Abstract
Background
Temporomandibular pain has multiple etiologies and a range of therapeutic options. In this pilot study, the authors assessed the feasibility of conducting a larger trial to evaluate chiropractic treatment of temporomandibular disorders (TMDs).
Methods
The authors assigned 80 participants randomly into one of the following four groups, all of which included a comprehensive self-care program: reversible interocclusal splint therapy (RIST), Activator Method Chiropractic Technique (AMCT) (Activator Methods International, Phoenix), sham AMCT and self-care only. They made assessments at baseline and at month 2 and month 6, including use of the Research Diagnostic Criteria for Temporomandibular Disorders.
Results
The authors screened 721 potential participants and enrolled 80 people; 52 participants completed the six-month assessment. The adjusted mean change in current pain over six months, as assessed on the 11-point numerical rating scale, was 2.0 (95 percent confidence interval, 1.1-3.0) for RIST, 1.7 (0.9-2.5) for self-care only, 1.5 (0.7-2.4) for AMCT and 1.6 (0.7-2.5) for sham AMCT. The authors also assessed bothersomeness and functionality.
Conclusions
The authors found the study design and methodology to be manageable. They gained substantial knowledge to aid in conducting a larger study. AMCT, RIST and self-care should be evaluated in a future comparative effectiveness study. Practical Implications. This pilot study was a necessary step to prepare for a larger study that will provide clinicians with information that should be helpful when discussing treatment options for patients with TMD.
Keywords: Temporomandibular disorder, chronic pain, chiropractic, Oral Health Impact Profile, randomized controlled trial
Pain and dysfunction associated with temporomandibular disorders (TMDs) affect more than 10 million Americans, or 5 to 12 percent of the population, with an annual cost estimated at $4 billion according to data from the National Institute of Dental and Craniofacial Research, National Institutes of Health (NIH).1 Of those affected, an estimated one-half to two-thirds will seek treatment.1 Although some of these conditions relate to structural or degenerative joint disease (for example, osteoarthritis), the majority are related to myofascial pain. Dentists and physicians commonly administer conservative treatments to ameliorate patients' symptoms, although, to date, no one treatment has emerged as the reference standard.2 Many cases of TMD will resolve with no or little treatment within a few months. However, TMD can become a chronic problem lasting several years, and patients receive little help from traditional forms of treatment. Consequently, some patients may seek complementary and alternative medicine (CAM) approaches to manage their TMD-related pain.3
Few studies of CAM for patients with TMD have been reported in the literature. Ritenbaugh and colleagues4 conducted a study composed of 168 participants in which they compared the effectiveness of traditional Chinese medicine with that of psychosocial care. La Touche and colleagues5 conducted a systematic review and meta-analysis of four randomized controlled trials (RCTs) of acupuncture for the treatment of pain in patients with TMD. Li and colleagues6 conducted a randomized, placebo-controlled trial composed of 55 participants who were treated with topical herbal ointment. In addition, the literature contains a few reports of different forms of manual therapy used for the treatment of TMD. Cuccia and colleagues7 compared osteopathic manual therapy with conventional conservative therapy (such as use of oral appliances, physical therapy, use of hot or cold packs or both, or transcutaneous electrical nerve stimulation) among 25 participants in each group. Kalamir and colleagues8 conducted an RCT with 93 participants using an intraoral myofascial form of chiropractic therapy. A case report by Houle and Descarreaux9 describes chiropractic treatment of TMD consisting of light spinal mobilizations of the upper cervical vertebrae along with ancillary methods. DeVocht and colleagues10 presented a case report and DeVocht and colleagues11 reported a case series in which patients with TMD exhibited improvement with another chiropractic approach: Activator Method Chiropractic Technique (AMCT) (Activator Methods International, Phoenix). Briefly, AMCT involves the use of a hand-held device to apply a precise mechanical adjustment; we describe it more fully in the Methods section below.
Although investigators in these studies reported some degree of reduction of TMD symptoms, the improvements were modest, with no definitive conclusion reached about which approach was best. Because AMCT showed promise in the case report10 and case series,11 an RCT was the logical next step to critically evaluate this chiropractic technique. Our study was part of a developmental center grant funded by the National Center for Complementary and Alternative Medicine, NIH. The purpose of this study was to assess the feasibility of conducting a full-scale RCT to evaluate the effectiveness of AMCT for the treatment of patients with chronic myofascial TMD. The specific aims included estimates of effects; an assessment of recruitment methods, compliance and retention of participants; and appropriateness of three different control groups (that is, reversible interocclusal splint therapy [RIST], self-care only or sham AMCT).
Methods
Study design and setting
In this prospective randomized controlled pilot trial, we compared the effects of AMCT plus self-care with three other conservative interventions: RIST plus self-care, a sham AMCT plus self-care, and self-care only for participants 21 years and older who had chronic myofascial TMD. We recruited participants from the eastern Iowa region.
The institutional review boards at the University of Iowa, Iowa City, and Palmer College of Chiropractic, Davenport, Iowa, approved the study protocol. Potential participants responding to recruitment efforts were screened (by L.T.) via an initial telephone call and two baseline clinical visits. Each study participant provided oral consent to participate in the telephone screening and written informed consent before the baseline evaluations and treatment allocation.
