Abstract
A vast amount of investigation has centered on how the endothelium and smooth muscle communicate. From this evidence, myoendothelial junctions have emerged as critical anatomical structures to regulate heterocellular cross talk. Indeed, there is now evidence that the myoendothelial junction serves as a signaling microdomain to organize proteins used to facilitate vascular heterocellular communication. This review highlights the evolving role of myoendothelial junctions in the context of vascular cell-cell communication.
Resistance arteries and arterioles are a specialized subset of blood vessels within the vascular tree. These vessels function to control blood flow and pressure to match organ metabolism with capillary perfusion supply. To carry out these processes, communication between endothelium and smooth muscle are highly orchestrated in response to physical forces, humoral factors, and sympathetic nerve input. Although identification of critical proteins and specific signaling pathways has been under intense investigation, a growing appreciation of spatially localized proteins and compartmentalized signaling cascades has emerged. The myoendothelial junction (MEJ) has surfaced as a critical anatomical microsignaling domain used to facilitate cross talk between the endothelium and smooth muscle in resistance arteries.
The MEJ: A Brief History
In 1957, Moore and Ruska were the first to describe MEJs at the ultrastructure level, as endothelial cell protrusions extending through fenestrations of the internal elastic lamina making direct contact with smooth muscle (41). Here, it was suggested that the occurrence of vesicles in arteriolar endothelial cells combined with endothelial and smooth muscle connections would facilitate cytopempsis (transmission by cell) to regulate circulating factors from the lumen to the vessel media (41). Subsequent work performed by Rhodin produced more resolvable images of MEJs in rabbit kidney arterioles (47). Additionally, Rhodin proposed that MEJs could serve as “conductive devices” for communication. Over 45 years later, these original hypotheses, based on transmission electron micrographs, have driven the field toward exploration of the functional roles of the MEJ in the setting of both physiology and disease.
The MEJ: A Connection to the Other Side
Morphologically, the MEJ is a cellular extension from an endothelial cell and to a lesser extent smooth muscle cell that juxtaposes the opposite cell type (FIGURE 1). The MEJ has structural resemblance to that of a synapse in the nervous system. Similar in size (∼0.5 μm in width, ∼0.5 μm in depth) and shape (club vs. flat), the MEJ comes in either direct or indirect contact with the adjacent cell type (extensively reviewed in Ref. 52). The incidence of MEJs is variable throughout the vascular tree depending on the organ and species, and tends to be inversely proportional to the lumen diameter (47, 49). Additionally, in humans, ∼40% of MEJs originate from smooth muscle cell (7), although in mice the derivation is almost exclusively from the endothelial cell (53). In a physiological setting, MEJs are typically observed in small arteries or arterioles; however, it has been noted that in veins and in the developing aorta MEJs are also observed (39, 59, 64). Currently, it is unclear why differences in MEJ numbers, size, shape, and origin have evolved, although a speculative explanation for these morphological variances may involve specific role(s) for each MEJ.
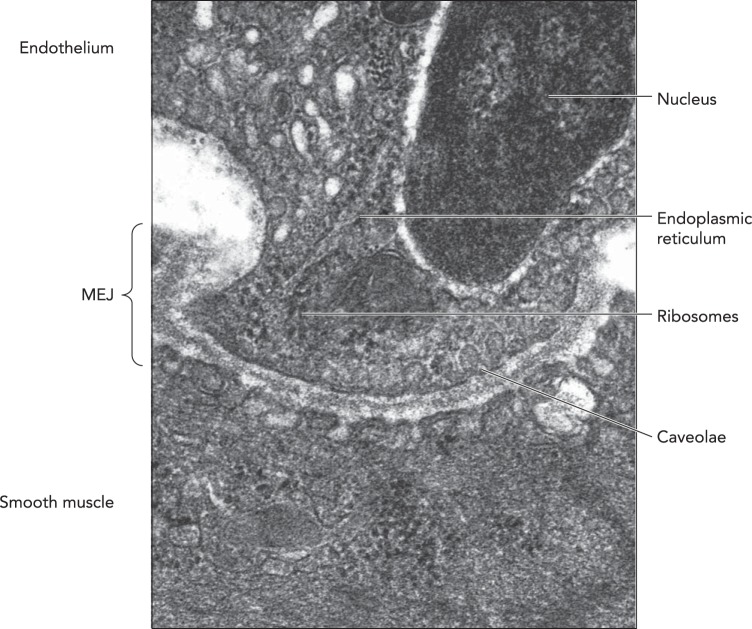
FIGURE 1.
