Abstract
Background and aim: Patients with genotype 4 (G4) chronic hepatitis C (CHC) are considered a difficult to treat population, although current data on G4 treatment responsiveness and duration are controversial. Greece represents a country with an intermediate prevalence of G4 infections, offering an opportunity to compare treatment outcomes by genotype and to identify potential prognostic factors for sustained virologic response (SVR).
Methods: All CHC patients from the HepNet.Greece, an ongoing nationwide cohort study on viral hepatitis, with known hepatitis C virus (HCV) genotype who received treatment with Peg-IFNa and ribavirin were analyzed.
Results: From 4443 patients, 951 (61.7% males, 78.4% Greeks, median age 40.6 years, 10% cirrhosis) fulfilled the inclusion criteria. G4 was found in 125 (13.1%) patients. Genotype distribution was not significantly different between Greeks and immigrants. Patients with G4 had similar odds of SVR compared to G1 but significantly lower compared to G2/G3. Age, treatment discontinuation, presence of cirrhosis and previous history of HCV-treatment were associated with lower probabilities of SVR. Ethnicity did not affect SVR for all genotypes while response to treatment was similar between Greek and Egyptian patients groups (35.7% vs 40.9%, p=0.660%) with G4 infection. The relation between SVR and genotype did not substantially change after adjustment for age, gender, cirrhosis, treatment interruption and history of HCV-treatment.
Conclusions: The findings of this large cohort of CHC patients with a well balanced genotype distribution further supports the idea of considering G4 as a difficult to treat genotype. Further investigation is needed to identify genotype specific prognostic factors.
Keywords: Viral hepatitis, HCV treatment, interferon, pegylated-interferon
Introduction
Chronic infection with hepatitis C virus (HCV) is an important public health problem, which affects 150 - 185 million persons worldwide1,2. It has been estimated that the incidence and the consequences of chronic liver disease due to HCV infection will be increased during the next decade as a result of the limited number of patients who are receiving anti-viral treatment3.
The high genetic heterogeneity (6 different HCV genotypes and a significant number of subtypes) represents an important feature of viral strain, which affects duration and response to treatment4. Patients infected with HCV genotype (G) 2 or 3 seem to respond faster and better to the combination of pegylated interferon-alpha (Peg-IFNa) and ribavirin compared to those infected with HCV G15-7. Although major advances with the development of direct-acting antiviral (DAA) agents have changed the optimal treatment regimen for patients with G1 infection8 the Peg-IFNa and ribavirin combination for 24 and 48 weeks remains the standard of care for patients with G2/3 and 4 respectively9. However, current data on G4 chronic hepatitis C (CHC) regarding treatment responsiveness and duration are limited and controversial10.
HCV genotypes show a large degree of geographically defined distribution11. G1, followed by G2 and 3, are the most common HCV genotypes in North and South America and Europe while G4 is the most prevalent in Egypt, Saudi Arabia, Middle East and North African countries12.
G4 is found in approximately 15% of all HCV infected individuals in Greece13-15. Thus, Greece represents a country with G4 prevalence higher than that observed in Western Europe and USA and lower than that in Middle East, offering an opportunity to compare anti-HCV treatment outcomes by genotype and to identify potential prognostic factors for Sustained Virologic Response (SVR).
For this purpose we used data from the HepNet.Greece network, a large collaboration cohort of individuals with CHC and chronic hepatitis B (CHB) infections throughout Greece14,16,17.
Patients and methods
In 2003 the HepNet.Greece network was established with the support of the Hellenic Center for Infectious Disease Control and Prevention (KEELPNO) aiming to collect and evaluate data on patients with CHB and CHC in Greece14,16,17. Twenty-five Centers throughout the country participated in the network, and enrolled all patients with CHB and CHC followed in the Centers from January 1997 to June 2003 and then prospectively followed them, along with all new cases, till March 2009.
A structured case report form was made including detailed data on demographic, clinical, biochemical, virological, serological and histological characteristics of the patients, as well as a detailed therapy history. Prior to the network establishment (i.e., before 2003) data were collected retrospectively from the patients’ medical records and prospectively updated twice per year thereafter.
The study protocol was reviewed and approved by the Governing Board of KEELPNO.
All individuals with CHC enrolled in the HepNet.Greece study, with known HCV genotype and treated by Peg-IFNa plus ribavirin for at least 2 weeks (to avoid including patients who have been prescribed but never started antiviral treatment), were considered for inclusion in the current analysis. Patients co-infected with HBV; aged less than 14 years at treatment initiation and those who were under treatment or they have not completed the 6 months follow-up period at the data frozen date were excluded from the analysis. History of previous treatment with non Peg-IFNa with or without ribavirin and with Peg-IFNa monotherapy was not considered as an exclusion criterion.
Diagnosis of cirrhosis was made histologically, when a baseline liver biopsy was available. Otherwise, classification was based on ultrasound features (presence of hepatic nodules, splenomegaly, diameter of portal vein >16mm)18. The non-invasive test APRI (AST to Platelet Ratio Index) was also used in order to assess liver fibrosis. APRI score was calculated according to the formula proposed by Wai et al19. Alcohol abuse was defined as consumption of more than one drink daily.
Decision for treatment initiation followed the national or international guidelines, using the standard clinical and laboratory criteria. Treatment discontinuation was based on physicians’ discretion when major side effects were observed or when patient could not fulfill the treatment schedule. Reasons for treatment discontinuation were classified according to the cause of interruption: no response, major side effects and patient’s willingness.
