Abstract
Aim: The aim of this study was to evaluate the analgesic effects of postoperative epidural administration of neostigmine and morphine in patients scheduled for caesarean section under epidural anaesthesia.
Material and Methods: Sixty ASA I-II patients, scheduled for caesarean section under epidural anaesthesia, were randomly allocated into three groups. Neostigmine (10 μg/kg), morphine (3 mg), and saline (6 mL) were administered to the neostigmine, morphine, and control groups, respectively, 30 minutes after the surgery via the epidural catheter. Afterwards, postoperative pain treatment was administered to all patients with a patient-controlled epidural analgesia (PCEA) device, using 0.125% bupivacaine. The patients were followed up for 24 hours. The total volume of local anaesthetics used, the time to first analgesic requirement, analgesic requirements, VAS scores, analgesia quality, first passage of bowel gas, ambulation times, haemodynamic parameters and side effects were evaluated.
Results: The time to first analgesic requirement was significantly longer in the morphine group than in the neostigmine and control groups (p<0.01), and in the neostigmine group compared to the control group (p<0.05). The total local anaesthetic consumption and the number of bolus injections were significantly higher in the control group than in the other groups (p<0.01). The first passage of bowel gas occurred significantly sooner in the neostigmine group than in the morphine (p<0.01) and the control (p<0.05) groups. Itching frequency was significantly higher in the morphine group than in the other two groups (p<0.05). VAS scores were similar in the morphine and neostigmine groups.
Conclusion: Postoperative single-dose epidural neostigmine reduced the 24-hour analgesic requirements but in the chosen doses presented an analgesic effect significantly lower than morphine. Hippokratia 2014; 18 (1): 44-48.
Keywords: Caesarean section, epidural analgesia, morphine, neostigmine
Introduction
Epidural anaesthesia is commonly used in elective caesarean sections, as it reduces mortality and morbidity, and provides a good way for postoperative pain control. Traditionally, opioids have been successfully used in bolus, continuous infusion, and patient-controlled epidural analgesia (PCEA) for postoperative epidural analgesia schemes1,2. A single dose of epidural morphine is frequently used, due to ease of application, low cost, and high patient satisfaction. However, morphine has sometimes has undesirable side effects, such as nausea and vomiting, urinary retention, itching, respiratory depression and decreased bowel motility3-9. Especially due to the later side effects of opioids given intrathecally and epidurally, such as respiratory depression4,6, non-opioid analgesics have been extensively investigated. Neostigmine, one of the non-opioid agents used for this purpose, has been reported in animal and human studies to induce analgesia without causing serious side effects except for a high incidence of nausea and vomiting when administered intrathecally10-13. In later publications, it has been shown to cause analgesia without serious and disturbing side effects when administered in the epidural space14-20.
The aim of this study was to evaluate and compare the analgesic and adverse effects of single doses of postoperative epidural neostigmine and morphine in patients undergoing caesarean sections under epidural anaesthesia.
Material and Methods
This prospective study was conducted at Gazi University Faculty of Medicine, Department of Anaesthesiology and Reanimation, after approval by the Ethical Committee of Gazi University Faculity of Medicine on 13 June 2001 (approval number: 2001/2). The study included 60 ASA I–II patients who were scheduled for elective caesarean sections. Only single-foetus pregnancies at term were included. The exclusion criteria were the presence of contraindications for epidural anaesthesia, allergy to local anaesthetics, history of opioid abuse, parturients with obstetric complications such as multiple pregnancy, premature labor, non-vertex presentation or preeclampsia and patients with decompensated heart failure, dysrhythmias, chronic obstructive pulmonary disease.
All patients were examined at the preoperative visit one day prior to surgery. They were informed about the epidural anaesthesia and the whole analgesic procedure and informed consent was obtained. The patients were also informed about the visual analogue scale (VAS), which is used for the evaluation of postoperative pain, as well as the PCEA device (Abbott Pain Management, Provider, Abbott Laboratories, Chicago, USA). The patients were randomly assigned to three groups according to the drug to be administered in the epidural space postoperatively: control group (Group C, n=20), morphine group (Group M, n=20), and neostigmine group (Group N, n=20). Patients were assigned to one of the three study groups using a computer-generated random number table.
