Abstract
Purpose
Novel strategies are needed to improve the long-term outcomes of patients with high-risk prostate cancer treated with androgen deprivation and external beam radiation therapy (XRT). Preclinical data suggest that angiogenesis inhibitors improve the therapeutic index of XRT. To assess the feasibility of combined vascular endothelial growth factor receptor (VEGFR)/platelet-derived growth factor receptor (PDGFR) inhibition in combination with androgen deprivation and XRT, a Phase I study with sunitinib was initiated.
Methods and Materials
Seventeen men with localized adenocarcinoma of the prostate with cT2c-cT4 or Gleason 8–10 or PSA > 20ng/ml received initial androgen deprivation (leuprolide 22.5mg every 12 weeks + oral bicalutamide 50mg daily) for 4–8 weeks prior to oral sunitinib 12.5, 25, or 37.5 mg daily for 4 weeks as lead-in, then concurrently with and 4 weeks following XRT (75.6 Gy in 42 fractions to prostate and seminal vesicles). A 3+3 sequential dose-escalation design was employed to assess the frequency of dose-limiting toxicity (DLT) and establish a maximal tolerated dose (MTD) of sunitinib.
Results
Sunitinib at 12.5 and 25 mg dose-levels was well tolerated. The first 4 patients enrolled at 37.5 mg experienced a DLT during lead-in and a drug-interaction between sunitinib and bicalutamide was suspected. The protocol was revised and concurrent bicalutamide omitted. Of the next 3 patients enrolled at 37.5 mg, 2 of 3 on concurrent therapy experienced DLTs during radiation: Grade 3 diarrhea and Grade 3 proctitis respectively. Only 1/7 patients completed sunitinib at 37.5mg daily whereas 3/3 patients (25mg as starting dose) and 3/4 patients (25mg as reduced dose) completed therapy.
Conclusions
The feasibility of combined VEGFR/PDGFR inhibitor therapy, androgen deprivation, and radiation therapy for prostate cancer was established. Using a daily dosing regimen with lead-in, concurrent and post-XRT therapy, the recommended Phase 2 dose of sunitinib is 25mg daily.
Keywords: Sunitinib, androgen deprivation therapy, radiation, Phase I, high-risk prostate cancer
INTRODUCTION
Among prostate-specific antigen (PSA) screened populations, metastatic disease accounts for less than 5% of diagnoses [1] and a significant proportion of mortality from prostate cancer may be attributed to the failure to effectively control high-risk localized disease. A standard of care in this setting remains external beam radiation therapy in combination with androgen deprivation therapy (ADT) [2, 3]. Since long-term treatment failure rates with this strategy approach 50% or higher, significant improvements in treatment outcome are required. Local persistence of disease following radiation therapy appears to account for a significant fraction of treatment failures and a late wave of metastases [4]. Therefore, adjuvant therapeutics that enhance local control beyond those obtained with optimized dose-schedules of radiation therapy and luteinizing hormone releasing hormone (LHRH) agonists have the potential to improve long-term survival.
A principal mode of tissue damage from radiation therapy is the induction of endothelial apoptosis in tissue microvasculature [5] and since the proliferative rate of endothelial cells in tumors vary up to 35-fold higher than the proliferative rate of endothelial cells found in normal tissues, they can serve as a preferential target for radiation therapy [6]. The enhanced therapeutic index of radiation therapy with VEGFR/PDGFR inhibitors in a range of experimental tumors may be explained by priming tumor vasculature for radiation-induced endothelial apoptosis as well as limiting VEGF-dependent revascularization after radiation-induced injury [7]. Hypoxia is a major determinant of radiation resistance in tumor tissues and angiogenesis inhibitors can remodel tumor vasculature to reduce interstitial pressure, improve tumor blood flow and reduce hypoxia [8,9]. Direct effects of VEGFR/PDGFR inhibitors on autocrine and paracrine survival pathways generated by irradiated tumor cells and stroma contribute to the complexity of therapeutic effects [7].
