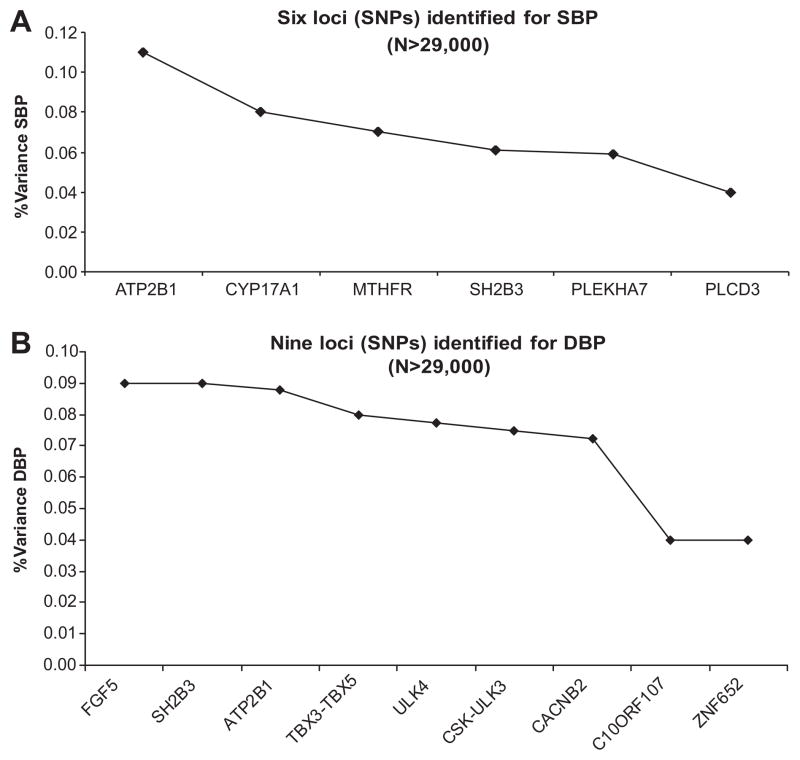
In spite of recent successes for other complex diseases, essential hypertension has been remarkably reluctant to reveal its underlying susceptibility genes through genome-wide association (GWA) studies. Only after shifting attention to the underlying quantitative traits (ie, systolic [SBP] and diastolic blood pressure [DBP]) and application of “brute force” GWA meta-analyses (n>29 000) of multiple population cohorts in the GlobalBPGen and CHARGE consortia was a tiny tip of the iceberg revealed.1,2 Six loci for SBP and 9 for DBP were discovered, of which 2 overlapped, yielding 13 independent genome-wide significant signals. However, without exception, the most significant single nucleotide polymorphisms (SNPs) representing these loci only explained a very small part of the total BP variance (≤0.11%; Figure). Heritability is well established for BP and typically ranges between 30% and 60%, as estimated in twin or family studies.3 This means that the majority of the BP heritability is still “missing” and remains at large.4 Expansion of GWA sample size (eg, in the International Consortium on BP-GWAS, a merger of GlobalBPGen and CHARGE) will likely uncover additional BP variants.5 However, they will have even smaller effect sizes, and a substantial increase in explained heritability of all loci combined is not expected. GWA studies in general experience a number of other inherent limitations, including a predominant focus on common variants (minor allele frequency: >5%) and a tendency for signals to map to noncoding sequence. Finding suitable answers to these challenges will to a large extent determine continued progress in chipping away at the missing BP heritability. The article by Tomaszewski et al6 in the present issue of Hypertension provides some intriguing clues as to the best way forward.
Figure.
