggesting effects beyond glycemic control. Eight GLP-1 treated patients had reduced requirements for diuretics. Four patients in the GLP-1 treated group and 2 in the control group experienced asymptomatic hypoglycemia (plasma glucose 50-70 mg/dl). Nevertheless, the diabetic patients who received GLP-1 had better glycemic control and reduced requirements for insulin or oral hypoglycemic agents compared to diabetic patients in the control group. A subsequent exploratory study evaluated three different doses of the long-acting GLP-1 agonist (albiglutide) in a 12-week, randomized, double-blind clinical trial including outpatients with stable NYHA class 2 or 3 HF and LVEF≤40%. The administration of albiglutide up to 30 mg/wk for 12 weeks to subjects with mild-moderate heart failure was generally well-tolerated. Compared with placebo, peak VO2 improved significantly by 1.51 mL/kg/min (95% CI 0.21- 2.82 ml/kg/min, p=0.024) in subjects on albiglutide 30 mg, but there were no treatment-related improvements in LV size, LV function, 6-minute walk or quality of life scores.[GlaxoSmithKline, unpublished data, posted at clinicaltrials.gov/show/NCT01357850, 2013]
On the basis of preclinical and early phase clinical data supporting the concept that GLP-1 induces favorable effects on myocardial glucose metabolism with a favorable safety profile in large numbers of patients with diabetes, we designed a randomized, blinded, proof-of-concept trial of GLP-1 therapy in HF patients already treated with evidence-based medications. Because preclinical data indicate that myocardial metabolism is particularly abnormal in more advanced HF (NYHA III-IV) and that GLP-1 appears more effective in high-risk settings (following ischemia and heart surgery), we postulate that patients with more advanced HF are most likely to benefit from GLP-1 agonist therapy. Moreover, because the months after AHFS hospitalization are a vulnerable period for adverse outcomes, we felt that patients hospitalized with AHFS are a particularly opportune group to target with sustained GLP-1 therapy. Accordingly, the NHLBI HF Network has designed and initiated a trial to test the hypothesis that sustained therapy with a subcutaneous GLP-1 agonist initiated during the post- AHFS discharge period will be associated with greater clinical stability through 180 days as assessed by a composite clinical endpoint. While very long-acting GLP-1 agonists have the advantage of ease of administration (one dose per week), a concern with these formulations is the long interval between their initiation and steady-state levels, particularly when incremental dose escalation is envisioned. Given the nontrivial risks of re-hospitalization during the first six weeks after discharge, we selected liraglutide because of its once daily dosing while allowing steady-state levels within a few days after initiating each dose.
Study Design and Patient Population
The Functional Impact of GLP-1 for Heart Failure Treatment (FIGHT) study is a randomized, double-blinded, placebo-controlled clinical trial in high-risk patients with reduced ejection fraction (LVEF ≤ 40%) and AHFS who are treated for 180 days with placebo or a GLP-1 agonist delivered by daily SQ injection (clinicaltrials.gov, NCT01800968). Recognizing that insulin resistance has been observed in advanced HF without diabetes,24, 25 FIGHT includes both diabetics and nondiabetics and consists of screening, study drug titration, and follow-up phases. A total of 300 patients meeting eligibility criteria are being enrolled and randomized in the study. Patients admitted to the hospital due to AHFS are screened for basic inclusion and exclusion criteria (Table 1) and consenting patients meeting these criteria are enrolled either in the hospital shortly before their anticipated discharge or as an outpatient within 14 days of discharge.
Table 1.