All patients were given 10 mL/kg of lactated Ringer’s solution before the procedure. An epidural block was performed in sitting position. After infiltrating the skin and subcutaneous tissue with 2 mL of 2% lidocaine at the L3–4 interspinous area, the epidural space was determined using the loss of resistance technique and the catheter was inserted 4–5 cm inside the epidural space through an 18 G Tuohy needle (Perifix; Braun, Melsungen, Germany). Then, 3 mL of 2% lidocaine including 5 μg/mL of epinephrine were administered epidurally as a testing dose. Additional doses of fractionated 0.5% bupivacaine were given via the epidural catheter until the upper level of sensory blockade tested with pinprick reached T4. Surgery began after the T4 sensory block level was reached. If the epidural block was still not achieved 30 min after epidural injection of the local anaesthetic, the patient was removed from the study group and general anaesthesia was administered. Patients who had a VAS score >3/10 during surgery were also removed from the study and general anaesthesia was administered.
Thirty minutes after surgery, which was considered as the starting time (0 hours), the following regimens were administered via epidural catheter: 10 μg/kg of neostigmine in 6 mL of saline in group N, 3 mg of morphine in 6 mL of saline in group M, and 6 mL of saline in group C. The 10 μg/kg dose of neostigmine was chosen as an effective analgesic dose based on Nakayama et al16. For treatment of postoperative pain, PCEA was set up as follows: a bolus dose of 6 mL 0.125% bupivacaine, lockout time limit of ten minutes, four-hour limit of 30 mL, and no background infusion was used. The patients were assisted in the use of the device during the first analgesic requirement and then PCEA device was left with them to be used for subsequent pain control.
Heart rate (HR), mean arterial pressure (MAP), respiratory rate (RR), postoperative pain, time to regression of sensory block to T10, and sedation scores19 [0=awake (absent); 1=drowsy but responding to verbal stimuli (mild); 2=responding to moderate touch (moderate); 3=responding to firm touch (severe)] were recorded at 0, 15, and 30 minutes and 1, 4, 12, and 24 hours. In addition, the presence of side effects such as itching, nausea, vomiting and their duration and intensity21 (0=none, 1=mild, 2=moderate, 3=severe) were recorded. Postoperative pain was assessed with visual analog scale (VAS), which consisted of a 10-cm line with 0 equaling “no pain at all” and 10 equaling “the worst possible pain.
Nausea and vomiting were treated with 10 mg metoclopramide IV and pruritus was treated with 20 mg diphenhydramine IV. Furthermore, when VAS scores were 3 or above despite PCEA, 1 g metamizole IV was used as an additional analgesic. The first passage of bowel gas and ambulation times were determined and recorded as well. The follow-up period for the patients lasted until they were discharged. The application of PCEA was terminated after 24 hours, and total volume used, frequency and time of demand, and first request times were recorded. The patients were asked to evaluate the efficacy of treatment using the 5-point scale for quality of analgesia (1=poor, 2=inadequate, 3=moderate, 4=good, 5=excellent).
Statistical analysis was performed using the SPSS for Windows, version 9.0 (SPSS Inc., Chicago, IL, USA). Data regarding age, body weight, height, gestational age, time to regression of sensory block T10, first ambulation time, time to first passage of bowel gas, and first analgesic requirement time were compared using one-way analysis of variance (ANOVA). When a difference was determined, the Tukey’s post-hoc test was also applied. Mean arterial pressure, HR, and RR data were assessed by a repeated measures ANOVA (Bonferroni adjustment was used in the comparisons of intragroup mean MAP, RR and HR values, in which the time factor was considered important through repeated measures of variance analysis), and the number of usages of the PCEA device and VAS data were evaluated by the Friedman test. Comparison of side effects, additional analgesia, and quality of analgesia were completed using chi-square and Fisher’s exact chi-square tests. A value of p<0.05 was considered statistically significant.
