Abstract
Objective
To examine the incidence, tumor burden, and risk factors for nonmelanoma and other skin cancer types in this heart transplant cohort.
Design
Retrospective review of patient medical records.
Setting
Tertiary care center.
Patients
All heart transplant recipients at Mayo Clinic from 1988 to 2006.
Main Outcome Measures
Cumulative incidence of skin cancer and tumor burden, with Cox proportional hazards regression models used to evaluate risk factors for posttransplant primary and secondary nonmelanoma skin cancer.
Results
In total, 312 heart transplant patients had 1395 new skin cancers in 2097 person-years (mean, 0.43 per year per patient) with a range of 0 to 306 for squamous cell carcinoma (SCC) and 0 to 17 for basal cell carcinoma (BCC). The cumulative incidence rates of any skin cancer were 20.4%, 37.5%, and 46.4% at 5, 10, and 15 years after heart transplant, respectively. Cumulative incidence of SCC after the first BCC was 98.1% within 7 years. Multivariate analysis showed that posttransplant nonskin cancer, increased age, and heart failure etiologic factors other than idiopathic disease were associated with increased risk of SCC. Posttransplant herpes simplex viral infection, increased age, and use of mycophenolate mofetil for immunosuppression were associated with increased risk of BCC.
Conclusions
With prolonged survival, many heart transplant patients have numerous skin cancers. Vigilant sun protection practices, skin cancer education, and regular skin examination are appropriate interventions in these high-risk patients.
Solid organ transplant recipients are at increased risk for skin cancers.1 The degree of sun exposure correlates with skin cancer development. Patients living in regions of limited sun exposure, such as the Netherlands, have 10- and 20-year posttransplant risks of skin cancer of 10% and 40%, respectively, and those living in areas of high sun exposure, such as Australia, have 11- and 20-year posttransplant risks of skin cancer of 45% and 70%, respectively.2,3 Skin cancers have been shown to be a major factor in morbidity and death over the long term for heart transplant recipients.4–6
Incidence, tumor burden, and risk factors for skin cancer are well documented in renal transplant recipients, However, these characteristics are documented to a lesser extent in heart transplant patients, who are at least twice as likely to have skin cancer compared with renal transplant recipients.7–9 Factors contributing to this increased risk may be the greater use of immunosuppression agents and an average age at transplant that is 15 years older being greater in heart transplant recipients than in patients undergoing renal transplant.10
We aimed to further delineate the incidence, tumor burden, and risk factors for the development of basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and other types of skin cancers in heart transplant recipients.
METHODS
Approval for this study was obtained from the Mayo Clinic institutional review board. The Mayo Clinic master diagnosis index was queried to identify all patients who received a heart graft from 1988 to 2006. We identified 312 of these patients who had given consent to research. We then retrospectively reviewed the patients’ medical charts, extracting information on patient characteristics, cancers, risk factors, and death. The cumulative incidence of skin cancer was derived by using an extension of the Kaplan-Meier method for the competing risk of death.11 Skin cancer burden summaries are reported as mean (SD) or percentages as appropriate.
Cox proportional hazards regression models were used to evaluate posttransplant risk factors for SCC and BCC. Posttransplant risk factors, such as cytomegaloviral infection, were considered time-dependent covariates, as appropriate. Effects of medications were considered in a time-dependent manner according to use at the most recent of 2 months, 6 months, 2 years, and 6 years. Stepwise selection was used to obtain final multivariate models, with covariates significant univariately at α=.10. Covariates were kept in the multivariate model when P≤.05.