FIGHT Inclusion and Selected Exclusion Criteria
| Inclusion Criteria |
| Age ≥ 18 years |
| AHFS (defined by both symptoms and signs) is the primary cause of hospitalization |
| Prior clinical diagnosis of HF |
| LVEF ≤ 40% during the preceding 3 months |
| On evidence-based medication for HF (including beta-blocker and ACE-inhibitor/ARB) or previously deemed intolerant |
| Use of at least 40 mg of furosemide total daily dose (or equivalent) prior to admission for AHFS |
| Exclusion Criteria |
| Acute coronary syndrome or PCI within the past 4 weeks |
| Ongoing hemodynamically significant arrhythmias |
| VAD or heart transplant likely within the next 6 months |
| Severe anemia (Hgb < 8.0 g/dl) |
| Severe renal, hepatic or pulmonary disease |
| Primary infiltrative or restrictive cardiomyopathy |
| Severe aortic or mitral stenosis |
| Active infection driving AHFS hospitalization |
| Terminal illness (other than HF) with expected survival of less than 1 year |
| Previous adverse reaction to or ongoing treatment with GLP-1 agonist Rx |
| Type I diabetes mellitus or very poor glycemic control at the time of randomization |
| History of gastroparesis, pancreatitis or medullary thyroid cancer |
Randomization, Stratification and Blinding
After providing informed consent and signing the ICF, all subjects who fulfill all the inclusion criteria and none of the exclusion criteria are randomized. Randomization to GLP-1 agonist/placebo (1:1 allocation ratio) is stratified by site and presence or absence of diabetes using a permuted block randomization scheme to ensure relatively equal distribution of subjects to each arm within each clinical site. Before study drug administration, patients undergo baseline laboratory evaluations (metabolic profiling, heart failure biomarkers), echocardiography, a 6-minute walk test, a quality of life assessment, and training in study drug self administration. Patients are observed as they self administer their first dose of study drug.
Drug Intervention, Dose Titration and Follow-Up
Participants are started on placebo or GLP-1 agonist (liraglutide; at 0.6 mg SQ daily for 7 days). Liraglutide (Victoza®) is an FDA-approved human GLP-1 analog with 97% homology to native GLP-1. To enhance patient tolerance and allow adjustments in other diabetes treatments as needed, the dose is increased to 1.2 mg after one week of therapy and then the target dose of 1.8 mg at day 30. The 1.8 mg dose continues from day 30 through day 180. All participants receive study visits at 30, 90 and 180 days post-randomization and study phone calls at intervening times. Repeat echocardiography and metabolic profiling will be performed at the 180 day visit. Participants are called at day 210 ± 7 to determine their adverse event status.
The most common adverse effects of liraglutide are gastrointestinal related and include nausea, vomiting, diarrhea, dyspepsia, weight loss and constipation. Gastrointestinal adverse reactions are dose-related and typically decrease over time. The hypoglycemic effects of GLP-1 agonists are glucose-dependent and effectively nil in the presence of a normal circulating glucose. Accordingly, the risk of hypoglycemia with study-drug is low in non-diabetics. Nevertheless, all participants are counseled on the signs and symptoms of hypoglycemia, and the appropriate treatment prior to discharge. In diabetic patients, plans for hypoglycemia risk reduction include adjustments to insulin or insulin secretagogues (sulfonylureas or meglitinide) dosing at the time of study drug initiation or up-titration, at least daily monitoring of blood sugar and close follow-up with the provider managing the subject’s diabetes.
The FIGHT protocol allows for adjustment of standard HF therapies, including attempted uptitration of neurohumoral antagonists if not at goal or maximally tolerated doses, during and after the subjects’ initial hospitalization. There are no established guidelines for the treatment of diabetes in patients with AHFS. However, thiazolidinedione use is not recommended in patients with symptomatic HF in the American Diabetes Association (ADA) 2012 Standards of Medical Care in Diabetes.44 Metformin may be used in patients with stable HF provided that renal function is normal. However, it should be avoided in unstable or hospitalized patients with HF.