Sunitinib is an orally bioavailable multi-tyrosine kinase receptor inhibitor with potent activity against VEGFR and PDGFR that has demonstrated enhanced efficacy of radiation therapy in experimental pre-clinical models [10–11]. Combined PDGFR and VEGFR inhibitor therapy facilitated optimal antiangiogenic and antiproliferative effects of radiation in prostate cancer and glioma models compared to either inhibitor alone [12]. The goal of the present study was to evaluate the feasibility of administering sunitinib in combination with ADT and external beam intensity-modulated radiation therapy in patients with localized high-risk prostate cancer. Based on the range of mechanistic concepts from preclinical models [7–12], a lead-in, concurrent and post-XRT dosing schedule of sunitinib was selected with ADT administered at least 8 weeks prior to XRT in the standard fashion. The primary endpoint was to define the safety and feasibility of three different dose-levels of sunitinib with this dosing-schedule and establish a Phase II dose for further study of efficacy.
METHODS
Patients
Patients were eligible to participate in this study if they had histologically confirmed adenocarcinoma of the prostate with any of the following high-risk features: clinical T2c, clinical or pathological T3 or T4 disease or Gleason 8–10 disease or PSA > 20ng/ml. Patients could not have metastatic disease as determined by bone scan and CT scan of the abdomen and pelvis. Additional inclusion criteria included an ECOG performance status of ≤1 and no standard contraindications to radiation therapy, chronically uncontrolled hypertension, left ventricular ejection fraction of <40%, calculated creatinine clearance < 35cc/min, an absolute neutrophil count < 1,500/mm3, platelets ≤ 100,000/mm3, AST/ALT > 2.5 × UNL, or an elevated total bilirubin. All patients provided written informed consent to participate in this trial, which was approved by the Institutional Review Boards at all participating institutions.
Study Design and Treatment (Figure 1)
Fig 1. Schematic of trial design and protocol treatment.
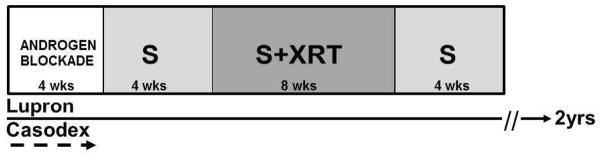
Pts were initiated on androgen blockade for 4 to 8 weeks (wks) prior to starting sunitinib (S). Oral S was administered for 4 wks prior to radiation (XRT) as a lead-in, concurrently with XRT for 8 weeks, and post XRT for 4 wks.
ADT
All patients were initiated on ADT with leuprolide acetate or goserelin acetate injections, administered every 12 weeks for a planned total of 2 years. When the trial was first opened, the use of an oral anti-androgen (preferably bicalutamide) concurrently with ADT was required for a period of at least 4 weeks to prevent a flare response. Subsequently, when an adverse interaction of bicalutamide with 37.5 mg of sunitinib was suspected in those patients among whom bicalutamide therapy was continued beyond the 4 week period, the protocol was amended such that administration of an oral anti-androgen could be used for a period not exceeding 14 days. In addition, the last day of anti-androgen administration had to be ≥ 14 days from the start of the first dose of sunitinib.
Sunitinib therapy
Treatment with sunitinib for a total of 16 continuous weeks starting 4 weeks before the initiation of radiation therapy (the “lead-in” period), during the 8 weeks of radiation therapy (the “concurrent” period) and 4 weeks beyond completion of radiation therapy (the “post” period) was planned. Three different dose levels of sunitinib were evaluated sequentially: 12.5mg daily, 25 mg daily and 37.5 mg using a standard 3+3 sequential dose-escalation design to assess the frequency of dose-limiting toxicity (DLT) and establish a maximal tolerated dose (MTD) of sunitinib.
Radiation therapy
External beam radiation therapy consisted of 75.6 Gy in 42 fractions delivered 5 days per week using IMRT. Gross target volume consisted of prostate and proximal seminal vesicles (defined as the portion from its origin with the prostate and extending 1 cm superiorly). The distal seminal vesicles (defined as that portion superior to the proximal seminal vesicles) received at least 50 Gy at 180 cGy per day. In cases of clinical or radiographic involvement, the seminal vesicle dose was taken to levels up to 75.6 Gy, in accordance with the tolerance parameters of the adjacent critical normal structures. Gross tumor volumes were expanded by 7–10mm in all directions except 4–7mm posteriorly to define PTVs. Dose limits to rectum were ≤80% receiving 30 Gy, ≤60% to 40 Gy, ≤20% to 70 Gy, ≤12% to 75.6 Gy, and ≤5% to 80 Gy. Other normal tissue constraints were: bladder to receive 70 Gy to no more than 20% of volume, femoral heads to receive 50 Gy to no more than 5% of volume, and sigmoid colon and small bowel maximum doses of 54 and 50 Gy, respectively.
