Abstract
Background
Maternal smoking during pregnancy is correlated with increased substance use in offspring. Research using rodent models shows that gestational nicotine exposure produces enduring alterations in the neurodevelopment of motivational systems, and that rats prenatally treated with nicotine have altered motivation for drug reinforcement on fixed-ratio (FR) schedules of reinforcement. Objective: The present study investigated methamphetamine (METH) self-administration in adult offspring prenatally exposed to intravenous (IV) nicotine or saline using a progressive-ratio (PR) schedule of reinforcement.
Methods
Pregnant rats were administered IV prenatal saline (PS) or nicotine (PN; 0.05 mg/kg/infusion), 3x/day during gestational days 8–21. At postnatal day 70, offspring acquired a lever-press response for sucrose (26%, w/v; FR1-3). Rats were trained with METH (0.05 mg/kg/infusion), and following stable FR responding, animals were tested using a progressive-ratio (PR) schedule for three different doses of METH (0.005, 0.025, and 0.05 mg/kg/infusion).
Results
METH infusion, active lever presses, and the ratio breakpoint are reported. PN-exposed animals exhibited more METH-maintained responding than PS controls, according to a dose × prenatal treatment interaction (e.g., infusions). PN rats self-administered more METH infusions between the range of 0.025 and 0.05, but not for the 0.005 mg/kg/infusion dose.
Conclusions
IV PN-exposure produced enhanced motivation to self-administer METH. These findings indicate that pregnant women who smoke tobacco may impart neurobiological changes in offspring’s motivational systems that render them increasingly vulnerable to drug abuse during adulthood.
Keywords: methamphetamine, prenatal, nicotine, rat, self-administration, motivation
1. INTRODUCTION
The incidence of substance abuse is higher in offspring who experienced prenatal tobacco smoke exposure. The initial clinical research demonstrated an association between maternal tobacco smoking and nicotine abuse in offspring (Buka et al., 2003; Cornelius et al., 2009; Kandel et al., 1994), and more recent findings extend this effect to multiple substances of abuse (Goldschmidt et al., 2012). In that study, adolescents exposed to tobacco smoke during gestation were 1.5× more likely to initiate drug use with multiple substances, i.e., tobacco, marijuana, and alcohol, compared to non-exposed controls (Goldschmidt et al., 2012). Moreover, activation of the ventral striatum, in anticipation of reward, was greater in offspring of mothers who smoked during pregnancy relative to controls (Muller et al., 2013), suggesting that maternal tobacco smoke exposure altered the development and functionality of the mesocorticolimbic dopamine system in those individuals (Kandel et al., 1994). Together, these data add to a growing body of literature linking maternal smoking with an increased vulnerability for substance abuse in offspring.
Preclinical models of prenatal nicotine (PN) exposure provide converging evidence that nicotine alone produces enduring effects on the behavior of offspring (Paz et al., 2007; Popke et al., 1997; Schneider et al., 2010; Slotkin, 1998; Tizabi et al., 1997). Of interest here are the findings that offspring of PN exposure exhibit alterations in motivation for drug reinforcement. Previous experiments that investigated the effects of PN and drug self-administration in offspring utilized a continuous PN exposure model (Franke et al., 2008; Levin et al., 2006). Adolescent offspring of continuous PN or prenatal saline (PS) exposure responded for nicotine on a fixed-ratio (FR) schedule of reinforcement. PN rats self-administered more nicotine than PS controls following a period of abstinence (Levin et al., 2006). Also, adolescent PN-exposed offspring required a higher dose of intravenous (IV) cocaine to acquire FR responding, and under the same continuous reinforcement schedule, PN offspring self-administered more cocaine than PS controls (Franke et al., 2008). These findings show that adolescent rats exposed to continuous PN exhibited altered responding for nicotine and cocaine, relative to controls, when FR schedules of reinforcement were used.
Our laboratory utilizes an IV model of PN exposure in order to mimic the absorption pharmacokinetics of inhaled nicotine in dams (Benowitz et al., 2009). In a recent study, adult offspring of IV PN and PS were trained to self-administer methamphetamine (METH) according to a FR3 schedule of reinforcement (Harrod et al., 2012). Once the animals met the response criterion, they were tested on various unit doses of METH (0.0005, 0.0025, 0.005, 0.025 and 0.05 mg/kg/infusion). The PN and PS rats’ pattern of responding represents an inverted “U”-shaped dose response curve that is characteristic for amphetamines (Yokel, 1987); however, the PN animal’s curve was shifted to the left, indicating that they were more sensitive to the reinforcing effects of METH compared to saline controls (Harrod et al., 2012). In a separate experiment, we assessed sucrose concentration response curves (0, 3, 10, 30, and 56% sucrose) with the FR3 schedule, using a similar approach as described above. The PS and PN rats’ response pattern was an inverted U-shaped sucrose concentration curve, however, there were no statistical differences attributable to prenatal treatment (Lacy et al., 2012). The motivation to respond for the same concentrations of sucrose was subsequently tested using a progressive-ratio (PR) schedule of reinforcement (PR; Richardson and Roberts, 1996), and in that experiment, PN rats exhibited greater motivation to take sucrose reinforcement relative to PS animals (Lacy et al., 2012). In the present study, we tested the hypothesis that offspring of IV PN exposure would exhibit greater motivation for METH on a PR schedule of reinforcement. Thus, after control and treated rats exhibited stable METH self-administration on the FR3, they were tested on various doses of METH using the PR schedule. The measures of METH infusion, ratio breakpoint, and active lever responding are reported.