Commercial PCR methods [bDNA, Limit of Detection (LOD) <615 IU/ml; Amplicor HCV Monitor, LOD <50 IU/ml; Versant TMA, LOD <15 IU/ml] were used to determine serum HCV RNA levels. Undetectable serum HCV RNA at the end of treatment was considered as End of Treatment Response (ETR), while SVR was defined as undetectable HCV RNA at the 6th month follow-up visit. Patients were classified in three groups according to their response to treatment: responders (R) when both ETR and SVR were observed, non responders (NR) when both ETR and SVR were absent and responders/relapsers (RR) when ETR, but not SVR, was observed.
Statistical methods
Description of the quantitative variables was based on the median and the interquartile range (IQR). For the qualitative variables counts and percents were used. Bivariate comparisons of SVR with demographic and medical characteristics of the patients were performed using the non-parametric Mann-Whitney test for the quantitative variables and the Pearson’s Chi-Square test for the qualitative ones. In order to investigate the effect of HCV genotype on SVR, adjusting also for potential confounders, we applied multiple logistic regression analysis. Missing SVR status was filled in using the multiple-imputations method20. Briefly, a multiple logistic model, including all, biologically and statistically, relevant covariates was fitted in patients with known SVR status. The fitted model was then used to impute missing cases 20 times. Logistic regression analysis was carried out on each imputed dataset and finally, results were combined using standard methods. The multiple-imputations method relies on the assumption that data are missing at random (MAR), which implies that the probability of missingness is independent of the true SVR status conditioning on other prognostic factors. Three sensitivity analyses were performed: firstly, analysis was restricted to those with known SVR status (complete case analysis); secondly, following the intend to treat principle, patients with missing SVR status were considered as not having achieved sustained virology response; thirdly, analysis was restricted to HCV treatment naïve patients.
Results
Demographic data
The HCV HepNet.Greece database, updated in May 2009, included data from 4.443 patients, of whom 1689 (38%) initiated treatment with Peg-IFNa and ribavirin. Excluded were: 39 HBV co-infected cases; 5 aged less than 14 years at treatment initiation and 482 whose follow-up time from treatment end till May 2009 was less than 6 months. Of the 1163 eligible patients, HCV genotype was available for 951 (81.8%). G4 was found in 125 (13.1%) of them, while the most frequent genotype was G1 (n=405, 42.5%). G2 and 3 were found in 81 (8.5%) and 340 (35.6%) patients, respectively. Genotype 5 was found in 3 patients only, who were excluded from further analysis. Descriptive characteristics of the final study population by HCV genotype and overall, are presented in Table 1.
Table 1. Characteristics of patients with chronic hepatitis C overall and by hepatitis C virus (HCV) genotype.
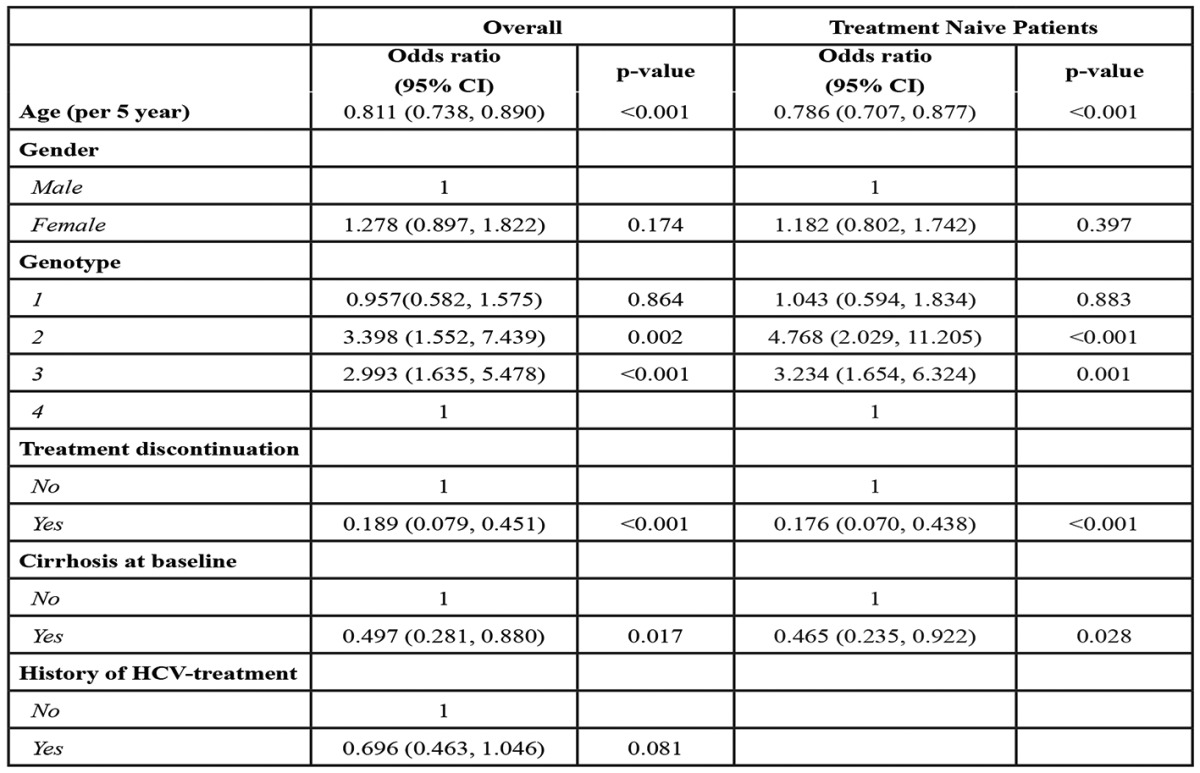
* Interquartile Range, †Alanine Aminotransferase, ‡Aspartate Aminotransferase, §Total Bilirubin, ¶Platelets.