Ultrastructure of the MEJ
An electron micrograph of an MEJ from a spinotrapezeous muscle arteriole. Arrows point to organelles localized to the MEJ, including endoplasmic reticulum, ribosomes, and caveolae. Original image is from Ref. 60 and used here with permission. Scale bar is 0.25 μm.
Recent work has focused on understanding key mechanisms that regulate MEJ formation in normal and pathological settings. Heberlein et al. first demonstrated that plasminogen activator inhibitor-1 (PAI-1), a protein involved in matrix degradation, plays a critical role in MEJ formation (18, 20). Because PAI-1 regulates cytoskeleton matrix stability and thus the growth of cellular extensions, disrupting the balance of the PAI-1 pathway resulted in decreased cellular invasion and thus MEJ formation. It was noted that an increase in circulating PAI-1 in knockout animals or localized translation of PAI-1 protein at the MEJ is critical for induction of MEJ formation (18, 20). Furthermore, there is evidence to support that, in an obese disease state, the number of MEJs positively correlates with PAI-1 expression, possibly to regulate or compensate for dysfunctional signaling that arises in the artery wall (18, 20).
Although these studies provide evidence of a regulatory pathway controlling MEJ formation and regulation, several questions still arise. 1) Is the extracellular matrix composition in small arteries and arterioles different than conduit arteries to allow for selective MEJ formation? 2) Are other matrix degrading enzymes critical for regulating MEJ formation? 3) What are the temporal dynamics of MEJ formation and retraction during physiological and pathological signaling processes? These questions will provide a basis for future studies focusing on structure-function relationships.
The MEJ: Hubs for Microdomain Organization and Localization in the Resistance Vasculature
The reason why MEJs are predominantly found in the resistance vasculature and the function of these connections are emerging areas of focus. Because the resistance vasculature represents a distinct population of blood vessels regulating blood flow and pressure, these arteries must maintain a balance between the conflicting needs of the organism and the functional demands of each tissue. Substantial evidence supports the view that various vascular components regulating blood flow and pressure are physiologically coupled. This coupling is apparent in the organization and distribution of the vascular network in conjunction with various manners of cell-cell communication between endothelium and smooth muscle. Anatomically situated at the interface between the endothelium and smooth muscle, the MEJ forms a unique coupling mechanism and microenvironment to localize, concentrate, and organize signaling proteins. Functionally, it is thought that gap junctions, located at the MEJ, are closely linked to fine-tuned control of vasomotor function (8, 10, 12, 14, 35). In fact, the number of MEJs and associated gap junctions increases in distal vs. proximal arteries, and this increase is linked to an elevation in the dependence of endothelial-derived hyperpolarizing factor (EDHF), a potent vasodilatory pathway (49, 56). Likewise, MEJs were found to be directly proportional to the time of endothelial cell response to second messengers from smooth muscle in an in vitro model of the MEJ, presumably through changes in the density of gap junctions (20). Gap junctions at the MEJ provide an important pathway for direct transfer of molecules between endothelium and smooth muscle; however, the MEJ also provides a signaling structure to sequester other signaling pathways and proteins involved in intracellular communication and paracrine-based signaling.