Assessment of Definition of DLT During Combination Therapy
During the lead-in and 8 week period of combined ADT, sunitinib, and radiation therapy, sunitinib was to be held for any intolerable Grade 3–4 non-hematological or Grade 4 hematological toxicities. The DLT for the purposes of dose-escalation was defined as any Grade 3–4 non-hematological or Grade 4 hematological toxicity that failed to resolve to ≤Grade 2 within 7 days after the sunitinib was held or any toxicity that resulted in interruption of radiation therapy for more than 7 days. The MTD was defined as the dose-level of sunitinib above which 2 or more DLTs were identified and this was planned as the recommended phase II dose. Grade 3 hypertension that was controlled by antihypertensive therapy was not assessed as a DLT. In order to justify dose-escalation independent of toxicity confined to the lead-in period, DLTs for each dose-level were operationally defined as those events occurring during concurrent treatment with ADT, sunitinib, and radiation therapy. For this reason, patients who demonstrated intolerance to sunitinib during the 4 week “lead-in” to the degree that they that required dose-reduction or requested cessation of treatment were not assessed towards the MTD estimation at their starting dose-level. All toxicity events are nevertheless reported. All toxicities were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version3 (CTCAEv3.0). Acute toxicities were defined as those occurring less than 90 days from the first day of radiation therapy, and late toxicities were defined as those occurring more than 90 days from the first day of radiation therapy.
RESULTS
Patient Characteristics
A total of 17 patients were enrolled on this study between May 2008 and September 2011 (Table 1). The median age was 65 years (range 52–82 years) and the median ECOG performance status was 0 (range 0 to 1). The median PSA was 17 ng/ml (range 3.4 to 136.8 ng/ml) and the median Gleason score was 9 (range 7 to 9). The clinical stage was T1c in 5 patients (30%), T2a in 1 patient (6%), T2b in 1 patient (6%), T2c in 6 patients (35%), and T3b in 4 patients (23%).
Table 1.
Patient Characteristics and PSA Outcomes.
| Pt | Age | Race | PS | BASELINE | ON TREATMENT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Gleason | Stage | PSA | Pre-S | Pre-XRT | Post-XRT | End-S | Nadir | Last | Months | Failure | ||||
| 1 | 74 | C | 1 | 9 | T3B | 3.4 | 2.8 | <0.1 | <0.1 | NA | <0.1 | <0.1 | 50.1 | No |
| 2 | 82 | C | 0 | 9 | T2C | 5.4 | 3.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 32.7 | No |
| 3 | 78 | C | 0 | 8 | T1c | 22.8 | 1.1 | 0.3 | 0.1 | <0.1 | <0.1 | <0.1 | 37.9 | No |
| 4 | 66 | C | 0 | 8 | T3B | 20.5 | 8 | 0.4 | 0.1 | <0.1 | <0.1 | <0.1 | 31.4 | No |
| 5 | 55 | C | 0 | 9 | T3B | 207 | 7 | 6 | 1 | 0.4 | <0.1 | <0.1 | 35.1 | No |
| 6 | 61 | C | 0 | 9 | T2B | 83.2 | 3.6 | 3.4 | 1.5 | 0.8 | 0.2 | 10.3 | 28.9 | Yes |
| 7 | 77 | AA | 1 | 8 | T2A | 36.3 | 2.2 | 0.5 | 0.1 | 0.1 | <0.1 | <0.1 | 6.7 | No |
|
| ||||||||||||||
| 8 | 52 | C | 0 | 9 | T2C | 136.8 | 30 | 21.5 | 3.8 | 3.5 | 2.6 | 9.8 | 13.3 | Yes |
| 9 | 52 | C | 0 | 7 | T2C | 32.6 | 18.6 | 18.4 | 1.2 | 0.9 | 0.2 | 0.2 | 22.5 | No |
| 10 | 62 | C | 0 | 8 | T1C | 18.1 | 3.7 | 0.9 | 0.2 | 0.1 | <0.1 | <0.1 | 24.7 | No |
|
| ||||||||||||||
| 11 | 59 | C | 0 | 9 | T1C | 11.3 | 1.4 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 19.6 | No |
| 12 | 61 | C | 0 | 9 | T1C | 8.3 | 2.6 | 0.9 | 0.2 | <0.1 | <0.1 | <0.1 | 18.7 | No |
| 13 | 62 | C | 0 | 8 | T2C | 4.2 | 1.1 | 0.2 | 0.1 | 0.2 | <0.1 | <0.1 | 16.9 | No |
| 14 | 76 | C | 0 | 9 | T2C | 4.4 | 0.8 | NR | NR | NR | <0.1 | <0.1 | 15.7 | No |
| 15 | 79 | C | 0 | 7 | T3 | 3.7 | 0.4 | 0.5 | 0.1 | 0.1 | <0.1 | <0.1 | 16.3 | No |
| 16 | 72 | C | 0 | 9 | T2C | 17 | 3.2 | 1.9 | 1.2 | 0.2 | <0.1 | <0.1 | 12.5 | No |
| 17 | 65 | C | 0 | 9 | T1C | 3.5 | 2.1 | 0.2 | <0.1 | <0.1 | <0.1 | <0.1 | 14.8 | No |
| dose (mg) |
|---|
| 12.5 |
| 25 |
| 37.5 |
C=Caucasian, AA=African American, Last=PSA at last follow up, Months=time on study, NR=none recorded, NA= Not applicable, Failure= biochemical failure [RTOG-ASTRO PHOENIX definition].
