A 59-year-old woman presented to her primary care physician with cough, exertional dyspnea, and foot swelling that had developed 2 weeks earlier while she was vacationing in Denmark. She had no rhinorrhea, pharyngitis, fever, rash, diarrhea, or new joint symptoms. Her medical history was notable for polyarthritis, for which her rheumatologist prescribed minocycline and meloxicam. She had undergone right total hip replacement 4 years previously, left total hip replacement 3 years previously, and left total knee replacement 1 year previously. There was no history of hypertension or diabetes. She had never used tobacco, illicit drugs, or herbal supplements and did not consume large amounts of alcohol. She was married and primarily had been a homemaker for her three children, and she did not have known occupational exposures. There was no family history of early-onset pulmonary or cardiovascular disease. Vital signs were within normal limits. She received a diagnosis of pneumonia and “travel-related edema” and was treated with a course of antibiotics; no chest imaging or blood tests were performed, and she did not receive diuretics.
New-onset dyspnea and cough in an otherwise healthy middle-aged person is most commonly due to acute bronchitis. However, the differential diagnosis is broad. Deepvein thrombosis with pulmonary embolus and endemic infectious diseases should be considered in someone who has recently traveled. Primary myocardial, pulmonary, and vascular diseases may also result in persistent cough, dyspnea, and edema.
Over the next week, the patient had progressive dyspnea, edema, and fatigue. She returned to her physician. Computed tomography (CT) of the chest was negative for a pulmonary embolus, but it showed cardiomegaly and changes consistent with pulmonary edema; these findings prompted admission to her local hospital. On physical examination, her weight was 100 lb (45 kg), height 59 in. (150 cm), heart rate 80 beats per minute, blood pressure 100/65 mm Hg, and oxygen saturation 94% while she was breathing ambient air. Her jugular venous pressure was 13 cm of water, with normal respiratory variation. There were no rales, rhonchi, or wheezes. Cardiac examination revealed a regular rhythm, normal S1 and S2 sounds, an S3 gallop, and a grade 2/6 holo-systolic murmur across the precordium. Neither hepatosplenomegaly nor ascites was detected. There was 1+ bilateral lower-leg edema. Blood tests revealed a normal complete blood count and normal electrolyte values; the creatinine level was 1.0 mg per deciliter (88 μmol per liter); liver-function tests and the thyrotropin level were normal. Electrocardiography showed a normal sinus rhythm, normal voltage, normal axis, and a QRS duration of 90 msec, with no Q waves. Chest radiography showed pulmonary vascular redistribution and degenerative changes of the spine. Echocardiography showed an estimated left ventricular ejection fraction (LVEF) of 25%, global hypokinesis, a left ventricular wall thickness of 0.9 cm (reference value, <1.1), normal right ventricular size and function, moderate mitral and mild tricuspid regurgitation, an estimated right ventricular systolic pressure of 35 mm Hg (reference value, <35), and moderate circumferential pericardial effusion. Coronary angiography was normal. Treatment with furosemide at a dose of 40 mg daily, carvedilol at a dose of 6.25 mg twice daily, and lisinopril at a dose of 2.5 mg daily was initiated.
New-onset heart failure, particularly in younger patients who do not have coexisting conditions, can be easily misdiagnosed. In this case, the elevated jugular venous pressure and third heart sound immediately suggested this diagnosis, and additional testing confirmed heart failure with a reduced ejection fraction. Cardiac catheterization ruled out coronary disease as the cause. In addition to the initiation of appropriate oral therapies for heart failure, further evaluation is needed to determine the primary cause and exacerbating factors.
Despite some initial improvement in symptoms, over the next 2 months, the patient had recurrent dyspnea, and the dose of oral furosemide was increased. The doses of her other medications could not be increased because of systolic blood pressures of 80 to 90 mm Hg and associated ligh-theadedness. Six months after the onset of symptoms, she was having difficulty climbing one flight of stairs because of progressive dyspnea. She was readmitted to her local hospital. Repeat echocardiography showed increased pericardial effusion. Right heart catheterization showed a central venous pressure of 15 mm Hg (reference range, 3 to 8), pulmonary-artery pressure of 46/25 mm Hg (reference range, 15 to 30 systolic, 4 to 12 diastolic), postcapillary wedge pressure of 14 mm Hg (reference range, 2 to 15), and cardiac output of 1.9 liters per minute (range, 4 to 8). Pericardiocentesis removed 440 ml of clear, amber-colored fluid. Vital signs were unchanged after this procedure. Analysis of the fluid and of a cell block showed no malignant cells, and Gram’s stain and routine culture did not reveal infectious organisms. The serum rheumatoid factor was 9 IU per milliliter (reference range, 0 to 14), a test for antinuclear antibody was negative, and the C-reactive protein level was less than 0.5 mg per deciliter. The patient subsequently received an implantable cardioverter–defibrillator (ICD).