Over the past 10 years, key subcellular organelles and scaffolds, particularly endoplasmic reticulum (ER) (26), caveolae (61), and cytosketetal components (18, 24), have been extensively localized within the MEJ. Identification of ER with ribosomes was initially showed via transmission electron microscopy in mouse cremasteric arterioles (19, 26), an observation that has been confirmed by multiple groups based on pharmacological and immunocytochemistry studies (e.g., Ref. 34). The localization of ER at the MEJ suggests two important roles: 1) a local intracellular pool of calcium for activation of cell signaling cascades and 2) the potential for local protein translation. A number of studies, both in vitro and ex vivo, have provided direct evidence for local calcium signaling via activation of inositol trisphosphate signaling (23, 34) and transient receptor potential 4 (TRPV4) cation channels (58). Because the ER is located particularly close to the plasma membrane, this provides a confined and concentrated pool of calcium needed for activation of specific signaling pathways and proteins.
The discovery of ribosomes on ER at the MEJ not only suggests a local calcium reservoir but also the potential for RNA trafficking and translation at the MEJ for local protein synthesis. Recent evidence supports this concept, demonstrating that localization of PAI-1 mRNA, via a nicotinamide phosphoribosyltransferase (NAMPT) and serpine1 RNA binding protein 1 (SERBP1)-dependent mechanism, regulates local PAI-1 protein production and subsequent increase in MEJ formation (18). This mechanism was dependent on the microtublule binding properties of NAMPT for localization to the MEJ. In sum, this is the first line of evidence for localized protein translation in endothelium, a function that may be applicable to other proteins that localize at the MEJ.
Caveolae are lipid-rich, flask-shaped structures of the plasma membrane that play a significant role in the mobilization and organization of signaling proteins (40). They are found in high density within endothelial cells (45), and it was demonstrated that caveolae and the coat-protein that make up caveolae, caveolin-1, are extensively localized at the MEJ (61). The localization of caveolin-1 at the MEJ provides evidence that this scaffolding domain is critical for organizing proteins at the MEJ. Indeed, caveolin-1 has been associated with multiple proteins such as IP3-Rs (27, 48), connexin 43 (33, 48), Na+-K+-ATPase (36, 65), SKca channels (1, 48), TRPV4 (48), and endothelial nitric oxide synthase (eNOS) (29), all of which localize to the MEJ (2, 11, 23, 24, 58, 61, 65). Functionally, knockout mice for caveolin-1 were found to have reduced EDHF-mediated vasorelaxation, presumably by modulating membrane localization of TRPV4 channels and connexins at the MEJ (48).
The need for structural support, organization, and transport of caveolae- and non-caveolae-associated proteins is required for localization to the MEJ. Two prominent cytoskeletal components have been identified, actin and microtubules (18, 24). These important cytoskeletal structures are likely required for specific trafficking of caveolae- and non-caveolae-associated proteins to and from the MEJ but may also be fundamentally important for organization of the MEJ micro-signaling domain. The interaction between caveolin-1 and microtubules could possibly be used to enhance accurate delivery of proteins to and from the MEJ.
Collectively, there is accumulating evidence supporting that the MEJ is a critical cell-signaling microdomain in the resistance artery wall. Although the MEJ harbors many structural components, scaffolds, and protein translational components, many questions still emerge. 1) Are there specific queues or “zip codes” that enable specific proteins to traffic to the MEJ? 2) Are there a subset of caveolae that specifically traffic proteins to the MEJ? 3) Does the plasma membrane lipid composition at the MEJ influence how or what proteins localize there? 4) What cellular signaling processes differentiate protein trafficking vs. protein translation at the MEJ? These questions should provide much needed insight into the organization and function of the MEJ.