Results
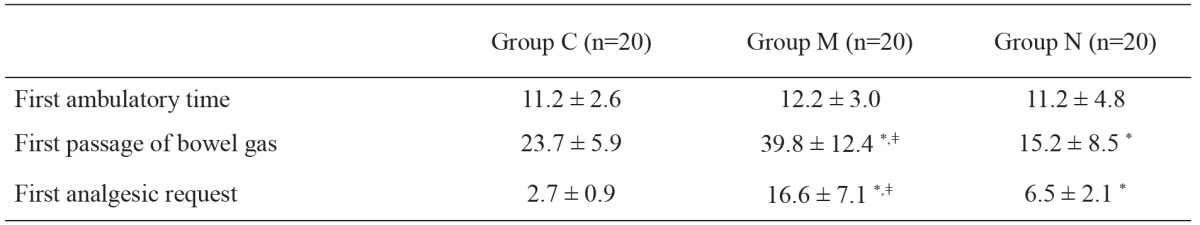
The groups did not differ significantly in terms of demographic data, gestational age, or duration of caesarean section (Table 1). There was also no significant difference in terms of the regression time of sensory block to T10 (group C: 283 ± 58 min; group M: 294 ± 83 min; and group N: 265 ± 64 min).
Table 1. Demographic data, gestational age and duration of cesarean section (mean ± Standard Deviation).
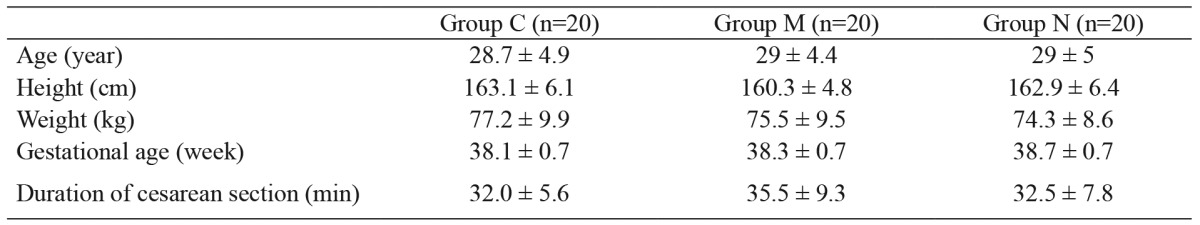
There were no significant differences among the groups in terms of postoperative HR. However, when the changes in HR were compared at different time points, there was a significant decrease in HR in group N at 1, 4, and 12 hours (p<0.0001, p<0.0001, p<0.0001, respectively), whereas there were no differences in groups C and M (Figure 1). There were also no significant differences in MAP among the groups. However, when the changes in MAP were compared at different time points, there was a significant increase in group C at 1, 4, and 12 hours (p<0.011, p<0.007, p<0.009, respectively), whereas there were no differences in groups M and N (Figure 2).
Figure 1. Heart rate values of the three groups. *: compared to the control value of the group p<0.05, Group C: Control group, Group M: Morphine group, Group N: Neostigmine group.
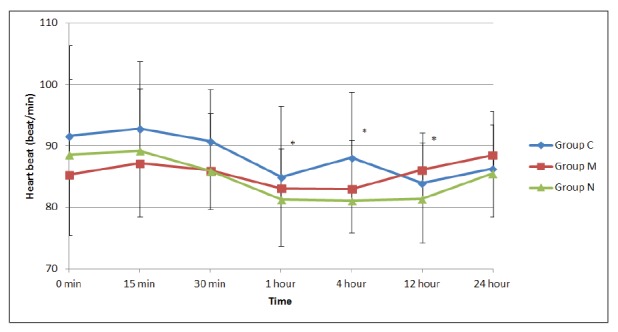
Figure 2. Mean arterial blood pressure values of the three groups. *: compared to the control value of the group p<0.05, Group C: Control group, Group M: Morphine group, Group N: Neostigmine group.