RESULTS
INCIDENCES AND TUMOR BURDEN
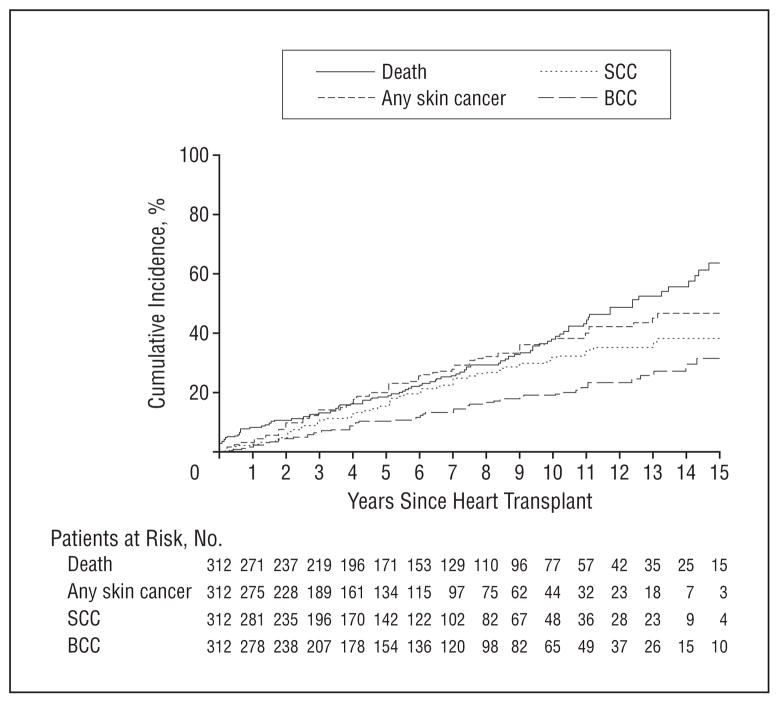
The medical records of 312 patients who received a heart graft were reviewed. Of these patients, 10% also received a renal graft either before or after receipt of a heart graft, and 9.3% had a history of diabetes mellitus (DM). The mean age at transplant was 47.4 years. Table 1 shows the characteristics of our patient cohort. The cumulative incidence of death was 18.4% (95% confidence interval [CI], 13.6%–23.0%) at 5 years, 37.9% (95% CI, 30.5%–44.5%) at 10 years, 63.5% (95% CI, 51.5%–72.5%) at 15 years, and 78.7% (95% CI, 57.7%–89.3%) at 18 years’ follow-up (Figure 1). Only 1 patient died of skin cancer—a malignant melanoma that caused death 8.6 years after heart transplant.
Table 1.
Characteristics of 312 Heart Transplant Recipients
| Characteristic | Valuea |
|---|---|
| Sex | |
| Male | 227 (72.8) |
| Female | 85 (27.2) |
| Age, y | |
| Mean (SD) | 47.4 (16.37) |
| Median (range) | 52.4 (0.1–73.3) |
| Year of transplant | |
| 1988–1993 | 62 (19.9) |
| 1994–1999 | 117 (37.5) |
| 2000–2006 | 133 (42.6) |
| Etiologic factors of cardiac failure | |
| Ischemic cardiomyopathy | 92 (29.5) |
| Idiopathic cardiomyopathy | 106 (34) |
| Congenital heart defect | 36 (11.5) |
| Amyloidosis | 33 (10.6) |
| Other | 45 (14.4) |
Categorical data are expressed as number (percentage) of patients unless stated otherwise.
Figure 1.
Cumulative incidence of squamous cell carcinoma (SCC), basal cell carcinoma (BCC), and any skin cancer after heart transplant that accounts for the competing risk of death.
A total of 1395 skin cancers developed in this cohort: 1236 SCCs (89%), 151 BCCs (11%), 5 malignant melanomas (<1%), 1 angiosarcoma, 1 atypical fibroxanthoma, and 1 pilomatrix carcinoma. Follow-up totaled 2097 person-years, for an average of 0.43 skin cancers per year per patient. Overall, 46.4% of patients had a skin cancer by 19 years of follow-up. The cumulative incidence of skin cancer is shown in Figure 1. At 5, 10, and 15 years of follow-up, 15.4%, 32.3%, and 38.2% of patients had an SCC; 10.3%, 19.2%, and 31.6% had a BCC; and 20.4%, 37.5%, and 46.4%, respectively, had a skin cancer of any kind.
When evaluating the posttransplant SCC tumor burden of the 312 patients, we found that 76 patients (24.4%) had at least 1 SCC, 24 patients (7.7%) had only 1 SCC, and 19 (6.1%) had 10 or more SCCs. The largest number of posttransplant SCCs in a sole patient was 306 in 8.4 years; another patient had 303 SCCs in 17.7 years. The mean (SD) number of SCCs that developed per patient per year after transplant was 0.37 (2.39), and the maximum number of SCCs that developed in a single year was 74. The mean number of SCCs per year after transplant is shown in 5-year periods in Table 2. In our analysis of patients who had at least 1 SCC within each 5-year block, we found that the mean (SD) number of SCCs per year was 1.71 (4.70), 2.65 (6.81), and 2.66 (5.08), respectively, for the blocks.