2. METHODS
2.1 Animal
A total of 60 female and 30 male adult, nulliparous Sprague-Dawley rats were purchased from Harlan Industries, Inc. (Indianapolis, IN). All rats were transported to the animal care facilities in the psychology department at the University of South Carolina and rodent food (ProLab Rat/Mouse/Hamster Chow 3000) and water were provided ad libitum throughout the course of the experiments, except when otherwise specified. All animal cages were provided with a Nylabone (Nylabone, Inc.; long lasting durable chew-original; Neptune, NJ) and Nestlets (NestletsT; Ancare, Bellmore, NY) throughout the duration of the study. A Nylabone was replaced if it was thoroughly chewed, and one Nestlet nesting product was placed in the animals’ cage when the cage was changed, which occurred 2×/week. The animal colony was maintained at 21 ± 2°C, 50% ± 10% relative humidity and a 12L:12D cycle with lights on at 0700 h. The protocol for this research methodology was approved by the Institutional Animal Care and Use Committee at the University of South Carolina.
2.2 Internalized jugular catheter surgeries
The catheterization procedure was performed at Harlan Industries (Indianapolis, IN), prior to arrival in the colony at the University of South Carolina, according to the methods of Mactutus et al. (1994). In brief, animals were anesthetized with a mixture of ketamine hydrochloride (100 mg/kg/ml) and xylazine (3.3 mg/kg/ml). Following anesthesia, a sterile Intracath IV catheter (Becton, Dickinson and Co., Franklin Lakes, NJ) with a Luer-Lok injection cap (Medex, Inc., Carlsbad, CA) was implanted dorsally in a subcutaneous pouch. The distal end of the catheter was inserted into the left jugular vein, advanced toward the heart, and then bound with a sterile suture. Animals were kept under periodic postoperative observation and returned to the colony upon recovery. On the day following surgery, catheters were flushed with 0.2 ml of heparinized saline to help maintain catheter patency.
2.3 Breeding
After arrival, all animals habituated to the colony for 7 days. Following this period, female rats were housed three per cage and one male was placed in the cage with the females, overnight, from approximately 1700 to 0900 for the purposes of breeding. Females were lavaged each morning to obtain vaginal samples, which were analyzed under a microscope to determine the results of breeding from the previous night. Following positive identification of sperm within a sample, the corresponding female was single-caged and that day was considered GD 0. The weight of the pregnant dams was recorded daily throughout pregnancy.
2.4 IV PN treatment
Pregnant dams were randomly assigned to either the PN (0.05 mg/kg/injection) or PS groups. Nicotine or saline was administered 3×/day via internalized IV catheters from GD 8–21. Intravenous injections were delivered through the Luer-Lok injection cap of the subcutaneously implanted injection port (20 seconds in duration). Following the first two daily injections, catheters were flushed with 0.2 ml of saline to flush the entirety of the catheter volume. Heparinized saline (2.5%) was used to flush the catheter after the third and final daily IV injection, to maintain catheter patency. All post-flush injections were 20 seconds in duration. The IV injections were performed during the light portion of the photoperiod, at approximately 1000, 1300, and 1600.
2.5 Surrogate fostering, litter composition, and postnatal testing
The day of birth was considered postnatal day (PND) 0. On PND 1, litters were culled to 10 pups with 5 males and 5 females whenever possible. All pups were surrogate-fostered to timed-pregnant, drug naïve dams on PND 1 to prevent poor maternal care (Vorhees, 1986). The developmental milestones of the righting reflex, negative geotaxis, and eye opening were assessed on PND 3–5, 8–10, and 13–17, respectively (Heyser, 2004). For all experiments, only one male and one female randomly selected from each litter were assigned to each treatment group (Holson and Pearce, 1992). The righting reflex was assessed by placing animals on their backs; upon releasing the animals, the latency to right themselves to their stomachs was recorded. The righting reflex was assessed in blocks of 3 trials across 3 consecutive days with a maximum latency of 25 seconds per trial. Negative geotaxis testing, consisted of placing animals on a wire mesh grid positioned on a 25° downward angle. The latency (30 seconds maximum) for animals to turn 180° to face up the slope was recorded. Negative geotaxis was measured in blocks of 3 trials across 3 consecutive days. The eyes of each animal (left and right) were checked for degree of openness across 5 consecutive days. The degree of openness was rated on a scale of 0–3: 0 = completely closed; 1 = any opening exposing the cornea; 2 = cornea and pupil exposed but eye lids are not fully open; 3 = fully open. All animals’ weights were recorded on PND 1, 7, 14, and 21. Rats were weaned and pair housed, same sex, on PND 21.
2.6. Operant Conditioning
2.6.1 Apparatus
Operant chambers (ENV-008; Med-Associates, St. Albans, VT), housed within sound-attenuating enclosures, were controlled by Med-PC computer interface software. The front and back panels of the chamber were stainless steel and the sides and top were constructed of polycarbonate. The front panel of the chamber allowed access to a recessed food dipper (ENV-202M) through a 5 × 5 cm opening. Two retractable metal levers (ENV-112BM) on either side of the magazine were located 7.3 cm above a metal grid floor. A dipper equipped with a 0.1 ml cup attached to the end of the arm was raised into the food receptacle, which allowed access to liquid sucrose following the completion of response requirements. A 28 V white cue light, 3 cm in diameter, located above each response lever, was used to signal timeouts. An infrared sensor (ENV-254-CB) was used to detect head entries into the magazine. During drug self-administration sessions a syringe pump (PHM-100) was used to deliver intravenous infusions through a water-tight swivel (PHM-115).
