Abstract
Endothelial dysfunction develops with age and increases the risk of age-associated vascular disorders. Nitric oxide insufficiency, oxidative stress, and chronic low-grade inflammation, induced by upregulation of adverse cellular signaling processes and imbalances in stress resistance pathways, mediate endothelial dysfunction with aging. Healthy lifestyle behaviors preserve endothelial function with aging by inhibiting these mechanisms, and novel nutraceutical compounds that favorably modulate these pathways hold promise as a complementary approach for preserving endothelial health.
Populations are aging worldwide. The number of older adults is projected to double over the next few decades, and we are ill-equipped to handle the attendant health care burden. Physiological dysfunction develops with advancing age, which increases the risk of clinical disease and leads to functional limitations. As such, we are facing a new epidemic of chronic degenerative diseases, disability, and associated health care costs driven by population aging (27, 136). An essential strategy for addressing this challenge is to identify and implement approaches that prevent or delay these events until an older age (“compression of dysfunction, morbidity, and disability”) (64, 92, 116). This would extend “healthspan”, i.e., the portion of the lifespan during which function is sufficient to maintain autonomy, control, independence, productivity, and well being (91, 101). The key to achieving this objective is establishing evidence-based approaches that preserve physiological function with aging.
Cardiovascular Disease and Endothelial Dysfunction
Despite declines in prevalence over recent decades, cardiovascular diseases (CVD) remain the leading cause of morbidity, disability, and death in modern societies. Atherosclerosis, characterized by recruitment of white blood cells to the arterial wall, a subsequent accumulation of lipids, and eventual lesion formation, is the main pathophysiological process underlying clinical CVD, and arterial endothelial dysfunction is the primary antecedent for atherosclerotic diseases (69, 77, 98).
The vascular endothelium is a single cell layer that forms the inner lining of arteries and represents the interface between the flow of blood in the lumen and the medial and adventitial layers of the arterial wall (FIGURE 1). The endothelium secretes a broad array of biologically active molecules that act in autocrine and/or paracrine fashion to modulate the function and health of the artery and surrounding tissue (28, 63, 117). In healthy arteries, the balance of the molecules synthesized and released by the endothelium favors vasodilation and inhibition of coagulation, proliferation, and inflammation, whereas endothelial dysfunction can be defined as any phenotype in which this normal functional state is altered (i.e., toward a vasoconstrictor, pro-coagulative, proliferative, and pro-inflammatory profile) (3, 55, 166). Nitric oxide (NO) exerts a vascular-protective influence on all of these processes and is therefore considered to be the most important endothelium-derived molecule. Indeed, sufficient bioavailability of NO is essential for optimal arterial function and health (63, 196).
FIGURE 1.
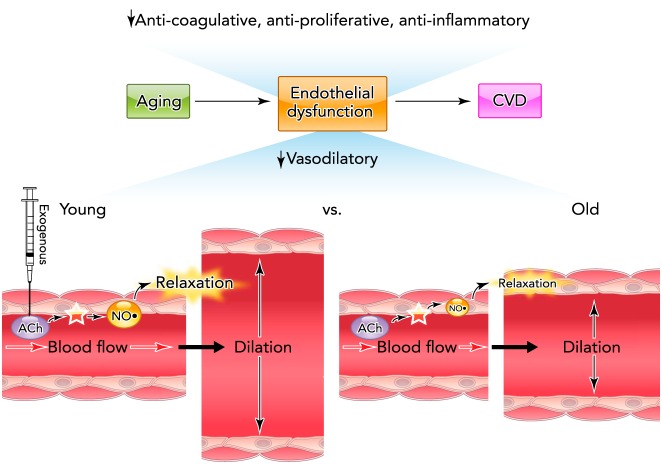
Vascular endothelial dysfunction and CVD risk with aging
Age-related vascular endothelial dysfunction is characterized by reductions in anti-coagulative, anti-proliferative, anti-inflammatory, and vasodilatory processes, all of which increase CVD risk. Nitric oxide (NO)-mediated endothelium-dependent dilation (EDD) in response to blood flow or pharmacological stimuli such as acetylcholine (ACh) is an important physiological indicator of endothelial function. EDD is impaired in older rodents and humans as a result of reduced NO bioavailability.
The most commonly used method of assessing endothelial function is to measure the dilation produced by a mechanical (increase in blood flow or shear rate) or chemical (typically acetylcholine) stimulus that activates endothelial NO synthase (eNOS) and increases the production of NO, resulting in cyclic guanosine monophosphate-induced vascular smooth muscle relaxation, a set of processes collectively termed NO-mediated “endothelium-dependent dilation” (EDD) (FIGURE 1). EDD can also be modulated by other endothelium-derived molecules and processes, including endothelin-1, vasodilatory and vasoconstrictor prostaglandins, and endothelium-dependent hyperpolarization (EDH), induced by an incompletely defined group of endothelium-derived hyperpolarizing factors (EDHF) and/or direct electrical coupling of vascular endothelial and smooth muscle cells (4, 50, 59, 192). As indicated by impaired EDD, vascular endothelial dysfunction is observed in most forms of clinical CVD, as well as in individuals with major risk factors for CVD (smoking, diabetes, dyslipidemia, hypertension, etc.) (12). Consistent with this, reduced EDD is a predictor of future CVD and CV events (141, 197, 214, 215) and is now viewed as a systemic circulatory disorder affecting multiple domains of organ and tissue function (102, 183).
Endothelial Dysfunction With Aging
Advancing age is the main risk factor for clinical CVD, and this relation is driven largely by the development of arterial dysfunction and disease (98, 113). Importantly for the present discussion, aging is associated with endothelial dysfunction characterized by reduced acetylcholine- and flow-mediated EDD (13, 25, 166, 182, 186) (FIGURE 1). Impaired EDD with aging is observed even in healthy adult humans and experimental animals free of other risk factors or clinical CVD, suggesting a primary effect of age (25, 65, 96, 104, 180, 182, 186). In addition to CVD, endothelial dysfunction is believed to play a major role in several other disorders of aging, including cognitive impairments (brain aging), Alzheimer's disease, motor dysfunction, insulin resistance, and sarcopenia (6, 76, 138, 174, 184, 205). This age-related endothelial dysfunction is mediated largely by reduced NO bioavailability (13, 55, 181, 186), although a shift to more vasoconstrictor prostaglandin production and increases in endothelin-1 also appear to contribute (46, 125). The impairment in EDD with aging also may be modulated by changes in EDH (122, 216).
Primary Mechanisms
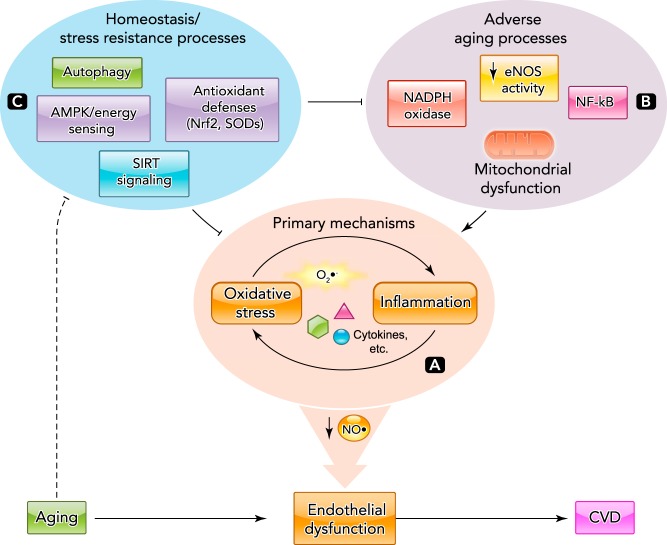
The primary pathophysiological process responsible for reduced NO bioavailability and endothelial dysfunction with aging is oxidative stress, as indicated by increased levels of cellular signatures of oxidative modification of biomolecules such as nitrotyrosine (3, 166). Vascular oxidative stress develops with aging as a result of excessive superoxide production and insufficient compensatory upregulation of antioxidant enzyme defenses (78, 104). Oxidative stress in arteries is bi-directionally associated with chronic low-grade inflammation; increases in the expression of pro-inflammatory factors, including cytokines and adhesion molecules, accompany increases in markers of vascular oxidative stress with age (54, 78). Thus insufficient NO bioavailability, superoxide-driven oxidative stress, and inflammation are the primary mechanisms of endothelial dysfunction with aging (FIGURE 2A).
FIGURE 2.
Mechanisms of age-related endothelial dysfunction
A: the primary mechanisms of endothelial dysfunction with aging are reduced NO bioavailability and increased oxidative stress/inflammation characterized by increased superoxide (O2−) and inflammatory mediators. B: various adverse cellular aging processes contribute to the development of age-associated oxidative stress and inflammation. C: cellular homeostasis and stress resistance systems protect endothelial function with aging by preventing adverse events and directly reducing oxidative stress/inflammation. Age-related impairments in these protective processes contribute to oxidative stress-/inflammation-mediated NO insufficiency and endothelial dysfunction with aging.
