Abstract
Prior studies of asthma in children with sickle cell disease (SCD) were based on reports of a doctor-diagnosis of asthma with limited description of asthma features. Doctor-diagnoses of asthma may represent asthma or wheezing unrelated to asthma. Objectives of this study were to determine if asthma characteristics are present in adults with a doctor-diagnosis of asthma and/or wheezing, and to examine the relationship between doctor-diagnosis of asthma, wheezing and SCD morbidity. This was an observational cohort study of 114 adults with SCD who completed respiratory symptom questionnaires and had serum IgE measurements. A subset of 79 participants completed pulmonary function testing. Survival analysis was based on a mean prospective follow-up of 28 months and data were censored at the time of death or loss to follow-up. Adults reporting a doctor-diagnosis of asthma (N = 34) were more likely to have features of asthma including wheeze, eczema, family history of asthma, and an elevated IgE level (all P < 0.05). However, there was no difference in pain or ACS rate, lung function, or risk of death between adults with and without a doctor-diagnosis of asthma. In contrast, adults who reported recurrent, severe episodes of wheezing (N = 34), regardless of asthma, had twice the rates of pain and ACS, decreased lung function and increased risk of death compared with adults without recurrent, severe wheezing. Asthma features were not associated with recurrent, severe wheezing. Our data suggest that wheezing in SCD may occur independently of asthma and is a marker of disease severity.
Introduction
Respiratory symptoms are common among individuals with sickle cell disease (SCD), and the extent to which respiratory symptoms represent asthma in this population has recently become an area of focus among investigators [1]. Ascertainment of symptoms that represent asthma is important for clinicians caring for this population. Among children with SCD, several studies have found that asthma is associated with increased rates of pain and acute chest syndrome (ACS) events [2–4]. A major limitation of these studies was that asthma was based on a parent or patient report of a doctor-diagnosis without supporting evidence of an asthma phenotype (i.e., wheezing, atopy, family history). The basis for reports of asthma diagnoses in these studies was not well defined. Furthermore, the significance of this diagnosis for adults with SCD has not been established.
In individuals with SCD, wheezing has been described independently of asthma diagnoses in the setting of ACS episodes and/or respiratory infections. Wheezing was present in 11% of individuals with SCD and ACS in the Cooperative Study of Sickle Cell Disease [5], and may be related to infections such as Chlamydia [6] or Mycoplasma [7] that are known triggers of ACS. Determining the underlying cause of wheezing is important to selecting the most appropriate therapy. We performed an observational cohort study of adults with SCD. The primary objective of this study was to determine whether adults with SCD who report a doctor-diagnosis of asthma are more likely to have features of asthma compared with adults with SCD but without a doctor-diagnosis of asthma. The secondary objective of the study was to examine the relationship between reports of a doctor-diagnosis of asthma, respiratory symptoms, and rate of hospitalizations for pain and ACS episodes. We tested the hypothesis that participants reporting a doctor-diagnosis of asthma would have increased morbidity compared to participants not reporting an asthma diagnosis.
Methods
For inclusion into our study cohort, we identified all adults with SCD ≥ 18 years of age who received SCD care exclusively at the Barnes-Jewish Hospital system and Washington University School of Medicine in order to accurately determine hospitalizations. We excluded individuals who were receiving chronic transfusion therapy because it could have altered their clinical phenotype. We invited all eligible patients to participate and, after informed consent was obtained, subjects completed questionnaires that included items from the American Thoracic Society and Division of Lung Diseases (ATS/DLD) Respiratory Symptom questionnaire (see Fig. 1) [8]. Pain and ACS hospitalizations were determined from a review of electronic medical records from January 1, 2004 to March 1, 2010. A second data extractor validated the accuracy of the morbidity data by re-examining all records. Enrollment into this study began in August 2006 and questionnaires were completed at the time of study entry. Laboratory studies were performed within 3 months of study entry and when the participant was at steady state, at least two weeks from a hospitalization for ACS or pain. This study was approved by the Human Research Protection Office at Washington University Medical Center.
Figure 1.
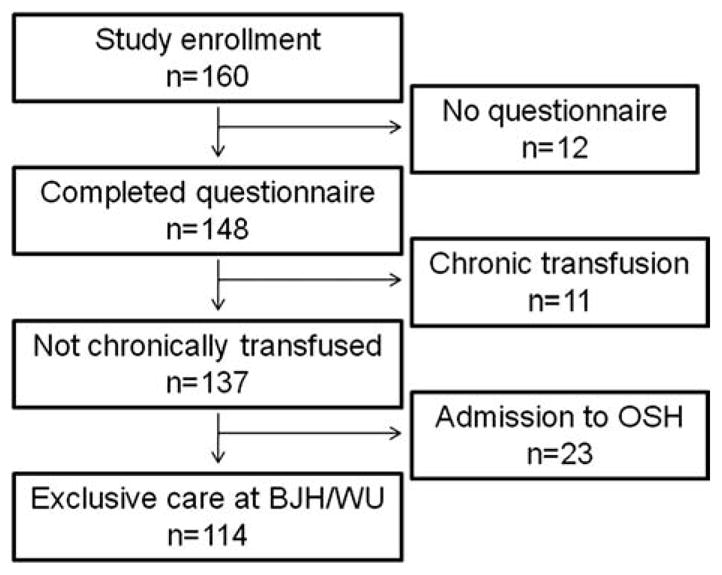
Enrollment into the adult sickle cell disease study cohort. OSH, outside hospital; BJH/WU, Barnes-Jewish Hospital/Washington University.