2.6.2 Preliminary training
Thirty-seven male and female, adult (PND 90+) animals, representing 23 litters, were used as subjects for this experiment. The groups were PN-exposed males (n = 8) and females (n = 10), and PS-exposed males (n = 9) and females (n = 10). Rats were food restricted (and housed singly) in order to maintain 85% of free-feeding weight for three days prior to the beginning of dipper training. Dipper training and autoshaping were conducted according to previous research (Harrod et al., 2012; Lacy et al., 2012; Reichel et al., 2008). Animals learned the lever-press response for 26% (w/v) sucrose. Following acquisition, animals responded for 26% sucrose, during 30-minute sessions, for one day, on the FR1 schedule; and then for two consecutive days on the FR3 schedule (Harrod et al., 2012). All animals learned the operant response during these three fixed-ratio sessions, prior to undergoing catheterization surgery. All operant conditioning and testing was performed during the light portion of the photoperiod.
2.6.3 Catheterization surgery for the drug self-administration procedure
Animals were allowed to feed without restriction for 5 days before undergoing catherization surgery. Rats were anesthetized with ketamine (100 mg/ml) and diazepam (5 mg/ml) then implanted with a catheter that was secured to the right jugular vein, which exited through a dental acrylic headmount. Headmounts were secured to the skull with metal screws according to the methods of Harrod et al. (2001). Animals were allowed to recover from surgery for a minimum of 6 days, during which time the catheters were flushed with heparinized saline (0.2%). Animals were monitored daily to ensure appropriate recovery.
2.6.4 Fixed-ratio training with METH
During 60-minute sessions both right and left levers were presented in the operant chamber. During FR1 training each response on the active lever resulted in a 5.9 second, IV infusion of METH (0.05 mg/kg/infusion), which delivered approximately 0.1 ml of drug. Thus, the concentration of METH was based on the animal’s weight so that each animal received approximately the same mg/kg dose. Responding on the inactive lever was recorded, but not reinforced. Each drug infusion was followed by a 20-second signaled timeout (both cue lights on) during which time the animals were not reinforced on either lever. The rats were maintained on the FR1 schedule for a minimum of 5 days or until a pattern of stable responding was exhibited and then they were transitioned to the FR3 schedule of reinforcement for a minimum of two days. Stable responding was operationally defined as less than 20% variability in active lever responding across two consecutive sessions, greater than 2:1 ratio of active to inactive responses, and a minimum of 10 infusions per session (Harrod et al., 2012). Assignment of the active lever (left or right) was balanced across groups and remained the active lever throughout the experiment for each animal.
2.6.5 Progressive-ratio testing
After animals displayed two consecutive days of stable responding on the FR3 schedule, animals began testing on the PR schedule with three different concentrations of METH. The concentrations were 0.005, 0.025, and 0.05 mg/kg/infusion, and they were presented according to a Latin-square procedure. Animals were returned to an FR3 schedule and responded for the training dose of METH for a minimum of two days between each PR test. The PR sessions were a maximum of 10 hours in length. Response requirements on the PR schedule were: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603…2012 according to the methods of Richardson and Roberts (1996). Both cue lights were illuminated for 20 seconds following completion of each ratio requirement. No responses were reinforced during the 20-second timeout. Progressive-ratio sessions ended prior to 10 hours if a ratio requirement was not met within an hour of previous reinforcement (Chiodo and Roberts, 2009).
2.7 Drugs
Nicotine hydrogen tartrate and METH hydrochloride were purchased from Sigma-Aldrich Pharmaceuticals (St. Louis, MO). Nicotine (base weight) and METH (salt weight) were dissolved in physiological saline (0.9%; Hospira, Inc. Lake Forest, IL). The pH of the nicotine solution was neutralized to ~ 7.0 with NaOH. Heparin (APP Pharmaceuticals, Schaumburg, IL) was added to saline and the heparinized saline solution (2.5%) was used to flush the IV catheters.
2.8 Data analysis
2.8.1 Litter Parameters. Maternal weight gain; pup weight gain; ratio of male to female pups; righting reflex, and negative geotaxis
Analysis of variance (ANOVA) techniques were used (SPSS 20, IBM). The between-subjects factors were Sex and Prenatal Treatment, and the within-subjects factors were PND and GD. A one-way analysis of variance (ANOVA) was conducted for the total number of pups born to PN and PS dams. A Sex × Prenatal Treatment factorial ANOVA was used to analyze the ratio of males to females born to nicotine treated and control dams. A Sex × Prenatal Treatment × PND mixed-factorial ANOVA was conducted for the pup weight gain, righting reflex, negative geotaxis and eye opening data. A Prenatal Treatment × GD mixed-factorial ANOVA determined if there were differences between nicotine treated and control dams on the measure of maternal weight gain.
2.8.2 Acquisition of sucrose-maintained responding using FR schedules of reinforcement
The dependent measure was active lever responding. Fisher’s exact tests were used to assess the rate that PN and PS rats acquired operant responding on the FR1 and FR3 schedules (Siegel and Castellan, 1988).
2.8.3 Acquisition of METH self-administration using FR schedules of reinforcement
The statistical approach used for the sucrose-maintained responding was also applied for METH self-administration on the FR1 and FR3 schedules of reinforcement.