Vascular NO insufficiency with aging is mediated in part by decreased NO production by eNOS as a consequence of inadequate bioavailability of the essential co-factor, tetrahydrobiopterin (BH4) (37, 57, 80), and possibly by increased activity of arginase, an enzyme that competes with eNOS for the critical substrate involved in NO production, L-arginine (8, 206, 213). Increased reaction of superoxide with NO due to excessive superoxide also contributes to reductions in NO with vascular aging (3, 79, 166). This reaction leads to accelerated formation of peroxynitrite, a highly reactive nitrogen species that oxidizes BH4 to its inactive form (BH2), reducing the bioavailability of this co-factor required for NO synthesis (193), while also causing nitration of tyrosine residues on proteins (nitrotyrosine) (3).
Excessive vascular superoxide production with aging is mediated by increased expression and activity of the potent oxidant-forming enzyme NADPH oxidase, as well as by eNOS uncoupling and increased superoxide formation by the electron transport chain associated with mitochondrial dysfunction (45, 53, 188, 212). Although the relative contributions of these mechanisms are unknown, inhibiting NADPH oxidase (53), inducing eNOS recoupling by increasing BH4 bioavailabilty (57, 130, 168), and scavenging of mitochondrial superoxide with mitochondrial-specific antioxidants (67) all improve/restore NO-mediated EDD in older adults and/or rodents.
Vascular inflammation with aging is mediated in part by activation of the master pro-inflammatory transcription factor nuclear factor κB (NF-κB) (44, 105), as inhibition of NFκB and/or its downstream mediators, such as tumor necrosis factor-α (TNF-α), reverses age-associated endothelial dysfunction in older rodents and humans (32, 105, 129, 145). Collectively, these events can be considered adverse vascular aging processes that induce and sustain oxidative stress, inflammation, and endothelial dysfunction (FIGURE 2B). Recent comprehensive reviews of these and other implicated mechanisms of vascular endothelial aging are available elsewhere (3, 55, 78, 166).
Critical Upstream Homeostatic and Stress Resistance Pathways
Alterations in several critical cellular homeostatic and stress resistance pathways modulate these mechanisms of endothelial dysfunction with aging (FIGURE 2C). One is autophagy, the vital process by which cells eliminate damaged proteins and dysfunctional mitochondria, which act to suppress oxidative stress and inflammation (81, 159). Autophagy is impaired with aging in arteries of old mice and in vascular endothelial cells obtained from older healthy adult humans, and this decline in autophagy contributes to age-related vascular oxidative stress and inflammation, as well as impaired NO-mediated EDD (100).
Cellular energy-sensing pathways fundamental to cellular homeostasis also appear to influence the primary mechanisms of endothelial dysfunction with aging. Activation of adenosine monophosphate protein-activated kinase (AMPK), a master regulator of metabolism, induces antioxidant effects in endothelial cell culture (202), and activation of AMPK is reduced in the aorta of mice with aging (107). Similarly, expression and activity of sirtuin-1 (SIRT-1), an NAD+-dependent protein deacetylase and ADP-ribosyltransferase with potent energy sensing/mobilizing and anti-aging effects (29, 35, 73), is reduced in aorta of mice and vascular endothelial cells of humans with aging (47, 48, 154). The reduction in arterial SIRT-1 activity accounts for the age-related impairment in EDD in mice (47), and decreases in SIRT-1 expression in arterial endothelial cells with aging in humans are related to corresponding reductions in EDD (47). The mechanisms linking AMPK and SIRT-1 signaling to endothelial function are incompletely understood. These pathways may directly influence eNOS activity (22, 139, 151), although modulation of prostaglandin and/or EDHF regulation of EDD also is a possibility (107).
Finally, cellular stress resistance pathways, including important vascular antioxidant defense systems, are either unchanged or reduced in arteries of rodents (38, 53, 154, 175, 185) and arterial endothelial cells of healthy humans (45, 143) with advancing age, despite marked increases in oxidative stress. Many of these endogenous antioxidant pathways are controlled by the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), which binds to the antioxidant response elements (ARE) in the promoter regions of genes to induce upregulation of antioxidant enzymes such as glutathione, manganese (mitochondrial) superoxide dismutase (SOD2), and heme oxygenase (86, 109). Recent work also indicates an important role for the activated protein-1 transcription factor JunD in regulating vascular superoxide, antioxidant pathways, NO bioavailability, and endothelial function with aging in mice (137).
Taken together, these observations suggest that strategies that act to suppress vascular oxidative stress and chronic low-grade inflammation and boost NO bioavailability and/or other endothelium-dependent vasodilatory signaling pathways should be effective for maintaining healthy endothelial function with aging.
Strategies for Optimizing Endothelial Function with Aging
Possible strategies for maintaining optimal endothelial function with aging in humans may have one or more of the following features: 1) evidence of vascular-protective effects based on previous preclinical, clinical, or even epidemiological observations; 2) favorably influence the primary mechanisms and/or modulating processes of endothelial dysfunction (FIGURE 2); 3) hold translational promise (feasibility) for use in human populations; 4) show potential to both prevent age-related declines in endothelial function as well as improve/restore endothelial function in middle-aged and older adults with existing dysfunction.
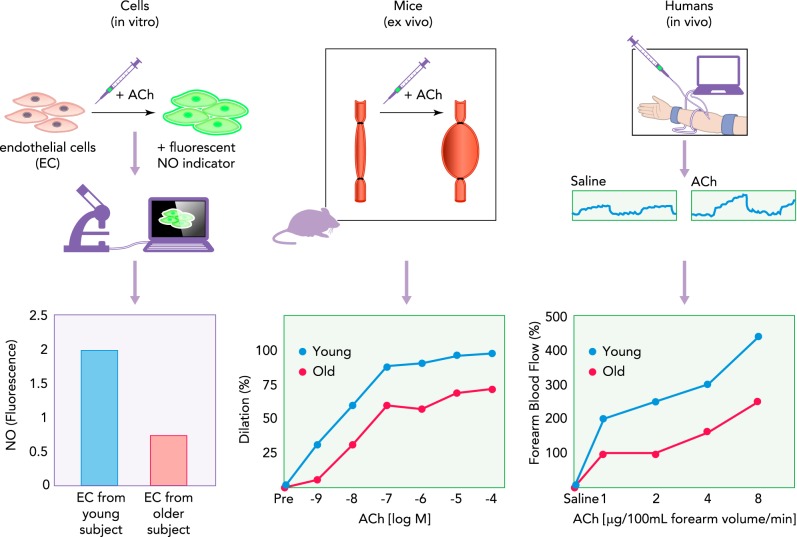
Several translational approaches are available to test candidate interventions (47, 100, 164). For example, stimulus-induced NO release in vitro in endothelial cell culture (18, 103), acetylcholine-evoked dilation of isolated arteries from rodents or humans studied ex vivo (2, 36, 156, 161), and dilation to intra-arterial infusions of acetylcholine in vivo in humans all are well established methods of assessing endothelial function (58, 88, 182, 207) (FIGURE 3). Similarly, “flow-induced” NO release/dilation can be studied in endothelial cell culture (26, 152), in isolated arteries of rodents (97, 133, 173), and in vivo in conduit arteries of humans (24, 74). “Pharmaco-dissection” of mechanisms influencing EDD also can be explored using these models. Moreover, the same molecular markers of processes of interest (nitrotyrosine, oxidant enzyme expression, inflammatory proteins) can be studied in primary or secondary endothelial cell culture, arteries of rodents, and vascular endothelial cells obtained from human subjects (43, 45, 47, 100, 187). Finally, promising prevention and therapeutic strategies, including lifestyle interventions, can be modeled and studied from cell culture to populations of humans (164).
FIGURE 3.
Example of translational assessment of endothelial function
Age-related differences in endothelial function can be examined in cell and animal models, and in human subjects. A: acetylcholine (ACh)-mediated NO production can be assessed in isolated and/or cultured endothelial cells (EC) using NO-specific fluorescent probes and microscopy. B: endothelium-dependent dilation (EDD) can be examined in arteries isolated from young and old rodents (or humans) by measuring increases in arterial diameter to cumulative addition of ACh. C: differences in EDD with aging can also be assessed in human subjects by monitoring changes in forearm blood flow in response to brachial artery infusions of ACh and/or other pharmacological agents. Another example would be flow/shear shear-stress stimulation of endothelial cells in culture, flow-mediated dilation (FMD) of arteries studied ex vivo, and in vivo assessments of FMD in humans (e.g., brachial artery FMD) (see text).
Lifestyle-Based Strategies
Aerobic exercise.