Table 2.
Number of Squamous Cell Carcinomas (SCCs) and Basal Cell Carcinomas (BCCs) per Patient-year Within 5-Year Blocks After Transplanta
| Type of Carcinoma | Years After Transplant
|
||
|---|---|---|---|
| 1–5 (n=312) | 6–10 (n=174) | 11–15 (n=82) | |
| SCC | 0.23 (1.80) | 0.79 (3.89) | 0.91 (3.19) |
| BCC | 0.05 (0.18) | 0.09 (0.37) | 0.14 (0.35) |
Data are presented as mean (SD).
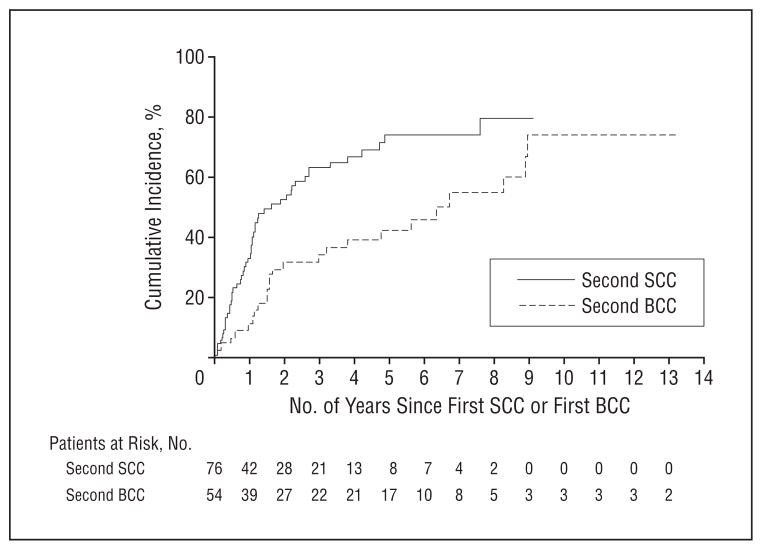
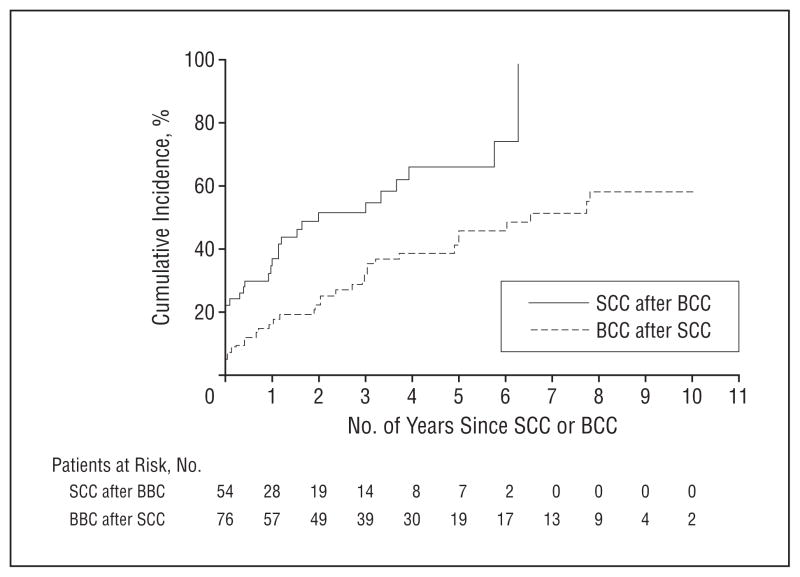
The cumulative incidence rates of a second SCC were 44.0%, 67.4%, and 75.9% at 1, 3, and 5 years after the first SCC (Figure 2). Finally, the cumulative incidence rates of having an SCC were 36.7%, 54.7%, and 65.9% at 1, 3, and 5 years after the first BCC and reached 98.1% within 7 years (Figure 3).
Figure 2.
Cumulative incidence of squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) (accounting for the competing risk of death) after a first SCC or a first BCC, respectively, in heart transplant recipients.
Figure 3.
Cumulative incidence of squamous cell carcinoma (SCC) after the first basal cell carcinoma (BCC) and of BCC after the first SCC (accounting for the competing risk of death) in heart transplant recipients.
