In a multicenter cohort, unmasking immune reconstitution inflammatory syndrome (IRIS) was observed in 12% of HIV-associated lymphomas. Presentation and survival for lymphoma IRIS were similar to non-IRIS, with possibly increased early mortality among IRIS cases.
Keywords: HIV/AIDS, lymphoma, Hodgkin lymphoma, non-Hodgkin lymphoma, immune reconstitution inflammatory syndrome
Abstract
Background. Lymphoma incidence is increased among human immunodeficiency virus (HIV)–infected individuals soon after antiretroviral therapy (ART), perhaps due to unmasking immune reconstitution inflammatory syndrome (IRIS). Clinical characteristics and survival for unmasking lymphoma IRIS have not been described.
Methods. We studied lymphoma patients in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) from 1996 until 2011. Unmasking lymphoma IRIS was defined as lymphoma within 6 months after ART accompanied by a ≥0.5 log10 copies/mL HIV RNA reduction. Differences in presentation and survival were examined between IRIS and non-IRIS cases.
Results. Of 482 lymphoma patients, 56 (12%) met criteria for unmasking lymphoma IRIS. Of these, 12 (21%) had Hodgkin lymphoma, 22 (39%) diffuse large B-cell lymphoma, 5 (9%) Burkitt lymphoma, 10 (18%) primary central nervous system lymphoma, and 7 (13%) other non-Hodgkin lymphoma. Median CD4 cell count at lymphoma diagnosis among IRIS cases was 173 cells/µL (interquartile range, 73–302), and 48% had suppressed HIV RNA <400 copies/mL. IRIS cases were similar overall to non-IRIS cases in histologic distribution and clinical characteristics, excepting more frequent hepatitis B and C (30% vs 19%, P = .05), and lower HIV RNA at lymphoma diagnosis resulting from the IRIS case definition. Overall survival at 5 years was similar between IRIS (49%; 95% confidence interval [CI], 37%–64%) and non-IRIS (44%; 95% CI, 39%–50%), although increased early mortality was suggested among IRIS cases.
Conclusions. In a large HIV-associated lymphoma cohort, 12% of patients met a uniformly applied unmasking lymphoma IRIS case definition. Detailed studies of lymphoma IRIS might identify immunologic mechanisms of lymphoma control.
Individuals infected with human immunodeficiency virus (HIV) have increased incidence of non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) during the first 6 months after antiretroviral therapy (ART) [1–3]. This may be due to immune reconstitution inflammatory syndrome (IRIS). IRIS is well described for opportunistic infections and Kaposi sarcoma (KS), and is classified as “unmasking” when it leads to a new HIV-associated condition that was not evident prior to ART, or “paradoxical” when it leads to worsening of a condition recognized and treated prior to ART [4–11]. Clinical characteristics and survival for lymphoma IRIS are unknown. We identified patients in a large HIV-associated lymphoma cohort meeting a uniformly applied unmasking IRIS case definition, and compared lymphoma IRIS and non-IRIS cases. Our data did not allow us to distinguish paradoxical lymphoma IRIS from refractory lymphoma due to aggressive tumor behavior alone, and we therefore focused on unmasking lymphoma IRIS.
METHODS
Patients
The Center for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) cohort includes >27 000 HIV-infected adults 18 years and older receiving care since 1 January 1995 at 8 United States CFAR sites [12]. We examined individuals with NHL or HL diagnosed between 1 January 1996 and 31 December 2011. Follow-up was administratively censored on 31 December 2011.
Procedures
CNICS captures comprehensive clinical data through electronic health records (EHRs) and other institutional data systems at each site. Historical information are collected upon cohort entry. Data quality is assessed prior to transmission to CNICS. After integration into the CNICS repository, data undergo extensive quality assurance. A standardized cancer verification procedure has been established [13], and cancer diagnoses are reviewed to collect information regarding type, histology, stage, and treatment. If >1 lymphoma diagnosis or relapse was recorded, we analyzed the first occurrence. Patients are enrolled in CNICS upon entering care at HIV clinics, but data may be available from EHRs prior to enrollment. To increase generalizability, we included individuals diagnosed with lymphoma before and after CNICS entry to avoid excluding patients newly diagnosed with HIV, out of care, or transferring HIV care at the time of lymphoma diagnosis. Mortality data are obtained from clinic sources and the Social Security Death Index.
Unmasking lymphoma IRIS was defined as NHL or HL diagnosed within 6 months after ART initiation with virologic suppression, defined as ≥0.5 log reduction in HIV RNA log10 copies/mL. This was modified from existing KS IRIS definitions, and IRIS definitions for tuberculous and cryptococcal disease taken from the International Network for the Study of HIV-associated IRIS [4, 5, 10, 11, 14]. For HIV RNA, the pre-ART measurement was the closest value to ART start date within 3 months before the ART start date. Post-ART HIV RNA at lymphoma diagnosis was the value closest to lymphoma diagnosis, which occurred after ART start date and up to 3 months after lymphoma diagnosis. For non-IRIS cases, HIV RNA at lymphoma diagnosis was the value closest to lymphoma diagnosis beginning 3 months before until 3 months after. Suppressed HIV RNA was defined as <400 copies/mL. CD4 count at lymphoma diagnosis was the value closest to lymphoma diagnosis beginning 3 months before until 3 months after. Nadir CD4 count was the lowest CD4 count at any time on or before the date of CD4 count at lymphoma diagnosis. Hepatitis B coinfection was defined as any positive hepatitis B surface antigen or DNA result, and hepatitis C coinfection as any positive hepatitis C antibody or RNA result, before or until 6 months after lymphoma diagnosis.