Quantitative Toxicity
All 17 patients received androgen ablation and were initiated on sunitinib (Table 2). Of the 17 patients, 7 were initiated on dose 12.5 mg sunitinib, 3 patients on 25 mg sunitinib, and 7 patients on 37.5 mg sunitinib. The first patient enrolled at 12.5 mg sunitinib experienced grade 4 fatigue during lead-in sunitinib. This patient did not receive any further sunitinib, recovered fully to complete ADT and radiation therapy without further limiting adverse events. The next 6 patients enrolled at 12.5 mg sunitinib completed protocol treatment without DLTs. The next 3 patients enrolled at 25 mg sunitinib completed protocol treatment without DLTs. The first 4 patients enrolled on 37.5 mg sunitinib unexpectedly experienced significant clinical toxicity during the lead-in phase. Specifically, with 2 events of Grade 3 neutropenia and a third event of Grade 3 thrombocytopenia, a drug-interaction with bicalutamide was suspected. Although prior drug interaction between bicalutamide and sunitinib has not been reported, in vitro data suggest that (R)-bicalutamide has the potential to inhibit CYP3A4 (13) which is the major metabolic pathway for sunitinib, resulting in increased plasma sunitinib levels. Of these first 4 patients, 3 were dose reduced to 25 mg and successfully completed protocol treatment without any DLTs with radiation therapy, while 1 patient withdrew from study during lead-in, recovered and completed ADT and radiation without further limiting adverse events. The protocol was revised to omit concurrent bicalutamide with sunitinib and accrue additional patients to a new 37.5mg dose-level.
Table 2.
Quantitative Toxicity by Sunitinib (S) Dose-level: pts (patients); LI (Lead-in); DLT (dose-limiting toxicity), Hem (hematological), GI (gastrointestinal);
| Sunitinib dose | # pts | LI Tox | Withdrew | Completed S+XRT | Post-XRT S completed | S Dose-reduced | DLT (S+XRT) | Grade/Type (n) |
|---|---|---|---|---|---|---|---|---|
| 12.5mg | 7 | 1 | 1 | 6 | 6 | 0 | 0 | G4 Fatigue (1) |
| 25mg | 3 | 0 | 0 | 3 | 3 | 0 | 0 | - |
| 37.5mg | 4 | 4 | 1 | 3 | 3 | 3 | 0 | G3 Hem(3) G2 Hem(1) |
| 37.5mg* | 3 | 0 | 0 | 3 | 2 | 2 | 2 | G3 GI (n=2) G3 Fatigue (n=1) |
| Totals | 17 | 5 | 2 | 15 | 14 | 5 | 2 | 8 events (7 pts) |
without concurrent bicalutamide.
As expected, of the next 3 patients subsequently enrolled at sunitinib 37.5 mg daily without concurrent bicalutamide, there were no lead-in toxicities. However, 2 of 3 experienced DLTs during radiation therapy with concurrent sunitinib 37.5mg daily. The first patient experienced abrupt onset Grade 3 diarrhea early in the first week of radiation therapy which was associated with biochemical evidence of hyperthyroidism. The second patient developed Grade 3 proctitis in the fifth week of radiation therapy. Both patients were dose-reduced to 25 mg and resumed treatment at recovery. The first patient went on to complete radiation therapy but discontinued the 25mg sunitinib on day 14 post radiation therapy for Grade 3 fatigue. The second patient went on to complete protocol treatment without any further toxicity.