As reviewed in detail previously (165, 167), regular aerobic exercise has a strongly favorable effect on vascular endothelial function with aging. In men, aerobic exercise appears to largely prevent the decline in EDD with age and to restore EDD in middle-aged and older, previously sedentary subjects to levels observed in young adults (40, 144, 167). The preservation of endothelial function with aging by aerobic exercise in men is mediated by improved NO bioavailability secondary to reduced vascular oxidative stress (56, 179). Observations in old male rodents are consistent with these findings and show an exercise-associated increase in eNOS expression and activation (53, 172). Restored NO-mediated dilation with voluntary aerobic exercise in old mice also is associated with reduced arterial nitrotyrosine and NADPH oxidase expression/activity, and an increase in superoxide dismutase activity (53). In support of these preclinical results, older sedentary men show an endothelial oxidative stress profile featuring increased endothelial cell nitrotyrosine and NADPH oxidase, and reduced circulating endothelium-derived (extracellular) superoxide dismutase activity compared with young controls, whereas older exercising men do not demonstrate these changes (143). Regular aerobic exercise also has potent anti-inflammatory effects on aging arteries (106) and vascular endothelial cells (143), and this may be mediated in part by suppression of NF-κB signaling (105, 106, 143, 145, 200).
The effects of regular aerobic exercise on endothelial function have been less well studied in women, and the results are not as consistent as those reported in men. Some studies in postmenopausal women have found significant increases in brachial artery FMD in response to an aerobic exercise program (9, 176, 217). In contrast, other recent work indicates that regular aerobic exercise is not associated with enhanced EDD in estrogen-deficient postmenopausal women (23, 144) but that estrogen replacement restores the ability to improve EDD with aerobic exercise training in this group by ameliorating oxidative stress (131).
Little is known about the intensity, frequency, and duration of exercise required to improve endothelial function in middle-aged/older adults. A single bout of aerobic exercise has been reported to improve brachial FMD in postmenopausal women (75). Moreover, low- to moderate-intensity aerobic exercise performed ≥3 days/wk appears to be a sufficient stimulus to improve EDD in the absence of changes in conventional risk factors for CVD (40, 131, 144, 176).
Last, recent work suggests that, in middle-aged and older adults, habitual aerobic exercise may exert direct endothelium-protective effects from adverse circulating factors such as elevated low-density lipoprotein cholesterol (198) and impaired fasting glucose levels (41), probably by increasing resistance to oxidative stress. Indeed, endothelial cell manganese SOD expression and circulating extracellular SOD activity, key antioxidant defense pathways involved in endothelial function (14, 89), are reduced with aging in sedentary healthy men but are fully preserved in older men who exercise (143). Consistent with this notion, aerobic exercise protects against Western diet-induced impairment in EDD in old mice by increasing resistance to superoxide-associated oxidative stress and preserving NO bioavailability (108). Although little information is available on vascular tissue per se, it is likely that some of the beneficial effects of aerobic exercise on arterial function with aging are mediated by activation of the homeostatic/stress resistance pathways mentioned above (17, 60, 111, 134, 153) (FIGURE 4A).
FIGURE 4.
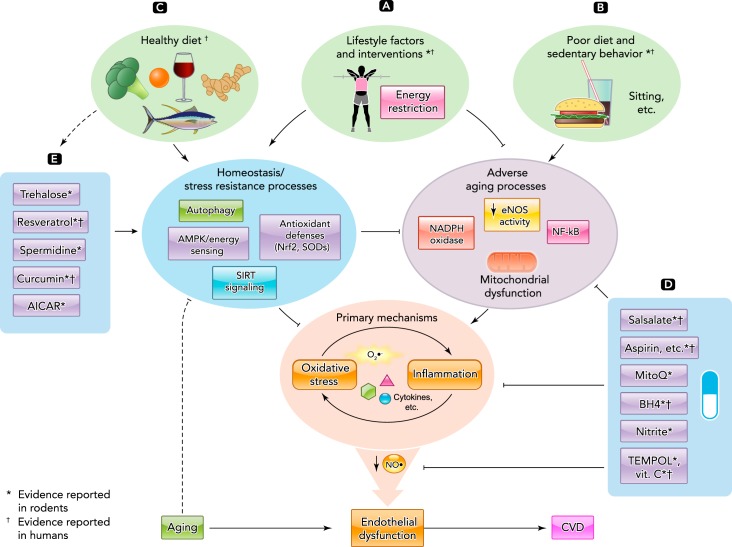
Translational strategies for preventing and treating age-related endothelial dysfunction
A: aerobic exercise and energy restriction are powerful lifestyle strategies for preserving endothelial function with aging by reducing adverse aging processes and enhancing homeostasis/stress resistance systems. B: poor dietary/lifestyle choices exert a negative influence on endothelial function by stimulating unfavorable signaling. C: healthy diets may exert some of their beneficial effects on endothelial function by enhancing the activity of homeostasis/stress resistance systems. D: certain pharmacological agents and/or nutraceuticals may preserve endothelial function with aging by directly suppressing adverse processes, and other novel compounds (E) may improve function by stimulating upstream protective pathways. Evidence for efficacy of these strategies comes from translational studies conducted in both preclinical models and human subjects.
Energy restriction and weight loss.
Energy restriction in the absence of malnutrition is associated with numerous beneficial effects on physiological function with aging (120, 203) and, therefore, would appear to be a candidate lifestyle strategy for preserving endothelial function. Consistent with this notion, in mice, lifelong restriction of energy intake preserves EDD with aging by maintaining NO bioavailability secondary to reduced vascular superoxide production and oxidative stress (31, 48, 189). The reduction in oxidative stress is associated with inhibition of NADPH oxidase isoform 2, increases in antioxidant defenses (total SOD and catalase activities), and normalization of stress resistance-nutrient sensing pathways (e.g., SIRT-1), all of which likely contribute to the favorable effects of energy restriction on EDD (48). These vascular endothelial-protective effects of lifelong energy restriction and related mechanisms of action can be recapitulated by short-term (8 wk) caloric restriction in mice begun late in life (154). Similarly, short-term energy restriction-based weight loss largely restores EDD in middle-aged and older overweight/obese adults by increasing NO bioavailability (142). Thus short- or long-term energy restriction appears to enhance NO-mediated endothelial function with aging, and this is associated with reduced oxidative stress and modulation of energy-sensing stress resistance pathways (FIGURE 4A).
Diet composition.
The composition of the diet also has an important modulatory influence on endothelial aging. Among middle-aged and older adults who are overweight/obese or have modestly elevated systolic blood pressure, lower sodium intake is associated with greater EDD (84), and dietary sodium restriction improves EDD (42, 85) by increasing NO secondary to reduced superoxide-associated oxidative stress, enhanced BH4 bioavailability, and increased circulating SOD activity (85) (FIGURE 4B). In addition to low sodium content, diets that are high in fruits, vegetables, fiber, and specific electrolytes (potassium, magnesium, and calcium), and low in total and saturated fat and cholesterol (e.g., Mediterranean and DASH diets) improve EDD in these same groups (11, 93, 123) (FIGURE 4C). Acute ingestion of whey-derived protein extract (5) and cocoa (128) also are reported to enhance EDD in middle-aged or older adults. In general, evidence is mounting for the CVD risk-lowering effects of consumption of seeds, including whole grains, nuts, legumes, cocoa products, and coffee (158), and this is likely to be mediated in part via preservation of endothelial function. Although current evidence is limited, many of these healthy dietary approaches may exert their beneficial endothelial actions in part via activation of cell homeostasis/stress resistance pathways that inhibit oxidative stress and inflammation (118, 119, 163) (FIGURE 4C).
Alternative Strategies
Healthy lifestyle behaviors should be considered the “first-line” strategies for prevention and treatment of endothelial dysfunction with aging and are likely to be the most effective overall approach when practiced. However, despite aggressive and informed public advocacy efforts for adopting healthy lifestyle choices, many middle-aged and older adults do not meet minimal guidelines for exercise and do not consume a healthy diet. As a result, there is growing interest in the use of pharmacological agents that induce at least some of the effects of healthy lifestyle behaviors. For simplicity, these will be broadly categorized as either pharmaceutical drugs or nutraceuticals.
Pharmaceutical drugs.