Statistical Analysis
Differences between IRIS and non-IRIS were assessed using χ2 or Fisher exact test, 1-way analysis of variance, and Kruskal-Wallis test. To assess CD4 and HIV RNA during the 12 months before and after lymphoma diagnosis, summative curves were derived by plotting median and interquartile values at each monthly time point across all patients within each group. Individual patient–level CD4 and HIV RNA curves were generated by plotting all available measurements for each patient over this time period and imputing values in between assuming a linear trajectory. To estimate trends over the entire 2-year period, first available measurements within the time period were carried backward to 12 months before lymphoma diagnosis, and last available measurements were carried forward to 12 months after lymphoma diagnosis. CD4 and HIV RNA values for each patient at each monthly time point were assigned based on the value at which individual curves intersected each monthly time point.
Mortality rates were calculated as number of deaths per 100 person-years of follow-up. Follow-up time was calculated from date of lymphoma diagnosis until administrative censoring, death, or loss to follow-up. Loss to follow-up date was based on last date of any clinical activity in CNICS. To minimize survival bias, patients with lymphoma diagnosed before HIV clinic attendance and CNICS enrollment were treated as late entries who contributed follow-up time only after CNICS entry [15]. Kaplan-Meier curves were used to estimate overall survival after lymphoma diagnosis, and Cox proportional hazards were used to assess differences in survival between IRIS and non-IRIS.
We conducted sensitivity analyses varying the IRIS definition to include (1) lymphoma within 3 months of ART accompanied by ≥0.5 log HIV RNA reduction; (2) lymphoma within 6 months of ART accompanied by ≥1 log HIV RNA reduction; and (3) lymphoma within 3 months of ART accompanied by ≥1 log HIV RNA reduction. We performed sensitivity analyses restricted to patients diagnosed with lymphoma after CNICS enrollment. All analyses were conducted using SAS version 9.3. A 2-sided α value of .05 was used to assess statistical significance. Patients were excluded from analyses that included variables for which data were missing.
RESULTS
Of 24 203 HIV-infected individuals in CNICS, 482 (2%) individuals were diagnosed with lymphoma between 1996 and 2011. Of these, 201 (42%) were diagnosed a median 4.3 months (interquartile range [IQR], 1.1–24.1) before CNICS enrollment. Patients with lymphoma diagnosed before CNICS enrollment were more likely to have any missing value for CD4 count at lymphoma diagnosis, nadir CD4 count, or HIV RNA at lymphoma diagnosis compared with patients for whom lymphoma was diagnosed after cohort entry (44% vs 12%, P < .0001). CD4 count and HIV RNA measurements at lymphoma diagnosis differed from lymphoma diagnosis date by a median of 12 days (IQR, 4–27) and 13 days (IQR, 4–29), respectively.
Fifty-six of 482 patients (12%) met the unmasking lymphoma IRIS case definition. Among non-IRIS cases, 9 patients were diagnosed with lymphoma within 6 months after ART, but without documented HIV RNA reduction as required by the unmasking IRIS case definition. Baseline characteristics for IRIS and non-IRIS cases are shown in Table 1. Among IRIS cases, 12 (21%) had HL, 22 (39%) diffuse large B-cell lymphoma (DLBCL), 5 (9%) Burkitt lymphoma (BL), 10 (18%) primary central nervous system lymphoma (PCNSL), and 7 (13%) other NHL. Median CD4 count at lymphoma diagnosis among IRIS cases was 173 cells/mL (IQR, 73–302), and 48% had HIV RNA <400 copies/mL. No significant differences were identified between IRIS and non-IRIS, excepting more frequent hepatitis B and C (30% vs 19%, P = .05), more frequent prior AIDS (91% vs 79%, P = .03), lower HIV RNA (2.7 vs 4.5 log10 copies/mL, P < .0001), and higher proportion with suppressed HIV RNA (48% vs 30%, P = .0009) among IRIS cases. HIV RNA differences resulted in part from the IRIS case definition, which required virologic response to ART. No significant differences in lymphoma subtype distribution were identified between IRIS and non-IRIS cases, although numerically higher proportions of HL (21% vs 16%, P = .30) and PCNSL (18% vs 10%, P = .09) were observed among IRIS. Unmasking lymphoma IRIS cases were on ART for a median 2.2 months (IQR, 0.9–3.5) before lymphoma diagnosis. Among 426 non-IRIS cases, 175 (41%) were on ART at lymphoma diagnosis for a median 20.3 months (IQR, 7.5–42.9), of whom 83 of 142 (58%) with an HIV RNA value recorded at lymphoma diagnosis had suppressed HIV RNA. Differences in ART duration between IRIS and non-IRIS cases were influenced by the IRIS case definition, which required lymphoma diagnosis within 6 months after ART initiation.