ATS/DLD wheezing questions
Participants were administered questions pertaining to chest symptoms from the ATS/DLD questionnaire [8]. Responses to the following questions about cough and wheezing were recorded for each participant: Do you usually have a cough with colds? Do you usually cough without having a cold? Does your chest ever sound wheezy when you have a cold? Does your chest ever sound wheezy apart from colds? Have you had an attack of wheezing after you have been exercising? Have you ever had an attack of wheezing that has made you feel short of breath? Have you had 2 or more such episodes?
Definitions
Asthma
An answer of “yes” to the question “Has a doctor ever said that you have asthma?” and/or reported use of anti-inflammatory asthma controller medications including inhaled corticosteroids and/or leukotriene modifiers. Reported use of bronchodilators alone was not sufficient for asthma classification in this cohort because of the increasing frequency of bronchodilator use in SCD independent of asthma, such as for ACS [9,10].
ACS episode
A new pulmonary infiltrate on chest radiograph in the context of an acute illness characterized by fever and respiratory symptoms [5].
Pain episode
A hospital admission with a primary diagnosis of pain related to SCD [11]. Hospitalizations within two weeks of a previous admission for pain and ACS are considered readmissions and counted as one event. Pain events that evolved into ACS were only counted as ACS episodes.
Recurrent, severe wheezing episodes. Two or more attacks of wheezing that made the patient feel short of breath (based on the ATS/ DLD question above).
Spirometry
Spirometry was performed in a subset of participants (N = 79) using a pneumotachograph-type spirometer interfaced with a personal computer system (Jaeger MasterScope, VIASYS, Hoechberg, Germany). All study participants were invited to perform spirometry without regard to asthma status, history of ACS, or other respiratory symptoms. Spirometry was performed at least 4 hr after the use of a short-acting bronchodilator [12]. NHANES III prediction equations for forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were used taking into account height, age, gender, and African American race [13].
Statistical analysis
Differences in baseline characteristics between adults with and without asthma were compared using Chi-Square tests for categorical variables and either Student’s t or Mann-Whitney U tests for continuous variables that were normally or non-normally distributed, respectively. Poisson regression adjusted for a negative binomial distribution was used to determine the relationship between history of asthma or respiratory symptoms and rates of pain and ACS. Rates of pain and ACS were analyzed from January 1, 2004 to March 1, 2010, last clinic visit or death. Age, SCD phenotype, gender, hemoglobin, hydroxyurea use, and tobacco smoking were included in multivariable negative binomial regression models for pain [2,14,15]. Age, SCD phenotype, white blood cell count (WBC), hemoglobin, hydroxyurea use and tobacco smoking were included in multivariable models for ACS [2,15,16]. Cox proportional-hazards regression was used to assess variables that could potentially be associated with risk of death. Survival was measured from date of consent through the date of death or March 1, 2010. Participants who were lost to follow up prior to the completion of the study were censored at the time of their last clinic visit. Variables that were analyzed for an association with risk of death included age, SCD phenotype, WBC, hemoglobin, hydroxyurea use, serum creatinine, ACS rate, asthma, and respiratory symptoms [17,18]. Data analysis was performed in SAS version 9.1 (SAS Institute, Cary, NC).
Results
Demographics and baseline characteristics
Our cohort was comprised of 114 adults with SCD (median age 32 years, range, 18–72 years) (Table I). Sixty-nine percent of the cohort had HbSS or HbSβ-thalassemia 0 and the remainder had HbSC, HbSβ-thalassemia+ and HbSC-Harlem. Forty-five percent of the cohort was male. Hydroxyurea was prescribed to 31% of study participants. Thirty percent (34/114) of the cohort reported a diagnosis of asthma and/or were taking daily anti-inflammatory asthma controller medications. Sixty-four percent of adults reported the presence of recurrent episodes of wheezing causing shortness of breath, wheezing in the absence of a cold, or wheezing with exercise. Of adults who reported wheezing symptoms, only 38% had a diagnosis of asthma.
TABLE I.