Baseline screening (by L.T.), the dental examination (by M.S., C.M.S.), the three assessments (that is, baseline, month 2, month 6), provision of instructions for self-care, and RIST treatment (by L.T., M.S., C.M.S.) took place in a dental unit of the Craniofacial Clinical Research Center at the University of Iowa. One member of the study team (W.S.) provided AMCT and sham AMCT in a local private chiropractic office. The Office of Data Management & Biostatistics at the Palmer Center for Chiropractic Research, Davenport, was the data coordinating center. Participants were masked to the nature of the sham intervention.
Baseline visit 1 (BL1) included administration of the informed consent document (by L.T.), a variety of surveys completed by the prospective participant and a brief dental screening examination (by M.S., C.M.S.). Baseline visit 2 (BL2) included the full examination, including use of the Research Diagnostic Criteria for Temporomandibular Disorders (RDC-TMD),12 the eligibility screening interview, final determination of eligibility, random allocation to one of the four treatment groups and instructions for self-care given to all participants. We allocated participants via a randomization algorithm stored in the Web-based system, with future allocations concealed. The clinicians who administered the RDC-TMD (C.M.S., M.S.) were trained at the University of Washington, Seattle, according to the International RDC-TMD training descriptions.13
Inclusion criteria
Inclusion criteria consisted of being at least 21 years of age, having had TMD symptoms for at least six months, the presence of more than seven teeth per dental arch, average self-reported TMD pain over the previous week of at least a 3 on an 11-point numerical rating scale (NRS),14,15 an RDC-TMD Axis I diagnosis of myofascial pain, and no changes in prescription medicine for pain in the preceding six months.
Exclusion criteria
Exclusion criteria included the following: current or pending litigation for a personal injury case, worker's compensation or disability; unstable periodontitis, untreated dental-related disease or both; Angle Class II malocclusion; the need for advanced diagnostic procedures to rule out pathology; systemic rheumatoid arthritis or similar autoimmune conditions; complete dentures; major psychological disorders; any treatment for TMD during the previous month (except nonprescription medication or a stable prescription medication regimen); inability to understand English; unwillingness to be enrolled in any of the four intervention groups; unwillingness to postpone other forms of treatment for TMD during the six-month active care phase; the intention to move away from the area during the next six months; or previous AMCT treatment for TMD at any time.
The authors compared the effects of a chiropractic technique plus self-care with three other conservative interventions.
We randomly allocated the screened participants who were eligible for the study to a treatment group.16 After two months, they received an RDC-TMD assessment by a clinician masked to the treatment group. The self-care–only group underwent the month 2 assessment eight weeks after the BL2. Participants in the other three groups underwent these assessments 10 weeks after the BL2. Six months after the BL2, a clinician masked to treatment (M.S. or C.M.S.) performed a final assessment that was identical to the BL2 and month 2 assessments. The clinicians who performed the TMD examination used the standardized RDC-TMD methodology.12 After the final assessment, all participants were informed as to which group they had been assigned. Those in the self-care–only group and the sham AMCT group were given the opportunity to receive one month of treatment with either RIST or AMCT at no cost to them. All visits, starting with the BL2 examination, were digitally recorded.
Self-care
All participants received a TMD self-care program. One member of the study team (C.M.S.) developed a TMD self-care guide in collaboration with the University of Washington. The self-care protocol was similar to the usual and customary recommendations that dentists give to patients with TMD, as well as to the self-care protocols used in other TMD studies.17,18 Briefly, self-care consisted of conservative and reversible strategies that required the dentist or dental care coordinator to review TMD with the participant; explain to him or her the current understanding of prognosis; and provide a standardized treatment checklist that identified recommendations for care (for example, jaw relaxation, reduction of parafunctional behaviors, use of thermal packs, use of over-the-counter pain medications, passive jaw-opening stretches and suggestions about stress reduction). All participants were given the same self-care instructions, regardless of their group assignment. The two-month intervention period for participants in the self-care–only group began at the end of BL2.
RIST
RIST is a relatively reversible intervention. For participants allocated to the RIST arm, the clinicians made maxillary and mandibular vinyl polysiloxane impressions (Position Penta Quick Impression Material, 3M ESPE, St. Paul, Minn.) at the end of the BL2 visit. They created interocclusal records with the use of a fast-setting vinyl polysiloxane bite registration material (Regisil Rigid, Dentsply Caulk, Milford, Del.) and an intraoral metal tray (Bite-Tray, Panadent, Colton, Calif.). A commercial laboratory then waxed and heat-processed a clear acrylic resin splint to capture the mandibular cusp tips in the occlusal plane of the splint. Two weeks later, patients returned to be fitted with a hard acrylic resin full-arch maxillary splint. The clinicians adjusted the splints to provide uniform posterior centric occlusal stops and to attain mandibular canine lateral guidance. (We should point out that we used canine guidance as a matter of convenience and consistency, and it was not a research objective of this study.) When necessary, the clinician added autopolymerizing resin to create canine disclusion or to bring posterior teeth into centric occlusal stops. The clinician then polished the splint and provided the patient with home care instructions. He instructed patients to wear the splint at night and for at least two hours during the day, thus beginning their two months of intervention. We did not see participants in this group again until the assessment at the end of the two-month intervention period.