The Myoendothelial Junction: Connections That Can Deliver the Message
Gap junctions, located at the points of cell-cell contact at the MEJ, are one communication mechanism used by endothelium and smooth muscle (25, 47, 49). Gap junctions permit the passage of second messengers and electrical signaling between cells, thereby coordinating signaling and thus unifying their function (10, 14, 38, 49, 53, 69). These studies suggest that gap-junction coupling between endothelium and smooth muscle is possible; however, it is important to point out that there is also evidence to suggest that gap-junction coupling may be limited or absent (5, 57). For example, membrane potentials and dye transfer studies between endothelium and smooth muscle were independent of each other in mouse cremasteric vascular bed (5, 57). These discrepancies may be explained by methodologies used [e.g., it was shown whether arterioles were prepped via cannulation or used in vivo that there were different vasoreactive properties indicative of open gap junctions at the MEJ (4)]. Because of the manipulation of the tissue and exposure to a host of different buffers and chemicals, the crux of this work could point to altered posttranslational modifications of proteins during ex vivo preparation, especially the proteins that compose the gap junctions at the MEJ, connexins.
There are four known vascular connexins (Cx37, Cx40, Cx43, Cx45) that make up gap junctions depending on species and vascular bed (24, 37, 50), of which only Cx37, Cx40, and Cx43 are present at the MEJ (17, 24, 51). Transcription regulation of connexin expression in various species and vascular beds likely influences the regulation of gap-junction expression at the MEJ. This area is currently unexplored. The chemical and electrical gating of molecules at the MEJ is a growing area of interest that may provide an explanation for these differences. Connexins are extensively phosphorylated on multiple serine/and or tyrosine residues that may be critical for gap-junction regulation at the MEJ, depending on tissue bed and species (reviewed in Ref. 32). For example, it was reported that phosphorylation of Cx43, via a protein kinase C-dependent mechanism, occurs on serine 368 at MEJs in in vivo mouse cremastric arterioles to reduce gap-junction communication via the MEJ (62). In addition, recent evidence demonstrated a critical role for connexin 43 S-nitrosylation/denitrosylation, which is the covalent attachment of a nitric oxide (NO) moiety to cysteine residue (61). It was found that this posttranslational modification occurred specifically on cysteine residue 271 at the MEJ to control gap-junction permeability via an eNOS- and S-nitrosoglutathione reductase-dependent mechanism. It is becoming increasingly clear that posttranslational modifications and transcriptional control of connexin protein may underlie the conflicting differences in reports between species and vascular beds (61, 62).
Myoendothelial Junctions: Smooth Muscle to Endothelial Cell Communication
Stimulation of smooth muscle with the α1D-adrenergic receptor agonist phenylephrine elicits vasoconstriction immediately followed by vasodilation. This signaling is thought to involve the molecular signaling from smooth muscle cells acting to increase endothelial cell calcium (10, 28, 31, 44, 54). The rise in calcium activates vasodilatory signaling such as NO and EDHF. This concept was first demonstrated by Dora et al., demonstrating that blockade of the eNOS pathway blunted ensuing vasodilation following phenylephrine stimulation (10). In line with this work, it was shown that endothelium calcium chelation inhibited the vasodilatory response after phenylephrine, implying that a molecule derived from smooth muscle could provoke a calcium response in endothelium (69). Furthermore, studies using gap-junction inhibitors reveal attenuated calcium increases following phenylephrine stimulation in endothelial cells, a result suggestive of gap-junction coupling at the MEJ (26, 30, 31). Together, these studies infer that a flux of molecules from smooth muscle to endothelium transferred at the MEJ can regulate vessel tone.
Stimulation of smooth muscle with phenylephrine signals through the α1D-adrenergic receptors, inducing the release of IP3 through a phospholipase C mechanism. It was demonstrated that inhibition of phospholipase C in smooth muscle and IP3 receptors in endothelium before application of vasoconstrictors resulted in decreased endothelial calcium (31). This work implied that IP3 diffusion from smooth muscle, rather than calcium diffusion, is critical for endothelium increases in calcium following constriction. Subsequent work in vitro showed that IP3 diffusion from smooth muscle selectively activates IP3-R1 on the endothelial cell side of the MEJ, which was later confirmed in arteries showing IP3-based signaling originating from holes in the internal elastic lamina (26, 34, 44). Based on this evidence, IP3 likely activates intracellular calcium stores directly through TRPV4, and this calcium flux is capable of triggering endothelium vasodilatory pathways such as NO pathway and EDHF (10, 48, 61, 63, 66, 67). Collectively, these data suggest a clear role for MEJ as a microsignaling domain in the regulation of IP3-mediated calcium increases in endothelium during vasoconstriction.