Qualitative Toxicities
There were no reported toxicities related to arterovenous thromboembolic events, congestive heart failure, gastrointestinal perforation, or fistulas. The most common acute toxicities as described in Table 3 were fatigue, neutropenia, anemia and hypertension. The most common late toxicities were fatigue and hypertension occurring in 12% of patients, with Grade 1 fatigue in two patients, Grade 1 hypertension in one patient and Grade 2 hypertension in a second patient. Grade 2 radiation proctitis occurred in one patient (6%) and Grade 3 cerebrovascular accident in one patient (6%). The patient with the cerebrovascular accident made a complete recovery. To date, there is no evidence of late-emerging bowel or bladder toxicity.
Table 3.
Qualitative Toxicity
| Acute Toxicity Events | ||||||
|---|---|---|---|---|---|---|
| Acute Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | # | % |
| Neutropenia | 2 | 8 | 2 | 0 | 12 | 70 |
| Thrombocytopenia | 0 | 3 | 1 | 0 | 4 | 24 |
| Hypertension | 2 | 1 | 3 | 0 | 6 | 35 |
| Hemoglobin | 10 | 0 | 0 | 0 | 10 | 58 |
| Nausea | 1 | 2 | 0 | 0 | 3 | 17 |
| Vomiting | 1 | 1 | 0 | 0 | 2 | 12 |
| Diarrhea | 0 | 1 | 1 | 0 | 2 | 12 |
| Fatigue | 14 | 0 | 2 | 1 | 17 | 100 |
| Proctitis | 0 | 0 | 3 | 0 | 3 | 17 |
| Hemorrhoids | 2 | 0 | 0 | 0 | 2 | 12 |
| Renal Insufficiency | 2 | 2 | 0 | 0 | 2 | 12 |
Treatment Outcomes
Treatment outcomes are juxtaposed with baseline risk characteristics in Table 1. The median post-treatment nadir PSA was <0.1 ng/ml (range <0.1 to 2.6 ng/ml) at a median follow up of 19.6 months (range 6.7 to 50.1 months). All patients have been followed for over 12 months except one lost to follow-up after 6.7 months. Two patients who had Gleason 9 disease, cT2b/c disease and PSA values of 83 and 136ng/ml experienced biochemical failure according to the RTOG-ASTRO PHOENIX definition (14) at 29 and 13 months respectively. Of the remaining 13/15 patients who completed concurrent sunitinib and XRT, the last follow-up post-treatment PSA value was <0.1ng/ml in 12/13 and 0.2ng/ml in 1/13 patients.
Discussion
Utilizing a lead-in, concurrent and post-XRT schedule of daily administration of an angiogenesis inhibitor, we have established the safety and feasibility of combination VEGFR and PDGFR inhibitor therapy with ADT and XRT in localized high-risk prostate cancer. The recommended Phase II dose for further study of this therapeutic strategy is 25mg daily. Evidence for the biological activity of the 25mg daily dose was suggested by the high-frequency of Grade 1–2 myelosuppression, fatigue and/or hypertension (15). Long-term follow-up and a larger experience at the recommended Phase II dose will be required to establish a fuller picture of the safety and efficacy of this therapeutic strategy. To date, with a median follow up of 19.6 months (range 6.7 to 50.1 months), there is no evidence of late-emerging bowel or bladder toxicity. In the absence of credentialed biomarkers that can predict efficacy of a VEGFR/PDGFR inhibitor with ADT and XRT, randomized Phase II clinical trials are required for an estimate of efficacy.