AMCT
The AMCT protocol is a structured method of chiropractic treatment that involves the use of a series of biomechanical tests in determining how, where and when (or when not) to perform a mechanically assisted manipulation. These biomechanical tests involve well-defined joint or joint complex (motor unit) movements involving the area of examination. Each specific manipulation—called an “adjustment”—is given with a hand-held, spring-loaded instrument (Activator IV, Activator Methods International) that delivers a quick, shallow thrust. The AMCT protocol can include the entire spine and extremities, as well as the temporomandibular joint. The entire AMCT protocol has been described in detail elsewhere.19
We scheduled participants for a maximum of 12 visits over two months: two visits per week for the initial four weeks and one visit per week for the second four-week period. Each visit followed a standard script of assessments and treatments. We scheduled the first visit for two weeks after BL2 to match the time required for those in the RIST group to receive their fabricated splint. One chiropractor (W.S.) performed all chiropractic treatment in a private practice setting.
Sham AMCT
The chiropractor used an Activator IV instrument in the sham AMCT group that was identical to the one used in the active AMCT group except for an undetectable clear plastic collar placed around the preloaded frame of the instrument. This collar prevented delivery of the mechanical thrust (the spring mechanism), although the audible “clicking” sound was the same, giving the impression that an impact had been produced. At the initial visit, these patients received the same chiropractic adjustment as patients in the active AMCT group received, except theirs was performed with the modified instrument.
We scheduled patient visits according to the same schedule as that outlined earlier for the active treatment group. The sham protocol followed the AMCT educational, examination and testing procedures of the active AMCT group. This involved patients' spending a similar amount of time with the chiropractor and in educational instruction, examination and testing, and treatment.
Outcomes and measurements
We measured outcome variables at month 2 (that is, end-of-treatment period) and at month 6. We captured patient-rated current TMD-related pain with an 11-point NRS (0 = no pain and 10 = pain as bad as it can be). We established an a priori two-point change in the NRS from baseline as a clinically meaningful change for patients with chronic TMD.20 We used the 14-item Oral Health Impact Profile (OHIP-14) to assess oral health–related quality of life.21-25 The OHIP-14 contains two questions about each of seven dimensions, indicating how often the participant had experienced each difficulty in the previous month; possible responses range from 0 (never) to 4 (very often). The OHIP score is obtained by summing the 14 ratings. It is intended to provide a measure of dysfunction, discomfort and disability arising from oral conditions.
Bothersomeness of TMD symptoms (adapted from Patrick and colleagues26) is a measure of the perceived impact of the TMD-related pain on daily living. Possible ratings range from 1 (not at all bothersome) to 5 (extremely bothersome) for five symptoms (facial pain, jaw clicking, jaw ache, grinding teeth and clenching jaw). We calculated the TMD bothersomeness index by summing the five symptom ratings, as reported by participants in one of the surveys we administered.
We measured participants' satisfaction with care on an 11-point NRS ranging from 0 (“not at all satisfied”) to 10 (“extremely satisfied”). With participants' permission, one of us (W.S.) digitally recorded their chiropractic treatment visits to allow us to compare the chiropractic clinician's interaction with participants in the active AMCT group with that in the sham AMCT group.
Sample size
We chose the sample size to determine feasibility and, therefore, the study was not powered to detect differences between groups. We targeted 20 participants per group to assess their willingness to be assigned randomly to one of the four treatment groups, especially given that one was sham and one was self-care only. In addition, the sample size allowed us to assess how well participants complied with the six-month protocol.
Statistical methods
We analyzed the data on an intention-to-treat basis. We performed multiple imputation for the missing outcomes of NRS, OHIP-14 and the TMD bothersomeness index by using the baseline outcome variables, other select baseline variables and the available month 2 and month 6 values for these outcomes (PROC MI in SAS, Version 9.2, SAS, Cary, N.C.). We used a one-way analysis of covariance (ANCOVA) to analyze the change in scores separately from baseline to month 2 and from baseline to month 6 for each outcome variable, adjusting for the baseline value of the respective outcome variable centered at its mean score. We combined the results across the analyses of multiple imputations to obtain the adjusted point estimates, with 95 percent confidence intervals from these ANCOVA models for both within-group changes and differences in changes between the AMCT group and the other three groups.