Clinically significant pericardial effusion is an uncommon finding among patients with heart failure, and it therefore raises the probability of conditions such as myopericarditis or autoimmune-related cardiomyopathy. The analysis of pericardial fluid and serum autoimmune markers did not provide support for such causes in this case. Cardiac magnetic resonance imaging (MRI) with contrast enhancement allows for detailed characterization of the degree, location, and pattern of inflammation, fibrosis, and infiltration within the myocardium. This form of imaging, which can aid in the diagnosis of ischemic heart disease, myocarditis, and a number of infiltrative cardiomyopathies, should be considered before the implantation of an ICD.
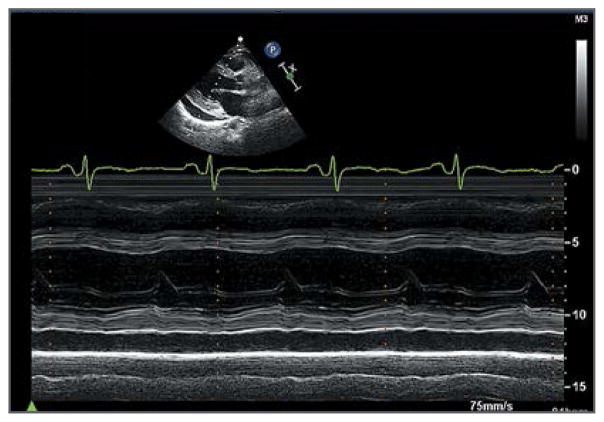
The patient was referred to a regional academic medical center. An evaluation for cardiac transplantation was completed. Repeat echocardiography showed no reaccumulation of pericardial fluid; the estimated left ventricular diastolic cavity was 59 ml (reference range, 56 to 104), and the LVEF was 31% (Fig. 1 and video, available with the full text of this article at NEJM.org). The B-type natriuretic peptide (BNP) level was 694 pg per milliliter (reference value, <100). On cardiopulmonary exercise testing, she stopped 3 minutes and 30 seconds into a protocol for congestive heart failure, with peak oxygen consumption of 15 ml per kilogram of body weight per minute at a respiratory exchange ratio of 0.95. The ferritin level was 117 ng per milliliter (reference range, 10 to 388), serum iron level 65 μg per deciliter (12 μmol per liter) (reference range, 30 to 160 [5 to 29]), and total iron-binding capacity 248 μg per deciliter (44 μmol per liter) (reference range, 210 to 390 [38 to 70]). The results of serum and urinary protein electrophoresis were normal. Serologic tests for the human immunodeficiency virus and hepatitis C virus were negative. Spironolactone was added to her medications. Over the ensuing weeks, she had worsening dizziness and fatigue, requiring reduced doses of lisinopril and carvedilol, and she was listed for cardiac transplantation.
Figure 1. Transthoracic Echocardiogram Obtained 3 Months before Placement of a Left Ventricular Assist Device.
The parasternal long-axis view shows reduced left ventricular systolic function in the absence of left ventricular dilatation or hypertrophy.
There is a window of time when advanced therapies (i.e., mechanical circulatory support and heart transplantation) can be considered: late enough that resources are used judiciously but before severe or permanent end-organ damage occurs. This patient’s symptomatic hypotension and the side effects of neurohormonal medications indicated the need to consider such therapies.
Three months after being listed for heart transplantation, the patient returned to the heart-failure clinic with worsening fatigue. Examination revealed a heart rate of 90 beats per minute, blood pressure of 70/54 mm Hg, and pale skin. The BNP level was more than 5000 pg per milliliter, creatinine level 1.5 mg per deciliter (130 μmol per liter), and aspartate aminotransferase level 459 U per liter. She was hospitalized. Additional testing revealed an arterial lactate level of 6.3 mmol per liter (reference range, 0.3 to 0.8) and venous oxygen saturation of 28% (reference range, 60 to 80); these findings prompted initiation of treatment with dobutamine and transfer of the patient to the intensive care unit. Repeat echocardiography showed an LVEF of 10%. Because of progressive evidence of low cardiac output and poor end-organ perfusion despite inotropic therapy, she was taken to the operating room, where a HeartMate II (Thoratec) left ventricular assist device (LVAD) was successfully implanted.
Early recognition of cardiogenic shock and timely implementation of appropriate stepwise interventions to restore adequate tissue perfusion are necessary to prevent multiorgan failure. If pharmacologic therapies do not restore adequate blood flow, mechanical circulatory support is indicated. When the cause of decompensation is potentially reversible, temporary support tiered to the hemodynamic need can be used; types of support include an intraaortic balloon pump, percutaneous LVAD, and extracorporeal membranous oxygenation. For this patient, in whom no reversible cause of heart failure was identified, right ventricular function was adequate, and no other contraindications were present, surgical implantation of a durable LVAD was indicated.
