Abstract
We review recent work on the role of intrinsic amygdala networks in the regulation of classically conditioned defensive behaviors, commonly known as conditioned fear. These new developments highlight how conditioned fear depends on far more complex networks than initially envisioned. Indeed, multiple parallel inhibitory and excitatory circuits are differentially recruited during the expression versus extinction of conditioned fear. Moreover, shifts between expression and extinction circuits involve coordinated interactions with different regions of the medial prefrontal cortex. However, key areas of uncertainty remain, particularly with respect to the connectivity of the different cell types. Filling these gaps in our knowledge is important because much evidence indicates that human anxiety disorders results from an abnormal regulation of the networks supporting fear learning.
Keywords: amygdala, medial prefrontal cortex, infralimbic, prelimbic, extinction, fear conditioning
This review focuses on learned fear and its regulation by intrinsic circuits of the amygdala. As biologists, we approach fear with an evolutionary perspective. We conceive fear as a set of innate response predispositions (behavioral, endocrine, autonomic, cognitive) to threatening stimuli. We assume that these response tendencies or rather, the underlying anatomical substrates and physiological mechanisms, have been retained by natural selection because they promote survival and reproductive success. Thus, the neuronal basis of fear should be well conserved across species, a corollary supported by congruent findings of animal and human studies (Phelps and LeDoux, 2005). We focus on observable correlates of fear like freezing behavior for two reasons. First, animals might not experience feelings of fear. Second, the subjective experience of fear and associated defensive behaviors likely depend on different mechanisms (LeDoux, 2014). Nevertheless, for simplicity, below we use the word fear when referring to defensive behaviors.
Building on innate fear, learned fear also represents an advantageous evolutionary adaptation: the ability to learn by experience that some stimuli or circumstances predict danger or safety is key to the survival of animals in the wild. In the laboratory, the paradigm most often used to study this process is Pavlovian fear conditioning where an initially neutral stimulus (conditioned stimulus–CS), such as tone, is paired with a noxious unconditioned stimulus (US), typically a mild foot shock. As a result, the CS acquires the ability to elicit conditioned fear responses (CRs; such as freezing) when later presented alone.
Pavlovian fear conditioning is used widely, in part because it is easy to implement: just a few (typically 4–5) CS-US pairings lead to the formation of a readily quantifiable memory that lasts the subjects’ lifetime (McAllister et al., 1986; Gale et al., 2004). Another factor behind this paradigm’s popularity is evidence that human anxiety disorders result from a dysregulation of normal fear learning mechanisms (Graham and Milad, 2011) and abnormal activity patterns in the cerebral networks that normally regulate fear learning (Shin et al., 2006a; Bremner et al., 2008).
Together, these factors have contributed to make fear learning mechanisms one of the most intensely studied questions in neuroscience. Indeed, during the last decade, ≈400 papers/year have been published on this question. Since this vast literature cannot possibly be reviewed here, we will focus on a line of investigation that has been particularly active lately: the intrinsic amygdala circuits that mediate learned fear. Although we concentrate on learned fear, it should be noted that the same circuits have been implicated in the acquisition of responses driven by positively valenced reinforcers (for instance, see Tye et al., 2008). The reader is referred to prior reviews for other aspects of fear conditioning such as mechanisms of synaptic plasticity (Pape and Pare, 2010; Johansen et al., 2011), memory consolidation and reconsolidation (Nader and Hardt, 2009), the impact of neuromodulators and stress (Rodrigues et al., 2009), or genetic factors (Hovatta and Barlow, 2008).
Anatomy and physiology of the amygdala
The amygdala is a critical component of the neural circuitry underlying fear learning (Davis, 2000; LeDoux, 2000). It is comprised of a heterogeneous collection of nuclei, some with properties reminiscent of cortex, others of striatum. In this review, we will focus on a subset of these because they are thought to regulate conditioned fear: the basolateral complex (BLA), which includes the lateral (LA), basolateral (BL), and basomedial (BM) nuclei, the central nucleus (CeA), commonly divided in lateral (CeL) and medial (CeM) sectors, and the intercalated cell masses (ICMs). In broad strokes, LA is the main point of entry for sensory inputs into the amygdala, whereas CeM is the main source of amygdala projections to brainstem fear effector structures. However, not all sensory inputs trigger fear, in part because impulse transfer from LA to CeM is flexibly gated depending on the specific pattern of environmental cues confronting the organism (Pare et al., 2003). It is thought that CeL and the ICMs fulfill this function because they receive glutamatergic inputs from BLA and send GABAergic projections to CeM. We now briefly consider the cell types and connectivity of these nuclei.
Basolateral complex
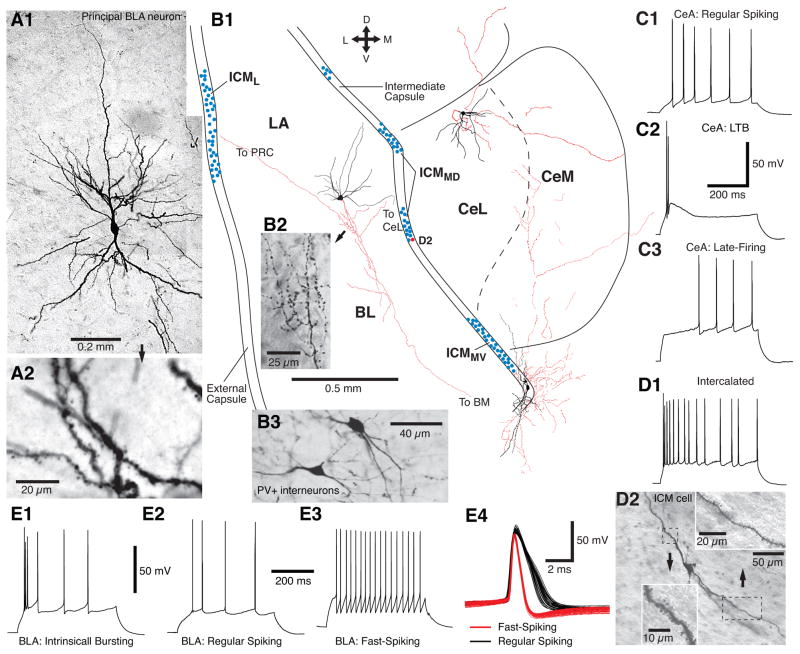
The cellular composition of the BLA is often likened to that of the cerebral cortex because it also contains a majority (≈80%) of spiny glutamatergic neurons (principal neurons; Fig. 1A, B) and a minority (≈20%) of sparsely spiny GABAergic interneurons (Fig. 1B3; McDonald, 1992; Spampanato et al., 2011)1. Although some intrinsically bursting principal cells exist (Fig. 1E1; Pare et al., 1995a), most are regular spiking neurons that exhibit a continuum of spike frequency adaptation due to the differential expression of voltage- and Ca2+-dependent K+ conductances (Fig. 1E2; Faber and Sah, 2002; Sah et al., 2003). Importantly, corticosterone and norepinephrine strongly reduce this adaptation thereby increasing the excitability of principal cells in emotionally arousing conditions (Duvarci and Pare, 2007; Tully et al, 2007).
Fig. 1.
Physiological and morphological properties of amygdala neurons. (A) LA projection cell at low (A1) and high (A2) magnification. (B1) Scheme of coronal section of the rat amygdala with camera lucida drawings of principal cells in LA, CeL, and ICMMV (black soma and dendrites; red, axons). Cells were labeled with biocityn during whole-cell recordings in vitro. Cross indicates orientation (D, dorsal; V, ventral; L, lateral; M, medial). Blue circles represent intercalated neurons. (B2) Micrograph showing varicose axon of LA neuron. (B3) Parvalbumin positive interneurons of the BL nucleus. (C–E) Repetitive firing behavior of (C) CeA, (D) intercalated, and (E) BLA neurons in response to supra-threshold depolarizing current pulses (C1, regular spiking; C2, Low-threshold bursting – LTB; C3, late-firing; E1, intrinsically bursting – IB; E2, regular spiking; E3, fast-spiking –FS). (E4) Superimposition of action potentials generated by BLA projection cell (black) and fast-spiking interneuron (red). (D2) Morphological property of an intercalated neuron in ICMMD (red circle in B1).
There are at least five types of GABAergic interneurons in the rodent BLA (McDonald and Betette, 2001; McDonald and Mascagni, 2001a, 2002; Mascagni and McDonald, 2003, 2007). Numerically, the two main classes express parvalbumin (PV+; Fig. 1B3) or somatostatin (SOM+). However, PV+ interneurons are not distributed homogenously in the BLA: they are more numerous in BA than LA (Muller et al., 2006). Different classes of interneurons regulate principal cells in distinct ways because they receive different inputs and target different postsynaptic domains (Smith et al., 2000; Muller et al., 2003, 2006, 2007; Bienvenu et al., 2012). For instance, PV+ interneurons receive strong inputs from principal cells, but very few from the cerebral cortex (Smith et al., 2000). They form inhibitory synapses with the soma, axon initial segment, and proximal dendrites of projection cells (Pitkänen and Amaral, 1993; Sorvari et al., 1995; Smith et al., 1998; McDonald and Betette, 2001). In contrast, SOM+ interneurons target the distal dendrites of principal cells (Muller et al., 2007) and they receive cortical inputs (Unal et al., 2013). Thus PV+ and SOM+ interneurons would be preferentially involved in feedback vs. feedforward inhibition, respectively.