Results
Screening, enrollment and follow-up
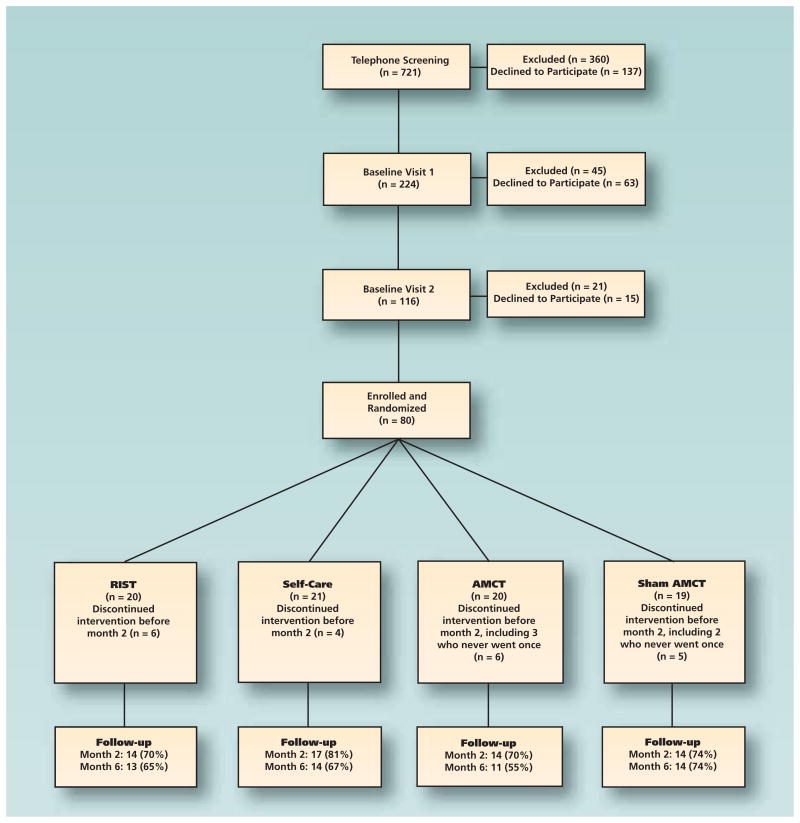
Enrollment over 18 months ended in July 2011; 721 patients were screened via telephone, 360 of whom were excluded and 137 declined to participate. Two hundred twenty-four were screened again at BL1 and 116 at BL2. We enrolled 80 participants, 52 of whom completed the six-month assessment. The most common single reason for exclusion at the telephone screening and at BL1 was an NRS pain score of less than 3. At BL2, the most common reason for exclusion was failure to meet the RDC for myofascial TMD. The figure shows the overall flow of the study.
The most successful recruitment method was use of the Internet, primarily bulk e-mails sent to the entire University of Iowa employee and student population, resulting in 34 of the 80 participants (42.5 percent). Other recruitment methods included bulk postcard mailings (n = 19), newspaper advertisements (n = 8), referrals from dentists (n = 6), word of mouth (n = 6) and other (n = 7).
Baseline characteristics
The mean age (standard deviation [SD]) of participants in the study was 35.0 years (SD, 11.7 years); 64 participants (80 percent) were female. The median duration of TMD symptoms was five to 10 years. Table 1 provides descriptive statistics for baseline characteristics, including baseline assessments of outcome variables.
Table 1.
Baseline characteristics of participants in TMD* pilot study.
| CHARACTERISTIC | No. (%) OF PARTICIPANTS† | |||
|---|---|---|---|---|
|
| ||||
| RIST‡ (n = 20) | Self-Care only (n = 21) | AMCT§ (n = 20) | Sham AMCT (n = 19) | |
|
| ||||
| Demographics | ||||
| Female | 17 (85) | 16 (76) | 16 (80) | 15 (79) |
| Mean (SD¶) age, in years | 36.9 (13.5) | 38.0 (12.7) | 31.7 (7.9) | 33.1 (11.4) |
| College education | 17 (85) | 18 (86) | 20 (100) | 17 (89) |
| Current full-time student | 6 (30) | 1 (5) | 6 (30) | 5 (26) |
| Annual income, in dollars | ||||
| < 20,000 | 5 (25) | 3 (14) | 3 (15) | 4 (21) |
| 20,000-40,000 | 4 (20) | 3 (14) | 7 (35) | 4 (21) |
| > 40,000-60,000 | 3 (15) | 5 (24) | 4 (20) | 4 (21) |
| > 60,000-80,000 | 5 (25) | 4 (19) | 1 (5) | 1 (5) |
| > 80,000 | 3 (15) | 6 (29) | 5 (25) | 6 (32) |
| Race: white | 18 (90) | 21 (100) | 19 (95) | 15 (79) |
| Previous treatment for TMD | 6 (30) | 6 (29) | 3 (15) | 9 (47) |
|
| ||||
| TMD Symptoms | ||||
| Median (IQR#) pain duration, in years | 10 (12.5) | 10 (11) | 7.5 (12.5) | 5 (8) |
| Quality of pain | ||||
| Persistent | 10 (50) | 9 (43) | 10 (50) | 11 (58) |
| Recurrent | 10 (50) | 12 (57) | 10 (50) | 8 (42) |
| Mean (SD) NRS**: current TMJ††pain | 3.7 (1.5) | 4.4 (1.4) | 4.2 (1.6) | 3.5 (1.9) |
| Mean (SD) NRS: worst pain in previous week | 4.0 (1.6) | 5.0 (1.5) | 4.7 (1.7) | 4.4 (1.7) |
| Mean (SD) NRS: worst pain in 6 months | 6.9 (1.6) | 7. 4 (1. 6) | 7. 5 (2.0) | 6 .7 (2.1) |
| Mean (SD) NRS: mean pain for 6 months | 4.2 (1.5) | 5.7 (1.9) | 4.6 (1.7) | 4.5 (2.0) |
| Mean (SD) OHIP‡‡ | 13.2 (8.2) | 15.8 (10.8) | 12.4 (8.2) | 10.7 (7.9) |
| Mean (SD) bothersomeness index§§ | 13.4 (3.6) | 13.7 (4.7) | 14.0 (3.1) | 12.4 (3.6) |
TMD: Temporomandibular disorder.