Prescription pharmaceutical drugs represent the category of pharmacological agents that have been most thoroughly tested for safety and efficacy in humans, usually in the setting of clinical trials on patients with existing clinical CVD or major risk factors for CVD. Several such agents, including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, aldosterone receptor antagonists, calcium channel blockers, statins, thiazolidinediones, and more recently developed beta blockers (e.g., nebivolol, carvedilol), generally improve endothelial function in middle-aged and older patients with cardiometabolic diseases or risk factors (10, 195). However, these medications generally are used to slow or prevent progression of an existing disorder and reduce the occurrence of adverse clinical outcomes. They are not indicated per se for primary prevention or treatment of endothelial dysfunction with aging in the absence of clinical disease. Moreover, there is relatively little information on the use of these agents in healthy middle-aged and older adults. Recent work shows that fenofibrate (199), combined fluvastatin and valsartan (statin and angiotension receptor blocker, ARB) (115), and prescription anti-inflammatory agents (discussed below) reduce oxidative stress and/or inflammation and improve EDD in healthy middle-aged and older subjects, independent of changes in lipids or blood pressure; in contrast, mineralcorticoid receptor blockade (82) and ARB treatment alone (148) had no effect in this population. Although these agents are not approved for treatment of endothelial dysfunction with primary aging, some may be considered for “repurposing” or “off-target” use in older adults without major risk factors/disease based either on currently available efficacy and safety data or on results from additional clinical testing in that population (132, 140). Thus, in the future, these FDA-approved medications may provide therapeutic options to prevent and treat age-associated endothelial dysfunction and its pathophysiological sequelae.
Nutraceutical compounds.
A nutraceutical (nutriceutical) is a food or food supplement with naturally occurring ingredients purported to have beneficial effects on human health. Categories of nutraceuticals include dietary supplements, functional foods, and medical foods (211). From a consumer standpoint, this class of compounds is gaining interest in part because of their lower cost (i.e., compared with nongeneric prescribed drugs), access (no medical prescription is needed), and, in many cases, their “natural” (nonsynthetic) origin. From a manufacturing standpoint, these compounds can be attractive because they require a far less costly, risky, and time-consuming regulatory approval process (albeit with less financial reward than a highly profitable patented drug). Nutraceuticals also are attractive in meeting the growing consumer demand for “anti-aging” strategies, and, in response, many compounds now are being developed based on observations from basic biology of aging research (83, 90, 135). Some of these compounds may mimic certain effects of vascular-protective lifestyle strategies. Although a number of clinical trials are underway (clinicaltrials.gov), in general, more uniform evidence for efficacy based on well controlled trials in humans is needed for this category of agents, including clinical outcomes. Other concerns include inconsistency in the composition of nutraceuticals among commercially available formulations and the overall less stringent regulations for use. A brief summary of current information on selective compounds reported to influence age-related endothelial dysfunction and their mechanisms of action is presented below (FIGURE 4, D AND E).
Inorganic nitrite.
Nitrite and nitrate, the metabolites of NO oxidation, are now viewed as potential precursor molecules for increasing NO bioavailability and preventing/treating physiological dysfunction in states of NO insufficiency, including settings of CV risk/disease such as essential hypertension (66, 114, 155, 169). Given the primary role of reduced NO bioavailability in endothelial aging, these molecules represent an intriguing strategy for improving NO-mediated endothelial function with advancing age. In agreement with this concept, short-term (3 wk) supplementation of inorganic nitrite (sodium nitrite in drinking water) delivered late in life rescues impaired EDD in mice by restoring NO bioavailability as a result of normalizing vascular oxidative stress (170). The latter is associated with reduced superoxide production via eNOS recoupling and inhibition of NADPH oxidase, as well as increases in antioxidant defenses and reversal of vascular inflammation (170). Results of a recent brief report suggest that increasing circulating nitrite levels via chronic supplementation with sodium nitrate modestly improves EDD in middle-aged/older adults without clinical CVD (149), and an initial clinical trial investigating the efficacy of sodium nitrite supplementation in this population is underway (clinicaltrials.gov). Boosting nitrite and NO bioavailability through increased dietary intake of foods high in nitrate (e.g., green leafy vegetables or beetroot) (15, 110, 218) may exert similar beneficial effects. Thus nitrite/nitrate supplementation is a promising area for preserving vascular health, in general, and endothelial function, in particular, with aging (169).
Antioxidants and other common supplements.
Based on the central influence of oxidative stress in mediating endothelial dysfunction with aging, antioxidant administration would seem to hold strong therapeutic potential. However, chronic supplementation of conventional oral antioxidants (vitamins C, E, etc.) generally has not shown efficacy in patients with CVD (52, 95), and their effectiveness for improving age-associated endothelial dysfunction is unclear. On the one hand, acute administration of supra-physiological concentrations of superoxide-scavenging agents (vitamin C, TEMPOL) essentially restores EDD in older humans and mice (56, 105, 181), as does single oral ingestion of an antioxidant “cocktail” containing vitamins C and E plus lipoic acid (209). Moreover, short-term (3 wk) oral treatment with TEMPOL, a SOD-mimetic, completely reverses endothelial dysfunction in old mice by restoring NO and mitigating vascular oxidative stress and inflammation (61), and 3 mo of vitamin C supplementation improves endothelial fibrinolytic function in overweight/obese middle-aged men (191). On the other hand, chronic (30 days) oral supplementation of vitamin C (500 mg/day) has no effect on EDD in older, otherwise healthy men with impaired baseline endothelial function (56). Given the emerging role of mitochondrial dysfunction and excessive mitochondrial superoxide production in vascular aging (34, 51, 94), mitochondrial-specific antioxidants (e.g., MitoQ), peptides, and/or other “mitochondrial health”-enhancing agents (67, 87, 171, 177, 204) represent a novel alternative approach for preserving endothelial function with aging. Consistent with this notion, short-term (4 wk) supplementation with MitoQ delivered in the drinking water completely restores endothelial function and NO bioavailability in old mice as a result of reduced vascular oxidative stress. These improvements are associated with normalization of vascular mitochondrial superoxide production and increased expression of markers of vascular mitochondrial health (67).
Finally, other dietary nutrients and vitamins such as omega 3 and 6 fatty acids, folate, and vitamin D, also exert protective effects on endothelial function in select populations. There is cross-sectional evidence that, in late middle-aged/older healthy adults, endothelial function is positively related to serum vitamin D [25(OH)D] concentrations and that impaired endothelial function in older adults with lower circulating vitamin D is mediated in part by NF-κB-dependent vascular inflammation (84a). However, the results from existing trials investigating these compounds in middle-aged/older adults without clinical disease are inconclusive (112, 121, 126, 190, 201, 220).
Anti-inflammatory agents.
Anti-inflammatory compounds represent a complementary strategy to antioxidant supplementation for prevention/treatment of endothelial dysfunction with aging. Short- or longer-term treatment with the nonsteroidal drugs aspirin or closely related salicylates improves NO-mediated EDD in middle-aged and older mice and rats, and this is associated with reductions in oxidative stress (16, 39, 105). Similarly, salsalate, a salicylate-based prescription drug used to treat inflammatory diseases and, more recently, Type 2 diabetes (71), is a potent inhibitor of NF-κB signaling that improves EDD in obese/overweight middle-aged and older adults by reducing endothelial oxidative stress and inflammation (145), although no effects have been reported in patients with Type 2 diabetes or coronary disease (70, 178). Other natural and synthetic anti-inflammatory agents currently available or under development also may prove effective in older adults. In this context, acute administration of the TNF-α inhibitor etanercept recently was shown to improve EDD in healthy estrogen-deficient postmenopausal women (129).
Tetrahydrobiopterin.
Given the role of reduced BH4 bioactivity in multiple settings of endothelial dysfunction, including older age (127, 162), BH4 is an attractive candidate to recouple eNOS and enhance enzymatic NO production with aging. In healthy middle-aged and older adults, a single dose of BH4 improves EDD by increasing NO bioavailability (57, 80). Chronic (4–8 wk) oral BH4 supplementation increases plasma BH4 concentrations, lowers blood pressure, improves EDD, and reduces circulating markers of oxidative stress in middle-aged and older patients with hypercholesterolemia or resistant hypertension (30, 146). No information is available on the effects of chronic BH4 supplementation on endothelial dysfunction in healthy older adults. Thus BH4-boosting interventions are presently an understudied area for prevention and treatment of endothelial dysfunction with primary aging.
Autophagy activators.
Stimulating autophagy can enhance physiological function in several nonvascular tissues with aging (147, 219) and, therefore, may well have similar beneficial effects on the vasculature. Indeed, recent work in mice supports the concept that reversing age-related impairments in vascular autophagy enhances endothelial function. Oral supplementation of trehalose, a natural disaccharide that stimulates autophagy (157, 160), restores vascular autophagy and rescues NO-mediated EDD in old mice by normalizing superoxide-related oxidative stress, while also ameliorating vascular inflammation (100). Similarly, the autophagy enhancer spermidine normalizes vascular markers of autophagy and reverses the age-associated impairment in EDD in old mice by restoring NO bioavailability secondary to reduced arterial superoxide production (99). These preclinical observations provide an experimental basis for determining the effects of trehalose, spermidine, and other autophagy-boosting compounds on endothelial dysfunction with aging in humans.
Modulators of energy sensing pathways.