In terms of electroresponsive properties, many BLA interneurons exhibit a fast-spiking phenotype characterized by very brief action potentials and little or no spike frequency accommodation (Fig. 1E3, E4; Spampanato et al., 2011). However, many other physiological types of interneurons have been described. In fact, even among neurochemically-homogeneous subtypes, the physiological properties of local-circuit cells are extremely diverse (Rainnie et al., 2006; Sosulina et al., 2006; Woodfruff and Sah, 2007; Jasnow et al., 2009).
Central nucleus of the amygdala
CeL and CeM each contain one main cell type (Hall, 1972; Kamal and Tömböl, 1975; McDonald, 1992) thought to be GABAergic (Paré and Smith, 1993a; McDonald and Augustine, 1993). Most CeM neurons have a large soma, dendrites that branch sparingly and exhibit a low to moderate density of dendritic spines. In contrast, most CeL neurons have a smaller soma, multiple primary dendrites that branch profusely and bear a high density of spines, similar to the main type of cells found in the striatum (Hall, 1972), the so-called medium spiny neurons. Also similar to the striatum, local-circuit cells appear to account for a much lower proportion of neurons in CeL than BLA. As to the physiological properties of principal CeL and CeM neurons, three subtypes have been described (Martina et al., 1999; Dumont et al., 2009; Lopez de Armentia and Sah, 2004): regular spiking (RS; Fig. 1C1), low-threshold bursting (LTB; Fig. 1C2) and late-firing (LF; Fig. 1C3).
Intercalated neurons
Intercalated neurons do not form a compact nucleus but occur as numerous small densely packed cell clusters (Fig. 1B1, blue circles), hence the designation intercalated cell masses (ICMs). Importantly, ICMs form distinct connections depending on their position. Indeed, intercalated cell clusters are found in two major fiber bundles of the amygdala: the external capsule, which borders it laterally, and the intermediate capsule, located in between BLA and CeA (Fig. 1B1). We will refer to the intercalated cell clusters located in the external and intermediate capsules as lateral ICMs (ICML) and medial ICMs (ICMM), respectively. Among the latter, we will distinguish between clusters located dorsally, near CeL (ICMMD) and those located ventrally, near CeM (ICMMV).
The vast majority of intercalated neurons are GABAergic (Nitecka and Ben-Ari, 1987; Pare and Smith, 1993a; McDonald and Augustine, 1993). They have a small soma (8–19 μm in diameter), a dendritic tree mostly confined to the fiber bundle where their soma is located, and a moderate to high density of dendritic spines (Fig. 1B1, D2; Millhouse, 1986). Compared to the rest of the amygdala, ICMs express very high levels of μ opioid and dopamine type-1 receptors (Herkenham and Pert, 1982; Jacobsen et al., 2006; Poulin et al., 2008). Physiologically, the main intercalated cell type exhibits a regular spiking firing pattern and a high intrinsic excitability due to a very high input resistance and modest spike frequency adaptation (Fig. 1D1; Royer et al., 2000b; Marowsky et al., 2005; Geracitano et al., 2007).
Intrinsic connectivity of the amygdala
Relative to other nucleated structures of the brain, such as the thalamus, the amygdala stands out for its very strong intra- and internuclear connectivity. For instance, principal BLA cells contribute multiple axon collaterals that form a high number (≈100–200/mm of axon) of en passant excitatory synapses with other BLA neurons (Fig. 1B2; Smith and Pare, 1994). Yet, paired recordings of closely spaced principal cells rarely provide evidence of connections (A Luthi and P Sah, personal communication). The explanation for this apparent contradiction resides in the spatial heterogeneity of the connections formed by principal cells with each other versus interneurons. Indeed, physiological studies have revealed that the axons of principal cells prevalently contact different types of neurons depending on the position of their targets: interneurons at proximity and other principal cells at a distance (Samson et al., 2003; Samson and Pare, 2006). Presumably, this arrangement allows the BLA network to prevent runaway excitation locally while allowing associative interactions between distant principal cells that receive different types of inputs.
Within CeA, principal neurons are also connected with each other, but via GABAergic synapses. For instance, local pressure application of glutamate in CEl evokes inhibitory postsynaptic potentials (IPSPs) in CEl neurons (Lopez de Amentia and Sah, 2004). Tracing studies have also revealed that CeL neurons project to CeM (Fig. 1B1) but that projections from CeM to CeL are weak or do not exist (Pretovich and Swanson, 1997; Jolkkonen and Pitkanen, 1998). More recently, it was found that distinct subtypes of CeL neurons contact CeM cells projecting to different brainstem sites. In particular, CeM cells that project to the periaqueductal gray (PAG) are contacted by CeL neurons expressing oxytocin receptors (OR+) whereas CeM cells projecting to the dorsal vagal complex (DVC) receive inputs from OR− CeL neurons (Viviani et al., 2011). It should be noted that many of the OR+ CeL neurons also express PKCδ but not SOM and conversely for OR− CeL neurons (Haubensak et al., 2010; Li et al., 2013).
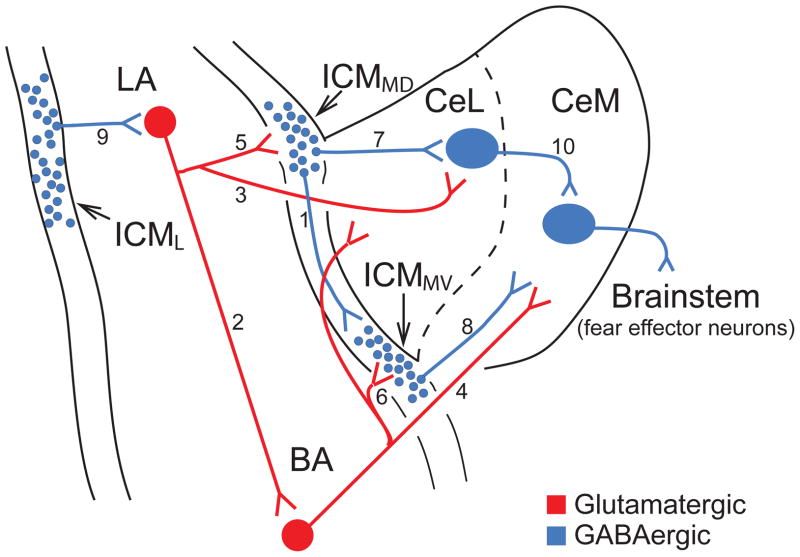
Locally within each intercalated cell cluster, individual neurons form inhibitory synapses with other intercalated cells, but these connections are rarely reciprocal (Geracitano et al., 2007, 2012). There are also connections between different intercalated cell clusters, at least between medially located ICMs (Fig. 2, link 1). However, these connections have a preferential direction from clusters located dorsolaterally (ICMMD) near CeL to those located ventromedially (ICMMV), near CeM (Fig. 2; Royer et al., 1999, 2000).
Fig. 2.
Intrinsic connectivity of the amygdala. Scheme of coronal section of the rat amygdala where all major internuclear connections are color coded (red, glutamatergic; blue, GABAergic). Numbers (1–10) refer to specific internuclear connections discussed in the main text.
Like the connections between different intercalated cell clusters, most internuclear amygdala connections have a preferential directionality. Within BLA, projections prevalently run dorsoventrally, from LA to BL and BM (hereafter collectively referred to as BA for basal nuclei; Fig. 2, link 2; Krettek and Price, 1978; Smith et al., 1994; Pitkanen et al., 1997). In addition, LA, BL, and BM project to CeA, a projection that is not reciprocated. Intriguingly, whereas LA exclusively projects to CeL (Fig. 2, link 3), the BA nuclei also project to CeM Fig. 2, link 4; Krettek and Price, 1978; Pare et al., 1995b; Pitkanen et al., 1997). Because CeM projections to brainstem fear effector neurons are much stronger than those originating from CeL (Hopkins and Holstege, 1978; Petrovich & Swanson, 1997), these differential connections are highly significant for the intra-amygdala mechanisms of conditioned fear.
On their way to CeA, the axons of principal BLA neurons form glutamatergic synapses with intercalated cells (Royer et al., 1999; Jungling et al., 2008). These projections are organized topographically such that neurons in LA vs. the BA nuclei preferentially contact intercalated cells in dorsally- (ICMMD; Fig. 2, link 5) vs. ventrally-located (ICMMV; Fig. 2, link 6) clusters, respectively. In turn, intercalated cells project to the region of CeA they are adjacent to (Fig. 2, link 7 and 8), generating feedforward inhibition (Pare and Smith, 1993b; Royer et al., 1999, 2000; Geracitano et al., 2007). Thus, there appears to be a repeated motif of connectivity between BLA, medial intercalated, and CeA neurons. Indeed, principal BLA neurons influence CeA neurons in two ways: via a direct glutamatergic projection, and indirectly, by exciting intercalated cells that then generate feedforward inhibition in CeA neurons. As we will see below, it was proposed that learned fear is regulated by modifying the relative efficacy of the direct vs. indirect limbs of this microcircuit.
In contrast with intercalated neurons located in the intermediate capsule, those located in the external capsule do not project to CeA but to BLA (Fig. 2, link 9; Marowsky et al., 2005). By virtue of their position, these intercalated cells are likely innervated by a variety of cortical fields. It is therefore likely that they allow for a flexible regulation of cortical influences over the BLA. This possibility remains to be tested however.