Myoendothelial Junctions: Endothelial to Smooth Muscle Communication
Among the many dilators that the endothelium can produce, including NO (15), prostaglandins(6), epoxyeicosatrienoic acid (EET) (42), hydrogen peroxide (68), and hydrogen sulfide (21), most studies have focused on the dilator EDHF (13). The EDHF pathway plays a major role in endothelium to smooth muscle vasodilation and has been linked to the MEJ (extensively reviewed in Ref. 16). Mechanistically, it is currently thought that EDHF invokes the release of endothelium intracellular calcium followed by an efflux of EETs, K+, and/or current to the smooth muscle, causing hyperpolarization and closure of voltage-dependent calcium channels in smooth muscle. Three potential overlapping mechanisms mediate smooth muscle hyperpolarization: 1) efflux of K+ through endothelium Kca channels into the myoendothelial extracellular space causing stimulation of Na+-K+-ATPase and possibly KIR channels, 2) transfer of EC-derived hyperpolarization via gap junctions located at the MEJ, and 3) release of EETs causing BKca-channel activation in smooth muscle. Recent work by Sonkusare et al., demonstrated that local calcium signaling in MEJs following acetylcholine stimulation activated single TRPV4 channels (58). This induced an intracellular calcium influx, causing maximal activation of intermediate conductase potassium channels (IK), small conductase potassium channels (SK), and subsequent smooth muscle hyperpolarization. The spread of EDHF may depend on Cx40 gap junctions located at the MEJ based on mimetic peptide studies (38). Functional studies suggest that the MEJ is a microdomain positioned to regulate endothelial to smooth muscle communication and to greatly influence vasodilation in the resistance vasculature.
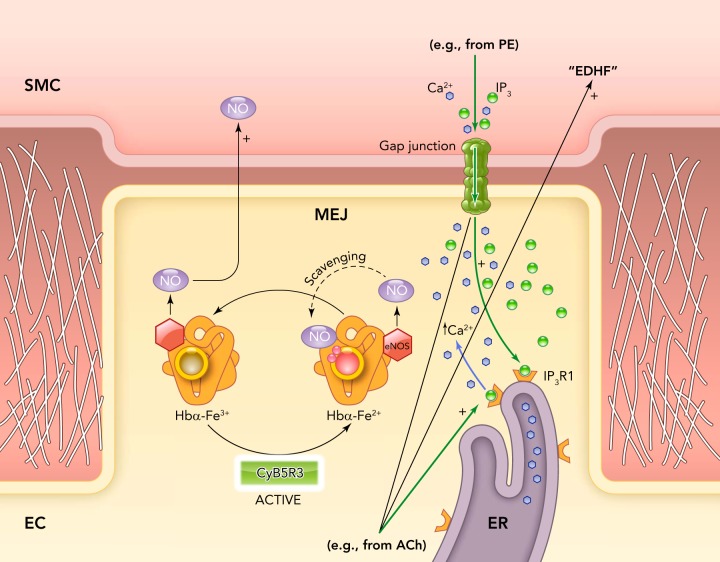
It has long been known that different signaling mechanisms control vessel tone along the vascular tree. For example, it has been shown by pharmacological inhibition of eNOS and in eNOS knockout mice that acetylcholine-induced vasodilation is mediated primarily by NO in conduit arteries (15, 22, 46). In contrast, resistance arteries primarily utilize the EDHF pathway for the vasodilatory response (9). The difference is likely not explained by variations in eNOS protein expression between the large and small arteries (43). One possible reason why the EDHF pathway may be predominant is to provide a more precise and controlled mechanism for blood flow and pressure regulation. However, it remains puzzling that eNOS, presumably capable of being activated by the same agonist (e.g., acetylcholine) in conduit as well as in resistance arteries, does not elicit the same if not more of an effect on dilation in the arterial microcirculation. Additionally, eNOS is also enriched in MEJs, reducing NO diffusion distance to smooth muscle cell in the arterial microcirculation compared with conduit arteries (61). Even though the NO pathway may not play a major role in EDHF-mediated vasodilation in resistance arteries, NO is required as an anti-inflammatory molecule, as a regulator of basal vascular tone, and for protein S-nitrosylation. Clearly, the NO in the arterial microcirculation is under tight control compared with in the conduit circulation. One mechanism inhibiting