A, Percentage of variance explained in SBP by the top SNPs of 6 loci identified in 2 large GWA meta-analysis studies.1,2 B, Percentage of variance explained in DBP by the top SNPs of 9 loci identified in 2 large GWA meta-analysis studies.1,2
We Should Find the Causal Variants
Tomaszewski et al6 used a custom-made gene-centric array with >30 000 common and rare SNPs from >2000 candidate loci to genotype 2020 European individuals from 520 nuclear families of the Genetic Regulation of Arterial Pressure of Humans in the Community Study with measures of 24-hour ambulatory BP available. A strong association with 24-hour DBP was found for an SNP (rs13306560) in the MTHFR/CLCN6/NPPB locus and subsequently replicated for clinic DBP in 2 additional cohorts (n>6000). Interestingly, a different SNP in the same locus was identified in the recent GWA studies for clinic BP (Figure).1,2 However, conditional analysis including both SNPs in the same model suggested that the association with 24-hour DBP is largely driven by the newly identified SNP. Additional bioinformatic analysis indicates a potential direct functional effect of rs13306560 (see also below). This finding illustrates an important limitation of GWA studies: genome-wide significant SNPs often merely tag but do not provide direct information on the causal variants. Fine mapping BP loci through sequencing and/or custom-made chip approaches will be required, not only to get a better estimate of the true (most likely larger) effect on the phenotype, but also to translate those signals to biological function.
We Should No Longer Focus on the Usual Suspects
A total of 105 candidate genes for BP with very good genetic coverage of common variants were present on the array tested by Tomaszewski et al.6 In spite of this, very little evidence was found for involvement of these most frequently investigated candidate genes for BP, such as those for the sympathetic nervous system and the renin-angiotensin system. These results are in line with those from the recent GWA studies, in which only 2 of the 13 gene loci discovered could have been regarded as candidate gene loci for BP (MTHFR and CYP17A1).5 Does this mean that we have been betting on the wrong horses for all these years? At least for common variants, the current evidence indeed seems to indicate that we may have more success if we start looking beyond the classic systems of BP regulation. Additional bioinformatic analysis by Tomaszewski et al6 indicates that signaling pathways that control cell survival may be one promising direction. It may be too early to entirely dismiss our beloved candidate genes, however. Future (meta-analytic) studies with larger sample sizes and exhaustive coverage of both common and more rare variants would be necessary for a more balanced evaluation of the evidence.
Low-Frequency/Rare Variants Are Important
Discoveries of GWA studies are inherently limited to common variants as a result of array design. Rare variants may, therefore, represent an important source of missing heritability.7 Interestingly, Tomaszewski et al6 found a significant overrepresentation of rare variants among polymorphisms showing at least nominal association with mean 24-hour BP. These results did not depend on the threshold of rare variant definition (minor allele frequency <5% or minor allele frequency <2%), significance level (P<0.05 or P<0.01), or the phenotype (24-hour SBP or DBP). Although these results indicate that a considerable proportion of its heritability may be explained by low-frequency variants, the overrepresented rare variants did not lead to amino acid substitutions pointing to more subtle regulatory mechanisms. Sample size is of the essence for reliable identification of individual rare variants contributing to BP, emphasizing the need for large meta-analyses of exactly the type of custom-made array used in the current study.
We Should Expand the Search From Clinic to Ambulatory BP
Ambulatory BP monitoring offers a number of advantages over clinic BP readings, including the ability to measure BP in real-life settings, track BP at night, and avoid the “white-coat” phenomenon. The added value of ambulatory BP measurements has been illustrated by studies showing that ambulatory BP is a better predictor of target organ damage and cardiovascular morbidity and mortality than BP measured in the clinic. The higher heritability of mean 24-hour compared with clinic BP measurements6 suggests that 24-hour BP monitoring may be particularly informative for gene discovery. However, we need to realize that different genes or sets of genes may contribute to BP regulation under different conditions. Our recent studies in twins showed that not only daytime and nighttime BP are influenced by partly different genes,8 the genes underlying mean 24-hour BP also differ from those for clinic BP to a large extent.9 Only 45% of the 24-hour SBP heritability and 49% of the 24-hour DBP heritability could be attributed to genes that also influenced clinic levels. These findings indicate that, to take full advantage of ambulatory BP recordings in gene-finding studies, they need to be used in both discovery and replication cohorts. In other words, GWA meta-analyses of ambulatory BP cohorts are urgently needed. Such studies will not only elucidate similarities and differences in genetic architecture with clinic BP but will also provide new insights into the mechanisms of BP regulation at night (including nocturnal BP fall)8 and in real-life settings.