Endpoints
Primary End Point
End-point selection for high-risk HF populations is critical and often debated. During the course of the study we expect that a certain number of subjects will achieve a clinical endpoint such as death or HF hospitalization. These clinically meaningful events must be included in the study endpoint, but relying on such events alone will not provide sufficient power in a phase 2 study of 300 subjects. As recently highlighted,45 there is increasing evidence that relative change in circulating natriuretic peptides over time helps stratify risk and provides a surrogate measure of HF severity, with decreases associated with improvement in clinical outcomes and responses to therapy.46-48 As such, we are measuring serial NT-proBNP levels in all subjects after discharge and using the proportional change in NT-proBNP over time to contribute to the study endpoint for subjects who do not experience a clinical event. Thus, the primary end point of FIGHT is a global rank endpoint in which all participants, regardless of treatment assignment, are ranked across three hierarchical groups: 1) time to death, 2) time to HF hospitalization, and 3) time-averaged proportional change in NT-proBNP (from baseline to 180 days). We will then compare the distribution of the ranks in the active and placebo arms to determine the overall effect of liraglutide on heart failure stability. Hospitalization for HF is distinguished from hospitalizations due to other causes based on the presence of both clinical manifestations of worsening HF and additional or increased therapy specifically for the treatment of worsening HF. An adjudication committee assesses the cause of hospitalizations in a uniform manner.
Secondary End Points
The principal secondary end points in this study include change in cardiac structure and function (by echocardiography) from baseline to 180 days. The most important metrics are left ventricular end-systolic volume, left ventricular end-diastolic volume, left-ventricular ejection fraction, and E/E’ ratio. Additional secondary endpoints include functional status based on the six-minute walk distances at 30, 90, and 180 days, changes in symptoms, based on the KCCQ, from baseline to 180 days, and individual components of the primary endpoint at 30, 90 and 180 days after randomization.
Tertiary Endpoints
Additional parameters assessed for efficacy include the change in AHFS biomarker panel (including aldosterone, cystatin C, hsCRP) from baseline to 30, 90 and 180 days. Several tertiary endpoints focus on the safety and efficacy of GLP-1 agonist therapy for diabetes in patients with advanced heart failure including: change in glycosylated hemoglobin at 30, 90 and 180 days after randomization, change in weight, change in insulin resistance (as assessed by HOMA-IR) in both diabetic and non-diabetic participants, and changes in fasting lipids.
Statistical Considerations
The primary analysis will be conducted on an intention-to-treat (ITT) basis. The ITT population includes all participants who are randomized. The analysis of the primary endpoint will be based on the Wilcoxon test statistic. For the primary comparison, participants randomized to liraglutide will be compared to placebo subjects using a Type I error rate of 0.05. For secondary and tertiary endpoints, general linear models and nonparametric approaches will be used to analyze the continuous outcomes. For binary outcomes, Chi-square tests and Fisher’s exact test will be used for unadjusted comparisons. For adjusted comparisons, logistic regression analysis will be used to compare liraglutide vs. placebo with the estimated odds ratio and associated 95% confidence interval. Unadjusted time-to-event comparisons will be conducted using Kaplan-Meier survival estimates and log-rank tests. For adjusted analyses, Cox proportional hazards regression models will be used to estimate hazard ratios.
Sample Size and Power Calculation
Data from the Diuretic Optimization Strategies Evaluation (DOSE) trial were used to estimate 60-day event rates for clinical endpoints including death, all-cause hospitalization, HF hospitalization, and composite endpoints including death or all-cause hospitalization and death or HF hospitalization (see Table 2).49 Data from the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial also provided relevant information regarding 6-month all-cause mortality and HF hospitalization event rates.50 In that population, the estimated 6-month all-cause mortality rate and HF hospitalization or all-cause mortality rates were approximately 13% and 30%, respectively. To account for the possible higher-risk patient population in FIGHT, we have assumed 180-day event rates of 15% for all-cause mortality and 35% for the composite of HF hospitalization or all-cause mortality.
Table 2.