Table 1.
Characteristics of 482 HIV-Infected Adults in the Center for AIDS Research Network of Integrated Clinical Systems Cohort With Lymphoma Between 1996 and 2011, Stratified by Unmasking Immune Reconstitution Inflammatory Syndrome Case Status
| Characteristic | IRIS | Non-IRIS | P Value |
|---|---|---|---|
| Total, No. (%) | 56 (11.6) | 426 (88.4) | … |
| Age at lymphoma diagnosis, y, mean (SD) | 41.1 (7.0) | 42.5 (9.0) | .19 |
| Male, No. (%) | 51 (91.1) | 375 (88.0) | .66 |
| Race/ethnicitya, No. (%) | |||
| White | 28 (50.0) | 211 (50.1) | .99 |
| Black | 21 (37.5) | 134 (31.8) | .39 |
| Other | 7 (12.5) | 76 (18.1) | .35 |
| Lymphoma diagnosis year, median (IQR) | 2003 (2000–2007) | 2003 (2000–2006) | .55 |
| Lymphoma category, No. (%) | |||
| HL | 12 (21.4) | 68 (16.0) | .30 |
| DLBCL | 22 (39.3) | 183 (43.0) | .66 |
| BL | 5 (8.9) | 52 (12.2) | .60 |
| PCNSL | 10 (17.9) | 44 (10.3) | .09 |
| Other NHL | 7 (12.5) | 79 (18.5) | .27 |
| Lymphoma stage I/II, No. (%)a | 9 (39.1) | 38 (24.2) | .13 |
| Hepatitis B/C coinfection | 17 (30.4) | 81 (19.0) | .047 |
| AIDS illness prior to lymphoma diagnosis, No. (%) | 51 (91.1) | 337 (79.1) | .032 |
| On ART at lymphoma diagnosis, No. (%) | 56 (100.0) | 175 (41.1) | <.0001 |
| CD4 count at lymphoma diagnosis, cells/µL, median (IQR)a | 173 (73–302) | 122 (35–288) | .22 |
| CD4 percentage at lymphoma diagnosis, median (IQR)a | 14.0 (6.8–21.0) | 12.0 (5.3–22.0) | .34 |
| CD4 count nadir, cells/µL, median (IQR)a | 69 (29–177) | 58 (15–167) | .54 |
| HIV RNA at lymphoma diagnosis, log10 copies/mL, median (IQR)a | 2.72 (1.48–3.98) | 4.52 (2.30–5.30) | <.0001 |
| HIV RNA <400 copies/mL at lymphoma diagnosis, No. (%)a | 27 (48.2) | 93 (30.3) | .0009 |
Abbreviations: ART, antiretroviral therapy; BL, Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; HIV, human immunodeficiency virus; HL, Hodgkin lymphoma; IQR, interquartile range; IRIS, immune reconstitution inflammatory syndrome; NHL, non-Hodgkin lymphoma; PCNSL, primary central nervous system lymphoma; SD, standard deviation.
a No. (%) of missing observations, IRIS/non-IRIS: race/ethnicity = 0 (0%)/5 (1.2%); lymphoma stage = 33 (58.9%)/269 (63.1%); CD4 count at lymphoma diagnosis = 0 (0%)/97 (22.8%); CD4 percentage at lymphoma diagnosis = 1 (1.8%)/122 (28.6%); CD4 count nadir = 0 (0.0%)/67 (15.7%); HIV RNA at lymphoma diagnosis = 0 (0.0%)/119 (27.9%).
Median CD4 count and HIV RNA with interquartile ranges during the 12 months before and after lymphoma diagnosis are shown in Figure 1 for IRIS, non-IRIS on ART, and non-IRIS off ART at lymphoma diagnosis. During the 24-month window, there were 3439 CD4 count measures for the study population, with a median of 8 (IQR, 5–10) per patient (IRIS, 10 [IQR, 8–12]; non-IRIS on ART, 6 [IQR, 5–9]; and non-IRIS off ART, 8 [IQR, 6–11]). During the 24-month window, there were 3266 HIV RNA measures for the study population, with a median of 7 (IQR, 5–10) per patient (IRIS, 9 [IQR, 8–11]; non-IRIS on ART, 6 [IQR 4–8]; and non-IRIS off ART, 8 [IQR, 6–11]). IRIS cases demonstrated marked reductions in HIV RNA after ART in the months before lymphoma diagnosis without significant CD4 increases, perhaps reflecting lymphoma-related lymphopenia. In the 12 months after lymphoma diagnosis, IRIS cases demonstrated modest CD4 count increases. Non-IRIS cases on ART demonstrated modest CD4 count increases and HIV RNA reductions after lymphoma diagnosis, perhaps from intensified ART, greater engagement in care, and/or enhanced adherence counseling. Non-IRIS cases off ART demonstrated robust CD4 count increases and HIV RNA reductions after lymphoma diagnosis, resulting from ART initiation by 6 months after lymphoma diagnosis in 198 of 251 (79%) patients.