The toxicities observed at the MTD of sunitinib at 37.5mg daily may be regarded in 3 categories. The first category describes systemic toxicities identified during the lead-in period principally related to those known to be associated with sunitinib and essentially these systemic toxicities were the most significant observed on study. In addition to elimination of concurrent bicalutamide and sunitinib therapy for a suspected drug interaction, a switch from ACE-inhibitor to a calcium-blocker for control of hypertension also ameliorated sunitinib-related Grade 2 azotemia and myelosuppression with enhanced blood pressure control. The second category of toxicities refers to those observed during the concurrent administration with XRT. One patient experienced Grade 3 diarrhea associated with profound fatigue and weakness. The unusually early onset of diarrhea within the first days of XRT raised doubts of a true association with XRT toxicity. Laboratory monitoring revealed the concordant onset of biochemical hyperthyroidism from sunitinib which may have been a contributing factor. Anti-motility agents were not used early in the course of the diarrhea to control these symptoms and it is conceivable that the severity of the symptoms may have been significantly mitigated had they been employed. A second patient experienced Grade 3 gluteal fold skin reaction in the 5th week of radiation therapy which necessitated a treatment interruption. There were no associated irritative bowel or bladder symptoms. The third category refers to toxicities in the post-XRT period. Only one patient who had been dose-reduced for Grade 3 diarrhea at 37.5mg daily to 25mg daily at recovery could not complete the planned 4-week post-XRT sunitinib therapy on account of recrudescent Grade 3 fatigue and failure to thrive. Furthermore, this was the only limiting toxicity observed at the 25mg dose-level. There were no other significant toxicities that were specifically observed in the post-XRT period in patients completing the study.
A recent Phase II study of the VEGF monoclonal antibody, bevacizumab, in combination with ADT and XRT in localized high-risk prostate cancer provides an important benchmark for the comparison of potent angiogenesis inhibitor strategies in combination with radiation therapy (16). Eighteen patients with cT2b-T4, Gleason 8–10 or PSA ≥20ng/ml and Gleason 7 disease were treated with ADT (bicalutamide and goserelin) and bevacizumab 10mg/kg every 2 weeks for 8 weeks and then concurrently in the same dose-schedule with XRT (77.9 Gy, 64.6 Gy, 57Gy to the prostate, seminal vesicles and regional nodes respectively over 38 fractions). After XRT, bevacizumab was continued at 15mg/kg every 3 weeks for 12 weeks and ADT (goserelin alone) for 2 years. The median PSA nadir was 0.01ng/ml at a median of 6.5 months and no patients have had biochemical failure by the ASTRO definition. As with sunitinib, hypertension and bone marrow suppression (leukopenia) were common Grade 2/3 acute toxicities, occurring in 72 and 28% of patients, respectively, and there were no Grade 4/5 toxicities, arterial/venous thromboses or gut perforations. Clinically significant late toxicities were noted in bevacizumab treated patients including Grade 4 prostatitis with prostatic abscess (n=1), Grade 3 radiation proctitis (n=1) and Grade 3 radiation proctitis and cystitis (n=1) with onset at 9, 13.5 and 18 months after completion of radiation therapy respectively. In addition, late bleeding complications were seen in 50% (n=9) patients; 3 with Grade 1epistaxis, 3 with Grade 1–2 hematuria and 3 with Grade 2–3 rectal bleeding. The authors concluded that late toxicities with this regimen were concerning and that in future studies a modified bevacizumab dose-schedule could mitigate such toxicity. While the conservative sunitinib dose-escalation schema together with the 4 week pre-radiation lead-in period that permitted dose-reductions for toxicity may have mitigated the emergence of late toxicities in our trial, longer follow up is required.
Improving the efficacy of radiation therapy is a major research imperative in prostate cancer. The current plateau in survival improvement obtained with traditional medical castration with LHRH agonists with XRT may be surpassed by the integration of novel hormonal therapeutics like abiraterone and enzalutamide that have enhanced castration effects in the tumor microenvironment. Aside from angiogenesis inhibitors, a growing number of non-hormonal therapeutics such as c-met (17) and src (18) inhibitors also deserve study as these agents may enhance the efficacy of ADT and radiation therapy through disruption of growth promoting epithelial-stromal interactions. Moving forward, the field requires a collective strategy to accelerate the study of such agents to improve efficacy while minimizing toxicity in patients with potentially curable disease.
Towards this goal, there is a need to develop biomarkers of antitumor efficacy that elucidate the complex effects of novel targeted agents on the tumor microenvironment. Blood soluble markers of angiogenesis inhibition are promising but have not been prospectively validated and experimental imaging techniques to detect blood flow or hypoxia are expensive and difficult to routinely incorporate into clinical trials. Despite earlier controversies, a candidate surrogate of efficacy worthy of further study is the template prostate biopsy obtained at the 2-year landmark after XRT. Residual tumor without evidence of treatment effect may strongly predict progression-free survival and less convincingly, metastasis-free and overall survival (19). In patients with high-risk localized disease treated with ADT and XRT, up to 35% of prostate biopsies will be positive for tumor at 2 years (20). Given the prolonged natural history of the disease state, accurate biomarkers of tumor fate derived from early landmark tissue sampling have the potential to accelerate the necessary integration of novel therapeutics such as sunitinib in combination with ADT and XRT.