Baseline Characteristics of 114 Adults with Sickle Cell Disease and Comparison of Those with and without a Report of a Doctor-Diagnosis of Asthma
| SCD cohort n = 114 |
SCD cohort by asthma diagnosis
|
|||
|---|---|---|---|---|
| Asthma n = 34 |
No asthma n = 80 |
P value | ||
| Demographics | ||||
| Age, median (IQR) | 31.8 (17) | 27.1 (12) | 34.9 (16) | <0.01 |
| Gender, % male | 45 | 47 | 44 | 0.75 |
| SCD characteristics | ||||
| Phenotype, % (HbSS/HbSB∘) | 69 | 77 | 66 | 0.28 |
| Hemogloblin (g/dL), mean (SD) | 8.9 (1.8) | 8.6 (1.5) | 9.1 (1.9) | 0.18 |
| WBC (cells/μL), mean (SD) | 10.8 (3.1) | 11.6 (3.7) | 10.5 (2.8) | 0.11 |
| LDH, median (IQR) | 327.5 (218.5) | 331.5 (174) | 325.5 (241) | 0.65 |
| Hydroxyurea, % | 31 | 41 | 26 | 0.11 |
| Allergic characteristics | ||||
| Total Serum IgE, median (IQR) | 40.6 (86) | 64.3 (262) | 33.2 (71) | 0.04 |
| Eosinophil, % WBC, median (IQR) | 2.5 (3.3) | 2.8 (4.5) | 2.2 (3.1) | 0.43 |
| Family history of asthma, % | 24 | 44 | 16 | <0.01 |
| History of eczema, % | 8 | 18 | 4 | 0.01 |
| Asthma symptoms | ||||
| ≥2 episodes of wheezing with SOB, % | 30 | 53 | 20 | <0.01 |
| Wheezes in presence of cold, % | 61 | 85 | 51 | <0.01 |
| Wheezes in absence of cold, % | 30 | 59 | 18 | <0.01 |
| Wheezes with exercise, % | 39 | 59 | 30 | <0.01 |
| Cough in presence of cold, % | 86 | 91 | 84 | 0.39 |
| Cough in absence of cold, % | 42 | 56 | 36 | 0.052 |
IQR, inter-quartile range; LDH, lactate dehydrogenase; SCD, sickle cell disease; SD, standard deviation; SOB, shortness of breath; WBC, white blood cell.
When subjects with severe wheezing and asthma were compared to subjects with severe wheezing but without asthma, there were no differences with regards to gender, markers of SCD severity (SCD type, hemoglobin, white blood cell count, lactate dehydrogenase (LDH)) or atopic features (serum IgE, family history of asthma, and history of eczema). Nonasthmatic patients with severe wheezing were older than those with asthma (median age 42 versus 27 years, P = 0.0005).
Asthma features
Despite the prevalence of respiratory symptoms in participants with and without asthma, those adults reporting a doctor-diagnosis of asthma were distinguished by typical features of asthma (Table I). Adults reporting an asthma diagnosis were more likely to have respiratory symptoms, a family history of asthma and features of an allergic diathesis compared to adults without a report of asthma. There was no association between reports of asthma and active smoking (P = 0.81).
Adults who reported respiratory symptoms (cough and/or wheezing in the presence or absence of a cold, wheezing with exercise) had some characteristics of asthma. Cough and wheeze in the absence of a cold and wheezing with exercise was associated with physician diagnosis of allergic rhinitis and wheezing in the presence or absence of a cold was associated with eosinophil count. However, severe recurrent wheezing was not associated with family history of asthma, diagnosis of allergic rhinitis or eczema, or serum IgE or eosinophil count. Additionally, there were no associations between respiratory symptoms and serum IgE, family history of asthma, or history of eczema. There was also no association between respiratory symptoms and active smoking with the exception of wheezing during exercise (P = 0.03; Supporting Information Table I).
Pulmonary function
Seventy-nine participants in our cohort (69%) completed spirometry. Participants with and without spirometry data were otherwise similar with regards to gender, SCD type, risk factors for asthma (family history, serum IgE, eosinophil count, and active smoking), and markers of disease severity (rates of pain and ACS, hemoglobin, white blood cell count, and hydroxyurea use). Those with missing spirometry data (N = 35) were older (mean 38.1 versus 33.2 years, P = 0.04) and were more likely to have died during the study period (20% versus 5%, P = 0.02).
There was no relationship between pulmonary function and reports of a doctor-diagnosis of asthma; however, lower FEV1 and FVC were associated with a history of recurrent, severe wheezing episodes, and wheezing in the presence of a cold (Tables II and IV). In addition, decreased FVC and FEV1 were each associated with a higher rate of ACS episodes (P < 0.01 in both cases). Based on prior studies describing the association between high LDH and morbidity and mortality in SCD, we dichotomized LDH at 315 U/L [19] and found that participants with high LDH levels had a lower FVC (84% versus 91%, P = 0.04), FEV1 (78% versus 87%, P = 0.01), and FEV1/FVC (78% versus 82%, P = 0.02).
TABLE II.