Figure 1.
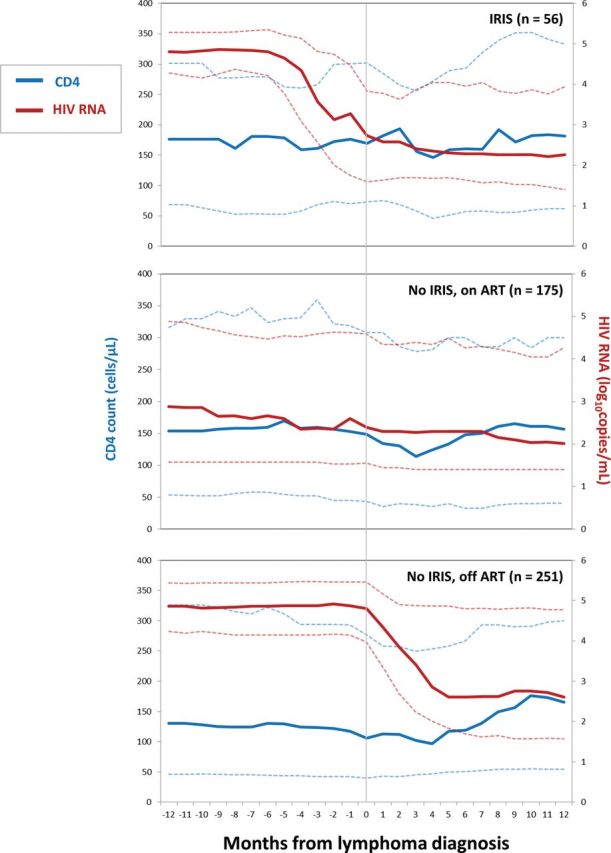
Median CD4, human immunodeficiency virus (HIV) RNA, and interquartile ranges for 482 HIV-infected adults in Centers for AIDS Research Network of Integrated Clinical Systems during the 12 months before and after lymphoma diagnosis, stratified by immune reconstitution inflammatory syndrome (IRIS) and antiretroviral therapy (ART) status. Solid lines indicate median values and dashed lines interquartile ranges.
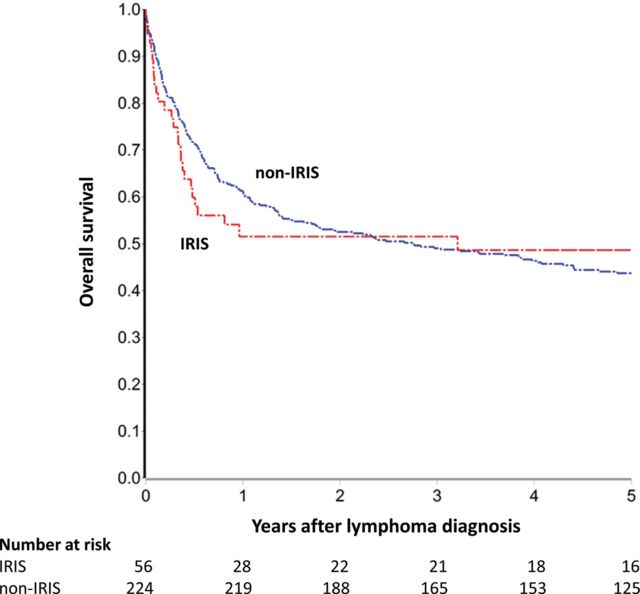
Among all 482 patients, 229 deaths occurred during 1571 person-years of follow-up, yielding a mortality rate of 14.6 deaths per 100 person-years (95% confidence interval [CI], 12.8–16.6; Table 2). Five-year survival was 44% for all lymphoma, 61% for HL, 51% for BL, 44% for DLBCL, 44% for other NHL, and 23% for PCNSL. Although not statistically significant, overall survival appeared worse at 6 months for IRIS than for non-IRIS cases (62% IRIS [95% CI, 50%–76%] vs 72% non-IRIS [95% CI, 67%–77%]), and also 1 year after lymphoma diagnosis (54% IRIS [95% CI, 42%–69%] vs 61% non-IRIS [56%–67%]). However, overall survival 5 years after lymphoma diagnosis was similar (49% IRIS [95% CI, 37%–64%] vs 44% non-IRIS [95% CI, 39%–50%]) (Figure 2). When follow-up time was partitioned at 6-month and 1-year time points, a pattern of increased early mortality followed by reduced late mortality was suggested for IRIS (hazard ratios [HRs], 1.42 [95% CI, .88–2.30] for 0–6 months; 0.62 [95% CI, .27–1.41] for 6 months–5 years; 1.28 [95% CI, .83–1.97] for 0–1 year; and 0.32 [95% CI, .08–1.31] for 1–5 years). This pattern of similar long-term survival but possibly increased early mortality was also observed when IRIS were separately compared with non-IRIS cases on ART at lymphoma diagnosis, and also to non-IRIS cases off ART. Despite limitations from small numbers of IRIS cases within histologic groups, possibly increased early mortality for IRIS was most strongly suggested for NHL, particularly BL and PCNSL.