Power Summary using the global-rank endpoint with all-cause death, HF hospitalization, and difference (Δ) in NT-proBNP
| Time- averaged Δ NT-proBNP |
Power for the Δ NT-proBNP Endpoint |
Global Rank Power (RRR* of 20%) |
Power for the Clinical Endpoint with RRR of 20% |
Global Rank Power (RRR of 25% |
Power for Clinical Endpoint with RRR of 25% |
|---|---|---|---|---|---|
| 0.4 SD | 92% | 74% | 21% | 83% | 31% |
| 0.5 SD | 98% | 86% | 21% | 92% | 31% |
| 0.6 SD | 99% | 93% | 21% | 97% | 31% |
RRR=relative risk reduction. SD=standard deviation. Δ=difference
To estimate the power of the primary endpoint for the FIGHT study, we have conducted a simulation study where the clinical events and biomarker changes were varied across a range of parameters. For the clinical events of all-cause death and HF hospitalizations, we assumed 20% and 25% reductions for the active treatment groups compared to the placebo group. For the NT-proBNP components, we assumed 0.4 to 0.6 standard deviation reductions compared to the placebo group. The estimated power shown in Table 2 was based on 1000 simulated data sets for each parameter setting. All simulations used 145 subjects per treatment group and assumed no missing data. Each computed test statistic was compared with the 2-sided 0.05 level. To allow for approximately 3-5% missing data for the time-averaged NT-proBNP component, the total sample size for FIGHT was increased to 300 subjects or 150 subjects per treatment group. This sample size provides 92% power under the assumptions of a 25% reduction in clinical events (both mortality and HF-hospitalizations) along with a 0.5 standard deviation reduction in time-averaged NT-proBNP from the time of enrollment to 180 days. With a 25% reduction in clinical events and a 0.4 standard deviation reduction in NT-proBNP, the estimated power would still be in excess of 80%.
Safety
Interim data analysis for efficacy and futility will not be conducted due to relatively small size and short duration of this phase-II clinical trial. Safety data, summarized at the treatment level, will be assessed approximately every 6 months by the NHLBI-appointed DSMB. The safety analyses will be based on the entire ITT population. Safety will be evaluated by comparing the occurrence of adverse events (AEs) and changes in laboratory values of the active arm compared to placebo. The number and percentage of participants experiencing treatment emergent AEs will be tabulated by treatment group, body system, and preferred term. The percentages between treatment groups will be compared using Fisher’s exact test.
Conclusions
A growing evidence base indicates that myocardial metabolic abnormalities, including reduced fatty acid oxidation and myocardial insulin resistance impairing glucose utilization, contribute to the syndrome of HF with reduced ejection fraction. Recognizing that no currently used HF therapy directly targets these fundamental metabolic derangements, the FIGHT study is designed to test the hypothesis that sustained therapy with a subcutaneous GLP-1 agonist therapy will improve clinical stability in patients with advanced HF by improving myocardial glucose utilization. This phase II proof-of-concept trial employs a randomized, placebo-controlled, double-blind design, a hierarchical composite endpoint, and an intention-to-treat analysis framework. We note that a particular novel aspect of the design is the targeting of patients hospitalized for AHFS to identify patients at high risk while facilitating enrollment, baseline testing and research subject training. Another novel feature of this study is its primary endpoint, which integrates clinically meaningful events as well as longitudinal measures of heart failure severity in subjects who do not experience events into a single hierarchical endpoint. We submit that positive results demonstrating an efficacy signal should motivate a Phase 3 study of GLP-1 agonists in high risk HFrEF patients powered for clinical events. A negative result will strongly suggest that such agents are ineffective in this population.
Acknowledgments
Sources of Funding This work was supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) (coordinating center: U10 HL084904; regional clinical centers: U01 HL084861, U10 HL110312, U109 HL110337, U01 HL084889, U01 HL084890, U01 HL084891, U10 HL110342, U10 HL110262, U01 HL084931, U10 HL110297, U10 HL110302, U10 HL110309, U10 HL110336, U10 HL110338).