Beyond the DNA Sequence: Epigenetics
Several epidemiological and clinical peculiarities of essential hypertension, such as the incomplete concordance between monozygotic twins (ranging from 38% to 52%), and its late onset and progressive nature are difficult to fully explain with traditional DNA sequence-based approaches. These observations may point to the involvement of epigenetic factors in hypertension development. Although epigenetics refers to all meiotically and mitotically heritable changes in gene expression that are not coded in the DNA sequence, epigenetic profiles are still affected by genetic variants to a certain extent. For example, DNA sequence variations could make certain loci more or less attractive to methylation. A recent study shows that ≈0.16% of SNPs in the human genome are associated with allele-specific methylation changes.10 The rs13306560 SNP identified in this study is a good example. It maps to a CpG island in the MTHFR/CLCN6 promoter with evidence of operation of selective pressure throughout mammalian evolution. One possible mechanism by which rs13306560 could, therefore, mediate the association with BP is through differential methylation of the MTHFR/CLCN6 promoter in the 2 alleles. Because methylation changes resulting from variations in DNA sequence can be discovered in genetic studies, they cannot be considered a source of missing heritability. In contrast, vertically transmitted DNA-independent epigenetic markers may contribute to the BP heritability that is still missing. Although it is well known that, during gametogenesis epigenetic reprogramming takes place, which consists of the removal of practically all methylation from the cell, some epigenetic signals can escape this process and are transmitted across generations. Recent advances in automated high-throughput array-based measurement have now made genome-wide methylation studies possible. Such studies are urgently needed not only to identify these epigenetic variants that might contribute to the missing heritability but, perhaps even more importantly, those that mediate differential gene expression resulting from environment influences.
The success of GWA studies has revolutionized the genetics of complex traits and diseases. However, the genetic architecture of BP regulation and essential hypertension has proved even more challenging than other complex traits and diseases, with most of the heritability still missing.4 As illustrated by Tomaszewski et al,6 future studies in BP genetics need to both build on and move beyond the successes of GWA studies to make continued progress.
Acknowledgments
We thank Gaifen Liu for making the figures.
Sources of Funding
X.W. was supported in part by grants from the National Heart, Lung, and Blood Institute (HL086530) and the American Heart Association (0730156N). The Georgia Cardiovascular Twin study is funded by grant HL56622 from the National Heart Lung and Blood Institute.
Footnotes
The opinions expressed in this editorial are not necessarily those of the editors or of the American Heart Association.
Disclosures
None.
References
- 1.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Kottgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677– 687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Doring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O’Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O’Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvanen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dorr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Volker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Volzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666– 676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Snieder H. Familial aggregation of blood pressure. In: Flynn JT, Ingelfinger JR, Portman RJ, editors. Clinical Hypertension and Vascular Diseases: Pediatric Hypertension. 2. Totowa, NJ: Humana Press Inc; 2010. [Google Scholar]
- 4.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Gutt-macher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehret GB. Genome-wide association studies: contribution of genomics to understanding blood pressure and essential hypertension. Curr Hypertens Rep. 2010;12:17–25. doi: 10.1007/s11906-009-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomaszewski M, Debiec R, Braund PS, Nelson CP, Hardwick R, Christ-ofidou P, Denniff M, Codd V, Rafelt S, van der Harst P, Waterworth D, Song K, Vollenweider P, Waeber G, Zukowska-Szczechowska E, Burton PR, Mooser V, Charchar FJ, Thompson JR, Tobin MD, Samani NJ. Genetic architecture of ambulatory blood pressure in the general population: insights from cardiovascular gene-centric array. Hypertension. 2010;56:1069–1076. doi: 10.1161/HYPERTENSIONAHA.110.155721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008 May;40:592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Ding X, Su S, Yan W, Harshfield G, Treiber F, Snieder H. Genetic influences on daytime and night-time blood pressure: similarities and differences. J Hypertens. 2009;27:2358–2364. doi: 10.1097/HJH.0b013e328330e84d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Ding X, Su S, Harshfield G, Treiber F, Snieder H. Genetic influence on blood pressure measured in the office, under laboratory stress and during real life. Hypertens Res. doi: 10.1038/hr.2010.218. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerkel K, Spadola A, Yuan E, Kosek J, Jiang L, Hod E, Li K, Murty VV, Schupf N, Vilain E, Morris M, Haghighi F, Tycko B. Genomic surveys by methylation-sensitive SNP analysis identify sequence-dependent allele-specific DNA methylation. Nat Genet. 2008;40:904–908. doi: 10.1038/ng.174. [DOI] [PubMed] [Google Scholar]