2.8.4 Tests of motivation for METH: PR schedule of reinforcement
METH infusion, breakpoint, and active lever responding were the dependent measures. Data were analyzed using a 2 × 2 × 3 mixed-factorial ANOVA. The between-subjects factors were Prenatal Treatment (2) and Sex (2), and the within-subjects factor was Dose (3). If the analysis revealed a nonsignificant main effect of Sex, and no interactions with the factor of sex, a subsequent Prenatal Treatment (2) × Dose (3) ANOVA was reported. Infusion, ratio breakpoint, and active lever data were analyzed. The Greenhouse-Geisser (G–G) correction for violations of sphericity was reported for main effects and interactions that contain the factor of Dose (i.e., significant Mauchly’s tests). Student’s t-tests were used for post hoc comparisons and the alpha level was adjusted for multiple comparisons (i.e., three comparisons equals an alpha level of 0.017). An alpha level of 0.05 was used to determine statistical significance for all other analyses.
3. RESULTS
3.1 Litter Parameters: Maternal weight gain; pup weight gain; ratio of male to female pups; righting reflex, and negative geotaxis
The weight for all dams increased throughout the gestational period, as indicated by the significant main effect of GD [F(3, 81) = 866.49, p<0.001]; however, there was no significant main effect of maternal treatment and no interaction between maternal treatment and GD. Analysis of the pup weight gain data resulted in a significant main effect of PND [F(3, 702) = 13992.42, p<0.001]. A significant main effect of Sex [F(1, 240) = 5.41, p<0.05] indicates that males gained slightly more weight than females [mean ±SEM: 18.79 ± 0.16 and 18.30 ± 0.15, respectively]; there was neither an effect of Prenatal Treatment nor a Prenatal Treatment × Sex interaction. There were no significant differences between PS and PN litters on the measures of male to female pup ratio, and the total number of pups born (data not shown). The assessment of the righting reflex showed a significant main effect of PND [F(2, 108) = 41.87, p<0.001]: animals exhibited the righting reflex more quickly across three days of testing (data not shown). There were no significant main effects of Sex or Prenatal Treatment and no interaction between these factors detected during testing of the righting reflex. Regarding the measure of negative geotaxis, a significant effect of PND was detected [F(2, 108) = 9.72, p<0.001], which indicates that animals improved across the days of testing with increasingly shorter latencies to orient in the upward direction (data not shown). There were no effects of Sex, Prenatal Treatment, or any interactions between these terms for negative geotaxis testing. The analysis of the eye-opening data revealed no significant main effects of Prenatal Treatment or Sex as well as no interaction between these factors on the days eye opening was assessed (data not shown). These data indicate that IV PN exposure did not produce a gross impairment of the postnatal milestone measures assessed in the present study.
3.2 Acquisition of sucrose-maintained responding
3.2.1 Sucrose-maintained responding during the initial three days of training
The Prenatal Treatment × Sex × Day ANOVA performed on active lever responding during 3 days of FR training revealed a significant main effect of Day [F(2,66) = 121.52, p<0.05] as animals increased responding across the FR1 and FR3 training sessions. Also, a significant main effect of Sex [F(1, 33) = 8.15, p<0.05] indicated that across three days of FR acquisition training the male animals (mean ±SEM: 242.01 ± 11.03) lever pressed significantly more than females (mean ±SEM: 199.30 ± 10.15). This difference was due to a standard sex difference in weight (see Section 3.1) and therefore consumption in males was greater than females due to an increased caloric intake that is associated with the sex difference in body weight. There were no significant interactions with Sex or any main effects or interactions with the factor of Prenatal Treatment. These results indicate that PS and PN animals showed equivalent responding for sucrose reinforcement during initial operant training.
3.2.2 Acquisition of METH self-administration using FR schedules of reinforcement: the initial 20 days of training
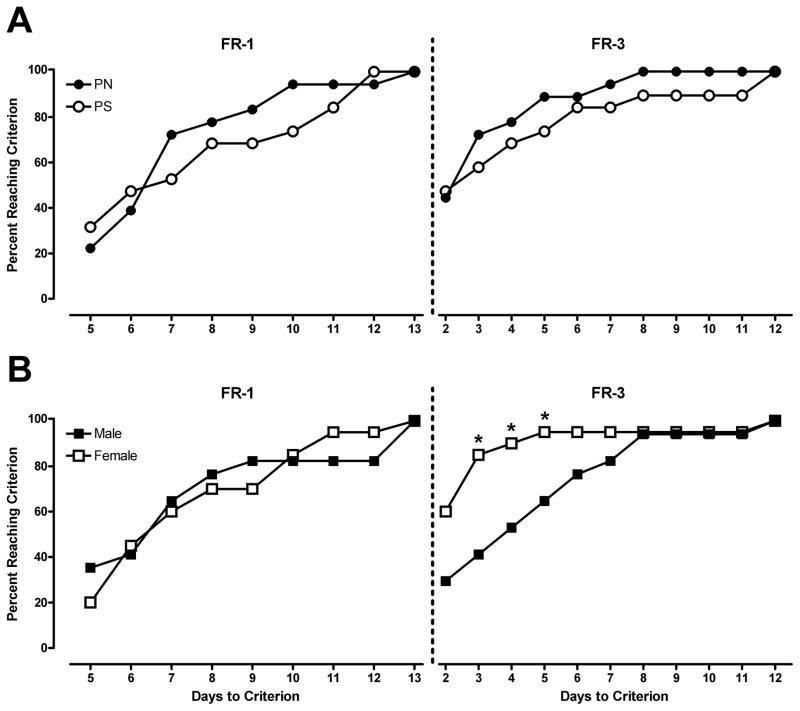
The total number of days for all rats to reach the criterion of stability on the FR1 and FR3 schedules of reinforcement was 9 and 11 days, respectively. Fisher’s exact tests were used to determine if there were differences between the PN and PS offspring, regardless of sex, in the number of animals that reached criterion across the 9 (FR1) and 11 days (FR3) period. No significant differences were observed between the PS and PN groups on the number of days to reach the criterion for the FR1 or FR3 schedule of reinforcement (Figure 1A). This non-parametric test was also used to determine potential sex differences, regardless of prenatal treatment, in the number of animals that reached criterion across the 9 (FR1) and 11 days (FR3) period. Females reached the FR3 criterion significantly faster than males, as indicated by a significant sex difference on the third, fourth, and fifth day of FR3 criterion days [p<0.05 on all three days; Figure 1B]. The proportion of females that reached criterion on those days was 85% (17/20), 90% (18/20), and 95% (19/20) compared to the proportion of males, which were 41% (7/17), 52% (9/17), and 65% (11/17), respectively. There were no significant sex differences during the remainder of the FR3 criterion period (i.e., days 6–11).