Association Between a Report of a Doctor-Diagnosis of Asthma, Wheezing, and Lung Function Among Adults with Sickle Cell Disease
| n = 79 | Asthma | No asthma | P value | Recurrent, severe wheezing | No recurrent, severe wheezing | P value |
|---|---|---|---|---|---|---|
| FEV1, % predicted, mean (SD) * | 81.1 (10.8) | 82.8 (17.9) | 0.66 | 77.1 (12.8) | 84.6 (16.7) | 0.047 |
| FVC, % predicted, mean (SD)* | 86.1 (11.7) | 87.7 (17.0) | 0.66 | 82.2 (12.7) | 89.7 (16.1) | 0.04 |
| FEV1/FVC, % predicted, mean (SD) * | 79.6 (6.5) | 79.3 (7.6) | 0.85 | 79.1 (7.2) | 79.5 (7.3) | 0.80 |
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SD, standard deviation.
Models adjusted for age, height, and gender.
TABLE IV.
Association Between Respiratory Symptoms and Biomarkers with Lung Function and Morbidity Among Adults with Sickle Cell Disease
| FEV1, % predicted
|
FVC, % predicted
|
FEV1/FVC
|
Rates of pain
|
Rates of ACS
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| βa | P | βa | P | βa | P | RRb (95% CI) | P | RRc (95% CI) | P | |
| Wheezing in presence of cold | −9.8 | 0.006 | −10.2 | 0.003 | −1.1 | 0.52 | 2.2 (1.4−3.5) | <0.001 | 1.5 (0.8−2.8) | 0.27 |
| Wheezing in absence of cold | −6.8 | 0.06 | −6.3 | 0.08 | −1.2 | 0.47 | 0.97 (0.6−1.6) | 0.92 | 0.8 (0.4−1.6) | 0.57 |
| Wheezing with exercise | −7.1 | 0.05 | −6.3 | 0.07 | −0.95 | 0.56 | 0.8 (0.5−1.3) | 0.40 | 0.7 (0.4−1.4) | 0.32 |
| Cough in presence of cold | −7.2 | 0.18 | −6.2 | 0.24 | 0.12 | 0.96 | 1.4 (0.7−2.9) | 0.41 | 1.0 (0.4−2.5) | 0.93 |
| Cough in absence of cold | −7.5 | 0.88 | 0.10 | 0.98 | 1.3 | 0.41 | 1.4 (0.9−2.2) | 0.19 | 0.9 (0.4−1.7) | 0.65 |
| Total serum IgEd | 1.5 | 0.28 | 2.0 | 0.14 | −0.34 | 0.59 | 1.1 (0.96−1.3) | 0.59 | 0.99 (0.8−1.3) | 0.96 |
| Eosinophilsd | −2.2 | 0.31 | −3.6 | 0.08 | 0.34 | 0.74 | 0.98 (0.7−1.4) | 0.90 | 1.04 (0.7−1.6) | 0.84 |
ACS, acute chest syndrome; CI, confidence interval; FEV1, forced expiratory volume in 1 sec; FVC, forced vital capacity; RR, relative risk.
Linear regression models of lung function were adjusted for age, gender, and height.
Negative binomial regression models for pain were adjusted for SCD phenotype, age, gender, hemoglobin, tobacco smoke exposure, hydroxyurea use.
Negative binomial regression models for ACS were adjusted for SCD phenotype, age, hemoglobin, white blood cell count, tobacco smoke exposure, hydroxyurea use.
Because of non-normally distributed data, IgE, and eosinophils were natural log transformed for all analyses.
Association of morbidity and mortality with asthma and respiratory symptoms
In adjusted multivariable models, severe recurrent wheezing—but not a report of a doctor-diagnosis of asthma—was associated with increased rates of hospitalization for pain or ACS (Table III). Wheezing in the presence of a cold was also associated with an increased rate of pain (rate ratio [RR] = 2.2, 95% confidence interval [CI] = 1.4–3.5, P = 0.009), but not ACS (RR = 1.5, 95% CI = 0.8–2.8, P = 0.27). There was no association between rates of hospitalization for pain or ACS and other respiratory symptoms, reported use of asthma controller agents, serum IgE or eosinophil count.
TABLE III.
Association of Recurrent, Severe Wheezing and Report of Doctor-Diagnosis of Asthma with Rates of Pain and Acute Chest Syndrome in Adults with Sickle Cell Disease
| Variable n = 114 |
Model 1: Effect of doctor-diagnosed asthma on the rates of pain and ACS events
|
Model 2: Effect of recurrent, severe wheezing on the rates of pain and ACS events
|
||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Paina | ||||
| Recurrent, severe wheezing | 2.0 (1.2–3.4) | 0.005 | ||
| Doctor-diagnosed asthma | 0.99 (0.6–1.7) | 0.46 | ||
| ACSb | ||||
| Recurrent, severe wheezing | 2.1 (1.1–4.0) | 0.03 | ||
| Doctor-diagnosed asthma | 1.3 (0.7–2.6) | 0.97 | ||
ACS, acute chest syndrome; SCD, sickle cell disease.
Pain model was adjusted for SCD phenotype, age, gender, hemoglobin, tobacco smoking, hydroxyurea use.