Table 2.
Mortality Rates and Survival for 482 HIV-Infected Adults in the Center for AIDS Research Network of Integrated Clinical Systems Cohort With Lymphoma Between 1996 and 2011, Stratified by Histology and Unmasking Immune Reconstitution Inflammatory Syndrome Case Status
| Case Status | Persons | Deaths | Person-years | Mortality Rate per 100 Person-years (95% CI) | 6-mo Survival % (95% CI) | 1-y Survival % (95% CI) | 5-y Survival % (95% CI) |
|---|---|---|---|---|---|---|---|
| All lymphoma | 482 | 229 | 1571 | 14.6 (12.8–16.6) | 70 (66–75) | 60 (55–65) | 44 (40–50) |
| IRIS | 56 | 30 | 169 | 17.7 (12.4–23.4) | 62 (50–76) | 54 (42–69) | 49 (37–64) |
| Non-IRIS | 426 | 199 | 1402 | 14.2 (12.4–16.3) | 72 (67–77) | 61 (56–67) | 44 (39–50) |
| HL | 80 | 25 | 302 | 8.3 (5.6–12.2) | 82 (73–92) | 77 (67–88) | 61 (50–75) |
| IRIS | 12 | 2 | 52 | 3.9 (1.0–15.4) | 82 (71–93) | 83 (65–100) | 83 (65–100) |
| Non-IRIS | 68 | 23 | 250 | 9.2 (6.1–13.8) | 82 (73–93) | 76 (65–89) | 57 (45–73) |
| All NHL | 402 | 204 | 1269 | 16.1 (14.0–18.4) | 68 (63–73) | 57 (51–62) | 41 (36–47) |
| IRIS | 44 | 28 | 118 | 23.9 (16.5–34.6) | 56 (43–73) | 46 (34–64) | 40 (27–58) |
| Non-IRIS | 358 | 176 | 1152 | 15.3 (13.2–17.7) | 70 (64–76) | 58 (53–65) | 42 (36–48) |
| BL | 57 | 23 | 157 | 14.6 (9.7–22.0) | 73 (61–88) | 63 (50–79) | 51 (38–68) |
| IRIS | 5 | 3 | 5 | 62.7 (20.2–194.5) | 60 (32–100) | 40 (16–99) | 40 (16–99) |
| Non-IRIS | 52 | 20 | 153 | 13.1 (8.5–20.3) | 75 (62–90) | 66 (53–82) | 53 (39–71) |
| PCNSL | 54 | 41 | 121 | 33.8 (24.9–45.9) | 38 (27–54) | 28 (18–43) | 23 (14–37) |
| IRIS | 10 | 10 | 2 | 417.6 (224.7–776.2) | 10 (2–4) | 0 (0–0) | 0 (0–0) |
| Non-IRIS | 44 | 31 | 119 | 26.1 (18.3–37.1) | 45 (32–63) | 34 (23–51) | 28 (18–44) |
| DLBCL | 205 | 101 | 692 | 14.6 (12.0–17.7) | 75 (69–83) | 64 (57–72) | 44 (37–53) |
| IRIS | 22 | 10 | 85 | 11.8 (6.3–21.9) | 72 (55–93) | 67 (50–90) | 54 (36–81) |
| Non-IRIS | 183 | 91 | 608 | 15.0 (12.2–18.4) | 76 (69–84) | 64 (56–72) | 43 (36–52) |
| Other NHL | 86 | 39 | 298 | 13.1 (9.6–17.9) | 71 (60–84) | 59 (47–73) | 44 (33–59) |
| IRIS | 7 | 5 | 24 | 19.7 (8.2–47.4) | 71 (46–100) | 57 (32–100) | 57 (32–100) |
| Non-IRIS | 79 | 34 | 273 | 12.5 (8.9–17.5) | 71(59–86) | 60 (47–75) | 43 (32–58) |
Abbreviations: BL, Burkitt lymphoma; CI, confidence interval; DLBCL, diffuse large B-cell lymphoma; HIV, human immunodeficiency virus; HL, Hodgkin lymphoma; IRIS, immune reconstitution inflammatory syndrome; NHL, non-Hodgkin lymphoma; PCNSL, primary central nervous system lymphoma.
Figure 2.
Overall survival for 482 HIV-infected adults in Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) with lymphoma between 1996 and 2011, stratified by unmasking immune reconstitution inflammatory syndrome (IRIS) case status. Patients enrolled in CNICS after lymphoma diagnosis were treated as late entries. Abbreviation: HIV, human immunodeficiency virus.