Figure 1.
Panel A: The percentage of PN and PS animals reaching criterion for stable METH self-administration during FR-1 and FR-3 training. Panel B: The percentage of male and female animals reaching criterion for stable METH self-administration during FR-1 and FR-3 training. On days 2, 3, and 4 of FR-3 training, females acquired responding significantly faster than males, * = p < 0.05. Stable criterion was defined as less than 20% variability in active lever responding across two consecutive sessions; greater than 2:1 ratio of active to inactive responses, and a minimum of 10 infusions per session.
3.3 PR schedule of reinforcement
3.3.1 Infusions
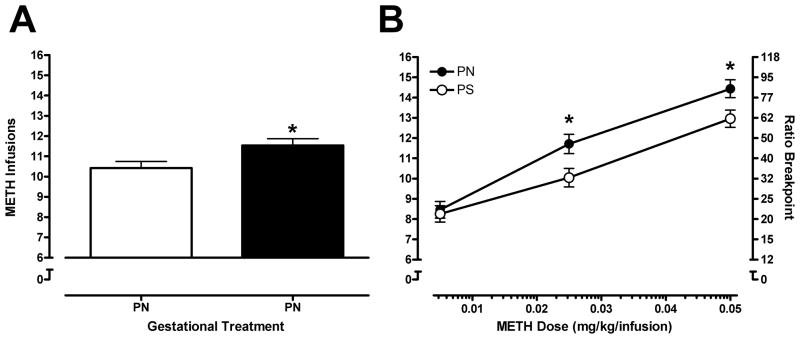
The main effect of Sex was non-significant (F<1.0) and no interactions with the factor of Sex were detected for the analysis of METH infusions. The Pretreatment × Dose ANOVA revealed a significant main effect of Prenatal Treatment that indicates PN offspring self-administered a greater number of METH infusions than PS animals [F(1, 35) = 6.09, p<0.05; Figure 2A]. The mean number of METH infusions received increased as a function of Dose [F(2, 70) = 220.733, p<0.001]: the mean (±SEM) values for the 0.005, 0.025, and 0.05 doses are 8.4 (±0.3), 10.9 (±0.3), and 13.7 (±0.3), and these data represent a linear increase in earned infusions. The Prenatal Treatment × Dose interaction [F(2, 70) = 3.174, p<0.05] indicates that as the unit dose of METH increased, the PN rats received more infusions than the PS animals. Figure 2B illustrates the Prenatal Treatment × Dose interaction. As can be seen in Figure 2B, there were no differences at the 0.005 dose; and received infusions are significantly greater at the 0.025 and 0.05 mg/kg/inf dose of METH (t-tests, p = 0.12 and p = 0.15, respectively). This indicates that PN rats exhibited greater motivation for METH, relative to PS rats, as defined by received infusions.
Figure 2.
Panel A shows the main effect of Prenatal Treatment [p<0.05] on the measure of METH infusions (PR testing) for the PN and PS groups (mean ±SEM). Panel B shows the mean (±SEM) number of METH infusions received during each PR session for PN and PS animals. Bonferroni-corrected post-hoc tests revealed significant differences between PN and PS animals at the 0.025 and 0.05 mg/kg doses of METH, * = p < 0.017.
3.3.2 Ratio Breakpoint
The main effect of Sex was non-significant (F<1.0) and no interactions with the factor of Sex were detected in the analysis on the ratio breakpoint data. The Prenatal Treatment × Dose ANOVA indicated that PN rats exhibited higher ratio breakpoint responding than PS controls [Pretreatment: F(1, 35) = 5.71, p<0.05]. The ratio breakpoint increased as the unit dose of METH increased [Dose: F(1.525, 66) = 89.71, p<0.001(G–G)]. The mean ratio breakpoints (±SEM) for the 0.005, 0.025, and 0.05 mg/kg/infusion doses of METH were 23.0 (±1.5), 42.9 (±3.3), and 78.0 (±4.8), respectively. The Pretreatment × Dose interaction was significant [F(1.525, 66) = 3.45, p< 0.05(G–G)], with PN animals exhibiting higher ratio breakpoints across METH doses relative to controls, in a pattern similar to the infusion data (Figure 2B).
3.3.3 Active lever responding
The main effect of Sex was non-significant (F<1.0) and no interactions with the factor of Sex were detected in the analysis on the breakpoint data. The Prenatal Treatment × Dose ANOVA revealed a main effect of Prenatal Treatment. Adult offspring exposed to PN exhibited greater active lever responding than the PS group [(F(1, 33) =6.37, p<0.05]. The main effect of Dose [(F(1.53, 66) = 93.36, p<0.001(G–G)] indicates that active lever responding increased in a linear manner as the unit dose of IV METH was increased: mean (±SEM) active lever responding was 98.9 (±7.5), 214.2 (±18.7), and 418.2 (±27.5) for the 0.005, 0.025, and 0.05 mg/kg/infusion doses, respectively. The Prenatal Treatment × Dose interaction [(F(1.53, 66) = 4.25, p<0.05(G–G)] suggests that the PN and PS groups responded similarly at the 0.005 mg/kg/infusion dose, but that the PN rats’ magnitude of responding increased to a greater degree than the PS group as the unit dose of IV METH increased. The main effect of Prenatal Treatment, which compared responding of the PN and PS rats for METH, regardless of dose, shows that PN animals were more motivated to respond for METH reinforcement compared to the PS rats.