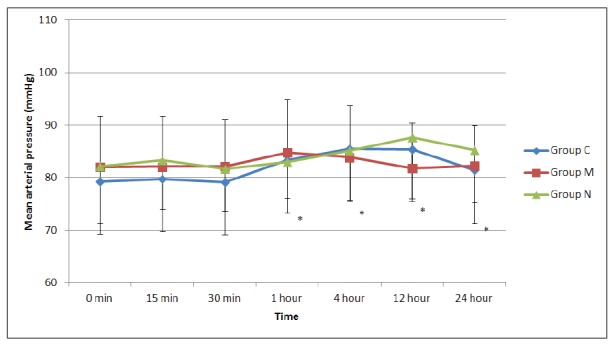
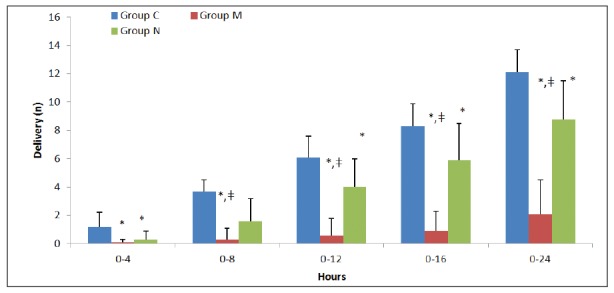
The number of analgesia request on the demand button of the PCEA for local anaesthetics and the number of boluses delivered were significantly lower in groups M and N than in group C at all time points (p<0.01) (Figure 3 and Figure 4). When group N was compared with group M, the number of requests for local anaesthetic and the number of boluses delivered were similar in the first four hours; however, both parameters were significantly lower in group M compared to group N (p<0.01) up to 24 hours postoperatively (Figures 3 and 4). The total local anaesthetic consumption levels from the PCEA device were 72.3 ± 9.6, 12.3 ± 14.1, and 53.1 ± 17.1 mL in groups C, M, and N, respectively. This level was significantly higher in group C than in the other two groups (p<0.01), and significantly lower in group M than in group N (p<0.01).
Figure 3. Number of local anesthetic of demands obtained from the patient-controlled epidural analgesia (PCEA) device (mean ± Standard Deviation). *: p<0.01, compared to the control group, ǂ: p<0.01, compared to the neostigmine group, Group C: Control group, Group M: Morphine group, Group N: Neostigmine group.
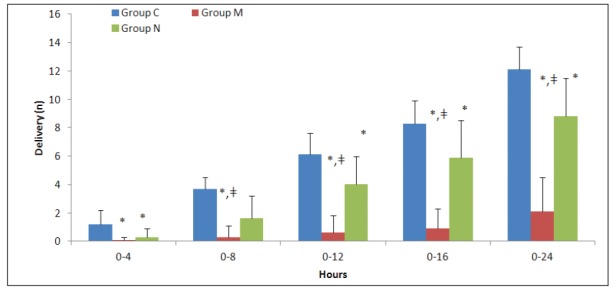
Figure 4. Number of delivery of local anesthetic boluses obtained from the patient-controlled epidural analgesia (PCEA) device (mean ± Standard Deviation). *: p<0.01, compared to the control group, ǂ: p<0.01, compared to neostigmine group, Group C: Control group, Group M: Morphine group, Group N: Neostigmine group.
The times until first analgesia was requested were 2.7 ± 0.9, 16.6 ± 7.1, and 6.5 ± 2.1 hours in groups C, M, and N respectively. The time was significantly longer in group M compared to the other two groups (p<0.01), and significantly longer in group N compared to group C (p<0.05) (Table 2).
Table 2. Time to first passage of bowel gas, time to ambulation and time to first analgesic request (hours) (mean ± Standard Deviation).
*: p<0.01, compared to the control group, ǂ: p<0.01, compared to neostigmine group.