The majority of patients were Greeks (681/869, 78.4%); among non Greeks the larger group (82/188, 43.6%) consisted of patients coming from former Soviet Union countries whereas Egyptians were the next group in frequency (29/188, 15.4%). The most commonly reported sources of infection were intravenous drug use (31.2%) and transfusion (22.0%). Source of infection was unknown in 288 (30.3%) patients. Histological evidence of cirrhosis was present in 68 (9.9%) out of 684 patients with available biopsy prior to treatment. In 27 out of 267 non biopsied patients (10.1%), diagnosis of cirrhosis was based on clinical signs or imaging.
Genotype distribution was not significantly different between patients of Greek origin and immigrants (p=0.115), although G4 was slightly less prevalent in Greeks compared to those of other nationalities (12.6% vs 16.5%). Twenty five out of 29 Egyptians (86.2%) were infected by G4. Age at treatment initiation did not differ between G1 and 4 patients [median (IQR) age: 46.1 (34.0, 58.9) vs 44.4 (37.0, 55.7) respectively], whereas the youngest among all patients were those infected with G3 [median (IQR) age 34.4 (26.6, 42.2) years].
Therapeutic outcome
Treatment related characteristics according to HCV genotype are presented in Table 2. One hundred and ninety two patients (20.2%) had received anti HCV treatment (interferon-alpha with or without ribavirin or Peg-IFNa monotherapy) before initiating standard combination therapy with Peg-IFNa and ribavirin.
Table 2. Antiviral treatment characteristics of patients with chronic hepatitis C overall and by hepatitis C virus (HCV) genotype.
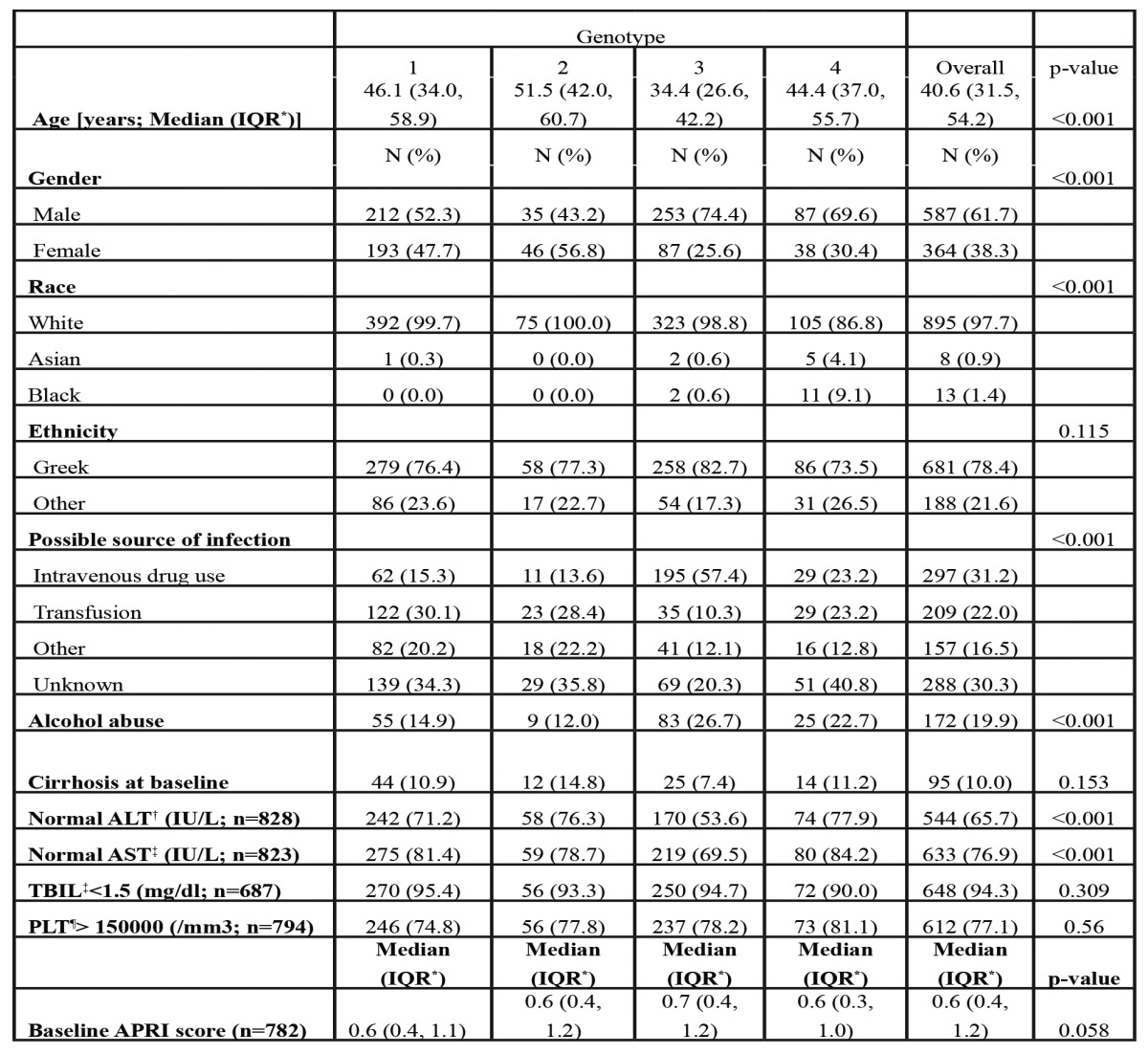
†N=687 for those with available laboratory or clinical information at the end of treatment and at 6 months afterwards, ‡N=799 for those with available laboratory or clinical information at 6 months after the end of treatment.