Sensitivity analyses varying the IRIS case definition were conducted as follows: (1) lymphoma within 3 months of ART with ≥0.5 log HIV RNA reduction (n = 33); (2) lymphoma within 6 months of ART with ≥1 log HIV RNA reduction (n = 41); and (3) lymphoma within 3 months of ART with ≥1 log HIV RNA reduction (n = 23). In these analyses, IRIS and non-IRIS were similar overall, with consistent demonstration of more frequent hepatitis B and C and lower HIV RNA at lymphoma diagnosis among IRIS cases, and consistently higher PCNSL proportions reaching statistical significance using IRIS case definitions (1) and (2). Similar overall survival between IRIS and non-IRIS cases 5 years after lymphoma diagnosis was also observed across varying IRIS case definitions, with possibly increased early mortality for IRIS consistently demonstrated. When analyses were restricted to 281 patients with lymphoma diagnosed after CNICS enrollment, 56 (20%) were classified as IRIS and 225 as non-IRIS, with findings otherwise similar.
DISCUSSION
In a multicenter HIV-associated lymphoma cohort, 12% of patients met a uniformly applied unmasking IRIS case definition. These results are descriptive, but important given that lymphoma IRIS has not been characterized in detail. Our findings support the concept of lymphoma IRIS as a distinct and definable entity, and extend previous cohort studies demonstrating increased lymphoma incidence during the first 6 months after ART [1–3].
Lymphomagenesis in HIV-infected individuals is complex, involving viral oncogens such as Epstein-Barr virus (EBV), immune surveillance in the germinal center, tumor microenvironment interactions, activation of cell signaling pathways such as nuclear factor–κB, and chronic B-cell activation, all of which vary in relative contribution across histologic subtypes [16–23]. Although no significant differences were observed in histologic distribution between lymphoma IRIS and non-IRIS, there was a suggestion of increased HL and PCNSL among IRIS cases. This should be interpreted with caution given small numbers of IRIS cases. However, HL and PCNSL are the HIV-associated lymphoma subtypes for which EBV is most consistently demonstrated in tumor specimens [16]. Examining EBV status among lymphoma IRIS patients may be informative, particularly since other herpesviruses, including KS-associated herpesvirus, cytomegalovirus, herpes simplex virus, and varicella zoster virus, are commonly implicated in IRIS presentations [6–8]. Possibly increased hepatitis B/C coinfection among IRIS cases may also be notable. Unlike EBV, which directly infects and transforms lymphocytes, hepatitis B and C increase NHL risk by inducing chronic immune activation and B-cell stimulation [24]. HIV-associated lymphomas, in which immune activation plays a pathogenically stronger role, may be more susceptible to IRIS effects.
Our results are consistent with the IRIS literature. First, 12% of HIV-associated lymphoma patients met an unmasking lymphoma IRIS case definition. IRIS is common after ART, occurring in 5%–15% of ART initiators with increased risk among those with advanced immunosuppression [6–8, 11, 14]. HIV-infected lymphoma patients present with advanced immunosuppression even in the ART era [25]. Identifying unmasking IRIS in a significant number of patients from a large HIV-associated lymphoma cohort may therefore not be surprising. Unmasking lymphoma IRIS cases were on ART for a median 2.2 months prior to lymphoma diagnosis, consistent with peak IRIS incidence within the first 3 months on ART [6, 7]. We also observed possibly increased early mortality among lymphoma IRIS cases, but similar long-term outcomes to non-IRIS, as with previous IRIS descriptions [6, 7]. This pattern was particularly observed for NHL. Notably, PCNSL IRIS cases had much worse outcomes than non-IRIS cases. Increased mortality has been described for other forms of IRIS involving the CNS, as excess intracranial inflammation leads to significant morbidity and mortality [6, 8, 26–28]. BL IRIS also appeared to have worse survival than non-IRIS, perhaps reflecting IRIS in a highly proliferative lymphoma subtype with frequent extranodal and CNS involvement [16, 29, 30].
Detailed lymphoma IRIS studies may elucidate immunologic mechanisms of lymphoma control. Treating human cancer by stimulating tumor-specific T lymphocytes is a promising therapeutic strategy. Immune activating cancer therapies include inhibitors of programmed death 1 (PD-1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) signaling [31, 32]. Activation of the PD-1 pathway is found in aggressive B-cell lymphomas, as well as virus- and immunodeficiency-associated malignancies [33]. Some immune-based cancer treatments are characterized by immunologic toxicities that may require systemic corticosteroids and immunomodulatory therapies not unlike severe IRIS, but a substantial rate of durable cancer remissions [34–36]. HIV-associated IRIS is characterized by a rapid increase in CD4 cells, lymphocyte proliferation responses, immune activation, and pathogen-specific delayed hypersensitivity [37–40]. Despite small numbers, we observed possibly increased early mortality followed by reduced late mortality among IRIS cases, with near plateauing of survival after 1 year. The similarity of this observation to findings from clinical trials of immune-based cancer treatments suggest that more detailed studies of lymphoma IRIS might yield immunobiologic insights with relevance even for HIV-uninfected patients.