NO diffusion is its reaction to superoxide anion, which has been shown in the pulmonary artery to limit NO diffusion and regulate vasoreactivity (3). Additionally, recent evidence has demonstrated that hemoglobin α (Hb α) regulates eNOS-derived NO diffusion and bioactivity in the resistance artery wall, but little is detected in large conduit arteries (63). Evidence from isolated small arteries and arterioles as well as in a vascular cell co-culture model showed that Hb α 1) is expressed by endothelial cells, 2) is enriched at MEJs, 3) regulates NO scavenging, 4) controls S-nitrosothiol formation, and 5) regulates vascular reactivity. It was observed that NO diffusion and bioactivity is dictated by heme iron (Fe) biochemistry, a mechanism similar to its activity within red blood cells (55). Cytochrome B5 reductase 3 (CytB5R3), a major methemoglobin reductase, was shown to balance the Hb α redox state and thus NOS-dependent signaling. Genetic and pharmacological inhibition of CytB5R3 used to maintain Hb α in the Fe3+state increased NO bioactivity and dramatically altered vasoreactivity (FIGURE 2). These results suggest that enriched Hb α in the MEJ of the resistance artery is a key element in the regulation of NO signaling, cell-cell communication, endothelial cell function, and vascular tone, and could explain why NO signaling in resistance arteries is different than in conduits.
FIGURE 2.
Possible mechanism of NO control at the MEJ
Hemoglobin α (Hbα) is synthesized by vascular ECs and is enriched at the MEJ, where it forms a complex with eNOS and the reductase CytB5R3. The NO generated by eNOS at the MEJ is able to diffuse to the overlying smooth muscle cell layer when Hbα resides in the Fe3+ state. Reduction of Hbα to the Fe2+ state by the activity of CytB5R3 promotes NO scavenging by Hbα, preventing NO diffusion. Presumably, the scavenging of NO by Hga in resistance arteries allows EDHF to predominate.
Conclusions and Future Directions
Taken together, the MEJ is an emerging area of interest in resistance artery biology. The initial hypothesis put forth by Rhodin over 45 years ago suggesting that the MEJ is a “conductive device” for communication may indeed be correct based on the accumulation of data over the years. In general, it is now widely accepted that the MEJ is a signaling microdomain critical for localizing, concentrating, and organizing cell-signaling components. Future directions focused on the mechanistic role(s) of the MEJ will be important for understanding the complex coupling that resistance arteries use to regulate localized blood flow and how this may translate to overall regulation of peripheral resistance and blood pressure. Furthermore, studies will be required to understand how the MEJ contributes to these processes, particularly in the context of pathophysiology, aging, gender, and epigenetics.
Footnotes
This work was supported by the National Heart, Lung, and Blood Institute Grants HL-088554 (B. E. Isakson), HL-107963 (B. E. Isakson), and HL-112904 (A. C. Straub). The study was supported by the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: B.E.I. and A.C.S. prepared figures; B.E.I., A.C.S., and A.C.Z. drafted manuscript; B.E.I. and A.C.S. edited and revised manuscript; B.E.I. approved final version of manuscript.