Of the 951 patients who initiated Peg-IFNα and ribavirin, 239 (25.1%) interrupted treatment, mainly due to side effects (n=128, 53.6%) or patients’ non-compliance (n=55, 23.0%; Table 2). The percentage of treatment interruption due to side effects was slightly higher in patients infected by G1 (17.2%) compared to those infected by G4 (13.6%). The overall genotype effect on treatment interruption due to side effects was not significant in the multivariable analysis (adjusted P=0.383) with an overall median time of treatment duration before discontinuation of 3.5 months.
In total, SVR was achieved in 414/799 (51.8%) patients, whereas information on ETR and SVR was not available for 218 (22.9%) and 152 (16.0%) patients respectively. SVR rates according to different patients characteristics are shown in Table 3. Patients with G1 and 4 achieved lower SVR rates (37.6% and 37.1% respectively) compared to those with G2 or 3 (64.8% and 72.9% respectively). Gender, race, ethnicity and type of PegIFNa used did not seem to affect SVR rates significantly. Patients with cirrhosis had lower response rates (25.9% vs 54.9% for non-cirrhotics, P<0.001). Patients with SVR were younger, had lower APRI score and a shorter duration of infection. Treatment naïve patients achieved more frequently SVR compared to those previously treated (57.2% vs 31.1%, P<0.001). The SVR rates among treatment naïve patients were 41.8%, 67.7%, 74.6% and 41.1% for patients with G1, 2, 3 and 4 CHC respectively.
Table 3. Sustained Virological Response (SVR) to antiviral treatment and chronic hepatitis C (CHC) patients characteristics.
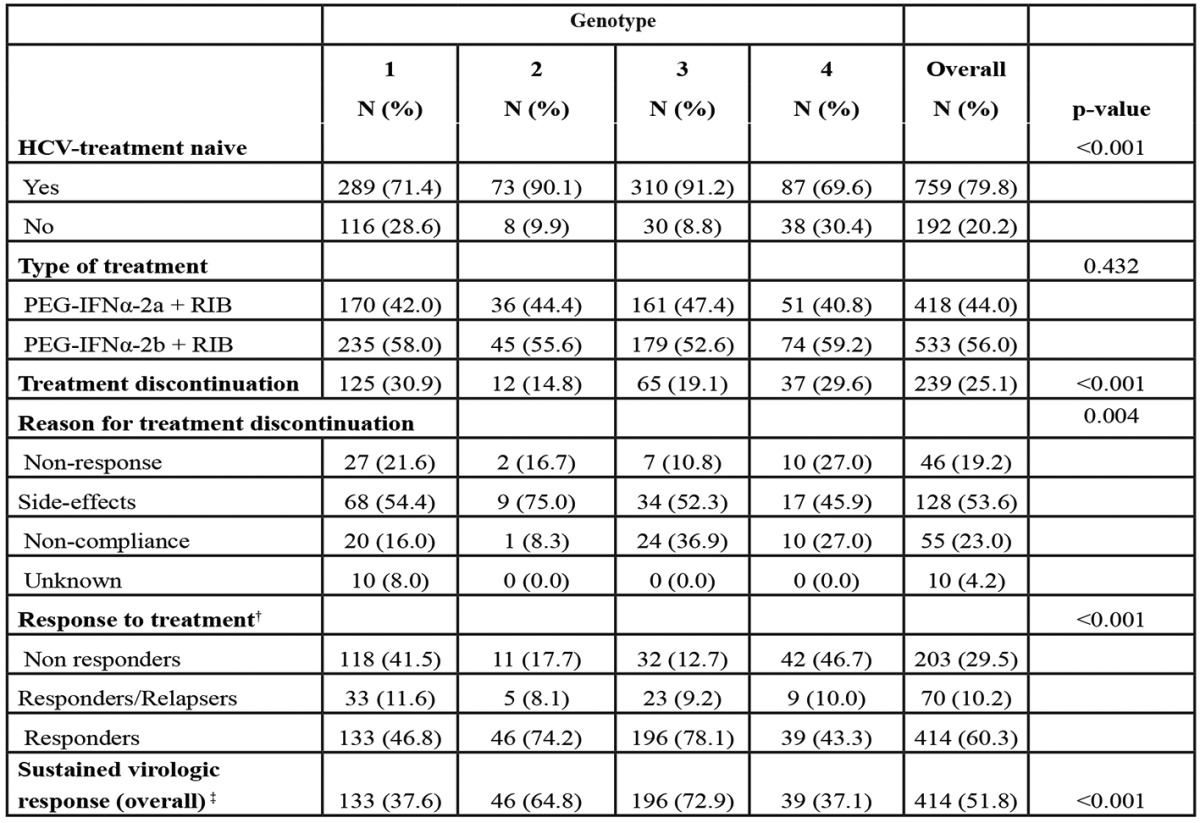
†Alanine Aminotransferase, ‡Aspartate Aminotransferase, §Total Bilirubin, ¶Platelets, ††N=799 for those with available laboratory or clinical information at 6 months after the end of treatment, * Interquartile Range.