Our research has several limitations. First, existing IRIS case definitions are not standardized. We applied a uniform case definition drawn from the literature, and varied this in sensitivity analyses yielding consistent results. Second, subclinical lymphoma leading to care-seeking behavior and ART initiation before lymphoma diagnosis could not be excluded. Third, data did not allow for a distinction between refractory lymphoma and paradoxical lymphoma IRIS, in which ART after lymphoma diagnosis may have led to clinical worsening. Paradoxical lymphoma IRIS would have been classified as non-IRIS, perhaps mitigating differences between groups. Fourth, data are observational and unadjusted, and associations may be due to measured and unmeasured confounding. Given small numbers of IRIS cases within histologic groups, we did not adjust for other covariates, although measured covariates were similar overall between IRIS and non-IRIS. Fifth, CNICS enrollment requires HIV clinic attendance, and patients with lymphoma diagnosis preceding CNICS enrollment are included, for whom data may have been incomplete. We sought to maintain generalizability to patients not receiving HIV care or newly diagnosed with HIV at lymphoma diagnosis. We minimized bias by analyzing follow-up time only after CNICS entry and sought to ensure that “immortal” person-time between lymphoma diagnosis and cohort entry was not inappropriately counted. We restricted analyses to patients with lymphoma diagnosis after CNICS enrollment and found consistent results. However, survival for patients with lymphoma before CNICS enrollment may not be accurately reflected. Sixth, detailed information regarding lymphoma presentation and treatment were not analyzed. We are implementing a centralized abstraction to collect these data. Finally, cause of death was unknown, and analyses focused on overall survival.
Despite limitations, our study has several strengths. To our knowledge, this is the first detailed description of HIV-associated lymphoma IRIS using a uniformly applied case definition. Although we identified only 56 cases of unmasking lymphoma IRIS, results are drawn from one of the largest multicenter HIV-associated lymphoma cohorts to date, providing a dataset from which characterizing a rare clinical entity like lymphoma IRIS is even possible. Patients studied represent a large and diverse HIV-infected population in routine care across the United States, undergoing regular assessment, in whom lymphoma diagnoses were extensively verified. Additionally, mortality assessment used active and passive surveillance.
In conclusion, 12% of HIV-associated lymphoma patients from a large multicenter cohort met a uniformly applied case definition for unmasking lymphoma IRIS. These patients were similar overall to non-IRIS cases, with similarities also to nonlymphoma IRIS descriptions. Five-year survival was comparable between IRIS and non-IRIS cases, although increased early mortality among IRIS cases was suggested. Detailed studies of lymphoma IRIS immunobiology may have implications for HIV-infected and HIV-uninfected individuals.
Notes
Acknowledgments. The authors thank all Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) investigators and data management teams from the 8 sites who contributed to the completion of this study at Case Western Reserve University; University of Alabama at Birmingham; University of California, San Francisco; University of Washington; University of California, San Diego; Fenway Health; University of North Carolina; and Johns Hopkins University. The authors additionally acknowledge Donna Porter for her significant contributions.
Financial support. This work was supported by CNICS, a program funded by the National Institutes of Health (NIH; R24AI067039). This research was also supported by NIH funding to the University of North Carolina (UNC) at Chapel Hill Center for AIDS Research (CFAR) (P30AI50410), Case Western Reserve University CFAR (P30AI36219), Johns Hopkins University (P30AI094189), and University of Alabama at Birmingham CFAR (P30AI027767). Additional support was provided by National Cancer Institute supplemental funding for HIV-associated malignancy research to the UNC Lineberger Comprehensive Cancer Center (LCCC; P30CA016086) and UNC CFAR, as well as an LCCC Developmental Research Award. S. G. received NIH support from the Fogarty International Center (K01TW009488), National Cancer Institute (R21CA180815), and AIDS Malignancy Consortium (U01CA121947).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lanoy E, Rosenberg PS, Fily F, et al. HIV-associated Hodgkin lymphoma during the first months on combination antiretroviral therapy. Blood. 2011;118:44–9. doi: 10.1182/blood-2011-02-339275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffe HW, De Stavola BL, Carpenter LM, Porter K, Cox DR. Immune reconstitution and risk of Kaposi sarcoma and non-Hodgkin lymphoma in HIV-infected adults. AIDS. 2011;25:1395–403. doi: 10.1097/QAD.0b013e3283489c8b. [DOI] [PubMed] [Google Scholar]
- 3.Yanik EL, Napravnik S, Cole SR, et al. Incidence and timing of cancer in HIV-infected individuals following initiation of combination antiretroviral therapy. Clin Infect Dis. 2013;57:756–64. doi: 10.1093/cid/cit369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meintjes G, Scriven J, Marais S. Management of the immune reconstitution inflammatory syndrome. Curr HIV/AIDS Rep. 2012;9:238–50. doi: 10.1007/s11904-012-0129-5. [DOI] [PubMed] [Google Scholar]