3.3.4 Inactive lever responding
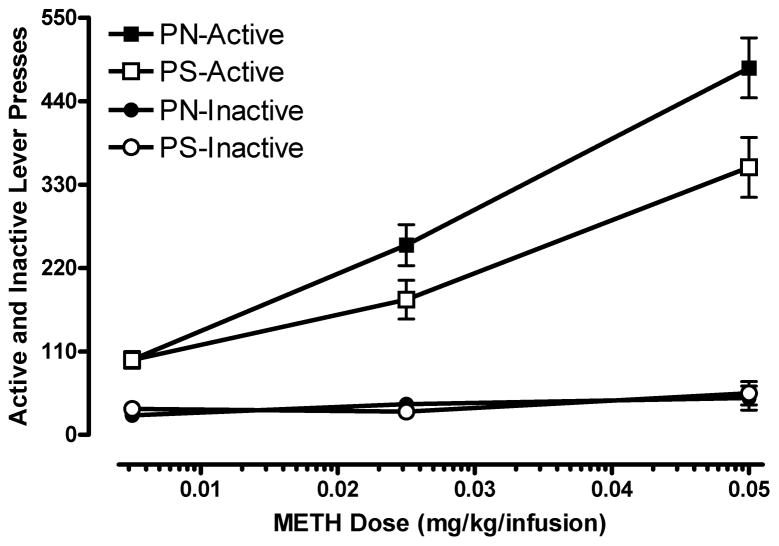
A Prenatal Treatment × Sex × Dose ANOVA revealed no significant effects (all p>0.05), which indicates that all rats exhibited a similar pattern of responding on the inactive lever during PR tests. The mean (±SEM) for responding was 39.0 (±6.0). Active and inactive lever responding is shown in Figure 3. This data suggests that the difference in active lever responding (and infusions) between the PN and PS groups is not due to a generalized increase in lever pressing for either lever; rather, responding was directed toward the active lever by both groups during PR testing.
Figure 3.
The PN and PS animals’ active and inactive lever responding (mean ±SEM) for each METH dose during the PR tests. There were no differences in inactive lever responding between the PN and PS rats.
4. DISCUSSION
The goal of the present study was to determine if adult offspring of prenatal IV nicotine exposure exhibit altered motivation for METH reinforcement. There was no effect of prenatal treatment on the acquisition of METH self-administration. PN and PS animals required the same number of training trials to acquire stable responding on the FR1 and FR3 schedules of reinforcement. During PR testing, PN-exposed animals exhibited greater motivation for METH reinforcement compared to controls. First, PN rats self-administered more METH infusions, exhibited higher breakpoints, and showed more active lever responding than PS rats, regardless of dose (i.e., main effects of prenatal treatment). PN animals also received more drug infusions on the PR schedule, depending on the dose of METH. PN rats were more motivated for METH in the dose range of 0.025 and 0.05 mg/kg/infusion. The results are in accord with previous studies from our laboratory which assessed the effect of IV PN exposure on motivation for sucrose reinforcement using the PR schedule (Lacy et al., 2012). In that study, adult animals showed enhanced motivation for sucrose reinforcement. Thus, IV PN exposure, administered 3×/day from gestational days 8–21, produces alterations in motivation to obtain sucrose and METH reinforcement, which suggests that this nicotine preexposure model imparts enduring changes in mesocorticolimbic function that affect motivation for sucrose and drug reinforcement.
There are various advantages and disadvantages to administering PN via the IV route of administration. The IV PN exposure model used in the current experiment allows for 100% bioavailability of nicotine and therefore mimics the pharmacokinetics of tobacco smoke inhalation (Benowitz et al., 2009). Also, the three daily IV injections, administered 3 hours apart, allows for accumulation of nicotine levels throughout the day (the half-life for 0.05 mg/kg/injection IV nicotine is ~49 minutes; Booze et al., 1999). Overall, less nicotine is needed to produce behavioral alterations in offspring, relative to other exposure models. The intentional administration of low overall concentrations of daily nicotine allows the experimenter to observe potential neurodevelopmental/neurobehavioral alterations in offspring without producing differences in litter composition, low birth weight, or any of the behavioral milestones tested, relative to PS controls (Harrod et al., 2012, 2011; Lacy et al., 2012, 2011; Morgan et al., 2013). Practically, this issue is important because it suggests that the modulation of METH reinforcement in PN-exposed offspring in the present study is due to PN-mediated alterations of neural development, rather than nicotine-induced changes in birth weight, and hence, growth anomalies. These findings are in accord with studies that administered PN continuously or via the oral route and report alterations in offsprings’ motivated behavior in the absence of changes in birth weight or various developmental milestones (Franke et al., 2008; Paz et al., 2007; Schneider et al., 2010). Given that a hallmark of maternal smoking is low birth weight, and this effect may differentially alter subsequent neurodevelopment (Comstock et al., 1971; Fried and O’Connell, 1987; Meyer and Comstock, 1972; Simpson, 1957), it will be important to conduct experiments that determine the effects of higher doses of IV PN, which also alter pup birth weight in order to fully understand the effects on gestational nicotine exposure on neural development and motivation in adolescent and adult offspring (e.g., LeSage et al., 2006). Regarding the disadvantages, the IV PN model does not mimic all of the characteristics of nicotine exposure via tobacco smoke inhalation. For example, focusing on the effects of nicotine alone precludes an understanding of how the 4,000 constituents found in tobacco smoke combine with those of nicotine to influence neurodevelopment (Franke et al., 2007). Also, the IV PN model is that it does not mimic the pulmonary uptake and delayed release of nicotine reported in tobacco smokers (Rose et al., 1999).