For all genotypes, ethnicity did not affect SVR, whereas response to treatment was similar between Greek and Egyptian patient groups (35.7% vs 40.9%, P=0.660) with G4 infection.
Multivariable analysis
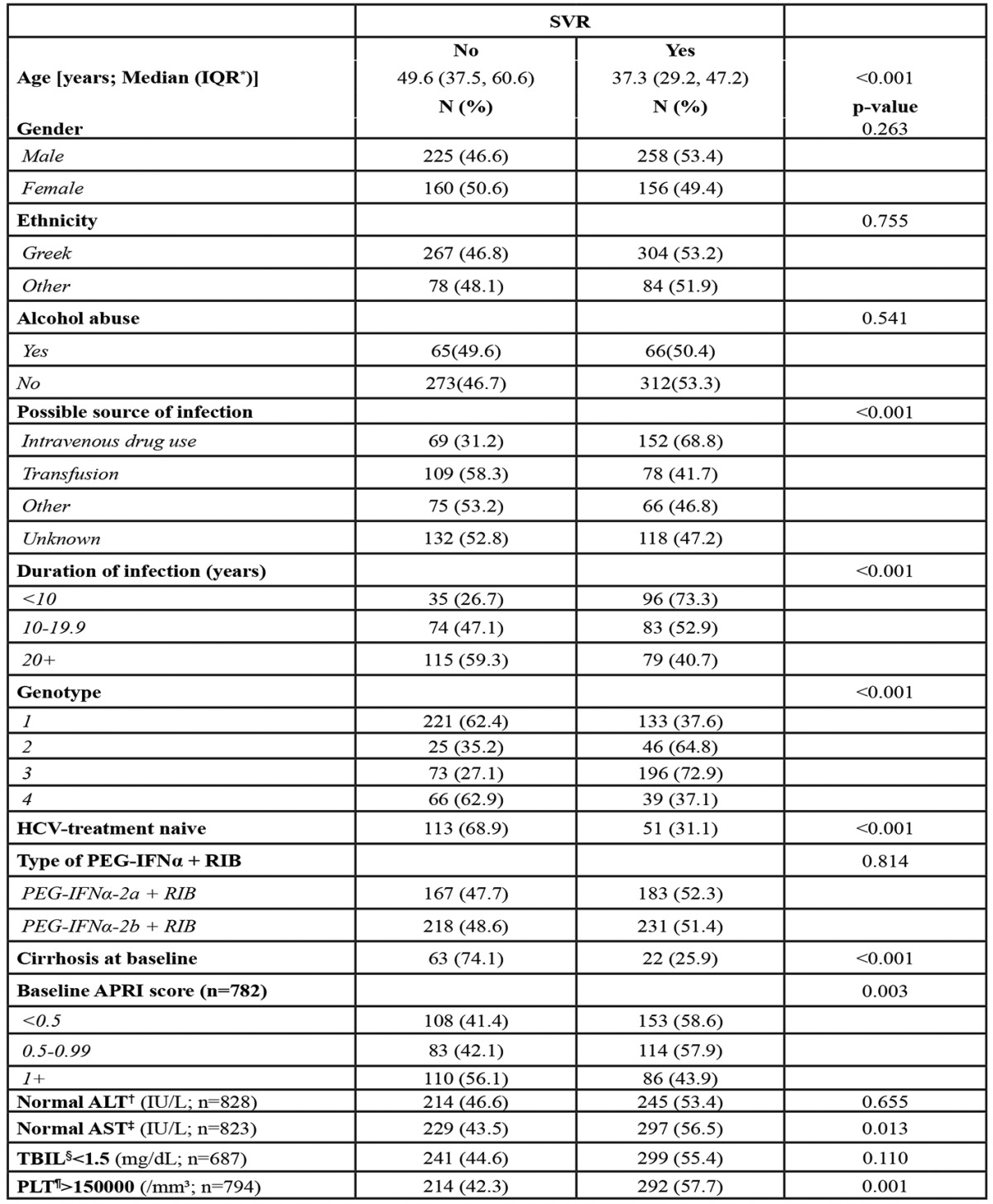
Results from the multivariable analysis of SVR are shown in Table 4. As mentioned before, the multiple imputations method was applied to deal with missing SVR status in 152 patients. The relation between SVR and genotype did not substantially change after adjustment for age, gender, cirrhosis status, treatment interruption status and previous history of HCV-treatment. G4 was associated with similar odds of SVR compared to G1, but significantly lower odds of SVR compared to G2 and 3. Older age at treatment initiation, presence of cirrhosis, treatment discontinuation and previous history of HCV-treatment were negatively associated with the probability of SVR. Results were almost identical when the analysis was restricted to patients with known SVR status (complete case analysis). Results from the sensitivity analysis considering patients with missing SVR status as non-responders confirmed the robustness of our main results. Restricting the analysis to the treatment of naïve patients gave similar to the main analysis results, regarding the by genotype differences (Table 4).
Table 4. Factors associated with Sustained Virologic Response (SVR) probability: Results from a multiple logistic regression model after applying the multiple-imputations method to deal with the missing SVR status.
Discussion
In this multicenter nationwide study, we analyzed data from a sizeable cohort of patients with CHC who were included in the HepNet.Greece cohort and were treated with the combination of Peg-IFNa plus ribavirin. Our data clearly showed identical response rates between patients with G4 and G1 HCV infections, which were significantly lower than those observed in G2/3 patients. In addition, we confirmed that cirrhotic patients, the elderly, those with a previous treatment failure and those who discontinued treatment are less likely to achieve SVR independently of HCV genotype.