Extrinsic connectivity of the amygdala
Consistent with the fact that the amygdala has access to information about all sensory modalities, mammals readily develop conditioned fear responses to auditory, olfactory, or visual CSs (Domjan, 2006). However, we will focus on auditory fear conditioning because it is the best understood form of fear learning. Multiple parallel routes exist for the transfer of CS and US information to the amygdala: via direct subcortical (pre-thalamic) routes, via the dorsal thalamus (generally posterior thalamic nuclei), and via the cerebral cortex, mainly associative cortical areas (LeDoux et al., 1990; Turner and Herkenham, 1991; McDonald, 1998; Linke et al., 2000). Whereas the main recipient of associative cortical inputs is LA (McDonald, 1998), thalamic and pre-thalamic inputs also target CeA and the BA nuclei (LeDoux et al., 1985, Turner and Herkenham, 1991; Linke et al., 2000). For instance, there is a major nociceptive pathway from the spinal cord and trigeminal sensory nuclei that reaches CeL via the pontine parabrachial nucleus and completely bypasses LA (Bernard and Besson, 1990; Bernard et al., 1993; Neugebauer et al., 2009). These findings suggest that both LA and CeA have the necessary connections to mediate CS-US associations during fear conditioning. It should also be mentioned that the BLA is reciprocally connected with the ventral hippocampus and that these connections have been implicated in contextual fear and anxiety (Narayanan et al., 2007; Felix-Ortiz et al., 2013).
On the output side, the targets of the amygdala are extremely diverse, with BLA and CeA axons usually projecting to different sets of brain regions (Pitkanen, 2000). Indeed, CeA supplies most amygdala projections to the brainstem nuclei that generate the behavioral and visceral correlates of conditioned fear, including the PAG, parabrachial nuclei, solitary nucleus and DVC (Hopkins and Holstege, 1978). In contrast, BLA contributes most amygdala projections to the striatum, thalamus, and cerebral cortex. Although many cortical regions are contacted by BLA axons, this review will only consider the medial prefrontal cortex (mPFC) because it plays a critical role in regulating conditioned fear (Sotres-Bayon and Quirk, 2010). Other amygdala outputs thought to contribute to conditioned fear include projections to BNST (Dong et al., 2001) and various hypothalamic nuclei (Pitkanen, 2000). However, in contrast with the above, both BLA and CeA contribute to these projections. Finally, the amygdala can also indirectly influence the excitability of the entire prosencephalon via its projections to neuromodulatory cell groups (releasing acetylcholine, noradrenaline – NA, and dopamine –DA) of the basal forebrain and brainstem (Steriade and Pare, 2007).
Acquisition and expression of conditioned fear
A series of lesion, inactivation, and unit recording studies performed in the 1990’s (reviewed in Pape and Pare, 2010) led to the view that LA is the critical site of synaptic plasticity for the acquisition of Pavlovian fear. In particular, it was proposed that convergence of synaptic inputs about the CS and US leads to the potentiation of synapses conveying CS information to LA (Davis, 2000; LeDoux, 2000). As a result, potentiated LA inputs about the CS would trigger conditioned fear by recruiting CeA neurons that project to downstream fear effector structures.
Below, we concentrate on the intrinsic amygdala networks that process CS information from LA to CeA. However, this focus does not imply that we consider the amygdala to be the sole site of plasticity for Pavlovian fear. In fact, auditory fear conditioning leads to widespread synaptic plasticity in the brain, not only the amygdala, but also in the auditory thalamus and cortex (Weinberger, 2011; Letzkus et al., 2011). Moreover, interfering with plasticity at these various sites prevents the acquisition of conditioned fear. Thus, there is incontrovertible evidence that plasticity in both the amygdala and its afferent neurons contributes to fear conditioning. The outstanding question is: what is their relative contribution?2
Factors intrinsic to LA regulate fear learning: role of neuronal excitability and synaptic inhibition
As mentioned above, LA receives inputs from thalamic and cortical neurons involved in processing auditory (CS) and somatosensory (US) information. Moreover, CS and US information can converge onto single LA neurons (Romanski et al., 1993). According to the cellular hypothesis of fear conditioning (Blair et al., 2001, Sigurdsson et al., 2007), CS inputs to LA are relatively weak prior to conditioning and hence the CS is unable to elicit fear responses. However, the potentiation of CS synapses as a result of conditioning would allow LA neurons to elicit fear by recruiting cells in fear effector structures such as CeM. This hypothesis predicts that CS-evoked responses should increase in LA following fear conditioning, a prediction that was confirmed by a number of extra- and intracellular recording studies (Quirk et al., 1995; Rogan et al., 1997; Collins and Pare, 2000; Repa et al., 2001; Rosenkranz and Grace, 2002; Goosens et al., 2003).3
In the unit recording studies mentioned above, relatively few LA neurons (≈20%) were seen to develop an increased CS responsiveness as a result of conditioning, despite the fact that most receive the necessary inputs (Han et al., 2007). This led to the suggestion that assignment of particular LA neurons to the fear memory trace engages a competitive process that preferentially recruits neurons with a higher intrinsic excitability (Han et al., 2007, 2009). In keeping with this, LA neurons expressing activated cAMP response element-binding protein (CREB), a property associated with increased neuronal excitability (Viosca et al., 2009; Zhou et al., 2009), are preferentially recruited into the memory trace (Han et al., 2007, 2009). Moreover, when CREB is overexpressed or downregulated in LA, the proportion of cells recruited into the memory trace does not change, suggesting that a competitive synaptic process is at play (Han et al., 2007).
Consistent with this, a recent modeling study (Kim et al., 2013b), revealed that LA neurons with a high intrinsic excitability were much more likely to acquire increased CS responses as a result of fear conditioning. Moreover, when the CREB overexpression or downregulation experiments were simulated by transforming a subgroup of cells with low excitability into more excitable neurons (or conversely), the number of model plastic cells was not altered. Thus, these results suggest that while higher intrinsic excitability biases principal LA neurons to become plastic, the number of plastic cells is constrained by synaptic interactions. In keeping with this, analysis of the connections of model plastic and nonplastic cells revealed that subgroups of principal LA neurons in effect band together via their excitatory interconnections to stifle plasticity in other principal cells by recruiting inhibitory interneurons.
Consistent with these observations, many experimental studies indicate that GABAergic transmission regulates fear conditioning and the underlying synaptic plasticity (reviewed in Pare et al., 2003; Ehrlich et al., 2009). As discussed above, principal LA neurons are under strong inhibitory control from both local-circuit cells as well as laterally located intercalated cells (ICML). Moreover, activity-dependent synaptic plasticity is more readily induced in LA neurons when GABAergic inhibition is reduced (Watanabe et al., 1995; Bissiere et al., 2003; Shaban et al., 2006; Shin et al., 2006b). Conversely, activation of GABA-A receptors in LA impairs acquisition of conditioned fear (Muller et al., 1997; Wilensky et al., 1999) and fear conditioning is associated with reduced BLA levels of GABA (Stork et al., 2002) and mRNA for GABA-synthesizing enzymes (Pape and Stork, 2003; Heldt and Ressler, 2007; Bergado-Acosta et al., 2008). Together, these results suggest that disinhibition of principal LA cells is an important permissive factor in fear conditioning.
What mechanisms could regulate intra-LA inhibitory circuits and thus the acquisition of conditioned fear? GABAergic neurons are important targets of neuromodulators, such as DA, NE, serotonin, gastrin releasing peptide (GRP) and endocannabinoids (Bissiere et al., 2003; Marowsky et al., 2005; Tully et al., 2007; Rainnie, 1999; Stutzmann and LeDoux, 1999; Shumyatsky et al., 2002; Marsicano et al., 2002). For instance, DA and NE suppress feedforward inhibition onto principal LA neurons through both inhibition of interneurons and lateral intercalated cells (Bissiere et al., 2003; Marowsky et al., 2005; Tully et al., 2007). Furthermore, DA and NE facilitate synaptic plasticity within LA (Bissiere et al., 2003; Tully et al., 2007). Since aversive learning activates neurons in the ventral tegmental area and locus coeruleus (Brischoux et al., 2009; Chiang and Aston-Jones, 1993), which respectively provide DA and NE inputs to LA, it is conceivable that these neuromodulators lead to disinhibition of principal cells, thereby facilitating the acquisition of conditioned fear. Consistent with this, NE and DA receptor activation in the amygdala has been implicated in the acquisition of conditioned fear (Bush et al., 2010; Greba et al., 2001; Guarraci et al., 1999; Nader and LeDoux, 1999). On the other hand, serotonin (Stutzmann and LeDoux, 1999) and GRP (Shumyatsky et al., 2002) excite inhibitory interneurons. By increasing inhibition of principal cells, these modulators likely constrain plasticity in LA (Schumyatsky et al., 2002).
As mentioned above, BLA interneurons show considerable diversity in terms of the peptides they express, inputs they receive, and cellular domains they target. This raises the question of how different interneuron subpopulations regulate fear conditioning. For example, PV+ interneurons mainly target the soma and proximal dendrites of principal cells and generate feedback inhibition, whereas SOM+ interneurons target their distal dendrites and provide feedforward inhibition. Therefore, these two interneuron subtypes likely mediate different aspects of information processing in the amygdala. Cell-type specific optogenetic manipulations will be required to dissect their respective roles in the acquisition and expression of conditioned fear.