References
- 1.Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SK(Ca) channels and caveolin-rich domains. Br J Pharmacol 151: 332–340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci USA 109: 18174–18179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billaud M, Marthan R, Savineau JP, Guibert C. Vascular smooth muscle modulates endothelial control of vasoreactivity via reactive oxygen species production through myoendothelial communications. PLos One 4: e6432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boettcher M, de Wit C. Distinct endothelium-derived hyperpolarizing factors emerge in vitro and in vivo and are mediated in part via connexin 40-dependent myoendothelial coupling. Hypertension 57: 802–808, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Budel S, Bartlett IS, Segal SS. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: unmasking an NO wave. Circ Res 93: 61–68, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bunting S, Gryglewski R, Moncada S, Vane JR. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins 12: 897–913, 1976 [DOI] [PubMed] [Google Scholar]
- 7.Chadha PS, Liu L, Rikard-Bell M, Senadheera S, Howitt L, Bertrand RL, Grayson TH, Murphy TV, Sandow SL. Endothelium-dependent vasodilation in human mesenteric artery is primarily mediated by myoendothelial gap junctions intermediate conductance calcium-activated K+ channel and nitric oxide. J Pharmacol Exp Ther 336: 701–708, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J Physiol 508: 561–573, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Suzuki H, Weston AH. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol 95: 1165–1174, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci USA 94: 6529–6534, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res 102: 1247–1255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dora KA, Martin PE, Chaytor AT, Evans WH, Garland CJ, Griffith TM. Role of heterocellular Gap junctional communication in endothelium-dependent smooth muscle hyperpolarization: inhibition by a connexin-mimetic peptide. Biochem Biophys Res Commun 254: 27–31, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res 87: 474–479, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 16.Garland CJ, Hiley CR, Dora KA. EDHF: spreading the influence of the endothelium. Br J Pharmacol 164: 839–852, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol 291: H2047–H2056, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Heberlein KR, Han J, Straub AC, Best AK, Kaun C, Wojta J, Isakson BE. A novel mRNA binding protein complex promotes localized plasminogen activator inhibitor-1 accumulation at the myoendothelial junction. Arterioscler Thromb Vasc Biol 32: 1271–1279, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heberlein KR, Han J, Straub AC, Best AK, Kaun C, Wojta J, Isakson BE. A novel mRNA binding protein complex promotes localized plasminogen activator inhibitor-1 accumulation at the myoendothelial junction. Arterioscler Thromb Vasc Biol 32: 1271–1279, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heberlein KR, Straub AC, Best AK, Greyson MA, Looft-Wilson RC, Sharma PR, Meher A, Leitinger N, Isakson BE. Plasminogen activator inhibitor-1 regulates myoendothelial junction formation. Circ Res 106: 1092–1102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377: 239–242, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Isakson BE. Localized expression of an Ins(1,4,5)P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. J Cell Sci 121: 3664–3673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isakson BE, Best AK, Duling BR. Incidence of protein on actin bridges between endothelium and smooth muscle in arterioles demonstrates heterogeneous connexin expression and phosphorylation. Am J Physiol Heart Circ Physiol 294: H2898–H2904, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res 97: 44–51, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res 100: 246–254, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Isshiki M, Anderson RG. Function of caveolae in Ca2+ entry and Ca2+-dependent signal transduction. Traffic 4: 717–723, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Jackson WF, Boerman EM, Lange EJ, Lundback SS, Cohen KD. Smooth muscle alpha1D-adrenoceptors mediate phenylephrine-induced vasoconstriction and increases in endothelial cell Ca2+ in hamster cremaster arterioles. Br J Pharmacol 155: 514–524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 272: 18522–18525, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium 44: 135–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamboley M, Pittet P, Koenigsberger M, Sauser R, Beny JL, Meister JJ. Evidence for signaling via gap junctions from smooth muscle to endothelial cells in rat mesenteric arteries: possible implication of a second messenger. Cell Calcium 37: 311–320, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys 384: 205–215, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol Biol Cell 19: 912–928, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Little TL, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res 76: 498–504, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Mohammadi K, Aynafshar B, Wang H, Li D, Liu J, Ivanov AV, Xie Z, Askari A. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol 284: C1550–C1560, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol 97: 1152–1158, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res 97: 399–407, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Michel RP. Arteries and veins of the normal dog lung: qualitative and quantitative structural differences. Am J Anat 164: 227–241, 1982 [DOI] [PubMed] [Google Scholar]