The majority of data regarding HCV treatment are derived from clinical studies focusing on patients with G1, 2 and 3 infections. Despite the high prevalence of G4 in many parts of the world, data on treatment outcome, optimal duration of treatment and factors that may affect response rates in CHC patients with G4, are limited. The wide geographic variation in HCV genotypes prevalence, as reflected by the small percentage of G4 in Western countries, in association with the higher proportion of G4 in African and Arabic countries, may explain the scarcity of the existed data. It is of note that, approximately, only 3% of the patients participating in large clinical trials had G4 infection5,6. As a result, the recommendations by international panels for the treatment of G4 patients are based on limited and not robust data. Treatment duration, response guided treatment, new pharmaceutical agents, SVR predictors and clinical management guidelines remain a matter of consideration in G4 patients21.
In this study we analyzed data from 951 patients with chronic hepatitis C receiving combination of Peg-IFN-α and ribavirin in 25 centers throughout the country. In contrast to most previously published studies22-28, in our report, we present data after a meticulous analysis from the whole cohort in order to reveal possible differences in response rates among HCV genotypes. Since Greece seems to have the highest prevalence of G4 infections in Europe, we were able to compare treatment outcome and factors that may affect it, across all genotypes. Indeed, in our study, G4 infection was found in 13.1% of CHC patients while G1 was detected in 42.5% of them.
In this analysis, the average rate of SVR in patients with G4 infection was 37.1%, being 41.1% in treatment naïve patients. These results are compatible with those reported from a recent Greek study28 but in contrast to those from clinical trials conducted in Egypt and Middle East, where combination treatment with Peg-IFNa and ribavirin resulted in SVR rates up to 65-69% in G4 patients22-25. The reasons for this discrepancy remain unclear; variations in epidemiological, clinical and virological features, associated with poor response to treatment, may explain the finding. However, these comparisons should be treated cautiously due to the lack of a head to head comparison and the small number of patients included in these trials22-25.
A study from France investigated a relatively large cohort of European, Egyptian and African ΕCV G4 patients and showed that Egyptian patients had higher SVR rates compared to European and African ones26. In another French study it was found that among patients with G4, those with subtype 4a infection27, which is more common in Egypt28, had higher response rate. Interestingly, a Greek molecular epidemiology study showed that 4a is the most common subtype among Greek patients with G4 (78%) as well13. Therefore, one might expect G4 studies from Greece to report SVR rates similar to those from Egypt, which however does not appear to be the case29. It could be hypothesized that different treatment susceptibility due to heterogeneity within subtype 4a30 may explain the observed differences.
Another hypothesis could be based on a different immune response due to different host genetic background. In fact, according to recent studies, IL28 C/C polymorphism is a strong predictor of treatment outcome to Peg-IFN plus ribavirin in G4 infected patients31-33. Thus, a different pharmacogenomic profile between people of Greek and Egyptian origin might explain the differences in SVR rates, but population data confirming this hypothesis are lacking. Of note however, Egyptian origin was not associated with higher SVR rates in our multivariate analysis, but this could be due to the relatively small number of Egyptian individuals included in the study (n=29 of whom 23 with G4).
Previous studies revealed that Peg-IFNalpha-2a is significally more efficient than Peg-IFNalpha-2b on G4 CHC34,35. However, our analysis did not reveal any difference on SVR between the two pegylated interferons, both in univariable and multivariable analysis. The different result could be possibly explained by the non randomized design of our study, although the distribution of genotypes was similar between the available pegylated interferons.
In our cohort, treatment naïve patients achieved significantly higher SVR response rates compared to experienced ones. Recognizing that patients, who have been treated in the past by IFN, with or without ribavirin, do not have the same odds of SVR with treatment naïve cases, we adjusted our statistical analysis for history of treatment. There is no evidence of an interaction between G4 and previous treatment history, regarding SVR (p-value=0.67). Furthermore, when we restricted the analysis only to naïve patients (sensitivity analysis) the results regarding differences by HCV genotype remained practically unchanged.
Despite the fact that in G2/3 treatment duration is significantly shorter, we did not find any genotype specific difference in treatment discontinuation due to adverse events or no compliance. In our study, discontinuation rate due to reasons not related to treatment response (20.3%) is higher than that observed in clinical trials. However we believe that our study reflects the real life clinical experience of CHC treatment. Actually, in the multivariable analysis, the only factor significantly associated with discontinuation of treatment was low educational level (data not shown).
Using a rigorous statistical analysis we did not reveal any virus, host or treatment related factor, whose impact on SVR was differentiated according to the genotype. Older age, presence of cirrhosis, previous treatment failure and discontinuation of treatment schedule were associated with treatment failure similarly across all genotypes.
However, our study has several limitations. First of all, the retrospective design is associated with recall, recording and selection bias. Additionally, in this study we were unable to analyze factors that may affect SVR, such as body mass index, baseline or on treatment virological parameters, because our data were derived from the daily clinical practice where availability of laboratory examinations is sometimes limited. Nevertheless, we believe that the multicenter nature of our network, the large size of our cohort and the rigorous analysis with the multiple imputations method, minimized potential biases and enhanced the power of our analysis.