Unless otherwise specified.
RIST: Reversible interocclusal splint therapy.
AMCT: Activator Method Chiropractic Technique (Activator Methods International, Phoenix).
SD: Standard deviation.
IQR: Interquartile range.
NRS: Numerical rating scale (0 = no pain and 10 = pain as bad as it can be).
TMJ: Temporomandibular joint.
OHIP: Oral Health Impact Profile (consisting of 14 items, with responses ranging from 0 [never] to 4 [very often]). Sources: Locker and Slade,21 Slade and Spencer,22 Slade,23 Slade24 and Locker and Allen.25
The maximum score is 25, indicating “extremely bothersome” ratings on five TMD symptoms (adapted from Patrick and colleagues26).
Study outcomes
Table 2 shows the within-group adjusted mean changes from baseline to month 2 and from baseline to month 6. Participants in all groups experienced improvement on the NRS, OHIP and bothersomeness index at both assessments. All participants experienced more improvement at month 6 than at month 2, except for those in the sham AMCT group on the NRS.
Table 2.
Adjusted mean changes in outcome variables from baseline to month 2 and baseline to month 6, according to treatment group.*
| VARIABLE | ADJUSTED† MEAN CHANGE (95% CONFIDENCE INTERVAL) | |||
|---|---|---|---|---|
| RIST‡ (n = 20) | Self-Care only (n = 21) | AMCT§ (n = 20) | Sham AMCT (n = 19) | |
| Change in NRS¶ for Current Pain at Month 2 | 1.4 (0.3 to 2.5) | 0.7 (-0.1 to 1.5) | 0.9 (-0.1 to 1.8) | 1.6 (0.6 to 2.6) |
| Change in NRS for Current Pain at Month 6 | 2.0 (1.1 to 3.0) | 1.7 (0.9 to 2.5) | 1.5 (0.7 to 2.4) | 1.6 (0.7 to 2.5) |
| Change in OHIP# at Month 2 | 3.6 (0.1 to 7.2) | 2.8 (-0.4 to 6.1) | 1.3 (-2.3 to 5.0) | 2.0 (-1.8 to 5.8) |
| Change in OHIP at Month 6 | 4.8 (1.7 to 8.0) | 4.0 (1.2 to 6.8) | 4.0 (1.0 to 7.0) | 3.8 (0.5 to 7.0) |
| Change in Bothersomeness Index at Month 2 | 2.4 (0.6 to 4.2) | 1.8 (0.3 to 3.3) | 2.3 (0.5 to 4.1) | 2.3 (0.7 to 4.0) |
| Change in Bothersomeness Index at Month 6 | 3.6 (2.0 to 5.2) | 2.6 (1.2 to 4.0) | 3.6 (2.2 to 5.0) | 2.7 (1.2 to 4.2) |
Missing data were replaced with imputed values.
Adjusted for baseline value of outcome variable centered at its mean score.
RIST: Reversible interocclusal splint therapy.
AMCT: Activator Method Chiropractic Technique (Activator Methods International, Phoenix).
NRS: Numerical rating scale.
Table 3 shows the adjusted mean differences between the change in the AMCT group and the changes in each of the other three groups at month 2 and month 6. As expected at the onset of the trial, none of the differences was statistically significant at the .05 level.
Table 3.
| VARIABLE | ADJUSTED MEAN DIFFERENCE (95% CONFIDENCE INTERVAL) | ||
|---|---|---|---|
| AMCT Versus RIST§ | AMCT Versus Self-Care only | AMCT Versus Sham AMCT | |
| NRS¶ at Month 2 | -0.5 (-1.7 to 0.7) | 0.1 (-1.0 to 1.3) | -0.7 (-1.8 to 0.5) |
| NRS at Month 6 | -0.5 (-1.7 to 0.7) | -0.2 (-1.3 to 0.9) | -0.1 (-1.2 to 1.1) |
| OHIP# at Month 2 | -2.3 (-6.9 to 2.4) | -1.5 (-6.4 to 3.3) | -0.6 (-5.3 to 4.1) |
| OHIP at Month 6 | -0.8 (-5.2 to 3.6) | 0.0 (-4.1 to 4.2) | 0.3 (-4.3 to 4.8) |
| Bothersomeness Index at Month 2 | -0.1 (-2.4 to 2.2) | 0.5 (-1.8 to 2.8) | -0.1 (-2.3 to 2.2) |
| Bothersomeness Index at Month 6 | 0.0 (-2.1 to 2.1) | 1.0 (-1.0 to 2.9) | 0.9 (-3.1 to 1.3) |
Adjusted for baseline value of outcome variable centered at its mean score.
AMCT: Activator Method Chiropractic Technique (Activator Methods International, Phoenix).
Missing data were replaced with imputed values.
RIST: Reversible interocclusal splint therapy.
NRS: Numerical rating scale.
Participants' mean (SD) satisfaction with care, as measured on the 11-point NRS, at month 2 was 7.2 (1.4) for the RIST group, 4.9 (2.3) for the self-care–only group, 6.4 (2.0) for the AMCT group and 5.9 (2.9) for the sham AMCT group. Participants' mean satisfaction at month 6 increased to 8.2 for the RIST group and 6.6 for the self-care–only group. Scores for the other two groups remained approximately the same at month 6.