- 40.Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol 285: L1179–L1183, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Moore DH, Ruska H. The fine structure of capillaries and small arteries. J Biophys Biochem Cytol 3: 457–462, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mugge A, Lopez JA, Piegors DJ, Breese KR, Heistad DD. Acetylcholine-induced vasodilatation in rabbit hindlimb in vivo is not inhibited by analogues of L-arginine. Am J Physiol Heart Circ Physiol 260: H242–H247, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Nakajima S, Ohashi J, Sawada A, Noda K, Fukumoto Y, Shimokawa H. Essential role of bone marrow for microvascular endothelial and metabolic functions in mice. Circ Res 111: 87–96, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Nausch LW, Bonev AD, Heppner TJ, Tallini Y, Kotlikoff MI, Nelson MT. Sympathetic nerve stimulation induces local endothelial Ca2+ signals to oppose vasoconstriction of mouse mesenteric arteries. Am J Physiol Heart Circ Physiol 302: H594–H602, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol 8: 185–194, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Rees DD, Palmer RM, Hodson HF, Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol 96: 418–424, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res 18: 181–223, 1967 [DOI] [PubMed] [Google Scholar]
- 48.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res 86: 341–346, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res 60: 643–653, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels [K(Ca)] and connexins: possible relationship to vasodilator function? J Anat 209: 689–698, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. Myoendothelial contacts, gap junctions, and microdomains: anatomical links to function? Microcirculation 19: 403–415, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res 90: 1108–1113, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Schuster A, Oishi H, Beny JL, Stergiopulos N, Meister JJ. Simultaneous arterial calcium dynamics and diameter measurements: application to myoendothelial communication. Am J Physiol Heart Circ Physiol 280: H1088–H1096, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Sharma VS, Traylor TG, Gardiner R, Mizukami H. Reaction of nitric oxide with heme proteins and model compounds of hemoglobin. Biochemistry 26: 3837–3843, 1987 [DOI] [PubMed] [Google Scholar]
- 56.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Siegl D, Koeppen M, Wolfle SE, Pohl U, de WC. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res 97: 781–788, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sosa-Melgarejo JA, Berry CL. Myoendothelial contacts in the thoracic aorta of rat fetuses. J Pathol 166: 311–316, 1992 [DOI] [PubMed] [Google Scholar]
- 60.Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler Thromb Vasc Biol 31: 399–407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler Thromb Vasc Biol 31: 399–407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Straub AC, Johnstone SR, Heberlein KR, Rizzo MJ, Best AK, Boitano S, Isakson BE. Site-specific connexin phosphorylation is associated with reduced heterocellular communication between smooth muscle and endothelium. J Vasc Res 47: 277–286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature 491: 473–477, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Svendsen E, Austarheim AM, Haugen B, Dalen H, Dregelid E. Myoendothelial junctions in human saphenous veins. Acta Anat (Basel) 138: 150–153, 1990 [DOI] [PubMed] [Google Scholar]
- 65.Tian J, Xie ZJ. The Na-K-ATPase and calcium-signaling microdomains. Physiology 23: 205–211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brigdan M, Welsh DG. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol 302: C1226–C1242, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 97: 908–915, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Wei EP, Kontos HA. H2O2 and endothelium-dependent cerebral arteriolar dilation. Implications for the identity of endothelium-derived relaxing factor generated by acetylcholine. Hypertension 16: 162–169, 1990 [DOI] [PubMed] [Google Scholar]
- 69.Yashiro Y, Duling BR. Integrated Ca2+ signaling between smooth muscle and endothelium of resistance vessels. Circ Res 87: 1048–1054, 2000 [DOI] [PubMed] [Google Scholar]