There is accumulating evidence that activation of cellular energy sensing pathways may be an effective strategy for preserving endothelial function with aging. These pathways are believed to be involved in mediating many of the benefits of energy restriction on longevity and physiological function (7, 19). Thus compounds that stimulate these cellular processes have the potential to exert similar effects. Daily administration of the AMPK-activator aminoimidazole carboxamide ribonucleotide (AICAR) for 2 wk restores arterial AMPK protein expression and state of activation, and reverses the age-associated impairment in EDD in old mice by ameliorating excessive superoxide suppression of endothelial function, independent of NO or prostaglandin signaling (107). These preclinical findings provide support for conducting translational studies in middle-aged and older humans targeting chronic AMPK activation.
Similarly, activation of SIRT-1 by the nonflavonoid polyphenol, resveratrol, or with red wine improves EDD with aging in rodents fed normal chow or high-fat diets (33, 139), an effect associated with increased eNOS and reduced vascular NADPH oxidase activity, oxidative stress, and inflammation (139). Resveratrol also improves EDD assessed ex vivo in surgically removed superior thyroid arteries of middle-aged and older patients with hypertension and dyslipidemia (22). The improvements were associated with modulation of NO metabolism via AMPK-activation of eNOS, increased bioavailability of BH4, and reduced vascular oxidative stress associated with a Nrf2-dependent increase in the expression of manganese SOD. However, no effects of resveratrol on EDD were observed in age-matched control subjects without these disorders. Acute resveratrol administration is reported to improve brachial artery EDD in middle-aged and older overweight and obese adults with elevated blood pressure (208), and chronic resveratrol supplementation enhances EDD in older obese adults (207a). Thus SIRT-1 activation with resveratrol and perhaps other activators of this energy-sensing molecular network (e.g., NAD+-boosting agents) may improve endothelial function with aging, but more evidence is needed from trials in humans, including middle-aged and older adults without clinical disease or elevated disease risk.
Endogenous antioxidant-enhancing compounds.
With the largely failed trials of oral antioxidant supplementation in patients with CVD (95), there has been growing interest in nutraceuticals that boost endogenous antioxidant stress resistance pathways. Several such nutraceuticals upregulate endogenous antioxidant enzymes through activation of the Nrf2-ARE pathway (20, 90).
One of the strongest nutraceutical activators of Nrf2 is curcumin, a nonflavonoid polyphenol found in the curry spice turmeric (21, 194). Ingestion of curcumin-supplemented chow for 4 wk restores EDD in old mice to levels of young controls by increasing NO bioavailability and normalizing superoxide production and oxidative stress (62). The latter were associated with reduced expression of the NADPH oxidase subunit p67 and restoration of manganese SOD (62). Consistent with these preclinical findings, oral supplementation of curcumin improves EDD and lowers circulating C-reactive protein in healthy postmenopausal women without changing plasma lipids or blood pressure (1).
Many other nutracueticals are reported to have Nrf2-activating actions and may, therefore, enhance endothelial function, including epigallocatechin 3-gallate (EGCG) from green tea, the sulfur-containing nutraceuticals, allicin (garlic) and L-sulforaphane (broccoli), quercitin (apples), rosmarinic acid or carnosic acid (carnosol) (both from rosemary), coffee-derived diterpenes, lycopene (tomato products), shogaol (ginger), and capsaicin (90). Commercially available nutraceutical products, such as Protandim, containing a blend of herbal ingredients, also have purported Nrf2-activating properties and could be effective (49, 194). Establishing the safety and efficacy of Nrf2-/ARE-activating nutraceutical compounds for promoting endothelial health with aging in humans will be important, particularly given the recent termination of a clinical trial on bardoxolone methyl, a synthetic triterpenoid Nrf2 activator that was withdrawn because of toxicity (150).
Future Directions
Given the broad impending societal effects of the changing demographics of aging, preservation of endothelial function with age is a highly clinically relevant area of physiological research with strong demand for additional investigation. Much more needs to be understood about the genetic/environmental interactions and biological mechanisms that modulate endothelial function with aging. One of the many open issues on this topic is how environmental factors to which we are exposed with aging (e.g., stress, diet, physical activity) interact with homeostatic/stress resistance pathways to modulate oxidative stress/inflammation and thereby influence endothelial function. Such research should provide insight into additional therapeutic targets.
Although there is good evidence that healthy lifestyle behaviors, when practiced, are the most potent “medicine” for preserving endothelial function with aging, many fundamental questions remain unanswered. What type (mode), intensity, frequency, and duration of exercise is necessary to optimize endothelial function across adulthood? What components of a healthy diet are most important, and how much do we need to consume, or is our overall diet the most influential factor rather than specific components (as some recent findings in nutrition research suggest)?
A better understanding of the safety and efficacy of already approved pharmaceutical agents known to improve endothelial function in patients with cardiometabolic risk factors and diseases is required to determine their potential application to middle-aged/older adults without clinical disorders. It has been suggested that aging and clinical CVD involve the same pathophysiological processes and that such clinical states are an accelerated form of aging (98). If so, one could reasonably postulate that established pharmacological therapies in CVD should be effective in primary aging, as this certainly seems to be true for lifestyle factors.
A major developing area of investigation is the use of established or novel nutraceutical compounds with potential “healthy lifestyle-mimicking” effects. The benefits, safety, formulaic consistency, and mechanisms of action of such compounds will need to be demonstrated in well controlled clinical trials. Many compounds show promise in preclinical models, but will they prove effective in aging humans? It is unlikely that such compounds will have the broad health-promoting benefits of healthy lifestyle practices, but selective beneficial effects certainly are possible. Moreover, understanding the interaction between lifestyle, pharmaceutical agents, and nutraceutical agents will be a vital part of future investigation in this area. Potentially negative interactions between statins, antioxidant treatments, and resveratrol with aerobic exercise have been reported (68, 72, 124, 210), suggesting the possibility of unfavorable interactions between certain pharmacological agents and healthy lifestyle strategies. Relatedly, if we successfully identify efficacious lifestyle and pharmaceutical and nutraceutical therapies, do we need to employ several of these approaches or a select few (or only one)? Understanding the pathways activated by these diverse stimuli will be necessary to guide investigation on this issue. Comparative effectiveness analyses and prospective trials likely will be required to address these issues.
Finally, strategies shown to be effective in the prevention and treatment of age-associated endothelial dysfunction might prove beneficial for other clinically important expressions of vascular aging, such as large elastic artery stiffening. For any approach demonstrating promise, it will be essential to establish efficacy (including dose-response) in both middle-aged/older men and postmenopausal women given the unique interactions of sex hormone changes and aging in the two groups. Fortunately, contemporary translational physiological research approaches are now available to pursue these and other important issues concerning arterial health with human aging.
Conclusions
As summarized in FIGURE 4, vascular endothelial dysfunction develops with advancing age and plays a key role in the increased risk of CVD and several other major disorders of aging. Age-associated endothelial dysfunction is mediated largely by NO insufficiency linked to vascular oxidative stress and chronic low-grade inflammation, as modulated by altered cellular homeostasis/stress resistance processes. Physical activity and diet exert a strong influence on endothelial function with aging, but lifestyle-based strategies are limited by low adherence in most human populations. Continued advocacy for broad engagement in these healthy behaviors is essential, because these approaches will have the greatest impact on endothelial function, as well as numerous other (nonvascular) physiological declines with aging. Selective use of established vascular-protective agents also needs to be explored. Last, the development of novel nutraceutical compounds that favorably modulate homeostatic stress resistance pathways and/or adverse aging processes to reduce oxidative stress and inflammation, and enhance bioavailability of NO, holds considerable promise as a complementary approach for maintaining optimal endothelial health with aging.
Acknowledgments
We thank Natalie De Picciotto, Lauren Cuevas, and Sierra Hill for technical assistance in reviewing the published literature cited. TheraVasc and Verdure Sciences are providing nutraceutical compounds for ongoing clinical studies of the authors' laboratory.
Footnotes
This work was supported by National Institutes of Health awards AG-013038, HL-107120, HL-107105, AG-000279, AG-039210, AG-044031, and TR-001082.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: D.R.S., R.E.K., R.A.G.-R., and T.J.L. prepared figures; D.R.S., R.E.K., R.A.G.-R., and T.J.L. drafted manuscript; D.R.S., R.E.K., R.A.G.-R., and T.J.L. edited and revised manuscript; D.R.S., R.E.K., R.A.G.-R., and T.J.L. approved final version of manuscript.