Relay of CS information from LA to CeM
In order for conditioned fear to be expressed, information about the CS must reach brainstem-projecting CeM cells. However, LA does not project to CeM (Krettek and Price, 1978b; Smith and Pare, 1994; Pitkanen et al., 1995). Instead, CS information from LA can reach CeM indirectly, either via glutamatergic neurons of the BA nuclei or GABAergic neurons of CeL (Fig. 2, links 2 and 3, respectively). However, these two paths are expected to exert opposite effects on CeM cells: an excitation via the BA nuclei and an inhibition via CeL. Which of these two paths is critical for fear expression?
Following fear conditioning, CeM neurons show sustained elevations in firing rates during CS presentations (Ciocchi et al., 2010; Duvarci et al., 2011). Consistent with this, optogenetically activating CeM neurons elicits freezing whereas inactivation of these neurons impairs expression of conditioned freezing (Ciocchi et al., 2010). Since CeM neurons show excitatory responses to the CS, these results suggest that glutamatergic inputs from BA might be critical for relaying CS information downstream of LA. Indeed, BA nuclei are ideally situated to mediate this function as they receive strong inputs from LA and project heavily to CeM (Krettek and Price, 1978; Smith and Pare, 1994; Pare et al., 1995b; Pitkanen et al., 1997).
The first attempts to test the involvement of the BA nuclei in fear conditioning yielded negative results: pre-training BA lesions had little or no effect on conditioned fear (Amorapanth et al., 2000; Goosens and Maren, 2001; Nader et al., 2001). In contrast, post-conditioning BA lesions were later shown to abolish conditioned fear responses (Anglada-Figueroa and Quirk, 2005). Together, these results suggested that in the intact brain, the BA nuclei are indeed required for relaying the CS-evoked LA responses to CeM. However, when training occurs in their absence, CS information reaches CeM via another route.
In agreement with the dramatic effects of post-training lesions, BA neurons develop increased CS responses as a result of fear conditioning (Herry et al., 2008; Amano et al., 2011). Moreover, BA inactivation largely reduces conditioned fear responses (Amano et al., 2011). Another observation supporting the notion that BA neurons are critical for relaying LA inputs to CeM is the mismatch between the duration of conditioned fear responses and the tone responses of LA neurons. Indeed, most LA neurons show only transient responses at CS onset (Quirk et al., 1995; Repa et al., 2001). In contrast, most BA neurons show sustained responses that last for the entire CS duration and in some cases beyond, mirroring the persistence of conditioned fear responses (Amano et al., 2011). Interestingly, prelimbic (PL) neurons show a similar response pattern (Burgos-Robles et al., 2009; Fitzgerald et al., 2013; Courtin et al., 2014). Moreover, PL inactivation impairs fear expression (Corcoran and Quirk, 2007), and BLA inactivation abolishes tone responses in PL (Sotres-Bayon et al., 2012). In keeping with this, a recent study utilizing projection-specific optogenetic identification showed that CS responsive BA neurons indeed project to PL (Senn et al., 2014). Together, these results suggest that reciprocal connections between PL and the BA nuclei might contribute to prolong the transient tone signal generated by LA neurons into persistent responses (Fig. 3A). Furthermore, these observations imply that BA neurons are not passive relays of CS information from LA to CeM, but that they also actively extend these signals in time, through interactions with each other and/or with PL. Future research investigating the contribution of PL to CS-evoked BA responses –using projection specific optogenetic manipulations– will be important to unravel how these structures interact.
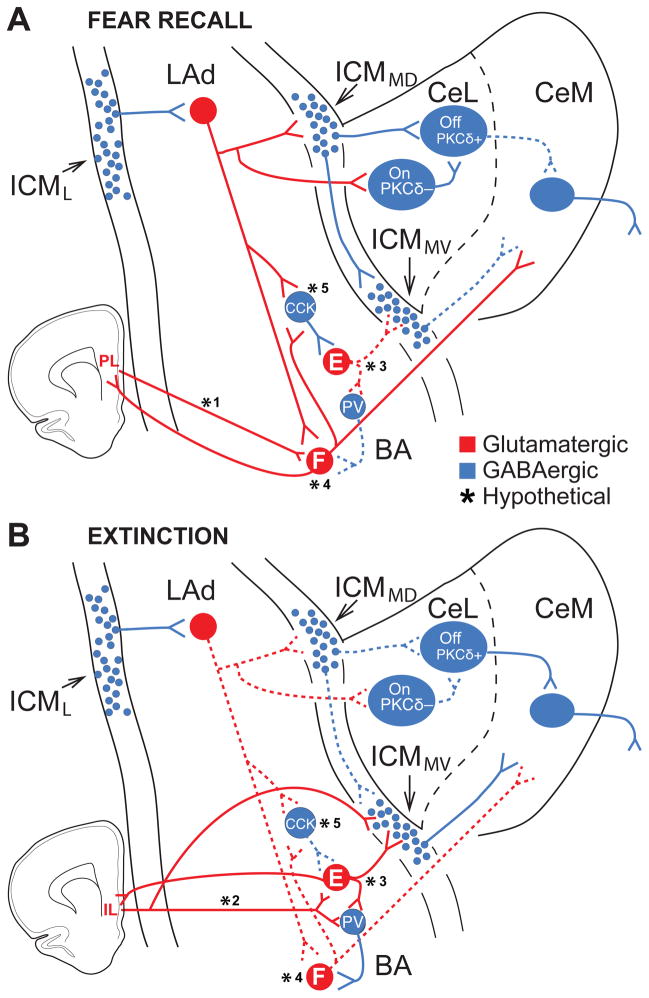
Fig. 3.
Intra-amygdala interactions supporting expression and extinction of conditioned fear. The model includes known pathways and hypothetical links (marked by asterisks) that collectively account for most of the available evidence. Solid and dashed lines represent connections that are more or less active, respectively. (A) The increased CS responsiveness of CeM output neurons after conditioning likely results from two parallel mechanisms: excitation by glutamatergic BA neurons plus disinhibition from CeL and ICMMV inputs. CeM excitation: CS-induced LA activation causes a BA neuron subtype (“Fear neurons”, F) to fire and excite CeM cells whereas another type of BA neurons (“Extinction cells”, E) are inhibited, possibly by CCK+ interneurons. Although LA neurons respond transiently to the CS, BA fear neurons, through excitatory interactions with each other and/or with prelimbic (PL) cells (lower left), would prolong the transient tone signal emanating from LA into persistent CS responses. CeM disinhibition: The excitation of LA cells also leads to the recruitment of ICMMD neurons and of a subgroup of CeL cells, likely PKCδ− (CeL-On) cells. ICMMD neurons would then inhibit ICMMV cells, disinhibiting CeM neurons. In addition, ICMMD cells would inhibit subgroups of CeL neurons, possibly PKCδ+ (CeL-Off) cells. The recruitment of PKCδ− (CeL-On) cells by LAd neurons would cause a further inhibition of PKCδ+-neurons and disinhibition of CeM cells. (B) The decreased CS responsiveness of CeM output neurons after extinction likely depends on two parallel mechanisms: disfacilitation of CeM cells and increased feedforward inhibition of CeM neurons. CeM disfacilitation: The rapid extinction of LAd responses to the CS results in a diminished recruitment of BA fear neurons, disfacilitation of CeM neurons, and, possibly, of CCK+ interneurons. As a result, BA extinction cells are disinhibited. Reciprocal excitatory interactions between IL (lower left) and BA might also contribute to enhance the excitability of extinction cells. The disinhibition of extinction cells causes increased excitation of a different set of BA interneurons, possibly PV+ interneurons, controlling fear cells. CeM inhibition: the reduced CS responsiveness of LAd neurons would cause a disfacilitation of ICMMD neurons and consequent disinhibition of ICMMV neurons. This effect would coincide with an increased excitation of ICMMV cells by inputs from BA extinction cells, thus resulting in an increased feedforward inhibition of CeM cells. The disfacilitation of ICMMD neurons would also cause a disinhibition of subsets of CeL cells, possibly corresponding to PKCδ+ neurons. This effect would be reinforced by the reduced activation of PKCδ− cells secondary to reduced LAd inputs. Hypothetical connections (marked by asterisks). (*1, *2) While it was shown that BA fear and extinction cells differentially project to PL and IL, respectively, whether return mPFC projections are similarly segregated is unknown. (*3, *4) Currently, there is no data available on the connections of fear and extinction neurons with other amygdala neurons. The differential connections shown are hypotheses based on the available literature. (*5) Little data is available on the connectivity of CCK cells with different types of BA neurons. Trouche et al. (2013) reported that they contact fear (not shown here) and extinction-resistant neurons. CCK synapses to extinction-resistant (but not fear neurons) showed an up-regulation of CB1 receptors after extinction training. The input and output connections of CCK interneurons shown in the figure are all hypothetical. It is possible that other subtypes of interneurons are differentially connected to fear and extinction neurons.
Multiple disinhibitory networks control CeM output
As mentioned above, preconditioning BA lesions have no effect on the expression of conditioned fear. This raises the question of how CS information is relayed from LA to CeM in the absence of BA? CeL neurons have the necessary connections (Fig. 2, links 7 and 10) but at first glance appear unlikely candidates for this role because they are expected to generate feed-forward inhibition in CeM. However, recent studies reviewed below suggest that this reasoning is incorrect and that CeL indeed plays a key role in fear conditioning.