In the coming years, treatment against HCV may change as several agents are under investigation with promising results. Currently, the use of protease inhibitors (boceprevir or telaprevir) in combination with the standard of care is the optimal therapy only for patients with G1 infection. Boceprevir does not seem to be effective against G4 with the present regimen, while limited evidence in a small proof of concept study has shown that telaprevir may have some antiviral activity in patients with G4 infection36. In addition, new agents with pangenotypic activity are under investigation in combination or not with Peg-IFN and ribavirin. Until the new treatment regimens against HCV G4 infection are clarified, the Peg-IFN plus ribavirin combination would be the standard of care.
In conclusion, in this large sized cohort of CHC patients we showed clearly that Caucasian patients with G4 HCV infection should be considered as a difficult to treat population. Further investigation is needed to identify genotype specific prognostic factors and to clarify the nature of the differences observed among different ethnicity groups of patients infected by HCV G4.
Funding
This work has been funded by the Hellenic Center for Disease Control and Prevention (HCDCP, KEELPNO, Greece).
Conflict of Interest
None of the authors has any financial or personal relationships with people or organizations that could inappropriately influence this work, although most of them have, at some stage in the past, received funding from a variety of pharmaceutical companies for research, travel grants, speaking engagements or consultancy fees.
Acknowledgments
We thank the Hellenic Center for Disease Control and Prevention (HCDCP, KEELPNO, Greece) for sponsoring the HepNet.Greece network. We also thank all HepNet.Greece network collaborators.
References
- 1.World Health Organization Hepatitis C. WHO fact sheet 164. Available at http://www.who.int/mediacentre/factsheets/fs164/en/, accessed 10 November 2013.
- 2.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 3.Sypsa V, Touloumi G, Papatheodoridis GV, Tassopoulos NC, Ketikoglou I, Vafiadis I, et al. Future trends of HCV-related cirrhosis and hepatocellular carcinoma under the currently available treatments. J Viral Hepat. 2005;12:543–550. doi: 10.1111/j.1365-2893.2005.00588.x. [DOI] [PubMed] [Google Scholar]
- 4.Simmonds P, Bukh J, Combet C, Deléage G, Enomoto N, Feinstone S, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–973. doi: 10.1002/hep.20819. [DOI] [PubMed] [Google Scholar]
- 5.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 6.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 7.Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 8.Welsch C, Jesudian A, Zeuzem S, Jacobson I. New direct-antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut. 2012;61 Suppl 1:i36–i46. doi: 10.1136/gutjnl-2012-302144. [DOI] [PubMed] [Google Scholar]
- 9.Antaki N, Craxi A, Kamal S, Moucari R, Van der Merwe S, Haffar S, et al. The neglected hepatitis C virus genotypes 4, 5 and 6: an international consensus report. Liver Int. 2010;30:342–355. doi: 10.1111/j.1478-3231.2009.02188.x. [DOI] [PubMed] [Google Scholar]
- 10.Ghany MG, Strader DB, Thomas DL, Seeff LB;American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen MH, Keeffe EB. Prevalence and treatment of hepatitis C virus genotypes 4, 5 and 6. Clin Gastroenterol Hepatol. 2005;3:S97–101. doi: 10.1016/s1542-3565(05)00711-1. [DOI] [PubMed] [Google Scholar]
- 13.Katsoulidou A, Sypsa V, Tassopoulos NC, Boletis J, Karafoulidou A, Ketikoglou I, et al. Molecular epidemiology of hepatitis C virus (HCV) in Greece: temporal trends in HCV genotype-specific incidence and molecular characterization of genotypes 4 isolates. J Viral Hepat. 2006;13:19–27. doi: 10.1111/j.1365-2893.2005.00649.x. [DOI] [PubMed] [Google Scholar]
- 14.Raptopoulou M, Touloumi G, Tzourmakliotis D, Nikolopoulou G, Dimopoulou M, Giannoulis G, et al. Significant epidemiological changes in chronic hepatitis C infection: results of the nationwide HEPNET-GREECE cohort study. Hippokratia. 2011;15:26–31. [PMC free article] [PubMed] [Google Scholar]
- 15.Savvas SP, Koskinas J, Sinani C, Hadziyannis A, Spanou F, Hadziyannis SJ. Changes in epidemiological patterns of HCV infection and their impact on liver disease over the last 20 years in Greece. J Viral Hepat. 2005;12:551–557. doi: 10.1111/j.1365-2893.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- 16.Manesis EK, Papatheodoridis GV, Touloumi G, Karafoulidou A, Ketikoglou J, Kitis GE, et al; HEPNET. Greece Cohort Study. Natural course of treated and untreated chronic HCV infection: results of the nationwide Hepnet.Greece cohort study. Aliment Pharmacol Ther. 2009;29:1121–1130. doi: 10.1111/j.1365-2036.2009.03974.x. [DOI] [PubMed] [Google Scholar]
- 17.Raptopoulou M, Papatheodoridis G, Antoniou A, Ketikoglou J, Tzourmakliotis D, Vasiliadis T, et al. Epidemiology, course and disease burden of chronic hepatitis B virus infection. HEPNET study for chronic hepatitis B: a multicentre Greek study. J Viral Hepat. 2009;16:195–202. doi: 10.1111/j.1365-2893.2008.01057.x. [DOI] [PubMed] [Google Scholar]