In addition to PCEA, the additional analgesic requirements (metamizole) of the patients in groups M and N were similar; the additional analgesic requirements of the patients were found to be significantly lower in the neostigmine (p<0.05) and morphine groups (p<0.01) when compared to the control group. The number of patients with additional analgesic requirements were 10 (10 g) in group C, 3 (3 g) in group N, and 0 in group M.
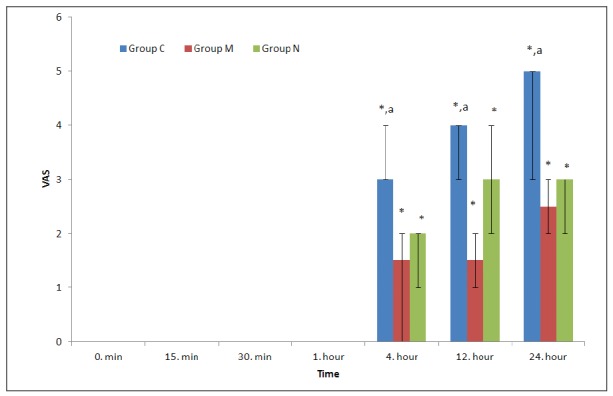
In the postoperative intragroup comparisons in terms of VAS, the values at 12 and 24 hours were significantly higher in all three groups compared to the control values (p<0.05). The VAS values were significantly higher at 12 and 24 hours in the control group compared to the morphine and neostigmine groups (p<0.05). However, there was no significant difference between the morphine and neostigmine groups (Figure 5).
Figure 5. Visual analogue scale (VAS) values of the groups [Median (%25-75)]. *: p<0.05, compared to the control value of the groups, a: p<0.05, compared to the groups N and M, Group C: Control group, Group M: Morphine group, Group N: Neostigmine group.
The first passage of bowel gas was at 23.7 ± 5.9, 39.8 ± 12.4, and 15.2 ± 8.5 hours in groups C, M, and N respectively. The time was significantly shorter in the neostigmine group compared to the morphine (p<0.01) and control groups (p<0.05) (Table 2). In addition, when compared with the control, it was significantly longer in the morphine group (p<0.01). No significant differences were detected among the groups in terms of first ambulation time (Table 2).
Except for nausea and pruritus, no other side effects were encountered in any of the groups. Two patients in group M, one patient in group N, and no patients in group C had nausea. All three patients responded well to a single dose of intravenous metoclopramide. When the patients were compared in terms of itching, this side effect was significantly higher in the morphine group compared to the other two groups (p<0.05). In the morphine group, 15 cases had mild to moderate itching which did not require any treatment, and two cases had severe itching that required treatment.
No significant differences were determined in terms of change in postoperative respiratory rate values over time among the groups, nor within the groups. The sedation scores were identified as 0 at all times and were excluded from statistical analysis.
Regarding the quality of analgesia, 5 patients in group C, 20 patients in group M (15 patients excellent, 5 patients good), and 17 patients in group N (17 patients good) expressed that the quality was good or excellent. There were no significant differences between the morphine and neostigmine groups in terms of quality of analgesia, whereas it was significantly lower in the control group compared to the other two groups (p<0.0001, p<0.0001, respectively). However, the patients who rated the quality of analgesia as excellent were significantly more in Group M than the patients in Groups C and N (p<0.0001, p<0.0001, respectively).
Discussion
In recent years, the use of epidural neostigmine for postoperative analgesia has been widely discussed. However, detailed studies comparing its effect to that of epidural morphine are limited. In this study, we investigated the analgesic efficacy and side effects of epidural neostigmine and morphine in the postoperative period.
In our study, there were no significant differences between the groups in terms of haemodynamic parameters. In the first 12 hours postoperatively, in the neostigmine group, there was a significant decrease in HR compared to its baseline values, while a significant increase in MAP was observed in the control group. However, there was no difference between the groups, and clinical bradycardia requiring treatment was not observed in any of the cases. These results suggest that neostigmine can be used in the epidural space, even at a relatively high dose of 10 μg/kg. In other studies examining the effects of epidural neostigmine, there is no data regarding its haemodynamic effects15-17. Harjai et al22 used epidural neostigmine in doses of 100 and 200 µg and indicated that it had no effect on HR or MAP. Although there is an insufficient number of reports on the haemodynamic effects of epidural neostigmine, our results indicate that epidural administration of 10 μg/kg neostigmine has no significant effect on haemodynamic parameters.