Initially, CeA was conceived as a passive output station of the amygdala for fear expression (reviewed in Samson et al., 2005). However, subsequent studies suggested that CeA is in fact necessary for both, the acquisition and expression of conditioned fear (Goosens and Maren, 2003; Wilensky et al., 2006). Following up on these findings, a recent study selectively inactivated either CeL or CeM to identify their respective contribution. This revealed a functional dissociation between the two sub-nuclei: whereas inactivation of CeL selectively impaired fear acquisition, inactivation of CeM impaired fear expression (Ciocchi et al., 2010).
These findings raised the question of how could CeL, via its GABAergic projections to CeM, elicit increases in the firing rate of CeM neurons? Interestingly, two populations of neurons exist in CeL, one showing inhibitory (CeL-Off) and the other excitatory (CeL-On) responses to the CS after fear conditioning (Ciocchi et al., 2010; Duvarci et al., 2011). Moreover, it was further shown that CeL-Off cells correspond to the PKCδ+ neurons mentioned earlier and that they express oxytocin receptors (Ciocchi et al., 2010, Haubensak et al., 2010). These findings led to the hypothesis that under baseline conditions, CeL-Off neurons exert a tonic inhibitory influence onto CeM cells. Excitation of CeL-On cells during the CS would cause the inhibition of CeL-Off neurons, resulting in the disinhibition of CeM fear output neurons (Ciocchi et al., 2010; Haubensak et al., 2010; Fig. 3A). Supporting this view, release of endogenous oxytocin in CeL attenuates conditioned freezing (Knobloch et al., 2012), presumably through the activation of CeL-Off cells. However, CeL-On and CeL-Off neurons both project to CeM and reciprocally inhibit each other (Ciocchi et al., 2010; Haubensak et al., 2010). It is therefore not clear how one population could become dominant as a result of fear conditioning.
A possible solution comes from another study where recordings of rat CeL neurons revealed that from the training day to the recall test one day later, the incidence of CeL-Off neurons triples with no change in the proportion of CeL-On cells (Duvarci et al., 2011). A potential explanation for these results is that CeL-On to CeL-Off synapses are potentiated as a result of fear conditioning. Another is that a different inhibitory input, extrinsic to CeL, is involved. We will return to this idea below.
Support for the first possibility comes from a recent study that selectively manipulated the activity of a subpopulation of CeL neurons expressing SOM (Li et al., 2013). Inactivation of SOM+ neurons impaired acquisition of fear conditioning whereas optogenetically activating them elicited freezing behavior. Interestingly, fear conditioning potentiated LA synapses onto SOM+ neurons while weakening these inputs onto SOM− cells. This suggests that fear conditioning may bias the competition between mutually inhibitory CeL neuron subtypes (Li et al., 2013). However, whether the SOM+ neurons correspond to CeL-On cells remains to be determined. Indeed, unlike CeL-On cells (Ciocchi et al., 2010; Haubensak et al., 2010), SOM+ cells do not project to CeM (Li et al., 2013).
Another possible explanation for the marked increase in the incidence of CeL-Off cells from conditioning to fear recall (Duvarci et al., 2011) is the involvement of an inhibitory input extrinsic to CeL. Consistent with this notion, CeL inactivation does not affect fear expression (Ciocchi et al., 2010). What could this alternative disinhibitory pathway be? As discussed earlier, BLA can influence CeA by exciting ICMMV cells (Fig. 2, link 6) that then generate feedforward inhibition in CeA neurons (Fig. 2, link 8). Since LA projects to ICMMD (Fig. 2, link 5), but not ICMMV, CS presentations should cause the glutamatergic activation of ICMMD neurons, leading to the inhibition of ICMMV cells (via link 1 in Fig. 2) as well as CeL-Off neurons (via link 7 in Fig. 2), with the final result of disinhibiting CeM (Fig. 3A). Supporting this hypothesis, expression of the immediate-early gene Zif268 increases during fear recall in ICMMD, but not in ICMMV, cells (Busti et al., 2011). An important challenge for future research will be to identify the targets of ICMMD cells in CeL: do they preferentially end on CeL-Off neurons, as predicted here?
Overall, the findings reviewed in this section suggest that multiple parallel disinhibitory circuits exist within the amygdala and that their dynamic interactions ultimately determine fear expression.
Fear extinction
In the previous sections, we considered how intrinsic amygdala networks enable animals to learn that some stimuli predict danger. However, animals can also learn that stimuli previously associated with adverse outcomes no longer represent a threat. The most studied form of such safety learning is fear extinction in which repeated presentations of the CS alone lead to a gradual reduction of conditioned fear responses.
There is compelling behavioral evidence that extinction training does not erase or reverse the original CS-US association. Rather, extinction leads to the formation of a new inhibitory memory that competes with the initial fear memory for control of behavior (Bouton et al., 2006; Myers and Davis, 2007). First, fear extinction is not permanent but decays with time, a process known as spontaneous fear recovery (Brooks and Bouton, 1993). Second, conditioned fear responses can be restored by presenting the US alone in the context in which extinction training occurred (reinstatement) (Rescorla and Heth, 1975). Third, extinction is context-dependent such that fear responses return if the CS is presented in a different context than the one where extinction training occurred, a phenomenon known as fear renewal (Bouton, 2002, 2004). In other words, during extinction training, animals learn that the CS is associated with safety in a particular context.
Together, these findings suggest that fear and extinction memory traces co-exist and can be retrieved independently. A large body of evidence suggests that fear extinction is mediated by a distributed network that includes the amygdala, mPFC and hippocampus (see Pape and Pare, 2010; Herry et al., 2010; Milad and Quirk, 2012; Maren et al., 2013). However, we will focus on the intrinsic circuits of the amygdala that support extinction learning and expression.
Amygdala outputs parallel fear expression
There is a strong correlation between the CS-responsiveness of CeM neurons and levels of fear expression (Ciocchi et al., 2010; Duvarci et al., 2011). For instance, during extinction training, the firing of CeM neurons and the fear responses elicited by the CS decrease in parallel (Duvarci et al., 2011). Since extinction does not erase the initial CS-US association, this reduction is likely caused by inhibitory circuits that suppress the CS-evoked firing of CeM neurons and, consequently, fear expression. Based on their GABAergic projections to CeM, CeL and/or ICMMV neurons are good candidates to fulfill this role. However, increased inhibition within BLA could also be involved. Below, we review the evidence supporting these various possibilities, beginning with the BLA.
Fear and extinction circuits co-exist in the BLA
Depending on their location, LA neurons are differentially affected by extinction training. In the dorsal subdivision of LA (LAd), the main termination site of thalamic inputs about the CS, extinction causes a rapid reduction of CS-evoked responses (Repa et al., 2001). In contrast, in ventrally-located LA (LAv) neurons, CS responses persist despite extinction (Repa et al., 2001), as seen in the auditory cortex (Quirk et al., 1997; Armony et al., 1998). There is evidence that the rapid reduction of CS-responsiveness in LAd neurons occurs through depotentiation of thalamic inputs (Kim et al., 2007). Irrespective of the underlying mechanisms however, the persistence of CS-evoked responses in LAv neurons despite their loss in LAd raises the intriguing possibility that extinction training causes a shift in the networks responsible for transferring CS information to the amygdala. Consistent with this, during fear renewal, some LA neurons show a resurgence of CS-elicited firing, which depends on dorsal hippocampal activity (Hobin et al., 2003; Maren and Hobin, 2007). Thus, even though extinction training does not abolish CS-US associations, it causes a re-organization of the fear memory. In this new representation, LAv neurons likely contribute to maintain the original CS-US association.
Extinction training also causes drastic changes in the CS responsiveness of BA neurons, consistent with the finding that BA inactivation impairs extinction (Herry et al., 2008; Amano et al., 2011; Sierra-Mercado et al., 2011; Livneh and Paz, 2012). In previous unit recording studies (Herry et al., 2008; Amano et al., 2011), three main types of BA neurons were distinguished based on task-related changes in CS responsiveness: “Fear cells” that develop excitatory CS responses as a result of fear conditioning but lose them following extinction training, “Extinction cells” that only become CS responsive following extinction training, and “Extinction-resistant neurons” that acquire CS responses during conditioning but continue to be CS responsive after extinction training. The latter cell type is reminiscent of LAv neurons and might also be involved in the maintenance of CS-US association after extinction. In contrast, fear cells are similar to CeM neurons in that their CS responsiveness correlates with the level of fear expression, diminishing with extinction but returning during fear renewal. However, extinction cells have no counterpart in other amygdala nuclei.4
The existence of fear and extinction neurons in BA suggests that different circuits, mediating fear and extinction, co-exist within the amygdala. Cell-type specific projections of fear and extinction neurons (within and/or outside the amygdala) likely underlie their contrasting functions. Indeed, prior tracing studies have revealed that BA neurons send strong projections to the mPFC, particularly the PL and infralimbic (IL) areas (Krettek and Price, 1977). Whereas PL supports fear expression (see above), IL was implicated in extinction (Sotres-Bayon and Quirk, 2010). For instance, IL inactivation interferes with the acquisition of extinction (Sierra-Mercado et al., 2011) and IL neurons show high-frequency bursting immediately after extinction training (Burgos-Robles et al., 2007), as well as CS-evoked responses during extinction recall (Milad and Quirk, 2002). Moreover, electrical stimulation of IL paired with CS onset reduces conditioned fear responses and accelerates the acquisition of extinction (Milad and Quirk, 2002).