ACS model was adjusted for SCD phenotype, age, hemoglobin, white blood cell count, tobacco smoking, hydroxyurea use.
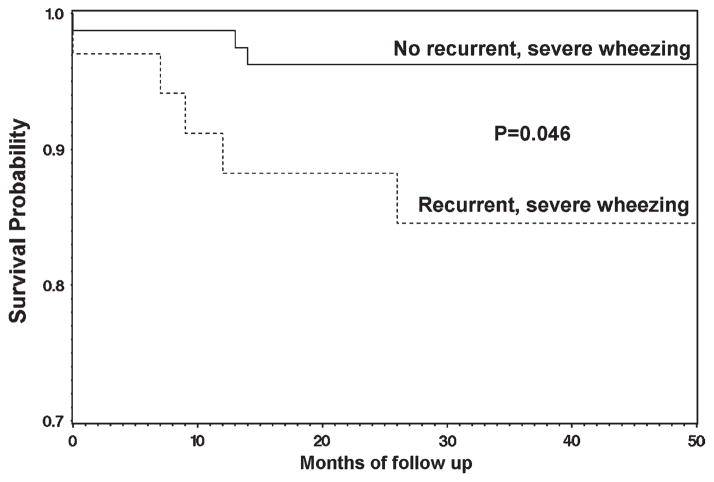
Eleven participants (10%) died during the prospective observation period (mean length of prospective follow up 2.3 ± 0.7 years). The causes of death were as follows: sudden death during a pain episode (N = 5), stroke (N = 2), liver failure (N = 1), and multisystem organ failure (N = 3). There was no association between reports of asthma and risk of death (hazard ratio (HR) = 2.4, 95% CI = 0.6–9.7, P = 0.21). A history of recurrent, severe wheezing episodes, however, was associated with premature death (HR = 4.2, 95% CI = 1.0–17.5, P = 0.046) (see Fig. 2). Univariate analyses were also performed for the variables age, SCD phenotype, WBC, hemoglobin, hydroxyurea use, serum creatinine, IgE, eosinophils, use of asthma controller agents, LDH, ACS rate, and other ATS/DLD respiratory symptoms. With the exception of recurrent, severe wheezing episodes, no variables were associated with an increased risk of death.
Figure 2.
Kaplan-Meier survival curve comparing adults with sickle cell disease with and without recurrent, severe wheezing episodes.
Discussion
Few studies have examined whether individuals with SCD who report a doctor-diagnosis of asthma have typical asthma features. Since many etiologies cause wheezing, the presence of asthma features in those who report a doctor-diagnosis of asthma adds substantial validity to that classification. We examined a cohort of adults with SCD and found that those who report a doctor-diagnosis of asthma were more likely to have typical features of asthma (i.e., respiratory symptoms, family history, allergic features) compared with those without asthma. Contrary to our expectation, and unlike children with SCD, there was no association between reports of a doctor-diagnosis of asthma and an increased rate of hospitalization for pain or ACS. Regardless of asthma diagnosis, asthma-like symptoms (i.e., wheezing) were common in our cohort, and there was an association between recurrent, severe wheezing episodes and an increased rate of pain, ACS episodes, and premature death. Only a subset of adults who reported recurrent, severe wheezing episodes had a doctor-diagnosis of asthma; this group did not otherwise have typical features of asthma.
In our cohort, a report of a doctor-diagnosis of asthma was associated with presence of respiratory symptoms, high serum IgE, a history of eczema and a family history of asthma. All of these factors have been related to asthma in the general population [20–24]. More recently, IgE has been associated with asthma in children with SCD [25]. These data suggest that there was not significant misclassification of asthma diagnoses in our cohort. There were few associations with allergic features or a family history among adults with SCD who reported respiratory symptoms implying many cases of wheezing are not related to asthma. The higher prevalence of respiratory symptoms compared to reported doctor-diagnoses of asthma in our study further supports the notion that many individuals with SCD and wheezing may not have asthma.
In some cases, reports of respiratory symptoms in our cohort may be related to pulmonary complications of SCD. Airway hyper-responsiveness and pulmonary function abnormalities are present in up to 78% [26] and 90% [27] of individuals with SCD, respectively. The high prevalence of airway hyper-responsiveness and pulmonary function abnormalities—which are much higher than the prevalence of asthma among African Americans in the general population [28,29] imply that factors other than asthma may be causing respiratory symptoms in individuals with SCD. Chronic dyspnea has also been reported in adults with SCD [30,31], but has been associated with pulmonary vascular dysfunction rather than airway obstruction [30].