Evaluation of the BCC tumor burden of heart transplant recipients showed that 54 patients (17.3%) had at least 1 BCC, 23 patients (7.4%) had only 1 BCC, and 2 patients (0.6%) had 10 or more BCCs. The largest number of BCCs that developed in a sole patient after transplant was 17 in 8.4 years. The mean (SD) number of BCCs that developed per year after transplant was 0.06 (0.19), with a maximum number of 2 BCCs in 1 year. In 5-year blocks, Table 2 shows the mean number of BCCs after transplant. In patients with at least 1 BCC in each 5-year block, the mean number of BCCs per year was 0.50 (0.37), 0.64 (0.80), and 0.62 (0.50).
The cumulative incidence rates of a second BCC were 32.1%, 48.6%, and 51.4% at 1, 3, and 5 years after the first BCC (Figure 2). In addition, the cumulative incidence rates of a BCC developing was 16.3%, 31.8%, and 45.7% at 1, 3, and 5 years after the first SCC and reached 51.1% at 7 years (Figure 3).
RISK FACTORS
The univariate Cox proportional hazards regression models for associations with SCC development are summarized in Table 3. Specifically, increased age at transplant (10-year hazard ratio [HR], 2.57; P<.001), presence of herpes simplex virus (HSV) (HR, 2.71; P=.02), and posttransplant nonskin cancer (HR, 2.32; P=.001) were associated with increased risk of SCC. Female patients (HR, 0.42; P=.01) and patients who received a heart graft from 1994 to 1999 (HR, 0.54; P=.02) vs from 1988 to 1993 had a decreased risk of SCC after transplant. Patients who underwent heart transplant because of idiopathic (HR, 0.39; P<.001) or congenital heart failure (HR, 0.04; P=.002), compared with those who had the procedure because of ischemic cardiomyopathy, had a significantly lower risk of SCC after transplant. In the combination of causes, ischemic cardiomyopathy led to an increased risk of SCC vs all others (HR, 2.64; P<.001), and congenital heart failure led to a decreased risk of SCC vs all others (HR, 0.07; P=.01). Similarly, heart transplant patients who had a mismatch of 1 or more (HR, 0.39; P=.003), compared with patients with a mismatch of 0, were associated with a lower risk of SCC after transplant. Medications that were not significantly associated with the development of SCC included azathioprine (HR, 0.99; P=.97), cyclosporine (HR, 1.43; P=.41), mycophenolate mofetil (HR, 0.96; P=.88), sirolimus (HR, 0.19; P=.10), corticosteroids (HR, 0.93; P=.80), and tacrolimus (HR, 1.31; P=.61).
Table 3.
Univariate Cox Proportional Hazards Regression Model Summaries for Squamous Cell Carcinoma (SCC) and Basal Cell Carcinoma (BCC) After Heart Transplant
| Variable | SCC
|
BCC
|
||
|---|---|---|---|---|
| HR (95% CI) | P Valuea | HR (95% CI) | P Valuea | |
| Posttransplantb | ||||
| Azathioprine use | 0.99 (0.58–1.69) | .97 | 0.56 (0.32–1.00) | <.05 |
| Mycophenolate mofetil use | 0.96 (0.52–1.75) | .88 | 2.32 (1.28–4.21) | .005 |
| HSV infection | 2.71 (1.17–6.28) | .02 | 3.55 (1.39–9.03) | .008 |
| Non–skin cancer | 2.32 (1.40–3.83) | .001 | 1.70 (0.92–3.14) | .09 |
| Age, 10-y unit | 2.57 (1.93–3.42) | <.001 | 1.77 (1.35–2.31) | <.001 |
| Female sex vs male sex | 0.42 (0.22–0.82) | .01 | 0.63 (0.31–1.28) | .20 |
| Years of transplant | ||||
| 1988–1993 | 1 [Reference] | NA | 1 [Reference] | NA |
| 1994–1999 | 0.54 (0.32–0.90) | .02 | 0.67 (0.35–1.27) | .22 |
| 2000–2006 | 0.80 (0.42–1.51) | .48 | 1.00 (0.45–2.22) | .99 |
| Etiologic factors | ||||
| Ischemic cardiomyopathy | 1 [Reference] | NA | 1 [Reference] | NA |
| Idiopathic cardiomyopathy | 0.39 (0.23–0.66) | <.001 | 0.56 (0.30–1.04) | .07 |
| Congenital heart defect | 0.04 (0.01–0.30) | .002 | 0.36 (0.12–1.04) | .06 |
| Ischemic vs other causes | 2.64 (1.68–4.16) | <.001 | 1.82 (1.05–3.15) | .03 |
| Congenital heart failure vs other causes | 0.07 (0.01–0.49) | .008 | 0.50 (0.18–1.38) | .18 |
| HLA mismatches (≥1 vs 0) | 0.39 (0.21–0.72) | .003 | 0.55 (0.27–1.09) | .09 |
Abbreviations: CI, confidence interval; HR, hazard ratio; HSV, herpes simplex virus; NA, not applicable.