Both male and female offspring were investigated in the present experiment. Females acquired METH self-administration more readily than males on the FR1 schedule of reinforcement; however, this effect was ameliorated when animals progressed to the FR3 phase of acquisition. In an experiment that explicitly investigated sex differences in METH self-administration, Roth and Carroll (2004) showed that females acquired METH self-administration more rapidly than males. Moreover, females were more motivated to self-administer METH, relative to males, when a PR schedule was used. In the present experiment, sex was not a significant factor in the analysis of the PR data. This finding is consistent with previous research, which reported no sex differences for sucrose maintained responding in adult offspring of IV PN exposure (Harrod et al., 2012; Lacy et al., 2012). Notably, Cox et al., (2013) also directly investigated sex differences in METH self-administration and report that female rats exhibited more active lever responses and higher breakpoints for METH compared to males, but no sex difference in number of METH infusions were reported. These differential outcomes between studies regarding sex differences and PR responding for METH may be due, in part, to methodological differences in housing and training procedures.
To date, no clinical or epidemiological studies have tested if the offspring of maternal tobacco smoke exposure show an increased likelihood of abusing amphetamines or cocaine (Goldschmidt et al., 2012). The finding that maternal tobacco smoke exposure is associated with increased substance abuse across different classes of drugs (nicotine, marijuana, alcohol), however, suggests that the developmental effects on the mesocorticolimbic dopamine system may be to alter the reinforcing properties of all drugs of abuse. The findings from the preclinical literature investigating these psychostimulant drugs predict that human offspring of prenatal nicotine exposure will exhibit an increased vulnerability to cocaine and methamphetamine abuse (Harrod et al., 2012; Franke et al., 2008).
In conclusion, the findings of the present study show that offspring of IV PN exposure exhibited increased motivation to self-administer METH compared to controls. These data are consistent with previous results from our laboratory, which indicate enduring behavioral and/or neurophysiological consequences of IV PN exposure (Harrod et al., 2012, 2011; Lacy et al., 2012, 2011; Morgan et al., 2013). Furthermore, these data support a small, but growing literature showing that PN exposure alters motivated behavior in offspring; particularly in regard to drug self-administration (Buka et al., 2003; Franke et al., 2008; Kandel et al., 1994; Levin et al., 2006; Weissman et al., 1999). Although low, daily administration of IV nicotine produced changes in offsprings’ motivation to self-administer METH, this procedure did not produce growth abnormalities in the offspring. This suggests that mothers who simply reduce tobacco smoking during pregnancy in order to decrease the somatic deficits, e.g., low birth weight, but do not exhibit complete nicotine cessation, may still impart significant, long-term alterations in neurophysiological substrates that mediate drug abuse vulnerability. Thus, in this context, strict reductions in nicotine content in tobacco cigarettes, as discussed by Benowitz and Henningfield (1994), and suggested by The Family Smoking Prevention and Tobacco Control Act (H.R. 1256), may be an important step to prevent PN-induced modulation of motivated behavior in offspring exposed to maternal smoking.
Acknowledgments
Role of Funding Source: The funding for this research was provided by the NIDA Grant DA021287.
We would like to thanks Drs. Rick Bevins and Nichole Neugebauer for their expert assistance in programming the operant contingencies used in the present experiment.
Footnotes
Conflict of Interest: No conflict of interest is declared.