An analysis of the digital recording of participants' visits to the chiropractic clinician indicated some differences (such as subtle differences in body language) between his interaction with participants in the active AMCT group and his interaction with those in the sham AMCT group. The specifics of that analysis have been presented27 and will be provided in detail elsewhere.
Discussion
We designed this pilot study to evaluate pragmatic aspects of conducting a multisite randomized controlled trial. TMD is a multifaceted disorder that includes various etiologies, with complex interactions of genetic, psychological, physiological, emotional and functional aspects. The use of minimally invasive approaches with an emphasis on patient self-awareness and self-management may be a logical approach to managing TMD. Many minimally invasive approaches to managing chronic pain incorporate a significant therapeutic alliance between the clinician and the patient. These complex personal interactions overlap with clinical or therapeutic interventions, often making it difficult for the clinician to establish a definite benefit from one specific procedure or technique. Therefore, we selected an expertise-based RCT for this study. To promote consistency in the trial design and operations across project managers, outcomes assessors and clinicians, we designed a Web-based manual of procedures, and the clinicians who performed the RDC-TMD were trained and underwent technique calibration.
The information gained during this pilot study allowed us to assess the feasibility of conducting a full-scale trial and to provide estimates of effect sizes and variability of outcome measures to use in planning future trials. Key milestones achieved included the development of an integrated research team across two institutions and a private practice; use of Web-based data collection forms that allowed for real-time eligibility assessments; training and calibration of masked evaluators; practical patient flow across sites; achievable recruitment strategies; and expertise building in the area of controlled interactions between clinicians and patients who had chronic pain (also known as a “therapeutic alliance”). Strengths of this study included enrolling the targeted 80 participants in less time than anticipated and performance of outcomes assessments by evaluators masked to the participant's treatment assignment.
The changes in pain and function seen in this study were smaller and more evenly spread out than expected across treatment groups. The minimum NRS pain score of 3 for eligibility may have been too low for participants to exhibit much improvement. Requiring a higher level of pain for inclusion in the study might have resulted in a greater therapeutic response, but it also would have created additional challenges in participant recruitment.
We included three different control groups in this study to identify an appropriate control for a larger study. We chose RIST because it is a commonly used conservative treatment approach for TMD in dental practice.28 We included the self-care group because it is the most conservative form of treatment and has been shown to result in at least some benefit.17 We included the sham AMCT group to determine whether the clinician's interaction with the participant was a major factor in the study results.
Requiring a higher level of pain for inclusion in the study might have resulted in a greater therapeutic response, but it also would have created additional challenges in participant recruitment.
Members of the study team evaluated the three control groups and concluded that it would be most appropriate to have two control groups in the next, multisite study: RIST and self-care alone. Inasmuch as RIST is in common use, it would be helpful for practicing clinicians to know how it compares with the alternate method being considered (in this case, AMCT). In this study, self-care alone was a confounder when combined with the other interventions. By including this intervention in a totally separate manner (that is, in a control group), researchers will be able to determine any specific effects. We do not recommend including the sham AMCT group in future studies. Because the AMCT protocol relies on patient and practitioner feedback and responsiveness to biomechanical test and treatment protocols, we found it difficult to achieve truly consistent clinician-patient interactions between the active and sham AMCT groups.
One of the purposes of this pilot study was to evaluate the various methods of recruitment for participants. The most successful method used was sending bulk e-mails to the university community. The clinical coordinator (L.T.) sent bulk e-mails on multiple occasions to 40,000 employees and students at minimal cost. However, this option may not be available in some locations, and recruiting from this healthy population may have contributed to the relatively low levels of baseline TMD pain found in our sample. We also sent 27,000 postcards in a targeted mailing. Although the return rate was low, some people responded to each mailing, at a cost of $1,028 per enrolled participant. Newspaper advertisements resulted in eight enrolled participants, at a cost of $473 per enrolled participant.
The sample of enrolled patients had, on average, moderate levels of self-reported current pain (4.0 on the 11-point [0-10] NRS scale). The changes in NRS scores over time were quite small. The change in current pain level may not be the most appropriate outcome measure to use. The median duration of TMD symptoms was nine years, and current pain may not capture the burden of the condition in patients with chronic pain. If investigators use NRS as an outcome, we believe it would be better to use some type of composite score of multiple variations of the NRS for pain, such as characteristic pain intensity as the primary outcome, which is the mean of the present, average and worst TMD-related pain in the previous two months.17 Given the complexity of chronic pain perception, a more holistic, multidimensional approach may be warranted for future studies. In addition to the NRS, pain-related disability measures, such as the OHIP and bothersomeness index, likely are informative as secondary measures to aid in the evaluation of symptom improvement.
Study limitations
This study had several limitations. First, we provided a self-care program in all four treatment groups but did not monitor self-care in a daily fashion during the six months of the study; therefore, we could not determine the degree of compliance by participants. It is possible that participants in the self-care–only group were more compliant than those in other groups, in which self-care was an addition to another intervention.