P ≤ .05 was considered to be statistically significant.
Variables measured after transplant are time-dependent predictors; nonsignificant items are not included.
Patients with a history of DM had no significant difference in risk of SCC (P=.18), although the estimated risk was greater for a patient with DM (HR, 1.72; P=.18). Specific immunosuppressants used after transplant also were not significantly associated with a change in the risk of SCC development (P=.97 [azathioprine], P=.41 [cyclosporin], P=.88 [mycophenolate mofetil], P=.97 [sirolimus], and P=.61 [tacrolimus]). However, the estimated risk of SCC was less when sirolimus was used (HR, 0.19; P =.10). No significant association with SCC development was found for corticosteroid use (P=.80) or for infection with varicellazoster virus (P=.06), Epstein-Barr virus (P=.17), or cytomegalovirus after transplant (P=.29).
Sex- and age-adjusted HRs are summarized in Table 4. These adjusted HRs showed that HSV infection (HR, 2.90; P=.01) and the presence of a nonskin cancer (HR, 1.80; P=.02) were associated with an increased risk of SCC.
Table 4.
Cox Proportional Hazards Regression Model Summaries for Squamous Cell Carcinoma (SCC) and Basal Cell Carcinoma (BCC) After Heart Transplant and Adjusting for Age and Sex
| Variable | SCC
|
BCC
|
||
|---|---|---|---|---|
| HR (95% CI) | P Valuea | HR (95% CI) | P Valuea | |
| Posttransplantb | ||||
| Azathioprine use | 0.93 (0.54–1.59) | .79 | 0.55 (0.31–0.97) | .03 |
| Mycophenolate mofetil use | 1.05 (0.57–1.94) | .86 | 2.74 (1.50–5.04) | .001 |
| HSV infection | 2.90 (1.24–6.77) | .01 | 3.38 (1.32–8.66) | .01 |
| Nonskin cancer | 1.80 (1.09–2.99) | .02 | 1.31 (0.70–2.43) | .40 |
| HLA mismatches (≥1 vs 0) | 0.53 (0.28–0.98) | .04 | 0.72 (0.36–1.45) | .36 |
Abbreviations: CI, confidence interval; HR, hazard ratio; HSV, herpes simplex virus.
P ≤ .05 was considered to be statistically significant.
Variables measured after transplant are time-dependent predictors.
Multivariate analysis showed that the most significant risk factors for SCC after heart transplant were post-transplant nonskin cancer (HR, 2.03; P = .007), increased age at transplant (10-year HR, 2.55; P<.001), and heart transplant due to idiopathic heart failure (HR, 0.59; P=.04), compared with all other causes (Table 5).
Table 5.
Final Multivariate Cox Proportional Hazards Regression Model Summaries for Squamous Cell Carcinoma (SCC) and Basal Cell Carcinoma (BCC) After Heart Transplant
| Variable | HR (95% CI) | P Value |
|---|---|---|
| SCC | ||
| Nonskin cancer after transplanta | 2.03 (1.21–3.39) | .007 |
| Age, 10-y unit | 2.55 (1.90–3.42) | <.001 |
| Cause (idiopathic vs all other causes) | 0.59 (0.35–0.97) | .04 |
| BCC | ||
| HSV infection after transplanta | 2.97 (1.16–7.59) | .02 |
| Age, 10-y unit | 1.81 (1.38–2.37) | <.001 |
| Mycophenolate mofetil use after transplanta | 2.62 (1.42–4.82) | .002 |
Abbreviations: CI, confidence interval; HR, hazard ratio; HSV, herpes simplex virus.
Variables measured after transplant are time-dependent predictors.