In our study, the time to first analgesic request was significantly longer in groups M and N than in group C. In addition, total local anaesthetic doses administered via PCEA were significantly lower in groups M and N than in group C. In a similar study, Nakayama et al16 compared 5 and 10 μg/kg of postoperative epidural neostigmine and reported that 10 μg/kg of epidural neostigmine significantly prolonged the time to first analgesic request. However, they found that 24-hour total analgesic demand was similar with 5 and 10 μg/kg neostigmine. The reasons for this discrepancy might be the choice of analgesic drug and route of administration.
The intrathecal use of neostigmine is severely restricted in practice, due to the high incidence of nausea. However, it does not cause nausea when administered through the epidural space18,19. Nakayama et al16 reported that patients with abdominal hysterectomy who received 10 μg/kg neostigmine had similar rates of nausea and vomiting as the control group. These findings are in accordance with our results. There were varying rates of nausea and vomiting associated with neostigmine, which is probably due to the methodology and the limited number of cases. In addition, the fact that nausea and vomiting are affected by gender, smoking, and location and type of operation may also explain the differences in results between the studies.
Sedation has been reported as one of the major side effects of epidural neostigmine. Studies have reported dose-independent mild sedation to varying degrees of dose-dependent sedation19,22. However, in this study, despite the high doses of neostigmine administered, we did not observe any sedation, nor did we detect any sedation in the morphine group, despite adding diphenhydramine for control of nausea in most patients of this group. Similar to our study, Chia et al17 reported that all patients were fully awake after neostigmine was administered through the thoracic epidural space, and they indicated that the sedation scores were zero. In contrast to our findings, Harjai et al22 reported dose-dependent sedation in patients treated with neostigmine. However, their results might have been affected by the general anaesthesia which was administered in their study. Kaya et al19 also reported dose-independent mild sedation after administration of neostigmine together with the use of other agents that can cause sedation, such as intrathecal fentanyl or intravenous patient-controlled analgesia with morphine. As it was the only agent used in our study, we agree with Chia that sedation is not a serious side effect of neostigmine17.
Another striking finding in our study is that the time until passage of gas was prolonged with epidural morphine compared to the control group, whereas it was shortened by the use of neostigmine compared to the other two groups. Morphine is known to slow passage in the gastrointestinal tract when administered systemically or epidurally9. In our study, the first passage of bowel gas was found to be 39.8 ± 12.4 hours. In a series of patients with abdominal hysterectomy, Thoren et al9 found the average bowel gas passage time to be 56 hours. Similarly, we observed the first passage of bowel gas as late as 60 hours in a patient who was given morphine. In the neostigmine group, this period was 15.2 ± 8.5 hours. In a study conducted in patients undergoing abdominal aorta surgery, neostigmine was found to shorten the time for passage of bowel gas23. Considering that neostigmine is used in the treatment of acute colonic pseudo-obstruction24, we believe that satisfactory results can be achieved with a combination of epidural neostigmine and morphine.
Epidural morphine has already been known to be superior to other methods in terms of patient satisfaction8,25. In our study, 75% of the patients in the control group assessed the quality of analgesia as “moderate”, whereas 85% of the patients in the neostigmine group as “good”, and 75% of the patients in the morphine group evaluated the quality of analgesia as “excellent”.
In the current study, epidural neostigmine was found to be effective for postoperative pain management after caesarean section. In a study in rats, in which it was investigated whether there was any difference in efficacy of intrathecal neostigmine between genders, it was found that the efficacy of intrathecal neostigmine differed significantly in favour of female rats26. Based on this study and considering the fact that the patients in our study were all women, it is probable that this efficacy of neostigmine is not valid for every surgery or for both genders. This matter should be further investigated.