Second, a considerable number of participants were lost to follow-up, yet these numbers were fairly consistent across the four groups (five to nine per group). Investigators in future studies should use more aggressive efforts at retention such as sending e-mail reminders before treatment and assessment appointments, as well as making prompt and repeated efforts to contact those who miss appointments.
Conclusions
The purpose of this study was to assess the feasibility of conducting a full-scale RCT to evaluate the effectiveness of AMCT for the treatment of patients with chronic myofascial TMD. We found the study logistics to be manageable, and the lessons learned will improve the design of a larger-scale study. The next step should be a comparative effectiveness study of three groups—AMCT, RIST and self-care—that involves the use of a composite score of multiple variations of the NRS for pain as the primary outcome, as well as secondary outcomes that are more functionally oriented.
Figure.
Flow diagram of screening, enrollment and follow-up of participants through six months. AMCT: Activator Method Chiropractic Technique (Activator Methods International, Phoenix). RIST: Reversible interocclusal splint therapy.
Acknowledgments
This study was supported by grant U19AT004663 from the National Center for Complementary and Alternative Medicine (NCCAM), National Institutes of Health (NIH), Bethesda, Md. The contents of the manuscript of this article are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM or NIH.
This study also was supported by the National Center for Advancing Translational Sciences, NIH, through grant 2 UL1 TR000442-06 at the University of Iowa, Iowa City. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
This investigation was conducted partially in a facility constructed with support from Research Facilities Improvement Program grant C06 RR15433-01 from the National Center for Research Resources, NIH.
The two Activator IV instruments for active and sham Activator Method Chiropractic Technique treatments were provided by Activator Methods International, Phoenix.
The authors thank the participants for their valuable contributions to this project. They acknowledge Lynne Carber and Karen O'Rourke for their invaluable assistance in project management, as well as Kimberly Huggins, RDH, BS, and Edward Truelove, DDS, University of Washington, Seattle, for the Research Diagnostic Criteria for Temporomandibular Disorders instrument training and advice in the early design phase of the study.
Abbreviation Key
- AMCT
Activator Method Chiropractic Technique
- BL1
Baseline visit 1
- BL2
Baseline visit 2
- CAM
Complementary and alternative medicine
- NCCAM
National Center for Complementary and Alternative Medicine
- NIH
National Institutes of Health
- NRS
Numerical rating scale
- OHIP-14
Oral Health Impact Profile
- RCT
Randomized controlled trial
- RDC-TMD
Research Diagnostic Criteria for Temporomandibular Disorders
- RIST
Reversible interocclusal splint therapy
- TMD
Temporomandibular disorder
- TMJ
Temporomandibular joint
Footnotes
Disclosure. Dr. Schaeffer instructs occasionally for Activator Methods International, Phoenix. None of the other authors reported any disclosures.
Contributor Information
Dr James W. DeVocht, Palmer Center for Chiropractic Research, Palmer College of Chiropractic, Davenport, Iowa.
Dr Christine M. Goertz, Research and Health Policy, and a professor, Palmer Center for Chiropractic Research, Palmer College of Chiropractic, Davenport, Iowa.
Dr Maria A. Hondras, Palmer Center for Chiropractic Research, Palmer College of Chiropractic, Davenport, Iowa.
Dr Cynthia R. Long, Palmer Center for Chiropractic Research, Palmer College of Chiropractic, Davenport, Iowa.
Dr Wally Schaeffer, Schaeffer Chiropractic, Coralville, Iowa.
Ms Lauren Thomann, Craniofacial Clinical Research Center, Dows Institute for Dental Research, College of Dentistry, University of Iowa, Iowa City.
Dr Michael Spector, Craniofacial Clinical Research Center, Dows Institute for Dental Research, College of Dentistry, University of Iowa, Iowa City.
Dr Clark M. Stanford, Email: Clark-Stanford@uiowa.edu, Dows Institute for Dental Research, College of Dentistry, University of Iowa, N419 Dental Science Building, 801 Newton Road, Iowa City, Iowa 52242.