The univariate Cox proportional hazards regression models for time to a BCC are summarized in Table 3. Older age at transplant (10-year HR, 1.77; P<.001), posttransplant infection with HSV (HR, 3.55; P=.008), and mycophenolate mofetil use (HR, 2.32; P=.005) were significantly associated with an increased risk of BCC. Medications that were not significantly associated with the development of BCC included cyclosporine (HR, 2.52; P=.20), sirolimus (HR, 1.11; P=.86), corticosteroids (HR, 0.91; P=.80), and tacrolimus (HR, 0; P=.06). Interestingly, azathioprine use (HR, 0.56; P<.05) was significantly associated with a decreased risk of BCC development.
We also considered a dual transplantation with a heart and kidney to be a possible time-dependent predictor of SCC and found that risk of SCC was no different after a heart and kidney transplant compared with a heart transplant solely (HR, 1.31; P=.62).
History of DM (HR, 0.87; P=.81) and female sex (HR, 0.63; P=.20) were both estimated to decrease the risk of BCC, although these associations were not statistically significant. The time frame of heart transplant also was not statistically significant in relation to risk of BCC, although the patients who received a graft from 1994 to 1999 had an estimated decreased risk (HR, 0.67; P=.22) compared with those who received a graft from 1988 to 1993. In addition, no significant association with BCC development was found for infection with varicellazoster virus (P=.88), Epstein-Barr virus (P=.65), or cy-tomegalovirus after transplant (P=.46). When causes were combined, ischemic cardiomyopathy led to an increased risk of BCC compared with all other causes (HR, 1.82; P=.03). When HRs were adjusted for sex and age (Table 4), mycophenolate mofetil use (HR, 2.74; P=.001) and the presence on an HSV infection (HR, 3.38; P=.01) were associated with an increased risk of BCC in this patient population.
A multivariate analysis of risk factors for BCC development after heart transplant found that older age at transplant (10-year HR, 1.81; P<.001), posttransplant infection with HSV (HR, 2.97; P=.02), and mycophenolate mofetil use (HR, 2.62; P =.002) were the most important predictors of BCC risk (Table 5).
COMMENT
Organ graft recipients are at a substantially increased risk of posttransplant skin cancers, with the relative risk of nonmelanoma skin cancer (NMSC) in heart transplant patients at 108.6 compared with the general population.12 Skin cancer in the case of organ transplant can be a clinically significant cause of morbidity and death.4–6,13–16 In previous studies, the factors found to contribute to development of skin cancer include age at transplant,17,18 duration and degree of immunosuppressive therapy,19,20 a history of increased UV exposure,1,17 male sex,11,17 infection with human papillomavirus,21 fair complexion with blue eyes and blond hair,1 pretransplant disease (eg, poly-cystic kidney disease and cholestatic liver disease),22 decreased awareness of skin cancer risk,23 and a history of a prior NMSC.12 A previous study8 has also shown that transplant recipients with SCC tend to have more subsequent SCCs, which may metastasize in 5% to 8% of this patient subset. Heart transplant recipients are particularly prone to the development of these skin cancers because of the intensive immunosuppression needed to prevent rejection of the transplanted organ graft, as well as being, on average, older at transplant. These findings would suggest a similar mortality rate in this patient population; however, the present study suggests that with aggressive screening and treatment of NMSC, the mortality rate associated with NMSC can be substantially decreased.
Although a considerable tumor burden was found in this study, the rate of death due to skin cancer was surprisingly low. Only 1 patient died of skin cancer, of a melanoma. This finding is in contrast to a study performed in Australia of patients with heart transplant and NMSC, which reported a 42% mortality rate.6 It should be noted that in all types of skin cancer cases, death due to NMSC should be preventable in most instances. Countries with high rates of skin cancer and low numbers of dermatologists who are concentrated in medical centers are more likely to see death due to NMSC. The low mortality rate seen in the patient population of this study shows that when aggressive early management occurs, even in patients with a high level of immunosuppression, death can be avoided for most patients. Health care providers and patients at our center have been educated for more than 10 years about the risk, early detection, and treatment of skin cancer, which is apparent from the low mortality rate seen in the patients of this study.
Another interesting finding of this study is the steady number of NMSCs noted per year after transplant. When assessing the mean number of SCCs per year for patients who had this neoplasm, we found that the mean did not change considerably in the early years after transplant compared with more than a decade later.