Contributors: RTL performed all experiments and prepared the initial manuscript. AJM assisted in performing experiments and contributed to editing the manuscript for submission. SBH performed the catheterization surgeries, provided editorial support during the preparation of the manuscript, and assisted in the interpretation of statistical analyses. All authors have approved of the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331:123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am J Psychiatry. 2003;160:1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- Chiodo KA, Roberts DC. Decreased reinforcing effects of cocaine following 2 weeks of continuous D-amphetamine treatment in rats. Psychopharmacology (Berl) 2009;206:447–456. doi: 10.1007/s00213-009-1622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock GW, Shah FK, Meyer MB, Abbey H. Low birth weight and neonatal mortality rate related to maternal smoking and socioeconomic status. Am J Obstet Gynecol. 1971;111:53–59. doi: 10.1016/0002-9378(71)90926-4. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Goldschmidt L, DeGenna N, Day NL. Smoking during teenage pregnancies: effects on behavioral problems in offspring. Nicotine Tob Res. 2007;9:739–750. doi: 10.1080/14622200701416971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38:2343–2353. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke RM, Belluzzi JD, Leslie FM. Gestational exposure to nicotine and monoamine oxidase inhibitors influences cocaine-induced locomotion in adolescent rats. Psychopharmacology (Berl) 2007;195:117–124. doi: 10.1007/s00213-007-0876-y. [DOI] [PubMed] [Google Scholar]
- Franke RM, Park M, Belluzzi JD, Leslie FM. Prenatal nicotine exposure changes natural and drug-induced reinforcement in adolescent male rats. Eur J Neurosci. 2008;27:2952–2961. doi: 10.1111/j.1460-9568.2008.06253.x. [DOI] [PubMed] [Google Scholar]
- Fried PA, O’Connell CM. A comparison of the effects of prenatal exposure to tobacco, alcohol, cannabis and caffeine on birth size and subsequent growth. Neurotoxicol Teratol. 1987;9:79–85. doi: 10.1016/0892-0362(87)90082-1. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Cornelius MD, Day NL. Prenatal cigarette smoke exposure and early initiation of multiple substance use. Nicotine Tob Res. 2012;14:694–702. doi: 10.1093/ntr/ntr280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. Lobeline attenuates d-methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2001;298:172–179. [PubMed] [Google Scholar]
- Harrod SB, Lacy RT, Morgan AJ. Offspring of prenatal IV nicotine exposure exhibit increased sensitivity to the reinforcing effects of methamphetamine. Front Pharmacol. 2012;3:116. doi: 10.3389/fphar.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Lacy RT, Zhu J, Hughes BA, Perna MK, Brown RW. Gestational IV nicotine produces elevated brain-derived neurotrophic factor in the mesocorticolimbic dopamine system of adolescent rat offspring. Synapse. 2011;65:1382–1392. doi: 10.1002/syn.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ. Assessment of developmental milestones in rodents. Curr Protoc Neurosci. 2004;Chapter 8(Unit 8–18) doi: 10.1002/0471142301.ns0818s25. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;84:1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Hord LL, Morgan AJ, Harrod SB. Intravenous gestational nicotine exposure results in increased motivation for sucrose reward in adult rat offspring. Drug Alcohol Depend. 2012;124:299–306. doi: 10.1016/j.drugalcdep.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Mactutus CF, Harrod SB. Prenatal IV nicotine exposure produces a sex difference in sensorimotor gating of the auditory startle reflex in adult rats. Int J Dev Neurosci. 2011;29:153–161. doi: 10.1016/j.ijdevneu.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Gustaf E, Dufek MB, Pentel PR. Effects of maternal intravenous nicotine administration on locomotor behavior in pre-weanling rats. Pharmacol Biochem Behav. 2006;85:575–583. doi: 10.1016/j.pbb.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence S, Petro A, Horton K, Seidler FJ, Slotkin TA. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacol Biochem Behav. 2006;85:669–674. doi: 10.1016/j.pbb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mactutus CF, Herman AS, Booze RM. Chronic intravenous model for studies of drug (Ab)use in the pregnant and/or group-housed rat: an initial study with cocaine. Neurotoxicol Teratol. 1994;16:183–191. doi: 10.1016/0892-0362(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Meyer MB, Comstock GW. Maternal cigarette smoking and perinatal mortality. Am J Epidemiol. 1972;96:1–10. doi: 10.1093/oxfordjournals.aje.a121427. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Harrod SB, Lacy RT, Stanley EM, Fadel JR. Intravenous prenatal nicotine exposure increases orexin expression in the lateral hypothalamus and orexin innervation of the ventral tegmental area in adult male rats. Drug Alcohol Depend. 2013;132:562–570. doi: 10.1016/j.drugalcdep.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller KU, Mennigen E, Ripke S, Banaschewski T, Barker GJ, Buchel C, Conrod P, Fauth-Buhler M, Flor H, Garavan H, Heinz A, Lawrence C, Loth E, Mann K, Martinot JL, Pausova Z, Rietschel M, Strohle A, Struve M, Walaszek B, Schumann G, Paus T, Smolka MN. Altered reward processing in adolescents with prenatal exposure to maternal cigarette smoking. JAMA Psychiatry. 2013;70:847–856. doi: 10.1001/jamapsychiatry.2013.44. [DOI] [PubMed] [Google Scholar]
- Paz R, Barsness B, Martenson T, Tanner D, Allan AM. Behavioral teratogenicity induced by nonforced maternal nicotine consumption. Neuropsychopharmacology. 2007;32:693–699. doi: 10.1038/sj.npp.1301066. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Tizabi Y, Rahman MA, Nespor SM, Grunberg NE. Prenatal exposure to nicotine: effects on prepulse inhibition and central nicotinic receptors. Pharmacol Biochem Behav. 1997;58:843–849. doi: 10.1016/s0091-3057(97)98985-1. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacol Biochem Behav. 2008;89:463–472. doi: 10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Coleman RE. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug Alcohol Depend. 1999;56:99–107. doi: 10.1016/s0376-8716(99)00025-3. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Schneider T, Bizarro L, Asherson PJ, Stolerman IP. Gestational exposure to nicotine in drinking water: teratogenic effects and methodological issues. Behav Pharmacol. 2010;21:206–216. doi: 10.1097/fbp.0b013e32833a5bb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill; New York: 1988. [Google Scholar]
- Simpson WJ. A preliminary report on cigarette smoking and the incidence of prematurity. Am J Obstet Gynecol. 1957;73:807–815. [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Tizabi Y, Popke EJ, Rahman MA, Nespor SM, Grunberg NE. Hyperactivity induced by prenatal nicotine exposure is associated with an increase in cortical nicotinic receptors. Pharmacol Biochem Behav. 1997;58:141–146. doi: 10.1016/s0091-3057(96)00461-3. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Principles of behavioral teratology. In: Riley EP, Vorhees CV, editors. Handbook of Behavioral Teratology. Plenum Press; New York, NY US: 1986. pp. 23–48. [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38:892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Yokel RA. Intravenous self-administration: response rates, the effects of pharmacological challenges, and drug preference. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer-Verlag; New York: 1987. pp. 1–33. [Google Scholar]