Disclosures Dr. Margulies reports unpaid advisory committee membership for Novo Nordisk; and research grant support from Juventis Therapeutics, Celladon Corporation, Thoratec Corporation and Innolign Biomedical, LLC. Dr Anstrom has received research support from AstraZeneca, Eli Lilly & Company, and Medtronic; has served as a consultant for Abbott Vascular, AstraZeneca, Bristol-Meyers Squibb, Pfizer (modest), GSK, and Ikaria; and has served on Data Monitoring Committees for Pfizer and Vertex. Dr Hernandez reports consulting for Novartis, Janssen, and Bristol-Myers Squibb and that his institution has received grants from Novartis, Janssen, and Bristol-Myers Squibb. Dr Redfield reports receiving royalties from Anexion and payment for educational presentations from the Heart Failure Society of America and reports unpaid advisory committee membership for Novartis. Dr Braunwald reports grants to his institution from AstraZeneca, Johnson & Johnson, Merck, sanofi-aventis, Daiichi Sankyo, GlaxoSmithKline, Bristol-Myers Squibb, Beckman Coulter, Roche Diagnostics, and Pfizer; uncompensated personal fees from Merck; consulting for Genzyme, Amorcyte, Medicines Co, CardioRentis, and sanofi-aventis; uncompensated lectures for Merck & CVRx; and payment for lectures from Eli Lilly, Daiichi Sankyo, Menarini International, Medscape, and Bayer, outside the submitted work. Dr. Cappola reports consulting for TEVA Pharmaceuticals and being a coinventor on pending patents for neuregulin and sFlt-1 as heart failure biomarkers and inventor on a patent for gene expression biomarkers of heart transplant rejection.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143–52. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munger MA, Carter O. Epidemiology and practice patterns of acute decompensated heart failure. Am J Health Syst Pharm. 2003;60(Suppl 4):S3–6. doi: 10.1093/ajhp/60.suppl_4.S3. [DOI] [PubMed] [Google Scholar]
- 4.Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF, Jr., Gheorghiade M, O’Connor CM. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10:460–466. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 6.Chin MH, Goldman L. Correlates of early hospital readmission or death in patients with congestive heart failure. Am J Cardiol. 1997;79:1640–1644. doi: 10.1016/s0002-9149(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 7.Ross JS, Mulvey GK, Stauffer B, Patlolla V, Bernheim SM, Keenan PS, Krumholz HM. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168:1371–1386. doi: 10.1001/archinte.168.13.1371. [DOI] [PubMed] [Google Scholar]
- 8.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 10.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr., Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 11.Cuffe MS, Califf RM, Adams KF, Jr., Benza R, Bourge R, Colucci WS, Massie BM, O’Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 12.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 13.Konstam MA, Gheorghiade M, Burnett JC, Jr., Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 14.Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Poder P, Kivikko M. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 15.Stiles S. [accessed November 26, 2010];ASCEND-HF: Nesiritide safe but of limited dyspnea benefit in acute HF. TheHeart.Org. 2010 http://www.theheart.org/article/1147999.do.
- 16.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, Lewinter MM, Konstam MA, Huggins GS, Rouleau JL, O’Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O’Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Davila-Roman VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM, NHLBI Heart Failure Clinical Research Network Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–43. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Haehling S. Recent developments in the treatment of heart failure: highlights from the American Heart Association’s Scientific Sessions, Los Angeles, California, 3 - 7 December 2012. Expert opinion on investigational drugs. 2013;22:933–937. doi: 10.1517/13543784.2013.798301. [DOI] [PubMed] [Google Scholar]
- 18.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 19.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102:808–813. doi: 10.1073/pnas.0408962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolwicz SC, Jr., Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res. 2013;113:603–616. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 22.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 23.Abel ED. Myocardial insulin resistance and cardiac complications of diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:219–226. doi: 10.2174/1568008054064869. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJ. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30:527–532. doi: 10.1016/s0735-1097(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 26.Fields AV, Patterson B, Karnik AA, Shannon RP. Glucagon-like peptide-1 and myocardial protection: more than glycemic control. Clin Cardiol. 2009;32:236–243. doi: 10.1002/clc.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle ME, Egan JM. Glucagon-like peptide-1. Recent Prog Horm Res. 2001;56:377–399. doi: 10.1210/rp.56.1.377. [DOI] [PubMed] [Google Scholar]