References
- 1.Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R, Maeda S. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr Res 32: 795–799, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Angulo J, Vallejo S, El Assar M, Garcia-Septiem J, Sanchez-Ferrer CF, Rodriguez-Manas L. Age-related differences in the effects of alpha and gamma peroxisome proliferator-activated receptor subtype agonists on endothelial vasodilation in human microvessels. Exp Gerontol 47: 734–740, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Bachschmid MM, Schildknecht S, Matsui R, Zee R, Haeussler D, Cohen RA, Pimental D, Loo B. Vascular aging: chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med 45: 17–36, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 202: 271–284, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard KD, Kupchak BR, Volk BM, Mah E, Shkreta A, Liptak C, Ptolemy AS, Kellogg MS, Bruno RS, Seip RL, Maresh CM, Kraemer WJ, Volek JS. Acute effects of ingestion of a novel whey-derived extract on vascular endothelial function in overweight, middle-aged men and women. Br J Nutr 109: 882–893, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Balletshofer BM, Rittig K, Enderle MD, Volk A, Maerker E, Jacob S, Matthaei S, Rett K, Haring HU. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with Type 2 diabetes in association with insulin resistance. Circulation 101: 1780–1784, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov 11: 443–461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108: 2000–2006, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis 203: 325–330, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med 170: 126–135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23: 168–175, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res 66: 286–294, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol 27: 1941–1946, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med 45: 468–474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulckaen H, Prevost G, Boulanger E, Robitaille G, Roquet V, Gaxatte C, Garcon G, Corman B, Gosset P, Shirali P, Creusy C, Puisieux F. Low-dose aspirin prevents age-related endothelial dysfunction in a mouse model of physiological aging. Am J Physiol Heart Circ Physiol 294: H1562–H1570, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Cacicedo JM, Gauthier MS, Lebrasseur NK, Jasuja R, Ruderman NB, Ido Y. Acute exercise activates AMPK and eNOS in the mouse aorta. Am J Physiol Heart Circ Physiol 301: H1255–H1265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai H, Dikalov S, Griendling KK, Harrison DG. Detection of reactive oxygen species and nitric oxide in vascular cells and tissues: comparison of sensitivity and specificity. Methods Mol Med 139: 293–311, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Canto C, Auwerx J. Calorie restriction: is AMPK a key sensor and effector? Physiology 26: 214–224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardozo LFMF, Pedruzzi LM, Stenvinkel P, Stockler-Pinto MB, Daleprane JB, Leite M, Jr, Mafra D. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie 95: 1525–1533, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Carmona-Ramirez I, Santamaria A, Tobon-Velasco JC, Orozco-Ibarra M, Gonzalez-Herrera IG, Pedraza-Chaverri J, Maldonado PD. Curcumin restores Nrf2 levels and prevents quinolinic acid-induced neurotoxicity. J Nutr Biochem 24: 14–24, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Carrizzo A, Puca A, Damato A, Marino M, Franco E, Pompeo F, Traficante A, Civitillo F, Santini L, Trimarco V, Vecchione C. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension 62: 359–366, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Casey DP, Pierce GL, Howe KS, Mering MC, Braith RW. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol 100: 403–408, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292: H1209–H1224, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 374: 1196–1208, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91: 3527–3561, 1998 [PubMed] [Google Scholar]
- 29.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Cosentino F, Hurlimann D, Delli Gatti C, Chenevard R, Blau N, Alp NJ, Channon KM, Eto M, Lerch P, Enseleit F, Ruschitzka F, Volpe M, Luscher TF, Noll G. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart 94: 487–492, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev 130: 518–527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol 170: 388–398, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Luz PL, Tanaka L, Brum PC, Dourado PM, Favarato D, Krieger JE, Laurindo FR. Red wine and equivalent oral pharmacological doses of resveratrol delay vascular aging but do not extend life span in rats. Atherosclerosis 224: 136–142, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res 110: 1109–1124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the ‘magnificent seven,’ function, metabolism and longevity. Ann Med 39: 335–345, 2007 [DOI] [PubMed] [Google Scholar]
- 36.De Mey JG, Vanhoutte PM. Role of the intima in cholinergic and purinergic relaxation of isolated canine femoral arteries. J Physiol 316: 347–355, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586: 1161–1168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demaree SR, Lawler JM, Linehan J, Delp MD. Ageing alters aortic antioxidant enzyme activities in Fischer-344 rats. Acta Physiol Scand 166: 203–208, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Demirci B, Demir O, Dost T, Birincioglu M. Antioxidative effect of aspirin on vascular function of aged ovariectomized rats. Age (Dordr) 36: 223–229, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 41.DeVan AE, Eskurza I, Pierce GL, Walker AE, Jablonski KL, Kaplon RE, Seals DR. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clin Sci (Lond) 124: 325–331, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr 89: 485–490, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Doel JJ, Godber BL, Eisenthal R, Harrison R. Reduction of organic nitrates catalysed by xanthine oxidoreductase under anaerobic conditions. Biochim Biophys Acta 1527: 81–87, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805–812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol 589: 4545–4554, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 12: 772–783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donovan EL, McCord JM, Reuland DJ, Miller BF, Hamilton KL. Phytochemical activation of Nrf2 protects human coronary artery endothelial cells against an oxidative challenge. Oxid Med Cell Longev 2012: 132931, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dora KA. Coordination of vasomotor responses by the endothelium. Circ J 74: 226–232, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Dromparis P, Michelakis ED. Mitochondria in vascular health and disease. Annu Rev Physiol 75: 95–126, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10: 453–471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El Assar M, Angulo J, Rodriguez-Manas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 65: 380–401, 2013 [DOI] [PubMed] [Google Scholar]
- 55.El Assar M, Angulo J, Vallejo S, Peiro C, Sanchez-Ferrer CF, Rodriguez-Manas L. Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol 3: 132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eskurza I, Seals DR, Desouza CA. Pharmacologic versus flow-mediatd assessments of peripheral vascular endothelial vasodilatory function in humans. J Cardiol 88: 1067–1069, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 117: 139–155, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res 11: 139–150, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11: 269–276, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, Seals DR. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol 48: 269–276, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med 303: 130–135, 1980 [DOI] [PubMed] [Google Scholar]
- 65.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 27: 849–853, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Ghosh SM, Kapil V, Fuentes-Calvo I, Bubb KJ, Pearl V, Milsom AB, Khambata R, Maleki-Toyserkani S, Yousuf M, Benjamin N, Webb AJ, Caulfield MJ, Hobbs AJ, Ahluwalia A. Enhanced vasodilator activity of nitrite in hypertension: critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension 61: 1091–1102, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol 591: 5047–5059, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 105: 1567–1572, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Goldfine AB, Buck JS, Desouza C, Fonseca V, Chen YD, Shoelson SE, Jablonski KA, Creager MA. Targeting inflammation using salsalate in patients with Type 2 diabetes: effects on flow-mediated dilation (TINSAL-FMD). Diabetes Care 36: 4132–4139, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldfine AB, Fonseca V, Shoelson SE. Therapeutic approaches to target inflammation in Type 2 diabetes. Clin Chem 57: 162–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomez-Cabrera MC, Ristow M, Vina J. Antioxidant supplements in exercise: worse than useless? Am J Physiol Endocrinol Metab 302: E476–E477, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature 444: 868–874, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harvey PJ, Morris BL, Kubo T, Picton PE, Su WS, Notarius CF, Floras JS. Hemodynamic after-effects of acute dynamic exercise in sedentary normotensive postmenopausal women. J Hypertens 23: 285–292, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Heffernan KS, Chale A, Hau C, Cloutier GJ, Phillips EM, Warner P, Nickerson H, Reid KF, Kuvin JT, Fielding RA. Systemic vascular function is associated with muscular power in older adults. J Aging Res 2012: 386387, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001 [DOI] [PubMed] [Google Scholar]
- 78.Herrera MD, Mingorance C, Rodriguez-Rodriguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev 9: 142–152, 2010 [DOI] [PubMed] [Google Scholar]
- 79.Higashi Y, Kihara Y, Noma K. Endothelial dysfunction and hypertension in aging. Hypertens Res 35: 1039–1047, 2012 [DOI] [PubMed] [Google Scholar]