Some reports of respiratory symptoms in our cohort could be due to asthma. Although this study found an association between doctor-diagnosis of asthma and allergic features, suggesting that misclassification of asthma diagnoses was not significant, nonatopic asthma has been well described among adults in the general population who lack typical atopic features (i.e., elevated IgE, eczema, eosinophilia) [32] and may account for some of the individuals in our cohort with respiratory symptoms, but without allergic features. A major cause of nonatopic asthma that could be relevant in our study is cigarette smoking [33,34]; however, we did not find an association between cigarette smoking and respiratory symptoms in our study cohort. Separate from asthma is the question of whether respiratory symptoms in our cohort represent chronic obstructive pulmonary disease (COPD). Our data suggests this is unlikely. Only 7 of 79 patients with acceptable spirometry met the COPD definition for airflow obstruction [35], and there was no association between severe recurrent wheezing and airflow obstruction. Given the high prevalence of respiratory symptoms in our cohort, etiologies in addition to asthma may be contributing to wheezing among adults with SCD.
Lung function was not associated with a doctor-diagnosis of asthma or allergic features (IgE and eosiniophil count) in study participants, but it was associated with recurrent, severe wheezing episodes, and ACS episodes. Potentially, severe wheezing episodes occur during ACS events (as has been previously described [5]) causing infarcts to the lung and leading to decreased lung function. This study, however, did not assess the timing of severe wheezing episodes in relation to ACS episodes. Given the cross-sectional nature of the questionnaire and spirometry data obtained for this manuscript, we cannot speculate about the timing and causal direction of recurrent wheezing and reduced lung function among our study participants. Interestingly, higher LDH was also associated with decreased lung function. In patients with SCD, LDH has been described as a marker of hemolysis and nitric oxide depletion [19]. Alterations in nitric oxide homeostasis are important to the pathogenesis of airway inflammation in asthma in the general population [36–38]. Among children, LDH has been associated airway hyper-responsiveness in children with SCD [39]. The present data showing a relationship between LDH and lower lung function adds to the preliminary data suggesting that hemolysis may have a role in lung dysfunction in SCD, however, further studies are necessary.
The present study examined an adult cohort with SCD, whereas most existing literature describing asthma in SCD was performed in children. The lack of an association between a doctor-diagnosis of asthma and SCD morbidity in our adult cohort may be explained by an improvement in asthma symptoms from childhood to adulthood. Among individuals in the general population, childhood asthma symptoms typically improve in adulthood as airway size enlarges [40–42]. In a large prospective cohort study of children with asthma, 60% of children who had episodic asthma at age 10 reported no wheezing symptoms when reassessed at age 42 [40]. Alternatively, studies in children based solely on reports of a doctor-diagnosis of asthma may have had significant misclassification of asthma diagnoses. Children with SCD lacking asthma features, but with wheezing symptoms, may have been incorrectly classified as having an asthma diagnosis in these prior studies.
Careful consideration should be given to the etiology of wheeze in adults with SCD. If the clinical presentation is most consistent with asthma, treatment utilizing the National Asthma Education and Prevention Program (NAEPP) guidelines [43] may be beneficial, although there have been no randomized clinical trials to determine the best treatment for asthma in the context of SCD. Further, it is important to recognize that the risks of corticosteroids, a mainstay of treatment for asthma, may be accentuated in SCD. For example, although systemic corticosteroids have been shown to shorten hospital stay and prevent clinical deterioration during episodes of ACS [44], they have also been associated with an increased risk of vaso-occlusion [45,46] and increased rates of rehospitalization [44,45,47].
Limitations are present in this study. First, our assessment of asthma diagnoses and respiratory symptoms are based on questionnaire responses. The validity of respiratory questionnaires for the diagnosis of asthma in adults with SCD is not known. Although there has been significant experience with ATS/DLD and other similar questionnaires, validating asthma questions has been challenging because there is no gold standard measure of asthma. Validating against airway hyper-responsiveness, the sensitivity of “doctor-diagnosis of asthma” questions is approximately 36% and specificity is 94% in the general population [48], whereas the sensitivity of “wheezing” questions is approximately 53% and specificity is 80% [48]. Despite this limitation, the ATS/DLD questionnaire has been used in several large epidemiologic asthma studies [49,50] and the doctor-diagnosis of asthma response was used to establish the relationship between asthma and SCD-related morbidity in children [2,18]. A second limitation was that at the time of questionnaire administration, participants did not receive additional instruction about the definition of wheezing, nor did we train the participants to recognize wheezing. There are many potential etiologies of recurrent respiratory symptoms including restrictive lung disease with or without diffusion defects and cardiopulmonary dysfunction [27,30,51] and there may have been confusion between wheezing and other respiratory symptoms. Third, tricuspid regurgitant jet velocity, a known marker of mortality in SCD [52], was not measured in the majority of our cohort, and thus was not accounted for in our survival analysis. Finally, our small sample size could have limited our ability to detect an association between a diagnosis of asthma and morbidity and mortality.
In summary, this study demonstrates that in a group of adults with typical features of asthma, reports of a doctor-diagnosis of asthma are not associated with hospitalization for pain, ACS episodes or death. However, a history of recurrent, severe wheezing episodes is associated with an increased morbidity and mortality in a group of adults without many features of asthma. Recurrent, severe wheezing occurs in SCD, may be distinct from asthma and warrants further investigation. Potentially, there are multiple etiologies of wheezing in SCD. Clinical trials are necessary to determine the optimal treatment regimens for asthma and/ or wheezing symptoms in children and adults with SCD.