In conclusion, we achieved effective and safe analgesia with 10 μg/kg epidural neostigmine used in elective caesarean section. In this study, although neostigmine was compared with morphine, which has a prolonged and effective analgesic effect and high patient satisfaction, we determined that neostigmine is an effective analgesic without affecting haemodynamic parameters or causing significant side effects compared to morphine, and that the time until passage of bowel gas is shortened, which are important findings to emphasize.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Harrison DM, Sinatra R, Morgese L, Chung JH. Epidural narcotic and patient- controlled analgesia for post-cesarean section pain relief. Anesthesiology. 1988;68:454–457. doi: 10.1097/00000542-198803000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Yu PY, Gambling DR. A comparative study of patient-controlled epidural fentanyl and single dose epidural morphine for post-caesarean analgesia. Can J Anaesth. 1993;40:416–420. doi: 10.1007/BF03009509. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho B. Respiratory depression after neuraxial opioids in the obstetric setting. Anesth Analg. 2008;107:956–961. doi: 10.1213/ane.0b013e318168b443. [DOI] [PubMed] [Google Scholar]
- 4.Fuller JG, McMorland GH, Douglas MJ, Palmer L. Epidural morphine for analgesia after caesarean section: a report of 4880 patients. Can J Anaesth. 1990;37:636–640. doi: 10.1007/BF03006481. [DOI] [PubMed] [Google Scholar]
- 5.Sultan P, Gutierrez MC, Carvalho B. Neuraxial morphine and respiratory depression: finding the right balance. Drugs. 2011;71:1807–1819. doi: 10.2165/11596250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Gustafsson LL, Schildt B, Jacobsen K. Adverse effects of extradural and intrathecal opiates: report of a nationwide survey in Sweden. 1982. Br J Anaesth. 1998;81:86–93. doi: 10.1093/bja/81.1.86. [DOI] [PubMed] [Google Scholar]
- 7.Halpern SH, Arellano R, Preston R, Carstoniu J, O’Leary G, Roger S, et al. Epidural morphine vs hydromorphone in post-caesarean section patients. Can J Anaesth. 1996;43:595–598. doi: 10.1007/BF03011773. [DOI] [PubMed] [Google Scholar]
- 8.Roseag OP, Lindsay MP. Epidural opioid analgesia after caesarean section: a comparison of patient-controlled analgesia with meperidine and single bolus injection of morphine. Can J Anaesth. 1994;41:1063–1068. doi: 10.1007/BF03015655. [DOI] [PubMed] [Google Scholar]
- 9.Thorén T, Sundberg A, Wattwil M, Garvill JE, Jürgensen U. Effects of epidural bupivacaine and epidural morphine on bowel function and pain after hysterectomy. Acta Anaesthesiol Scand. 1989;33:181–185. doi: 10.1111/j.1399-6576.1989.tb02886.x. [DOI] [PubMed] [Google Scholar]
- 10.Hood DD, Eisenach JC, Tong C, Tommasi E, Yaksh TL. Cardiorespiratory and spinal cord blood flow effects of intrathecal neostigmine methylsulfate, clonidine and their combination in sheep. Anesthesiology. 1995;82:428–435. doi: 10.1097/00000542-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Almeida RA, Lauretti GR, Mattos AL. Antinociceptive effect of lowe-dose intrathecal neostigmine combined with intrathecal morphine following gynecologic surgery. Anesthesiology. 2003;98:495–498. doi: 10.1097/00000542-200302000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Ho KM, Ismail H, Lee KC, Branch R. Use of intrathecal neostigmine as an adjunct to other spinal medications in perioperative and peripartum analgesia: a meta-analysis. Anaesth Intensive Care. 2005;33:41–53. doi: 10.1177/0310057X0503300107. [DOI] [PubMed] [Google Scholar]