- 18.Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89–94. doi: 10.1148/radiol.2272020193. [DOI] [PubMed] [Google Scholar]
- 19.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 20.Rubin DB. Multiple Imputations for Nonresponse in Surveys. Wiley, New York. 1987 [Google Scholar]
- 21.Khattab MA, Ferenci P, Hadziyannis SJ, Colombo M, Manns MP, Almasio PL, et al. Management of hepatitis C virus genotype 4: recommendations of an international expert panel. J Hepatol. 2011;54:1250–1262. doi: 10.1016/j.jhep.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 22.El-Zayadi AR, Attia M, Barakat EM, Badran HM, Hamdy H, El-Tawil A, et al. Response of hepatitis C genotype-4 naïve patients to 24 weeks of Peg-interferon-alpha2b/ribavirin or induction-dose interferon-alpha2b/ribavirin/amantadine: a non-randomized controlled study. Am J Gastroenterol. 2005;100:2447–2452. doi: 10.1111/j.1572-0241.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 23.Kamal SM, El Tawil AA, Nakano T, He Q, Rasenack J, Hakam SA, et al. Peginterferon {alpha}-2b and ribavirin therapy in chronic hepatitis C genotype 4: impact of treatment duration and viral kinetics on sustained virological response. Gut. 2005;54:858–866. doi: 10.1136/gut.2004.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamal SM, El Kamary SS, Shardell MD, Hashem M, Ahmed IN, Muhammadi M, et al. Pegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: The role of rapid and early virologic response. Hepatology. 2007;46:1732–1740. doi: 10.1002/hep.21917. [DOI] [PubMed] [Google Scholar]
- 25.Hasan F, Asker H, Al-Khaldi J, Siddique I, Al-Ajmi M, Owaid S, et al. Peginterferon alfa-2b plus ribavirin for the treatment of chronic hepatitis C genotype 4. Am J Gastroenterol. 2004;99:1733–1737. doi: 10.1111/j.1572-0241.2004.40077.x. [DOI] [PubMed] [Google Scholar]
- 26.Moucari R, Ripault MP, Martinot-Peignoux M, Voitot H, Cardoso AC, Stern C, et al. Insulin resistance and geographical origin: major predictors of liver fibrosis and response to peginterferon and ribavirin in HCV-4. Gut. 2009;58:1662–1669. doi: 10.1136/gut.2009.185074. [DOI] [PubMed] [Google Scholar]
- 27.Roulot D, Bourcier V, Grando V, Deny P, Baazia Y, Fontaine H, et al; Observational VHC4 Study Group. Epidemiological characteristics and response to peginterferon plus ribavirin treatment of hepatitis C virus genotype 4 infection. J Viral Hepat. 2007;14:460–467. doi: 10.1111/j.1365-2893.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 28.Ray SC, Arthur RR, Carella A, Bukh J, Thomas DL. Genetic epidemiology of hepatitis C virus throughout Egypt. J Infect Dis. 2000;182:698–707. doi: 10.1086/315786. [DOI] [PubMed] [Google Scholar]
- 29.Papastergiou V, Dimitroulopoulos D, Skorda L, Lisgos P, Ketikoglou I, Kostas N, et al. Predictors of sustained virological response in Greek and Egyptian patients with hepatitis C genotype 4: does ethnicity matter? J Med Virol. 2012;84:1217–1223. doi: 10.1002/jmv.23324. [DOI] [PubMed] [Google Scholar]
- 30.Genovese D, Dettori S, Argentini C, Villano U, Chionne P, Angelico M, et al. Molecular epidemiology of hepatitis C virus genotype 4 isolates in Egypt and analysis of the variability of envelope proteins E1 and E2 in patients with chronic hepatitis. J Clin Microbiol. 2005;43:1902–1909. doi: 10.1128/JCM.43.4.1902-1909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asselah T, De Muynck S, Broët P, Masliah-Planchon J, Blanluet M, Bièche I, et al. IL28B polymorphism is associated with treatment response in patients with genotype 4 chronic hepatitis C. J Hepatol. 2012;56:527–532. doi: 10.1016/j.jhep.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 32.De Nicola S, Aghemo A, Rumi MG, Galmozzi E, Valenti L, Soffredini R, et al. Interleukin 28B polymorphism predicts pegylated interferon plus ribavirin treatment outcome in chronic hepatitis C genotype 4. Hepatology. 2012;55:336–342. doi: 10.1002/hep.24683. [DOI] [PubMed] [Google Scholar]
- 33.Derbala M, Rizk N, Shebl F, Alkaabi S, Eldweik N, John A, et al. Interleukin-28 and hepatitis C virus genotype-4: treatment-induced clearance and liver fibrosis. World J Gastroenterol. 2012;18:7003–7008. doi: 10.3748/wjg.v18.i47.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ascione A, De Luca M, Tartaglione MT, Lampasi F, Di Costanzo GG, Lanza AG, et al. Peginterferon alfa-2a plus ribavirin is more effective than peginterferon alfa-2b plus ribavirin for treating chronic hepatitis C virus infection. Gastroenterology. 2010;138:116–122. doi: 10.1053/j.gastro.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Rumi MG, Aghemo A, Prati GM, D’Ambrosio R, Donato MF, Soffredini R, et al. Randomized Study of peginterferon-alpha2a plus ribavirin vs peginterferon-alpha2b plus ribavirin in chronic hepatitis C. Gastroenterology. 2010;138:108–115. doi: 10.1053/j.gastro.2009.08.071. [DOI] [PubMed] [Google Scholar]
- 36.Benhamou Y, Moussalli J, Ratziu V, Lebray P, Gysen V, de Backer K, et al. Results of a proof of concept study (C210) of telaprevir monotherapy and in combination with peginterferon alfa-2a and ribavirinin treatment naïve G4 HCV patients. J Hepatol. 2009;50:S6. [Google Scholar]