Our analysis showed that male sex, as well as increased age at transplant, was a risk factor for development of SCCs after heart transplant. These characteristics are both most likely markers of increased UV exposure, compared with female sex and younger age. Of note, receipt of a heart graft due to an ischemic cardiomyopathy also conferred an increased risk of SCC. It has been suggested that a high-fat diet may increase the incidence of actinic keratosis, in addition to promoting the development of skin cancer at the promotional stage of UV carcinogenesis.24 Perhaps the influence of high-fat diets leading to ischemic cardiomyopathy, in combination with the immunosuppression of heart transplant, is what places this group of patients at greater risk than others receiving a heart transplant.
The present study also found an association of increased risk of both SCC and BCC in patients who had an HSV infection. The association of viral infections with skin cancer development may reflect a common pathogenesis caused by increased levels of immunosuppression or may implicate a causal relationship. Although the ability to quantify the degree of immunosuppression in the current study was limited, we suspect that the association of skin cancer and viral infections is indicative of the patients who had greater levels of immunosuppression.
Finally, our study documented an increased risk of BCC in patients taking mycophenolate mofetil compared with those taking azathioprine. Tacrolimus and sirolimus had nonsignificant effects for a decreased risk of BCC and SCC, respectively. Only the increased risk of BCC in patients taking mycophenolate mofetil remained significant in the multivariate analysis. Again, because of the complexities of modeling medication doses over time, these observations may or may not continue to hold in future studies. The limitations of this study include its retrospective nature, as well as the single-institution design, which may have prevented elucidation of medication effects more clearly.
Vigilant sun protection practices, skin cancer education, regular skin examinations, and daily vitamin D supplementation are appropriate interventions in these high-risk heart transplant patients.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Dr Brewer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Brewer, Phillips, Roenigk, Van de Beek, Dierkhising, Kremers, McGregor, and Otley. Acquisition of data: Brewer, Colegio, Van de Beek, Dierkhising, and Otley. Analysis and interpretation of data: Colegio, Jacobs, Van de Beek, Dierkhising, Kremers, and Otley. Drafting of the manuscript: Brewer. Critical revision of the manuscript for important intellectual content: Brewer, Colegio, Phillips, Roenigk, Jacobs, Van de Beek, Dierkhising, Kremers, McGregor, and Otley. Statistical analysis: Dierkhising and Kremers. Obtained funding: Van de Beek. Administrative, technical, and material support: Van de Beek and Otley. Study supervision: Phillips, Roenigk, and Otley.
References
- 1.España A, Redondo P, Fernandez AL, et al. Skin cancer in heart transplant recipients. J Am Acad Dermatol. 1995;32(3):458–465. doi: 10.1016/0190-9622(95)90069-1. [DOI] [PubMed] [Google Scholar]
- 2.Hartevelt MM, Bavinck JN, Kootte AM, Vermeer BJ, Vandenbroucke JP. Incidence of skin cancer after renal transplantation in the Netherlands. Transplantation. 1990;49(3):506–509. doi: 10.1097/00007890-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Sheil AG. Development of malignancy following renal transplantation in Australia and New Zealand. Transplant Proc. 1992;24(4):1275–1279. [PubMed] [Google Scholar]
- 4.Veness MJ. Aggressive skin cancers in a cardiac transplant recipient. Australas Radiol. 1997;41(4):363–366. doi: 10.1111/j.1440-1673.1997.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 5.Ong CS, Keogh AM, Kossard S, Macdonald PS, Spratt PM. Skin cancer in Australian heart transplant recipients. J Am Acad Dermatol. 1999;40(1):27–34. doi: 10.1016/s0190-9622(99)70525-6. [DOI] [PubMed] [Google Scholar]
- 6.Veness MJ, Quinn DI, Ong CS, et al. Aggressive cutaneous malignancies following cardiothoracic transplantation: the Australian experience. Cancer. 1999;85(8):1758–1764. [PubMed] [Google Scholar]