Consistent with these findings, a recent study reported that fear and extinction cells contribute complementary projections to the PL and IL areas (Senn et al., 2014). In particular, fear neurons project to PL whereas extinction neurons project to IL (Senn et al., 2014). Moreover, optogenetic silencing of IL-projecting BA neurons during extinction training results in poor extinction recall the next day (Senn et al., 2014).5 In contrast, little is known about the connections of fear and extinction neurons within the amygdala.
Extinction depends on a regulation of intra-BA inhibitory circuits
The rapid switching of activity between BA’s fear and extinction neurons (Herry et al., 2008) suggests that intra-BA inhibitory circuits gate expression of fear versus extinction. In agreement with this, strengthening of GABAergic transmission in BLA has been implicated in extinction learning. For instance, levels of mRNA for the GABA-A receptor clustering protein gephyrin as well as surface expression of GABA-A receptors are upregulated in BLA after extinction training (Chhatwal et al., 2005b; Heldt and Ressler, 2007). At the same time, mRNA levels for the GABA synthesizing enzyme GAD67 increase, whereas those for the GABA transporter GAT1 decrease in BLA (Heldt and Ressler, 2007). Moreover, mice deficient in the activity-dependent GAD isoform, GAD65, show impaired extinction (Sangha et al., 2009). Consistent with these findings, the frequency and amplitude of miniature inhibitory postsynaptic currents (mIPSCs) increase in principal BLA neurons after extinction (Lin et al., 2009). Together, these findings suggest that extinction is associated with an overall increase in GABAergic inhibition in the BLA.
Another line of evidence implicating intra-BA GABAergic inhibition in extinction comes from studies of endocannabinoid signaling (Lutz, 2007). In particular, extinction training results in increased endocannabinoids levels in the BLA. Moreover, cannabinoid receptor 1 (CB1)-deficient mice show impaired extinction (Marsicano et al., 2002). Consistent with this, systemic (Marsicano et al., 2002; Chhatwal et al., 2005a) and intra-BLA (Roche et al., 2007) administration of CB1 receptor antagonists impair extinction. At the cellular level, endocannabinoids cause a long-term depression of GABAergic synaptic transmission via activation of CB1 receptors (Marsicano et al., 2002), and hence reduce GABAergic inhibition of principal BLA neurons (Katona et al. 2001).
Different BA interneuron subtypes regulate switching between fear and extinction memory
The endocannabinoid findings are at odds with the notion that GABAergic inhibition is globally enhanced in the BLA during extinction. In fact, it would seem a priori that a general increase or decrease in GABAergic inhibition in BLA cannot mediate extinction since extinction is context-dependent. Rather, switching between extinction and fear expression likely depends on the differential recruitment of particular subpopulations of GABAergic interneurons. As mentioned earlier, interneurons in BLA show considerable diversity. It is therefore possible that different inhibitory circuits are recruited during expression of fear versus extinction. Indeed, CB1 receptors are only found on the axon terminals of a specific subpopulation of BLA interneurons, which express the peptide cholecystokinin (CCK; Katona et al., 2001).
These findings lead us to hypothesize that CCK interneurons are prevalently connected to extinction and/or extinction-resistant neurons. According to this model (Fig. 3B), CB1 receptor activation during extinction would result in decreased inhibition of extinction neurons and, as a consequence, increases in their CS responsiveness. Supporting this hypothesis, a recent study using contextual fear conditioning found interneuron subtype-specific remodeling of inhibitory synapses in the BLA following extinction (Trouche et al., 2013). Utilizing a c-fos-based transgenic mouse line, this study reported that synapses formed by PV+ and CCK+ interneurons undergo differential plasticity during extinction depending on whether they contact cells active only during expression of conditioned fear (presumed “fear cells”) or neurons active during expression of both fear and extinction (presumed “extinction-resistant” cells). Moreover, the same study revealed that extinction increases expression of CB1 receptors around the soma of neurons active during extinction (Trouche et al., 2013). Although extinction neurons were not addressed in this study, the involvement of CB1 receptors in extinction suggests that extinction neurons are likely contacted by CCK+ interneurons.
Extinction also depends on gating of BA inputs to CeM by intercalated cells
As reviewed above, principal BA neurons contribute glutamatergic projections to CeM’s fear output neurons (Krettek and Price, 1978; Pare et al., 1995b; Royer et al., 1999). The existence of extinction as well as extinction-resistant neurons in the absence of fear expression suggests that an inhibitory circuit prevents the activation of CeM cells by BA neurons. As mentioned earlier, CeL and ICMMV are possible candidates for this task. However, conditioning-induced changes in the CS responsiveness of CeL neurons, are reversed during extinction training (Duvarci et al., 2011), arguing against their involvement in gating CS-evoked BA inputs to CeM.6 In contrast, diverse lines of evidence support the notion that intercalated cells mediate this function.
First, extinction is associated with increased expression of the immediate-early genes Zif268 (Busti et al., 2011) and c-fos (Knapska and Maren, 2009) in ICMMV, but not ICMMD, cells. Second, selective ICM lesions (Likhtik et al., 2008) as well as pharmacological inhibition of BLA inputs to ICM cells by neuropeptide S (Jungling et al., 2008) interfere with extinction. Third, extinction training causes a potentiation of BA inputs to ICMMV cells, resulting in increased feedforward inhibition of CeM neurons (Amano et al., 2010). Last, this potentiation requires IL activity during and/or shortly after extinction training (Amano et al., 2010), consistent with the finding that IL neurons show high-frequency bursting immediately after extinction training (Burgos-Robles et al., 2007).
Indeed, IL sends a very dense glutamatergic projection to ICMMV cells (Cassell and Wright, 1986; McDonald et al., 1996). Consistent with this, IL stimulation triggers high-frequency spike bursts in intercalated cells (Amir et al., 2011). Overall, these findings suggest a model where the reduced CS responsiveness of LAd neurons leads to a decreased recruitment of ICMMD cells and, as a result, disinhibition of ICMMV neurons. This effect, coupled with the convergence of BA and IL inputs on intercalated cells during extinction would lead to the potentiation of BA synapses onto ICMMV neurons. As a result, subsequent CS presentations would elicit more feedforward inhibition in CeM neurons via ICMMV neurons, leading to reduced fear expression (Figure 3B).
Conclusion
Considerable progress has been made toward understanding the amygdala networks that support the acquisition and extinction of conditioned defensive behaviors. Collectively, the new evidence reviewed here demonstrates that conditioned fear depends on far more complex networks than initially believed. These include interactions between multiple parallel excitatory and inhibitory circuits of the amygdala, many of which are coordinated with mPFC activity.
Despite these advances however, key areas of uncertainty remain. In particular, we still know little about the inputs and targets of different subtypes of BLA, CeL, and ICM neurons. For instance, are the fear and extinction cells found in BA differentially connected with ICMMV and CeM neurons? Given their opposite CS responsiveness, one would expect extinction cells, not fear neurons, to contact ICMMV cells and conversely for CeM neurons. Also, are extinction and fear neurons reciprocally inhibiting each other via specific subtypes of interneurons, as hypothesized in figure 3? Do IL and PL neurons provide a complementary pattern of innervation to these putative interneuronal circuits (Figure 3)? Although such an arrangement is suggested by the differential projections of extinction and fear neurons to IL and PL as well as their opposite pattern of CS responsiveness, it remains to be established.
With respect to CeL neurons, whether there is a correspondence between SOM expression and CS responsiveness (positive or negative) remains unclear. However, the low degree of overlap between SOM+ and PKCδ+ suggest this is the case. Also to be examined is the possibility that ICMMD cells differentially innervate the various subtypes of CeL cells. Undoubtedly, recent technical advances will soon allow researchers to tackle these important questions.
Acknowledgments
This work was supported by R01 grants MH-083710, and MH-098738 from NIMH (to DP) and EMBO Long-Term Fellowship and Marie Curie Actions (to SD).
Footnotes
Although the vast majority of GABAergic neurons in the BLA are local-circuit cells, a recent study reported that a small subset of SOM+ neurons located in or near the external capsule projects to the basal forebrain (McDonald et al., 2012).
A recent study (Kim et al., 2013a) sheds new light on this question using a biologically realistic computational model of LA. The model allowed a series of experimentally impossible manipulations that probed the contributions of plasticity in CS afferent pathways versus LA to conditioned fear. The results of these simulations suggest that training-induced increases in the responsiveness of auditory afferent neurons are necessary for fear memory formation. However, once the memory has been formed, this factor is no longer required because the efficacy of auditory afferent synapses onto LA neurons has augmented enough to maintain the memory. New technological developments will be required to test these provocative conclusions.
It is unlikely that the enhanced CS responsiveness of LA neurons after fear conditioning is entirely due to the increased recruitment of auditory thalamic and/or cortical neurons. Indeed, even in brain slices kept in vitro, where afferent auditory axons to LA are cut from the somata contributing them, the efficacy of auditory synapses is enhanced after fear conditioning (McKernan and Shinnick-Gallagher, 1997; Rumpel et al., 2005). Moreover, fear conditioning occludes long-term potentiation of cortical inputs to LA (Tsvetkov et al., 2002).