- 80.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis 186: 390–395, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Hubbard VM, Valdor R, Macian F, Cuervo AM. Selective autophagy in the maintenance of cellular homeostasis in aging organisms. Biogerontology 13: 21–35, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Hwang MH, Yoo JK, Luttrell M, Kim HK, Meade TH, English M, Segal MS, Christou DD. Mineralocorticoid receptors modulate vascular endothelial function in human obesity. Clin Sci (Lond) 125: 513–520, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Imai S. A possibility of nutriceuticals as an anti-aging intervention: activation of sirtuins by promoting mammalian NAD biosynthesis. Pharmacol Res 62: 42–47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis 3: 347–356, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84a.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 57: 63–69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol 61: 335–343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36: 1199–1207, 2004 [DOI] [PubMed] [Google Scholar]
- 87.James AM, Cocheme HM, Smith RA, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem 280: 21295–21312, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol 91: 2431–2441, 2001 [DOI] [PubMed] [Google Scholar]
- 89.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res 93: 622–629, 2003 [DOI] [PubMed] [Google Scholar]
- 90.Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 15: 7792–7814, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol 48: 1–5, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kirkland JL, Peterson C. Healthspan, translation, and new outcomes for animal studies of aging. J Gerontol A Biol Sci Med Sci 64: 209–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klonizakis M, Alkhatib A, Middleton G, Smith MF. Mediterranean diet- and exercise-induced improvement in age-dependent vascular activity. Clin Sci (Lond) 124: 579–587, 2013 [DOI] [PubMed] [Google Scholar]
- 94.Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res 112: 1171–1188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation 110: 637–641, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Kung CF, Luscher TF. Different mechanisms of endothelial dysfunction with aging and hypertension in rat aorta. Hypertension 25: 194–200, 1995 [DOI] [PubMed] [Google Scholar]
- 97.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol 259: H1063–H1070, 1990 [DOI] [PubMed] [Google Scholar]
- 98.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part III: cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003 [DOI] [PubMed] [Google Scholar]
- 99.LaRocca TJ, Gioscia-Ryan RA, Hearon CM, Jr, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev 134: 314–320, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 590: 3305–3316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Larrick JW, Mendelsohn A. Applied Healthspan engineering. Rejuvenation Res 13: 265–280, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Le Brocq M, Leslie SJ, Milliken P, Megson IL. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal 10: 1631–1674, 2008 [DOI] [PubMed] [Google Scholar]
- 103.Leikert JF, Rathel TR, Muller C, Vollmar AM, Dirsch VM. Reliable in vitro measurement of nitric oxide released from endothelial cells using low concentrations of the fluorescent probe 4,5-diaminofluorescein. FEBS Lett 506: 131–134, 2001 [DOI] [PubMed] [Google Scholar]
- 104.Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 Mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci 64: 9–20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci 66: 409–418, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301: H1025–H1032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lesniewski LA, Zigler MC, Durrant JR, Donato AJ, Seals DR. Sustained activation of AMPK ameliorates age-associated vascular endothelial dysfunction via a nitric oxide-independent mechanism. Mech Ageing Dev 133: 368–371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lesniewski LA, Zigler ML, Durrant JR, Nowlan MJ, Folian BJ, Donato AJ, Seals DR. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol 48: 1218–1225, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr Comp Biol 50: 829–843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lidder S, Webb AJ. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br J Clin Pharmacol 75: 677–696, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J 27: 4184–4193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu ZM, Woo J, Wu SH, Ho SC. The role of vitamin D in blood pressure, endothelial and renal function in postmenopausal women. Nutrients 5: 2590–2610, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation 121: 1768–1777, 2010 [DOI] [PubMed] [Google Scholar]
- 114.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008 [DOI] [PubMed] [Google Scholar]
- 115.Lunder M, Janic M, Jug B, Sabovic M. The effects of low-dose fluvastatin and valsartan combination on arterial function: a randomized clinical trial. Eur J Intern Med 23: 261–266, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Lunenfeld B, Stratton P. The clinical consequences of an ageing world and preventive strategies. Best Pract Res Clin Obstet Gynaecol 27: 643–659, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luscher TF, Barton M. Biology of the endothelium. Clin Cardiol 20: 3–10, 1997 [PubMed] [Google Scholar]
- 118.Marin C, Yubero-Serrano EM, Lopez-Miranda J, Perez-Jimenez F. Endothelial aging associated with oxidative stress can be modulated by a healthy mediterranean diet. Int J Mol Sci 14: 8869–8889, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marotta F, Yadav H, Kumari A, Catanzaro R, Jain S, Polimeni A, Lorenzetti A, Soresi V. Cardioprotective effect of a biofermented nutraceutical on endothelial function in healthy middle-aged subjects. Rejuvenation Res 15: 178–181, 2012 [DOI] [PubMed] [Google Scholar]
- 120.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev 126: 913–922, 2005 [DOI] [PubMed] [Google Scholar]
- 121.Massaro M, Scoditti E, Carluccio MA, De Caterina R. Nutraceuticals and prevention of atherosclerosis: focus on omega-3 polyunsaturated fatty acids and Mediterranean diet polyphenols. Cardiovasc Ther 28: e13–e19, 2010 [DOI] [PubMed] [Google Scholar]
- 122.Matz RL, Andriantsitohaina R. Age-related endothelial dysfunction: potential implications for pharmacotherapy. Drugs Aging 20: 527–550, 2003 [DOI] [PubMed] [Google Scholar]
- 123.McCall DO, McGartland CP, McKinley MC, Patterson CC, Sharpe P, McCance DR, Young IS, Woodside JV. Dietary intake of fruits and vegetables improves microvascular function in hypertensive subjects in a dose-dependent manner. Circulation 119: 2153–2160, 2009 [DOI] [PubMed] [Google Scholar]
- 124.Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ, Thyfault JP. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol 62: 709–714, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mirea O, Donoiu I, Plesea IE. Arterial aging: a brief review. Rom J Morphol Embryol 53: 473–477, 2012 [PubMed] [Google Scholar]
- 126.Moat SJ, Lang D, McDowell IF, Clarke ZL, Madhavan AK, Lewis MJ, Goodfellow J. Folate, homocysteine, endothelial function and cardiovascular disease. J Nutr Biochem 15: 64–79, 2004 [DOI] [PubMed] [Google Scholar]
- 127.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol 26: 2439–2444, 2006 [DOI] [PubMed] [Google Scholar]
- 128.Monahan KD, Feehan RP, Kunselman AR, Preston AG, Miller DL, Lott ME. Dose-dependent increases in flow-mediated dilation following acute cocoa ingestion in healthy older adults. J Appl Physiol 111: 1568–1574, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moreau KL, Deane KD, Meditz AL, Kohrt WM. Tumor necrosis factor-alpha inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis 230: 390–396, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302: H1211–H1218, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D'Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women: 2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 57: 1404–1423, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 134.Nair U, Klionsky DJ. Activation of autophagy is required for muscle homeostasis during physical exercise. Autophagy 7: 1405–1406, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ogle WO, Speisman RB, Ormerod BK. Potential of treating age-related depression and cognitive decline with nutraceutical approaches: a mini-review. Gerontology 59: 23–31, 2013 [DOI] [PubMed] [Google Scholar]
- 136.Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q 87: 842–862, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Paneni F, Osto E, Costantino S, Mateescu B, Briand S, Coppolino G, Perna E, Mocharla P, Akhmedov A, Kubant R, Rohrer L, Malinski T, Camici GG, Matter CM, Mechta-Grigoriou F, Volpe M, Luscher TF, Cosentino F. Deletion of the activated protein-1 transcription factor JunD induces oxidative stress and accelerates age-related endothelial dysfunction. Circulation 127: 1229–1240, 2013 [DOI] [PubMed] [Google Scholar]
- 138.Paul V, Ekambaram P. Involvement of nitric oxide in learning and memory processes. Indian J Med Res 133: 471–478, 2011 [PMC free article] [PubMed] [Google Scholar]
- 139.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF, Jr, Smith SC, Jr, Stone NJ, Taubert KA. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases American Heart Association Science Advisory and Coordinating Committee. Circulation 106: 388–391, 2002 [DOI] [PubMed] [Google Scholar]
- 141.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104: 191–196, 2001 [DOI] [PubMed] [Google Scholar]
- 142.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension 52: 72–79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 10: 1032–1037, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 120: 13–23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-κB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 119: 1284–1292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Porkert M, Sher S, Reddy U, Cheema F, Niessner C, Kolm P, Jones DP, Hooper C, Taylor WR, Harrison D, Quyyumi AA. Tetrahydrobiopterin: a novel antihypertensive therapy. J Hum Hypertens 22: 401–407, 2008 [DOI] [PubMed] [Google Scholar]
- 147.Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, Jung S, Jung YK. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun 4: 2300, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rajagopalan S, Brook R, Mehta RH, Supiano M, Pitt B. Effect of losartan in aging-related endothelial impairment. Am J Cardiol 89: 562–566, 2002 [DOI] [PubMed] [Google Scholar]
- 149.Rammos C, Hendgen-Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T. Dietary nitrate reverses vascular dysfunction in old adults with moderately increased cardiovascular risk. J Am Coll Cardiol 63: 1584–1585, 2014 [DOI] [PubMed] [Google Scholar]
- 150.Reata Pharmaceuticals. Company statement: termination of the BEACON trial (Online). Irving, TX: Reata Pharmaceuticals. http://www.reatapharma.com/investors-media/news/news-timeline/2012/company-statement-termination-of-beacon-trial.aspx.