- 28.Egan JM, Meneilly GS, Habener JF, Elahi D. Glucagon-like peptide-1 augments insulin-mediated glucose uptake in the obese state. J Clin Endocrinol Metab. 2002;87:3768–3773. doi: 10.1210/jcem.87.8.8743. [DOI] [PubMed] [Google Scholar]
- 29.Perfetti R, Merkel P. Glucagon-like peptide-1: a major regulator of pancreatic beta-cell function. Eur J Endocrinol. 2000;143:717–725. doi: 10.1530/eje.0.1430717. [DOI] [PubMed] [Google Scholar]
- 30.Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, Shannon RP. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail. 2010;3:512–521. doi: 10.1161/CIRCHEARTFAILURE.109.900282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137:2968–2978. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 32.Wei Y, Mojsov S. Distribution of GLP-1 and PACAP receptors in human tissues. Acta Physiol Scand. 1996;157:355–357. doi: 10.1046/j.1365-201X.1996.42256000.x. [DOI] [PubMed] [Google Scholar]
- 33.Noyan-Ashraf MH, Shikatani EA, Schuiki I, Mukovozov I, Wu J, Li RK, Volchuk A, Robinson LA, Billia F, Drucker DJ, Husain M. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127:74–85. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz K, Siddiqi N, Singh S, Neil CJ, Dawson DK, Frenneaux MP. The breathing heart - mitochondrial respiratory chain dysfunction in cardiac disease. Int J Cardiol. 2014;171:134–143. doi: 10.1016/j.ijcard.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Chang G, Zhang D, Liu J, Zhang P, Ye L, Lu K, Duan Q, Zheng A, Qin S. Exenatide protects against hypoxia/reoxygenation-induced apoptosis by improving mitochondrial function in H9c2 cells. Exp Biol Med (Maywood) 2014;239:414–422. doi: 10.1177/1535370214522177. [DOI] [PubMed] [Google Scholar]
- 36.Monji A, Mitsui T, Bando YK, Aoyama M, Shigeta T, Murohara T. Glucagon-like peptide-1 receptor activation reverses cardiac remodeling via normalizing cardiac steatosis and oxidative stress in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;305:H295–304. doi: 10.1152/ajpheart.00990.2012. [DOI] [PubMed] [Google Scholar]
- 37.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 38.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 39.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 40.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 41.Nathanson D, Ullman B, Lofstrom U, Hedman A, Frick M, Sjoholm A, Nystrom T. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double-blind, randomised controlled clinical trial of efficacy and safety. Diabetologia. 2012;55:926–935. doi: 10.1007/s00125-011-2440-x. [DOI] [PubMed] [Google Scholar]
- 42.Halbirk M, Norrelund H, Moller N, Holst JJ, Schmitz O, Nielsen R, Nielsen-Kudsk JE, Nielsen SS, Nielsen TT, Eiskjaer H, Botker HE, Wiggers H. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1096–1102. doi: 10.1152/ajpheart.00930.2009. [DOI] [PubMed] [Google Scholar]
- 43.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 44.Standards of medical care in diabetes--2012. Diabetes Care. 2012;35:S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desai AS. Are serial BNP measurements useful in heart failure management? Serial natriuretic peptide measurements are not useful in heart failure management: the art of medicine remains long. Circulation. 2013;127:509–516. doi: 10.1161/CIRCULATIONAHA.112.120493. [DOI] [PubMed] [Google Scholar]
- 46.Fruhwald FM, Fahrleitner-Pammer A, Berger R, Leyva F, Freemantle N, Erdmann E, Gras D, Kappenberger L, Tavazzi L, Daubert JC, Cleland JG. Early and sustained effects of cardiac resynchronization therapy on N-terminal pro-B-type natriuretic peptide in patients with moderate to severe heart failure and cardiac dyssynchrony. Eur Heart J. 2007;28:1592–1597. doi: 10.1093/eurheartj/ehl505. [DOI] [PubMed] [Google Scholar]
- 47.Masson S, Latini R, Anand IS, Barlera S, Angelici L, Vago T, Tognoni G, Cohn JN. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial) J Am Coll Cardiol. 2008;52:997–1003. doi: 10.1016/j.jacc.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 48.Latini R, Masson S, Anand I, Judd D, Maggioni AP, Chiang YT, Bevilacqua M, Salio M, Cardano P, Dunselman PH, Holwerda NJ, Tognoni G, Cohn JN. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2002;106:2454–2458. doi: 10.1161/01.cir.0000036747.68104.ac. [DOI] [PubMed] [Google Scholar]
- 49.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr., Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]