- 7.Kinlen LJ, Sheil AG, Peto J, Doll R. Collaborative United Kingdom-Australasian study of cancer in patients treated with immunosuppressive drugs. Br Med J. 1979;2(6203):1461–1466. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Euvrard S, Kanitakis J, Pouteil-Noble C, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol. 1995;33(2 pt 1):222–229. doi: 10.1016/0190-9622(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 9.Jensen P, Hansen S, Moller B, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40(2 pt 1):177–186. doi: 10.1016/s0190-9622(99)70185-4. [DOI] [PubMed] [Google Scholar]
- 10.Wu JJ, Orengo IF. Squamous cell carcinoma in solid-organ transplantation. Dermatol Online J. 2002;8(2):4. [PubMed] [Google Scholar]
- 11.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Lindelöf B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143(3):513–519. [PubMed] [Google Scholar]
- 13.Lindelöf B, Jarnvik J, Ternesten-Bratel A, Granath F, Hedblad MA. Mortality and clinicopathological features of cutaneous squamous cell carcinoma in organ transplant recipients: a study of the Swedish cohort. Acta Derm Venereol. 2006;86(3):219–222. doi: 10.2340/00015555-0069. [DOI] [PubMed] [Google Scholar]
- 14.Cooper JZ, Brown MD. Special concern about squamous cell carcinoma of the scalp in organ transplant recipients. Arch Dermatol. 2006;142(6):755–758. doi: 10.1001/archderm.142.6.755. [DOI] [PubMed] [Google Scholar]
- 15.Otley CC, Berg D, Ulrich C, et al. Reduction of Immunosuppression Task Force of the International Transplant Skin Cancer Collaborative and the Skin Care in Organ Transplant Patients Europe. Reduction of immunosuppression for transplant-associated skin cancer: expert consensus survey. Br J Dermatol. 2006;154 (3):395–400. doi: 10.1111/j.1365-2133.2005.07087.x. [DOI] [PubMed] [Google Scholar]
- 16.Otley CC, Griffin MD, Charlton MR, Edwards BS, Neuburg M, Stasko T. Reduction of Immunosuppression Task Force of the International Transplant Skin Cancer Collaborative. Reduction of immunosuppression for transplant-associated skin cancer: thresholds and risks [published online October 4, 2007] Br J Dermatol. 2007;157(6):1183–1188. doi: 10.1111/j.1365-2133.2007.08203.x. [DOI] [PubMed] [Google Scholar]
- 17.Herrero JI, Espana A, Quiroga J, et al. Nonmelanoma skin cancer after liver transplantation: study of risk factors. Liver Transpl. 2005;11(9):1100–1106. doi: 10.1002/lt.20525. [DOI] [PubMed] [Google Scholar]
- 18.Euvrard S, Kanitakis J, Decullier E, et al. Subsequent skin cancers in kidney and heart transplant recipients after the first squamous cell carcinoma. Transplantation. 2006;81(8):1093–1100. doi: 10.1097/01.tp.0000209921.60305.d9. [DOI] [PubMed] [Google Scholar]
- 19.Otley CC, Coldiron BM, Stasko T, Goldman GD. Decreased skin cancer after cessation of therapy with transplant-associated immunosuppressants. Arch Dermatol. 2001;137(4):459–463. [PubMed] [Google Scholar]
- 20.Fortina AB, Piaserico S, Caforio AL, et al. Immunosuppressive level and other risk factors for basal cell carcinoma and squamous cell carcinoma in heart transplant recipients. Arch Dermatol. 2004;140(9):1079–1085. doi: 10.1001/archderm.140.9.1079. [DOI] [PubMed] [Google Scholar]
- 21.Nordin P, Hansson BG, Hansson C, Blohme I, Larko O, Andersson K. Human papilloma virus in skin, mouth and uterine cervix in female renal transplant recipients with or without a history of cutaneous squamous cell carcinoma. Acta Derm Venereol. 2007;87(3):219–222. doi: 10.2340/00015555-0235. [DOI] [PubMed] [Google Scholar]
- 22.Otley CC, Cherikh WS, Salasche SJ, McBride MA, Christenson LJ, Kauffman HM. Skin cancer in organ transplant recipients: effect of pretransplant end-organ disease [published online September 22, 2005] J Am Acad Dermatol. 2005;53 (5):783–790. doi: 10.1016/j.jaad.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 23.Tavadia S, Dawn G, Payne C, Ramrakha-Jones V, Murday A, Holmes S. Skin-cancer awareness in Scottish cardiac transplant recipients. Clin Exp Dermatol. 2006;31(3):354–357. doi: 10.1111/j.1365-2230.2006.02098.x. [DOI] [PubMed] [Google Scholar]
- 24.Black HS. Influence of dietary factors on actinically-induced skin cancer. Mutat Res. 1998;422(1):185–190. doi: 10.1016/s0027-5107(98)00191-2. [DOI] [PubMed] [Google Scholar]