- 7.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–10. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 8.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–61. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bower M, Nelson M, Young AM, et al. Immune reconstitution inflammatory syndrome associated with Kaposi's sarcoma. J Clin Oncol. 2005;23:5224–8. doi: 10.1200/JCO.2005.14.597. [DOI] [PubMed] [Google Scholar]
- 10.Letang E, Lewis JJ, Bower M, et al. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS. 2013;27:1603–13. doi: 10.1097/QAD.0b013e328360a5a1. [DOI] [PubMed] [Google Scholar]
- 11.Achenbach CJ, Harrington RD, Dhanireddy S, Crane HM, Casper C, Kitahata MM. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clin Infect Dis. 2012;54:424–33. doi: 10.1093/cid/cir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37:948–55. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achenbach CJ, Cole SR, Kitahata MM, et al. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS. 2011;25:691–700. doi: 10.1097/QAD.0b013e3283437f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddow LJ, Easterbrook PJ, Mosam A, et al. Defining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis. 2009;49:1424–32. doi: 10.1086/630208. [DOI] [PubMed] [Google Scholar]
- 15.Cole SR, Hudgens MG. Survival analysis in infectious disease research: describing events in time. AIDS. 2010;24:2423–31. doi: 10.1097/QAD.0b013e32833dd0ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood. 2012;119:3245–55. doi: 10.1182/blood-2011-08-373738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohlius J, Schmidlin K, Boue F, et al. HIV-1-related Hodgkin lymphoma in the era of combination antiretroviral therapy: incidence and evolution of CD4(+) T-cell lymphocytes. Blood. 2011;117:6100–8. doi: 10.1182/blood-2010-08-301531. [DOI] [PubMed] [Google Scholar]
- 18.Guech-Ongey M, Simard EP, Anderson WF, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. 2010;116:5600–4. doi: 10.1182/blood-2010-03-275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–91. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton LM, Kim CJ, Weiss LM, et al. Molecular characteristics of diffuse large B-cell lymphoma in human immunodeficiency virus-infected and -uninfected patients in the pre-highly active antiretroviral therapy and pre-rituximab era. Leuk Lymphoma. 2014;55:551–7. doi: 10.3109/10428194.2013.813499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao C, Silverberg MJ, Martinez-Maza O, et al. Epstein-Barr virus infection and expression of B-cell oncogenic markers in HIV-related diffuse large B-cell lymphoma. Clin Cancer Res. 2012;18:4702–12. doi: 10.1158/1078-0432.CCR-11-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breen EC, Hussain SK, Magpantay L, et al. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1303–14. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liapis K, Clear A, Owen A, et al. The microenvironment of AIDS-related diffuse large B-cell lymphoma provides insight into the pathophysiology and indicates possible therapeutic strategies. Blood. 2013;122:424–33. doi: 10.1182/blood-2013-03-488171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011;117:1792–8. doi: 10.1182/blood-2010-06-275818. [DOI] [PubMed] [Google Scholar]
- 25.Gopal S, Patel MR, Yanik EL, et al. Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. J Natl Cancer Inst. 2013;105:1221–9. doi: 10.1093/jnci/djt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torok ME, Yen NT, Chau TT, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)—associated tuberculous meningitis. Clin Infect Dis. 2011;52:1374–83. doi: 10.1093/cid/cir230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longley N, Harrison TS, Jarvis JN. Cryptococcal immune reconstitution inflammatory syndrome. Curr Opin Infect Dis. 2013;26:26–34. doi: 10.1097/QCO.0b013e32835c21d1. [DOI] [PubMed] [Google Scholar]
- 28.Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7:e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noy A. Controversies in the treatment of Burkitt lymphoma in AIDS. Curr Opin Oncol. 2010;22:443–8. doi: 10.1097/CCO.0b013e32833d7dbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montoto S, Wilson J, Shaw K, et al. Excellent immunological recovery following CODOX-M/IVAC, an effective intensive chemotherapy for HIV-associated Burkitt's lymphoma. AIDS. 2010;24:851–6. doi: 10.1097/QAD.0b013e3283301578. [DOI] [PubMed] [Google Scholar]
- 31.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riley JL. Combination checkpoint blockade—taking melanoma immunotherapy to the next level. N Engl J Med. 2013;369:187–9. doi: 10.1056/NEJMe1305484. [DOI] [PubMed] [Google Scholar]
- 33.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–73. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 37.Notermans DW, Pakker NG, Hamann D, et al. Immune reconstitution after 2 years of successful potent antiretroviral therapy in previously untreated human immunodeficiency virus type 1-infected adults. J Infect Dis. 1999;180:1050–6. doi: 10.1086/315013. [DOI] [PubMed] [Google Scholar]
- 38.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 39.Lederman MM, Connick E, Landay A, et al. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998;178:70–9. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 40.Antonelli LR, Mahnke Y, Hodge JN, et al. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood. 2010;116:3818–27. doi: 10.1182/blood-2010-05-285080. [DOI] [PMC free article] [PubMed] [Google Scholar]