Of note, a recent study suggests that extinction neurons might overlap with Thy-1 expressing neurons in BA (Jasnow et al., 2013).
Interestingly, a recent in vitro optogenetic study investigating the impact of mPFC inputs to BLA during extinction reported that extinction decreases the efficacy of mPFC inputs to BLA neurons (Cho et al., 2013). However, because both PL and IL axons were optogenetically activated in this study, it is unclear whether synapses contributed by neurons in PL, IL or both contributed to this effect. Recent studies suggest that these two mPFC regions are differentially recruited during fear expression vs. extinction (Orsini et al., 2011; Knapska et al., 2012).
It is currently unclear whether this reversal of CeL activity reflects the extinction-induced decrease in CS responsiveness of LAd neurons or plasticity in CeL. It will therefore be important for future studies to test whether extinction induces plasticity within the intra-CeL circuitry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Duvarci S, Popa D, Pare D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–15489. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir A, Amano T, Pare D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol. 2011;105:3054–3066. doi: 10.1152/jn.00136.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3:74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Quirk GJ, LeDoux JE. Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning. J Neurosci. 1998;18:2592–2601. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergado-Acosta JR, Sangha S, Narayanan RT, Obata K, Pape HC, Stork O. Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory. Learn Mem. 2008;15:163–171. doi: 10.1101/lm.705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontineparabrachial area to the amygdaloid complex: A Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Bienvenu TC, Busti D, Magill PJ, Ferraguti F, Capogna M. Cell-type-specific recruitment of amygdala interneurons to hippocampal theta rhythm and noxious stimuli in vivo. Neuron. 2012;74:1059–1074. doi: 10.1016/j.neuron.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissière S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DC, Bouton ME. A retrieval cue for extinction attenuates spontaneous recovery. J Exp Psychol Anim Behav Process. 1993;19:77–89. doi: 10.1037//0097-7403.19.1.77. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DE, Caparosa EM, Gekker A, Ledoux J. Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Front Behav Neurosci. 2010;4:154. doi: 10.3389/fnbeh.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busti D, Geracitano R, Whittle N, Dalezios Y, Mańko M, Kaufmann W, Sätzler K, Singewald N, Capogna M, Ferraguti F. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci. 2011;31:5131–5144. doi: 10.1523/JNEUROSCI.6100-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Wright DJ. Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res Bull. 1986;17:321–333. doi: 10.1016/0361-9230(86)90237-6. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005a;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005b;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Aston-Jones G. Response of locus coeruleus neurons to footshock stimulation is mediated by neurons in the rostral ventral medulla. Neuroscience. 1993;53:705–715. doi: 10.1016/0306-4522(93)90618-p. [DOI] [PubMed] [Google Scholar]
- Cho JH, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron. 2013;80:1491–1507. doi: 10.1016/j.neuron.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, Lüthi A. Encoding of conditioned fear in central amygdale inhibitory circuits. Nature. 2010;468:270–276. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Collins DR, Pare D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−) Learn Mem. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The Amygdala: a functional analysis. Oxford: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- Domjan M. The principles of learning and behavior: Active learning edition. 5. Belmont, California: Thomson, Wadsworth; 2006. [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the striaterminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dumont E, Martina M, Samson R, Drolet G, Pare D. Physiological properties of central amygdala neurons: species differences. Eur J Neurosci. 2002;15:545–552. doi: 10.1046/j.0953-816x.2001.01879.x. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Popa D, Pare D. Central amygdala activity during fear conditioning. J Neurosci. 2011;31:289–294. doi: 10.1523/JNEUROSCI.4985-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Physiological role of calcium -activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Whittle N, Flynn SM, Graybeal C, Pinard CR, Gunduz-Cinar O, Kravitz AV, Singewald N, Holmes A. Prefrontal single-unit firing associated with deficient extinction in mice. Neurobiol Learn Mem. 2013;S1074–7427:00219–00220. doi: 10.1016/j.nlm.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geracitano R, Kaufmann WA, Szabo G, Ferraguti F, Capogna M. Synaptic heterogeneity between mouse paracapsular intercalated neurons of the amygdala. J Physiol. 2007;585:117–134. doi: 10.1113/jphysiol.2007.142570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geracitano R, Fischer D, Kasugai Y, Ferraguti F, Capogna M. Functional expression of the GABA(A) receptor α2 and α3 subunits at synapses between intercalated medial paracapsular neurons of mouse amygdala. Front Neural Circuits. 2012;6:32. doi: 10.3389/fncir.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci. 2003;117:738–750. doi: 10.1037/0735-7044.117.4.738. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Hobin JA, Maren S. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: mnemonic code or fear bias? Neuron. 2003;40:1013–1022. doi: 10.1016/s0896-6273(03)00728-1. [DOI] [PubMed] [Google Scholar]
- Graham BB, Milad MR. The study of fear extinction: implications for anxiety disorders. Am J Psychiatry. 2011;168:1255–1265. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899:218–226. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Frohardt RJ, Kapp BS. Amygdaloid D1 dopamine receptor involvement in Pavlovian fear conditioning. Brain Res. 1999;827:28–40. doi: 10.1016/s0006-8993(99)01291-3. [DOI] [PubMed] [Google Scholar]
- Hall E. The amygdala of the cat: A Golgi Study. Z Zellforsch. 1972;134:439–458. doi: 10.1007/BF00307668. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowshi JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Lüthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Pert CB. Light microscopic localization of brain opiate receptors: a general autoradiographic method which preserves tissue quality. J Neurosci. 1982;2:1129–1149. doi: 10.1523/JNEUROSCI.02-08-01129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context -dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Hovatta I, Barlow C. Molecular genetics of anxiety in mice and men. Ann Med. 2008;40:92–109. doi: 10.1080/07853890701747096. [DOI] [PubMed] [Google Scholar]
- Jacobsen KX, Hoistad M, Staines WA, Fuxe K. The distribution of dopamine D1 receptor and mu-opioid receptor 1 receptor immunoreactivities in the amygdala and interstitial nucleus of the posterior limb of the anterior commissure: relationships to tyrosine hydroxylase and opioid peptide terminal systems. Neuroscience. 2006;141:2007–2018. doi: 10.1016/j.neuroscience.2006.05.054. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Ressler KJ, Hammack SE, Chhatwal JP, Rainnie DG. Distinct subtypes of cholecystokinin (CCK)-containing interneurons of the basolateral amygdala identified using a CCK promoter-specific lentivirus. J Neurophysiol. 2009;101:1494–506. doi: 10.1152/jn.91149.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Ehrlich DE, Choi DC, Dabrowska J, Bowers ME, McCullough KM, Rainnie DG, Ressler KJ. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J Neurosci. 2013;33:10396–10404. doi: 10.1523/JNEUROSCI.5539-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkkonen E, Pitkänen A. Intrinsic connections of the rat amygdaloid complex: Projections originating in the central nucleus. J Comp Neurol. 1998;395:53–72. doi: 10.1002/(sici)1096-9861(19980525)395:1<53::aid-cne5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Jüngling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal AM, Tombol T. Golgi studies on the amygdaloid nuclei of the cat. J Hirnforsch. 1975;16:175–201. [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, Kim H, Lee C, Sun W, Kim K, Shin KS, Choi S. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci USA. 2007;104:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Paré D, Nair SS. Mechanisms contributing to the induction and storage of Pavlovian fear memories in the lateral amygdala. Learn Mem. 2013a;20:421–430. doi: 10.1101/lm.030262.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Paré D, Nair SS. Assignment of model amygdala neurons to the fear memory trace depends on competitive synaptic interactions. J Neurosci. 2013b;33:14354–14358. doi: 10.1523/JNEUROSCI.2430-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Macias M, Mikosz M, Nowak A, Owczarek D, Wawrzyniak M, Pieprzyk M, Cymerman IA, Werka T, Sheng M, Maren S, Jaworski J, Kaczmarek L. Functional anatomy of neural circuits regulating fear and extinction. Proc Natl Acad Sci USA. 2012;109:17093–17098. doi: 10.1073/pnas.1202087109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonaloxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Coming to terms with fear. Proc Natl Acad Sci USA. 2014;111:2871–2878. doi: 10.1073/pnas.1400335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990a;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala infear conditioning. J Neurosci. 1990b;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Lüthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci. 2013;16:332–329. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. Block of gamma-aminobutyric acid-A receptor insertion in the amygdala impairs extinction of conditioned fear. Biol Psychiatry. 2009;66:665–673. doi: 10.1016/j.biopsych.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Linke R, Braune G, Schwegler H. Differential projection of the posterior paralaminar thalamic nuclei to the amygdaloid complex in the rat. Exp Brain Res. 2000;134:520–532. doi: 10.1007/s002210000475. [DOI] [PubMed] [Google Scholar]
- Livneh U, Paz R. Aversive-bias and stage-selectivity in neurons of the primate amygdala during acquisition, extinction, and overnight retention. J Neurosci. 2012;32:8598–8610. doi: 10.1523/JNEUROSCI.0323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez De Armentia M, Sah P. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J Neurophysiol. 2004;92:1285–1294. doi: 10.1152/jn.00211.2004. [DOI] [PubMed] [Google Scholar]
- Lutz B. The endocannabinoid system and extinction learning. Mol Neurobiol. 2007;36:92–101. doi: 10.1007/s12035-007-8004-x. [DOI] [PubMed] [Google Scholar]
- Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn Mem. 2007;14:318–324. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martina M, Royer S, Pare D. Physiological properties of central medial and central lateral amygdala neurons. J Neurophysiol. 1999;82:1843–1854. doi: 10.1152/jn.1999.82.4.1843. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ, Coleman JR. Corticoamygdaloid and corticocortical projections of the rat temporal cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience. 1993;57:697–715. doi: 10.1016/0306-4522(93)90016-9. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. A novel subpopulation of 5-HT type 3A receptor subunit immunoreactive interneurons in the rat basolateral amygdala. Neuroscience. 2007;144:1015–1024. doi: 10.1016/j.neuroscience.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister WR, McAllister DE, Scoles MT, Hampton SR. Persistence of fear-reducing behavior: relevance for the conditioning theory of neurosis. J Abnorm Psychol. 1986;95:365–372. doi: 10.1037//0021-843x.95.4.365. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 67–96. [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;52:281–294. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Betette RL. Parvalbumin-containing neurons in the rat basolateral amygdala: morphology and co-localization of Calbindin-D(28k) Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to theamygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Zaric V. Subpopulations of somatostatin-immunoreactive non-pyramidal neurons in the amygdala and adjacent external capsule project to the basal forebrain: evidence for the existence of GABAergic projection neurons in the cortical nuclei and basolateral nuclear complex. Front Neural Circuits. 2012;6:46. doi: 10.3389/fncir.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–51. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Neurosci. 2005;25:7366–7376. doi: 10.1523/JNEUROSCI.0899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nader K, LeDoux JE. Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behav Neurosci. 1999;113:891–901. doi: 10.1037//0735-7044.113.5.891. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Nat Neurosci. 2001;3:74–79. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, Pape HC. Dissociated theta phase synchronization in amygdalo- hippocampal circuits during various stages of fear memory. Eur J Neurosci. 2007;25:1823–1831. doi: 10.1111/j.1460-9568.2007.05437.x. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev. 2009;60:226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitecka L, Ben-Ari Y. Distribution of GABA-like immunoreactivity in the rat amygdaloid complex. J Comp Neurol. 1987;266:45–55. doi: 10.1002/cne.902660105. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Stork O. Genes and mechanisms in the amygdala involved in the formation of fear memory. Ann N Y Acad Sci. 2003;985:92–105. doi: 10.1111/j.1749-6632.2003.tb07074.x. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. Distribution of GABA immunoreactivity in the amygdaloid complex of the cat. Neuroscience. 1993a;57:1061–1076. doi: 10.1016/0306-4522(93)90049-l. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993b;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Pare D, Pape HC, Dong J. Bursting and oscillating neurons of the cat basolateralamygdaloid complex in vivo: electrophysiological properties and morphological features. J Neurophysiol. 1995a;74:1179–1191. doi: 10.1152/jn.1995.74.3.1179. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y, Pare JF. Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: Phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience. 1995b;69:567–583. doi: 10.1016/0306-4522(95)00272-k. [DOI] [PubMed] [Google Scholar]
- Pare D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Li B. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J Neurosci. 2014;34:2432–2437. doi: 10.1523/JNEUROSCI.4166-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ledoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. The connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. Oxford: Oxford University Press; 2000. pp. 31–116. [Google Scholar]
- Pitkänen A, Amaral DG. Distribution of parvalbumin-immunoreactive cells and fibers in the monkey temporal lobe: The amygdaloid complex. J Comp Neurol. 1993;331:14–36. doi: 10.1002/cne.903310103. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Castonguay-Lebel Z, Laforest S, Drolet G. Enkephalin co-expression with classic neurotransmitters in the amygdaloid complex of the rat. J Comp Neurol. 2008;506:943–959. doi: 10.1002/cne.21587. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, McDonald AJ. Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. J Comp Neurol. 2006;498:142–161. doi: 10.1002/cne.21049. [DOI] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdale cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process. 1975;27:115–124. [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Roche M, O’Connor E, Diskin C, Finn DP. The effect of CB(1) receptor antagonism in the right basolateral amygdala on conditioned fear and associated analgesia in rats. Eur J Neurosci. 2007;26:2643–2653. doi: 10.1111/j.1460-9568.2007.05861.x. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Martina M, Paré D. Polarized synaptic interactions between intercalated neurons of the amygdala. J Neurophysiol. 2000a;83:3509–3518. doi: 10.1152/jn.2000.83.6.3509. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Paré D. Bistable behavior of inhibitory neurons controlling impulse traffic through the amygdala: Role of a slowly deinactivating K+ current. J Neurosci. 2000b;20:9034–9039. doi: 10.1523/JNEUROSCI.20-24-09034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez de Armentia M, Power J. The amygdaloid complex: Anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Samson RD, Dumont EC, Pare D. Feedback inhibition defines transverse processing modules in the lateral amygdala. J Neurosci. 2003;23:1966–1973. doi: 10.1523/JNEUROSCI.23-05-01966.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RD, Duvarci S, Pare D. Synaptic plasticity in the central nucleus of the amygdala. J Neurosci. 2005;25:1847–1855. doi: 10.1523/JNEUROSCI.3713-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RD, Pare D. A spatially structured network of inhibitory and excitatory connections directs impulse traffic within the lateral amygdala. Neuroscience. 2006;141:1599–1609. doi: 10.1016/j.neuroscience.2006.04.077. [DOI] [PubMed] [Google Scholar]
- Sangha S, Narayanan RT, Bergado-Acosta JR, Stork O, Seidenbecher T, Pape HC. Deficiency of the 65 kDa isoform of glutamic acid decarboxylase impairs extinction of cued but not contextual fear memory. J Neurosci. 2009;29:15713–15720. doi: 10.1523/JNEUROSCI.2620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savander V, Miettinen R, Ledoux JE, Pitkänen A. Lateral nucleus of the rat amygdala is reciprocally connected with basal and accessory basal nuclei: A light and electron microscopic study. Neuroscience. 1997;77:767–781. doi: 10.1016/s0306-4522(96)00513-1. [DOI] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Gründemann J, Fadok JP, Müller C, Letzkus JJ, Lüthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Lüthi A. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci. 2006;9:1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann NY Acad Sci. 2006a;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin RM, Tsvetkov E, Bolshakov VY. Spatiotemporal asymmetry of associative synaptic plasticity in fear conditioning pathways. Neuron. 2006b;52:883–896. doi: 10.1016/j.neuron.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Doyère V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with post-embedding GABA and glutamate immunocytochemistry. J Comp Neurol. 1994;342:232–248. doi: 10.1002/cne.903420207. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D. Cat intraamygdaloid inhibitory network: Ultrastructural organization of parvalbumin-immunoreactive elements. J Comp Neurol. 1998;391:164–179. doi: 10.1002/(sici)1096-9861(19980209)391:2<164::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D. Differential innervation of parvalbumin-immunoreactive interneurons of the basolateralamygdaloid complex by cortical and intrinsic inputs. J Comp Neurol. 2000;416:496–508. [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76:804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorvari H, Soininen H, Paljärvi L, Karkola K, Pitkänen A. Distribution of parvalbumin-immunoreactive cells and fibers in the human amygdaloid complex. J Comp Neurol. 1995;360:185–212. doi: 10.1002/cne.903600202. [DOI] [PubMed] [Google Scholar]
- Sosulina L, Graebenitz S, Pape HC. GABAergic interneurons in the mouse lateral amygdala: a classification study. J Neurophysiol. 2010;104:617–626. doi: 10.1152/jn.00207.2010. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Polepalli J, Sah P. Interneurons in the basolateral amygdala. Neuropharmacology. 2011;60:765–773. doi: 10.1016/j.neuropharm.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Steriade M, Pare D. Gating in cerebral networks. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Stork O, Ji FY, Obata K. Reduction of extracellular GABA in the mouse amygdala during and following confrontation with a conditioned fear stimulus. Neurosci Lett. 2002;327:138–142. doi: 10.1016/s0304-3940(02)00387-7. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, LeDoux JE. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci. 1999;19:RC8. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Sasaki JM, Tu T, Reijmers LG. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron. 2013;80:1054–1065. doi: 10.1016/j.neuron.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Tully K, Li Y, Tsvetkov E, Bolshakov VY. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci USA. 2007;104:14146–14150. doi: 10.1073/pnas.0704621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: A test of the amygdala’s role in sensory processing. J Comp Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal G, Paré JF, Smith Y, Paré D. Cortical inputs innervate calbindin-immunoreactive interneurons of the rat basolateralamygdaloid complex. J Comp Neurol. 2013 doi: 10.1002/cne.23511. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of theamygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;15:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Viosca J, Lopez de Armentia M, Jancic D, Barco A. Enhanced CREB-dependent gene expression increases the excitability of neurons in the basal amygdala and primes the consolidation of contextual and cued fear memory. Learn Mem. 2009;16:193–197. doi: 10.1101/lm.1254209. [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Ikegaya Y, Saito H, Abe K. Roles of GABAA, NMDA and muscarinic receptors in induction of long-term potentiation in the medial and lateral amygdala in vitro. Neurosci Res. 1995;21:317–322. doi: 10.1016/0168-0102(94)00867-f. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. The medial geniculate, not the amygdala, as the root of auditory fear conditioning. Hear Res. 2011;274:61–74. doi: 10.1016/j.heares.2010.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi R, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]