References
- 1.National Institute of Dental and Craniofacial Research. [Accessed Aug. 20, 2013];Facial pain. www.nidcr.nih.gov/DataStatistics/FindDataByTopic/FacialPain/
- 2.Jerjes W, Upile T, Abbas S, et al. Muscle disorders and dentition-related aspects in temporomandibular disorders: controversies in the most commonly used treatment modalities. Int Arch Med. 2008;1(1):23. doi: 10.1186/1755-7682-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBar LL, Vuckovic N, Schneider J, Ritenbaugh C. Use of complementary and alternative me dicine for temporomandibular disorders. J Orofac Pain. 2003;17(3):224–236. [PubMed] [Google Scholar]
- 4.Ritenbaugh C, Hammerschlag R, Dworkin SF, et al. Comparative effectiveness of traditional Chinese medicine and psychosocial care in the treatment of temporomandibular disorders-associated chronic facial pain. J Pain. 2012;13(11):1075–1089. doi: 10.1016/j.jpain.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Touche R, Goddard G, De-la-Hoz JL, et al. Acupuncture in the treatment of pain in temporomandibular disorders: a systematic review and meta-analysis of randomized controlled trials. Clin J Pain. 2010;26(6):541–550. doi: 10.1097/AJP.0b013e3181e2697e. [DOI] [PubMed] [Google Scholar]
- 6.Li LC, Wong RW, Rabie AB. Clinical effect of a topical herbal ointment on pain in temporomandibular disorders: a randomized placebo-controlled trial. J Altern Complement Med. 2009;15(12):1311–1317. doi: 10.1089/acm.2009.0129. [DOI] [PubMed] [Google Scholar]
- 7.Cuccia AM, Caradonna C, Annunziata V, Caradonna D. Osteopathic manual therapy versus conventional conservative therapy in the treatment of temporomandibular disorders: a randomized controlled trial. J Bodyw Mov Ther. 2010;14(2):179–184. doi: 10.1016/j.jbmt.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Kalamir A, Bonello R, Graham P, Vitiello AL, Pollard H. Intraoral myofascial therapy for chronic myogenous temporomandibular disorder: a randomized controlled trial. J Manipulative Physiol Ther. 2012;35(1):26–37. doi: 10.1016/j.jmpt.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Houle S, Descarreaux M. Conservative care of temporomandibular joint disorder in a 35-year-old patient with spinal muscular atrophy type III: a case study. J Chiropr Med. 2009;8(4):187–192. doi: 10.1016/j.jcm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeVocht JW, Schaeffer W, Lawrence DJ. Chiropractic treatment of temporomandibular disorders using the activator adjusting instrument and protocol. Altern Ther Health Med. 2005;11(6):70–73. [PubMed] [Google Scholar]
- 11.DeVocht JW, Long CR, Zeitler DL, Schaeffer W. Chiropractic treatment of temporomandibular disorders using the activator adjusting instrument: a prospective case series. J Manipulative Physiol Ther. 2003;26(7):421–425. doi: 10.1016/S0161-4754(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 12.Dworkin SF, Huggins K, Wilson L, et al. A randomized clinical trial using Research Diagnostic Criteria for Temporomandibular Disorders-Axis II to target clinic cases for a tailored self-care TMD treatment program. J Orofac Pain. 2002;16(1):48–63. [PubMed] [Google Scholar]
- 13.International RDC-TMD Consortium. RDC for TMD Training. [Accessed Sept. 3, 2013]; www.rdc-tmdinternational.org/TMDAssessmentDiagnosis/RDCTMD.aspx.
- 14.Jaeschke R, Singer J, Guyatt GH. A comparison of seven-point and visual analogue scales: data from a randomized trial. Control Clin Trials. 1990;11(1):43–51. doi: 10.1016/0197-2456(90)90031-v. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 16.Devereaux PJ, Bhandari M, Clarke M, et al. Need for expertise based randomised controlled trials. BMJ. 2005;330(7482):88. doi: 10.1136/bmj.330.7482.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truelove E, Huggins KH, Mancl L, Dworkin SF. The efficacy of traditional, low-cost and nonsplint therapies for temporomandibular disorder: a randomized controlled trial. JADA. 2006;137(8):1099–1107. doi: 10.14219/jada.archive.2006.0348. [DOI] [PubMed] [Google Scholar]
- 18.Michelotti A, Iodice G, Vollaro S, Steenks MH, Farella M. Evaluation of the short-term effectiveness of education versus an occlusal splint for the treatment of myofascial pain of the jaw muscles. JADA. 2012;143(1):47–53. doi: 10.14219/jada.archive.2012.0018. [DOI] [PubMed] [Google Scholar]
- 19.Fuhr AW. The Activator Method. 2nd. St Louis: Mosby Elsevier; 2009. [Google Scholar]
- 20.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 21.Locker D, Slade G. Oral health and the quality of life among older adults: the oral health impact profile. J Can Dent Assoc. 1993;59(10):830–833. 837-388, 844. [PubMed] [Google Scholar]
- 22.Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11(1):3–11. [PubMed] [Google Scholar]
- 23.Slade GD. Derivation and validation of a short-form Oral Health Impact Profile. Community Dent Oral Epidemiol. 1997;25(4):284–290. doi: 10.1111/j.1600-0528.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 24.Slade GD. Assessing change in quality of life using the Oral Health Impact Profile. Community Dent Oral Epidemiol. 1998;26(1):52–61. doi: 10.1111/j.1600-0528.1998.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 25.Locker D, Allen PF. Developing short-form measures of oral health-related quality of life. J Public Health Dent. 2002;62(1):13–20. doi: 10.1111/j.1752-7325.2002.tb03415.x. [DOI] [PubMed] [Google Scholar]
- 26.Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine (Phila Pa 1976) 1995;20(17):1899–1908. doi: 10.1097/00007632-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 27.DeVocht JW, Salsbury S, Seidman M, et al. Equivalence of doctor interactions between activator methods and sham chiropractic protocols during an expertise-based randomized clinical trial. BMC Complement Altern Med. 2012;12(suppl 1):P146. [Google Scholar]
- 28.Al Ani MZ, Davies SJ, Gray RJ, Sloan P, Glenny AM. Stabilisation splint therapy for temporomandibular pain dysfunction syndrome. Cochrane Database Syst Rev. 2004;(1):CD002778. doi: 10.1002/14651858.CD002778.pub2. [DOI] [PubMed] [Google Scholar]