Supplementary Material
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant numbers: K12 HL08710, HL079937.
The authors thank Nicole White for collecting data for this manuscript. Also, they thank Kim Williams, ANP, for her clinical care of the adults with SCD.
Footnotes
Conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ross J, Bernaudin F, Strunk RC, et al. Asthma is a distinct co-morbid condition in children with sickle cell anemia with elevated total and allergen-specific IgE levels. J Pediatr Hematol Oncol. 2011;33:e205–e208. doi: 10.1097/MPH.0b013e31820db7b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd JH, Macklin EA, Strunk RC, et al. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108:2923–2927. doi: 10.1182/blood-2006-01-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd JH, Moinuddin A, Strunk RC, et al. Asthma and acute chest in sickle-cell disease. Pediatr Pulmonol. 2004;38:229–232. doi: 10.1002/ppul.20066. [DOI] [PubMed] [Google Scholar]
- 4.Glassberg J, Spivey JF, Strunk R, et al. Painful episodes in children with sickle cell disease and asthma are temporally associated with respiratory symptoms. J Pediatr Hematol Oncol. 2006;28:481–485. doi: 10.1097/01.mph.0000212968.98501.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vichinsky EP, Styles LA, Colangelo LH, et al. Acute chest syndrome in sickle cell disease: Clinical presentation and course. Cooperative Study of Sickle Cell Disease. Blood. 1997;89:1787–1792. [PubMed] [Google Scholar]
- 6.Dean D, Neumayr L, Kelly DM, et al. Chlamydia pneumoniae and acute chest syndrome in patients with sickle cell disease. J Pediatr Hematol Oncol. 2003;25:46–55. doi: 10.1097/00043426-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Neumayr L, Lennette E, Kelly D, et al. Mycoplasma disease and acute chest syndrome in sickle cell disease. Pediatrics. 2003;112(Part 1):87–95. doi: 10.1542/peds.112.1.87. [DOI] [PubMed] [Google Scholar]
- 8.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118(Part 2):1–120. [PubMed] [Google Scholar]
- 9.Shilo NR, Lands LC. Asthma and chronic sickle cell lung disease: A dynamic relationship. Paediatr Respir Rev. 2011;12:78–82. doi: 10.1016/j.prrv.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 11.Field JJ, Krings J, White NL, et al. Urinary cysteinyl leukotriene E(4) is associated with increased risk for pain and acute chest syndrome in adults with sickle cell disease. Am J Hematol. 2009;84:158–160. doi: 10.1002/ajh.21348. [DOI] [PubMed] [Google Scholar]
- 12.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 13.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 14.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 15.Cohen RT, DeBaun MR, Blinder MA, et al. Smoking is associated with an increased risk of acute chest syndrome and pain among adults with sickle cell disease. Blood. 2010;115:3852–3854. doi: 10.1182/blood-2010-01-265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: Incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994;84:643–649. [PubMed] [Google Scholar]
- 17.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 18.Boyd JH, Macklin EA, Strunk RC, et al. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92:1115–1118. doi: 10.3324/haematol.11213. [DOI] [PubMed] [Google Scholar]
- 19.Kato GJ, McGowan V, Machado RF, et al. Lactate dehydrogenase as a bio-marker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burrows B, Martinez FD, Halonen M, et al. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 21.Lange P, Parner J, Vestbo J, et al. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 22.Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 23.Wilson NM, Dore CJ, Silverman M. Factors relating to the severity of symptoms at 5 yrs in children with severe wheeze in the first 2 yrs of life. Eur Respir J. 1997;10:346–353. doi: 10.1183/09031936.97.10020346. [DOI] [PubMed] [Google Scholar]
- 24.Young S, Le Souef PN, Geelhoed GC, et al. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N Engl J Med. 1991;324:1168–1173. doi: 10.1056/NEJM199104253241704. [DOI] [PubMed] [Google Scholar]
- 25.An P, Barron-Casella EA, Strunk RC, et al. Elevation of IgE in children with sickle cell disease is associated with doctor diagnosis of asthma and increased morbidity. J Allergy Clin Immunol. 2011;127:1440–1446. doi: 10.1016/j.jaci.2010.12.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozbek OY, Malbora B, Sen N, et al. Airway hyperreactivity detected by meth-acholine challenge in children with sickle cell disease. Pediatr Pulmonol. 2007;42:1187–1192. doi: 10.1002/ppul.20716. [DOI] [PubMed] [Google Scholar]