- 151.Reihill JA, Ewart MA, Hardie DG, Salt IP. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys Res Commun 354: 1084–1088, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rezvan A, Ni CW, Alberts-Grill N, Jo H. Animal, in vitro, and ex vivo models of flow-dependent atherosclerosis: role of oxidative stress. Antioxid Redox Signal 15: 1433–1448, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J 418: 261–275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell 9: 304–312, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rocha BS, Gago B, Pereira C, Barbosa RM, Bartesaghi S, Lundberg JO, Radi R, Laranjinha J. Dietary nitrite in nitric oxide biology: a redox interplay with implications for pathophysiology and therapeutics. Curr Drug Targets 12: 1351–1363, 2011 [DOI] [PubMed] [Google Scholar]
- 156.Rodriguez-Manas L, El-Assar M, Vallejo S, Lopez-Doriga P, Solis J, Petidier R, Montes M, Nevado J, Castro M, Gomez-Guerrero C, Peiro C, Sanchez-Ferrer CF. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 8: 226–238, 2009 [DOI] [PubMed] [Google Scholar]
- 157.Rodríguez-Navarro JA, Rodríguez L, Casarejos MJ, Solano RM, Gómez A, Perucho J, Cuervo AM, García de Yébenes J, Mena MA. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol Dis 39: 423–438, 2010 [DOI] [PubMed] [Google Scholar]
- 158.Ros E, Hu FB. Consumption of plant seeds and cardiovascular health: epidemiological and clinical trial evidence. Circulation 128: 553–565, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell 146: 682–695, 2011 [DOI] [PubMed] [Google Scholar]
- 160.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282: 5641–5652, 2007 [DOI] [PubMed] [Google Scholar]
- 161.Schiffrin EL, Deng LY. Comparison of effects of angiotensin I-converting enzyme inhibition and beta-blockade for 2 years on function of small arteries from hypertensive patients. Hypertension 25: 699–703, 1995 [DOI] [PubMed] [Google Scholar]
- 162.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 113: 47–63, 2007 [DOI] [PubMed] [Google Scholar]
- 163.Scoditti E, Calabriso N, Massaro M, Pellegrino M, Storelli C, Martines G, De Caterina R, Carluccio MA. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys 527: 81–89, 2012 [DOI] [PubMed] [Google Scholar]
- 164.Seals DR. Translational physiology: from molecules to public health. J Physiol 591: 3457–3469, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol 105: 1323–1332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol 587: 5541–5549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sindler AL, DeVan AE, Fleenor BS, Seals DR. Inorganic nitrite supplementation for healthy arterial aging. J Appl Physiol 116: 463–477, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smith RA, Hartley RC, Murphy MP. Mitochondria-targeted small molecule therapeutics and probes. Antioxid Redox Signal 15: 3021–3038, 2011 [DOI] [PubMed] [Google Scholar]
- 172.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Spier SA, Delp MD, Stallone JN, Dominguez JM, 2nd, Muller-Delp JM. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol 292: H3119–H3127, 2007 [DOI] [PubMed] [Google Scholar]
- 174.Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab 32: 1207–1221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol 286: H2249–H2256, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Swift DL, Earnest CP, Blair SN, Church TS. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: results from the DREW study. Br J Sports Med 46: 753–758, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal 10: 601–619, 2008 [DOI] [PubMed] [Google Scholar]
- 178.Tabit CE, Holbrook M, Shenouda SM, Dohadwala MM, Widlansky ME, Frame AA, Kim BH, Duess MA, Kluge MA, Levit A, Keaney JF, Jr, Vita JA, Hamburg NM. Effect of sulfasalazine on inflammation and endothelial function in patients with established coronary artery disease. Vasc Med 17: 101–107, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000 [DOI] [PubMed] [Google Scholar]
- 180.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996 [DOI] [PubMed] [Google Scholar]
- 181.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001 [DOI] [PubMed] [Google Scholar]
- 182.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995 [DOI] [PubMed] [Google Scholar]
- 183.Thompson CS, Hakim AM. Living beyond our physiological means: small vessel disease of the brain is an expression of a systemic failure in arteriolar function: a unifying hypothesis. Stroke 40: e322–e330, 2009 [DOI] [PubMed] [Google Scholar]
- 184.Timmerman KL, Volpi E. Endothelial function and the regulation of muscle protein anabolism in older adults. Nutr Metab Cardiovasc Dis 23, Suppl 1: S44–S50, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 106: 1925–1934, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tschudi MR, Barton M, Bersinger NA, Moreau P, Cosentino F, Noll G, Malinski T, Luscher TF. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest 98: 899–905, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tucsek Z, Gautam T, Sonntag WE, Toth P, Saito H, Salomao R, Szabo C, Csiszar A, Ungvari Z. Aging exacerbates microvascular endothelial damage induced by circulating factors present in the serum of septic patients. J Gerontol A Biol Sci Med Sci 68: 652–660, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007 [DOI] [PubMed] [Google Scholar]
- 189.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102: 519–528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vafeiadou K, Weech M, Sharma V, Yaqoob P, Todd S, Williams CM, Jackson KG, Lovegrove JA. A review of the evidence for the effects of total dietary fat, saturated, monounsaturated and n-6 polyunsaturated fatty acids on vascular function, endothelial progenitor cells and microparticles. Br J Nutr 107: 303–324, 2012 [DOI] [PubMed] [Google Scholar]
- 191.Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. Acute and chronic effects of vitamin C on endothelial fibrinolytic function in overweight and obese adult humans. J Physiol 586: 3525–3535, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 196: 193–222, 2009 [DOI] [PubMed] [Google Scholar]
- 193.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Velmurugan K, Alam J, McCord JM, Pugazhenthi S. Synergistic induction of heme oxygenase-1 by the components of the antioxidant supplement Protandim. Free Radic Biol Med 46: 430–440, 2009 [DOI] [PubMed] [Google Scholar]
- 195.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care 32, Suppl 2: S314–S321, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Virdis A, Ghiadoni L, Giannarelli C, Taddei S. Endothelial dysfunction and vascular disease in later life. Maturitas 67: 20–24, 2010 [DOI] [PubMed] [Google Scholar]
- 197.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002 [DOI] [PubMed] [Google Scholar]
- 198.Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. Am J Hypertens 22: 250–256, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Walker AE, Kaplon RE, Lucking SM, Russell-Nowlan MJ, Eckel RH, Seals DR. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension 60: 1517–1523, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Walker AE, Pierce GL, Kaplon RE, Russell MJ, Lesniewski LA, Donato AJ, Seals DR. Absence of nuclear factor kappaB-mediated suppression of vascular endothelial function with aging in exercising adults. FASEB J 25: 821.6, 2011 [Google Scholar]
- 201.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res 111: 245–259, 2012 [DOI] [PubMed] [Google Scholar]
- 202.Wang Q, Liang B, Shirwany NA, Zou MH. 2-Deoxy-d-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLos One 6: e17234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med 337: 986–994, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Weissig V, Cheng SM, D'Souza GG. Mitochondrial pharmaceutics. Mitochondrion 3: 229–244, 2004 [DOI] [PubMed] [Google Scholar]
- 205.Welsch MA, Dobrosielski DA, Arce-Esquivel AA, Wood RH, Ravussin E, Rowley C, Jazwinski SM. The association between flow-mediated dilation and physical function in older men. Med Sci Sports Exerc 40: 1237–1243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension 47: 245–251, 2006 [DOI] [PubMed] [Google Scholar]
- 207.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol 52: 631–646, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207a.Wong RH, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I, Howe PR. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens 31: 1819–1827, 2013 [DOI] [PubMed] [Google Scholar]
- 208.Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis 21: 851–856, 2011 [DOI] [PubMed] [Google Scholar]
- 209.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O'Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 116: 433–441, 2009 [DOI] [PubMed] [Google Scholar]
- 211.Wrick KL. The impact of regulations on the business of nutraceuticals in the united states: yesterday, today, and tomorrow. In: Regulation of Functional Foods and Nutraceuticals. Hoboken, NJ: Blackwell, 2007, p. 1–36 [Google Scholar]
- 212.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297: H1829–H1836, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang Z, Ming XF. Arginase: the emerging therapeutic target for vascular oxidative stress and inflammation. Front Immunol 4: 149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007 [DOI] [PubMed] [Google Scholar]
- 215.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yildiz O. Vascular smooth muscle and endothelial functions in aging. Ann NY Acad Sci 1100: 353–360, 2007 [DOI] [PubMed] [Google Scholar]
- 217.Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H. Additive beneficial effects of lactotripeptides intake with regular exercise on endothelium-dependent dilatation in postmenopausal women. Am J Hypertens 23: 368–372, 2010 [DOI] [PubMed] [Google Scholar]
- 218.Zand J, Lanza F, Garg HK, Bryan NS. All-natural nitrite and nitrate containing dietary supplement promotes nitric oxide production and reduces triglycerides in humans. Nutr Res 31: 262–269, 2011 [DOI] [PubMed] [Google Scholar]
- 219.Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med 14: 959–965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zuchi C, Ambrosio G, Luscher TF, Landmesser U. Nutraceuticals in cardiovascular prevention: lessons from studies on endothelial function. Cardiovasc Ther 28: 187–201, 2010 [DOI] [PubMed] [Google Scholar]