Summary.
Angiogenesis inhibitors targeting the vascular endothelial growth factor receptor and platelet-derived growth factor receptor may improve the therapeutic index of ionizing radiation. In a Phase I trial of sunitinib in combination with androgen deprivation and external beam radiation therapy, the feasibility of this approach in localized high-risk prostate cancer was established and a Phase II dose recommended for further study.
Acknowledgments
This study was supported by Pfizer, Robert Lawrence Fund for Prostate Cancer Research and the Department of Defense funded Prostate Cancer Clinical Trials Consortium.
Footnotes
Previously presented at the 2012 American Association for Cancer Research Annual Meeting, in Chicago, IL.
There are no conflicts of interest to be disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paquette EL, Sun L, Paquette LR, et al. Improved prostate cancer-specific survival and other disease parameters: impact of prostate-specific antigen testing. Urology. 2002;60:756–759. doi: 10.1016/s0090-4295(02)01960-x. [DOI] [PubMed] [Google Scholar]
- 2.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomized trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 3.Pilepic MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive therapy in prostate carcinoma – long term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–90. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20:3199–205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 5.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 6.Denekamp J, Hobson B. Endothelial-cell proliferation in experimental tumours. Br J Cancer. 1982;46:711–720. doi: 10.1038/bjc.1982.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clinical Cancer Research. 2003;9:1957–1971. [PubMed] [Google Scholar]
- 8.Teicher A, Dupis N, Kusumoto T, et al. Antiangiogenic agents can increase tumor oxygenation and response to radiation therapy. Radiat Oncol Investig. 1995;2:269–276. [Google Scholar]
- 9.Rockwell S, Dobrucki IT, Kim EY, et al. Hypoxia and radiation therapy: past history, ongoing research and future promise. Curr Mol Med. 2009;9:442–458. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheuneman AJ, Himmelfarb E, Geng L, et al. SU11248 maintenance therapy prevents tumor regrowth after fractionated irradiation of murine tumor models. Cancer Research. 2003;63:4009–4016. [PubMed] [Google Scholar]
- 11.Brooks C, Sheu T, Bridges K, Mason K, Kuban D, Mathew P, Meyn R. Preclinical evaluation of sunitinib, a multi-tyrosine kinase inhibitor, as radiosensitizer for human prostate cancer. Radiation Oncology. 2012;7:154. doi: 10.1186/1748-717X-7-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timke C, Zieher H, Roth A, et al. Combination of vascular endothelial growth factor receptor/platelet-derived growth factor receptor inhibition markedly improves radiation tumor therapy. Clin Cancer Res. 2008;14:2210–2219. doi: 10.1158/1078-0432.CCR-07-1893. [DOI] [PubMed] [Google Scholar]
- 13.Cockshott ID. Bicalutamide: clinical pharmacokinetics and metabolism. Clin Pharmacokinetics. 2004;43:855–78. doi: 10.2165/00003088-200443130-00003. [DOI] [PubMed] [Google Scholar]
- 14.Roach M, 3rd, Hanks G, Thames H, Jr, Schellhammer P, Shipley WU, Sokol GH, Sandler H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–73. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuky J, Pham HT, Warren S, et al. Phase II study of long-term androgen suppression with bevacizumab and intensity-modulated radiation therapy (IMRT) in high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:e609–15. doi: 10.1016/j.ijrobp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 17.De Bacco F, Luraghi P, Medico E, Reato G, Girolami F, Perera T, Gabriel P, Comoglio PM, Boccaccio C. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst. 2011;103:645–661. doi: 10.1093/jnci/djr093. [DOI] [PubMed] [Google Scholar]
- 18.Fizazi K. The role of Src in prostate cancer. Annals of Oncology. 2007;18:1765–1773. doi: 10.1093/annonc/mdm086. [DOI] [PubMed] [Google Scholar]
- 19.Crook JM, Malone S, Perry G, et al. Twenty-four month post-radiation biopsies are strongly predictive of 7-year disease-free survival. Cancer. 2009;115:673–679. doi: 10.1002/cncr.24020. [DOI] [PubMed] [Google Scholar]
- 20.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Eng J Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]