- 27.Klings ES, Wyszynski DF, Nolan VG, et al. Abnormal pulmonary function in adults with sickle cell anemia. Am J Respir Crit Care Med. 2006;173:1264–1269. doi: 10.1164/rccm.200601-125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arif AA, Delclos GL, Lee ES, et al. Prevalence and risk factors of asthma and wheezing among US adults: An analysis of the NHANES III data. Eur Respir J. 2003;21:827–833. doi: 10.1183/09031936.03.00054103a. [DOI] [PubMed] [Google Scholar]
- 29.Moorman JE, Rudd RA, Johnson CA, et al. National surveillance for asthma–United States, 1980–2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 30.Delclaux C, Zerah-Lancner F, Bachir D, et al. Factors associated with dyspnea in adult patients with sickle cell disease. Chest. 2005;128:3336–3344. doi: 10.1378/chest.128.5.3336. [DOI] [PubMed] [Google Scholar]
- 31.Miller GJ, Serjeant GR. An assessment of lung volumes and gas transfer in sickle-cell anaemia. Thorax. 1971;26:309–315. doi: 10.1136/thx.26.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bush A, Menzies-Gow A. Phenotypic differences between pediatric and adult asthma. Proc Am Thorac Soc. 2009;6:712–719. doi: 10.1513/pats.200906-046DP. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhuri R, Livingston E, McMahon AD, et al. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med. 2003;168:1308–1311. doi: 10.1164/rccm.200304-503OC. [DOI] [PubMed] [Google Scholar]
- 34.Court CS, Cook DG, Strachan DP. Comparative epidemiology of atopic and non-atopic wheeze and diagnosed asthma in a national sample of English adults. Thorax. 2002;57:951–957. doi: 10.1136/thorax.57.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chronic obstructive pulmonary disease guidelines. British Thoracic Society; 2010. [Google Scholar]
- 36.Vercelli D. Arginase: Marker, effector, or candidate gene for asthma? J Clin Invest. 2003;111:1815–1817. doi: 10.1172/JCI18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann N, King NE, Laporte J, et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer A, Folkerts G, Geppetti P, et al. Mediators of asthma: Nitric oxide. Pulm Pharmacol Ther. 2002;15:73–81. doi: 10.1006/pupt.2001.0332. [DOI] [PubMed] [Google Scholar]
- 39.Field JJ, Stocks J, Kirkham FJ, et al. Airway hyperresponsiveness in children with sickle cell anemia. Chest. 2011;139:563–568. doi: 10.1378/chest.10-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964–1999. J Allergy Clin Immunol. 2002;109:189–194. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
- 41.Zeiger RS, Dawson C, Weiss S. Relationships between duration of asthma and asthma severity among children in the Childhood Asthma Management Program (CAMP) J Allergy Clin Immunol. 1999;103(Part 1):376–387. doi: 10.1016/s0091-6749(99)70460-4. [DOI] [PubMed] [Google Scholar]
- 42.Vonk JM, Postma DS, Boezen HM, et al. Childhood factors associated with asthma remission after 30 year follow up. Thorax. 2004;59:925–929. doi: 10.1136/thx.2003.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.NAEPP guidelines. Amercian Academy of Allergy Asthma and Immunology; 2009. [Google Scholar]
- 44.Bernini JC, Rogers ZR, Sandler ES, et al. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 1998;92:3082–3089. [PubMed] [Google Scholar]
- 45.Strouse JJ, Takemoto CM, Keefer JR, et al. Corticosteroids and increased risk of readmission after acute chest syndrome in children with sickle cell disease. Pediatr Blood Cancer. 2008;50:1006–1012. doi: 10.1002/pbc.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darbari DS, Castro O, Taylor JGT, et al. Severe vaso-occlusive episodes associated with use of systemic corticosteroids in patients with sickle cell disease. J Natl Med Assoc. 2008;100:948–951. doi: 10.1016/S0027-9684(15)31410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sobota A, Graham DA, Heeney MM, et al. Corticosteroids for acute chest syndrome in children with sickle cell disease: Variation in use and association with length of stay and readmission. Am J Hematol. 2010;85:24–28. doi: 10.1002/ajh.21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toren K, Brisman J, Jarvholm B. Asthma and asthma-like symptoms in adults assessed by questionnaires. A literature review. Chest. 1993;104:600–608. doi: 10.1378/chest.104.2.600. [DOI] [PubMed] [Google Scholar]
- 49.Enright PL, Kronmal RA, Higgins MW, et al. Prevalence and correlates of respiratory symptoms and disease in the elderly. Cardiovascular Health Study. Chest. 1994;106:827–834. doi: 10.1378/chest.106.3.827. [DOI] [PubMed] [Google Scholar]
- 50.Childhood Asthma Management Program (CAMP) Reserach Group. Reserach Group, Design, Rationale, and Methods. Clin Trial. 1999;20:91–120. [PubMed] [Google Scholar]
- 51.Powars D, Weidman JA, Odom-Maryon T, et al. Sickle cell chronic lung disease: Prior morbidity and the risk of pulmonary failure. Medicine (Baltimore) 1988;67:66–76. [PubMed] [Google Scholar]
- 52.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.