- 13.Krukowski JA, Hood DD, Eisenach JC, Mallak KA, Parker RL. Intrathecal neostigmine for post-caesarean section analgesia: dose response. Anesth Analg. 1997;84:1269–1275. doi: 10.1097/00000539-199706000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Eisenach JC. Epidural neostigmine: will it replace lipid soluble opioids for postoperative and labor analgesia? Anesth Analg. 2009;109:293–295. doi: 10.1213/ane.0b013e3181a891c2. [DOI] [PubMed] [Google Scholar]
- 15.Lauretti GR, de Oliviera R, Perez MV, Paccola CA. Postoperative analgesia by intraarticular and epidural neostigmine following knee surgery. J Clin Anesth. 2000;12:444–448. doi: 10.1016/s0952-8180(00)00189-6. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama M, Ichinose H, Nakabayashi K, Satoh O, Yamamoto S, Namiki A. Analgesic effect of epidural neostigmine after abdominal hysterectomy. J Clin Anesth. 2001;13:86–89. doi: 10.1016/s0952-8180(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 17.Chia YY, Chang TH, Liu K, Chang HC, Ko NH, Wang YM. The efficacy of thoracic epidural neostigmine infusion after thoracotomy. Anesth Analg. 2006;102:201–208. doi: 10.1213/01.ane.0000184812.94185.b3. [DOI] [PubMed] [Google Scholar]
- 18.Ross VH, Pan PH, Owen MD, Seid MH, Harris L, Clyne B, et al. Neostigmine decreases bupivacaine use by patient-controlled epidural analgesia during labor: a randomized controlled study. Anesth Analg. 2009;109:524–531. doi: 10.1213/ane.0b013e31819518e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaya FN, Sahin S, Owen MD, Eisenach JC. Epidural neostigmine produces analgesia but also sedation in women after cesarean delivery. Anesthesiology. 2004;100:381–385. doi: 10.1097/00000542-200402000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Roelants F, Lavand’homme PM. Epidural neostigmine combined with sufentanil provides balanced and selective analgesia in early labor. Anesthesiology. 2004;101:439–444. doi: 10.1097/00000542-200408000-00025. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso MM, Leite AO, Santos EA, Gozzani JL, Mathias LA. Effect of dexamethasone on prevention of postoperative nausea, vomiting and pain after caesarean section: a randomised, placebo-controlled, double-blind trial. Eur J Anaesthesiol. 2013;30:102–105. doi: 10.1097/EJA.0b013e328356676b. [DOI] [PubMed] [Google Scholar]
- 22.Harjai M, Chandra G, Bhatia VK, Singh D, Bhaskar P. A comparative study of two different doses of epidural neostigmine coadministered with lignocaine for post operative analgesia and sedation. J Anaesthesiol Clin Pharmacol. 2010;26:461–464. [PMC free article] [PubMed] [Google Scholar]
- 23.Caliskan E, Turkoz A, Sener M, Bozdogan N, Gulcan O, Turkoz R. A prospective randomized double-blind study to determine the effect of thoracic epidural neostigmine on postoperative ileus after abdominal aortic surgery. Anesth Analg. 2008;106:959–964. doi: 10.1213/ane.0b013e318163fbfe. [DOI] [PubMed] [Google Scholar]
- 24.Paran H, Silverberg D, Mayo A, Shwartz I, Neufeld D, Freund U. Treatment of acute colonic pseudo-obstruction with neostigmine. J Am Coll Surg. 2000;190:315–318. doi: 10.1016/s1072-7515(99)00273-2. [DOI] [PubMed] [Google Scholar]
- 25.Bonnet MP, Mignon A, Mazoit JX, Ozier Y, Marret E. Analgesic efficacy and adverse effects of epidural morphine compared to parenteral opioids after elective caesarean section: a systematic review. Eur J Pain. 2010;14:894e1–9. doi: 10.1016/j.ejpain.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Chiari A, Tobin JR, Pan H, Hood DD, Eisenach JC. Sex differences in cholinergic analgesia I: a supplemental nicotinic mechanism in normal females. Anesthesiology. 1999;91:1447–1454. doi: 10.1097/00000542-199911000-00038. [DOI] [PubMed] [Google Scholar]