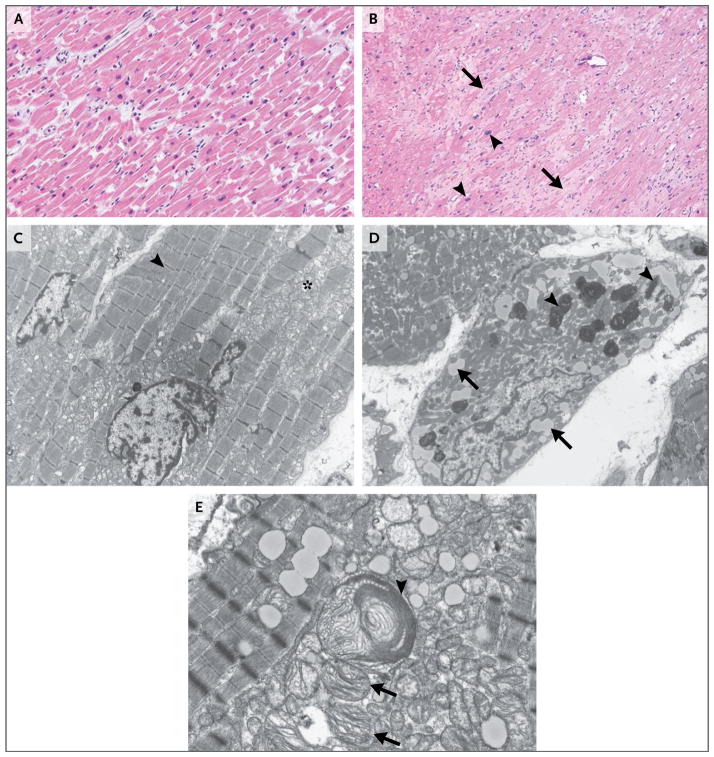
Histologic findings in a sample of the apical left ventricular tissue removed at the time of implantation of the LVAD inflow cannula showed moderate myocyte hypertrophy and extensive interstitial fibrosis (Fig. 2B). No amyloidosis, hemochromatosis, or myocarditis was identified. Transmission electron microscopy showed cardiomyocyte hypertrophy with substantial loss of contractile elements, increased lipid levels, and other degenerative features that were consistent with dilated cardiomyopathy (Fig. 2D). In addition, enlarged and atypical mitochondrial forms were noted (Fig. 2E); these findings indicated possible direct mitochondrial injury.
Figure 2. Micrographs of Specimens of Apical Left Ventricular Tissue.
Panel A shows a photomicrograph of a specimen of normal myocardium (hematoxylin and eosin) obtained from an autopsy specimen. Panel B shows a photomicrograph of a specimen of the patient’s left ventricular apical core (hematoxylin and eosin) obtained during implantation of a left ventricular assist device for cardiogenic shock. Moderate interstitial, pericellular fibrosis (arrows) and myocyte hypertrophy (arrowheads) are evident. Panel C shows a transmission electron micrograph of an autopsy specimen. Normal myocytes with abundant myofibrils (arrowhead) and morphologically normal mitochondria (asterisk) are present. Panel D shows a transmission electron micrograph of the patient’s left ventricular tissue. Myocytes have degenerative features, characterized by substantial loss of contractile units, intracytoplasmic lipid accumulation (arrows), and lipofuscin deposition (arrowheads). Panel E shows another transmission electron micrograph of the patient’s left ventricular tissue. Both a highly atypical, enlarged mitochondrion (arrowhead) and immediately beneath it several smaller mitochondria (arrows) containing abnormally configured cristae suggest direct mitochondrial injury.
Histopathological evaluation of myocardial tissue is not a standard part of the evaluation for most cardiomyopathies, but judicious use in certain clinical scenarios is indicated. The lack of ventricular dilatation in this case prompted the consideration of unusual causes of heart failure, and LVAD placement provided apical left ventricular tissue for evaluation. Amyloidosis and hemochromatosis can generally be ruled out on the basis of the histopathological findings. The findings of hypertrophy and fibrosis in this case are not specific to dilated cardiomyopathy and may be seen in hypertrophic or restrictive cardiomyopathies or in chronic ischemic, hypertensive, or valvular heart disease. Thus, correlating the findings with the clinical picture is imperative.
The patient was discharged home 2 weeks after LVAD implantation. A month later, she had episodes of ventricular tachycardia and was treated successfully with amiodarone. Three months after LVAD implantation, a suitable donor heart became available, and she underwent orthotopic heart transplantation. She was discharged on postoperative day 20 while receiving prednisone, tacrolimus, and mycophenolate mofetil.
Two months after transplantation, the patient was recovering as expected. On routine testing, the thyrotropin level was 58 mU per liter (reference range, 0.5 to 5.0), free thyroxine 0.62 ng per deciliter (8 pmol per liter) (reference range, 0.89 to 1.76 [11 to 23]), total triiodothyronine 54 ng per deciliter (0.8 nmol per liter) (reference range, 60 to 181 [0.9 to 2.8 nmol per liter]), and thyroglobulin antibody 36 U per milliliter (reference range, 0 to 23). Endocrinology consultation suggested that the hypothyroidism was most likely attributable to amiodarone. Thyroxine supplementation was initiated. Cataracts also developed in the patient and progressively worsened in the subsequent months.
Hypothyroidism is seen in approximately 10 to 20% of patients who receive amiodarone, although the appearance in this patient months after drug discontinuation is atypical. Cataracts are a recognized side effect of glucocorticoids; other concerns with glucocorticoid use include bone loss, weight gain, diabetes, and increased blood pressure.
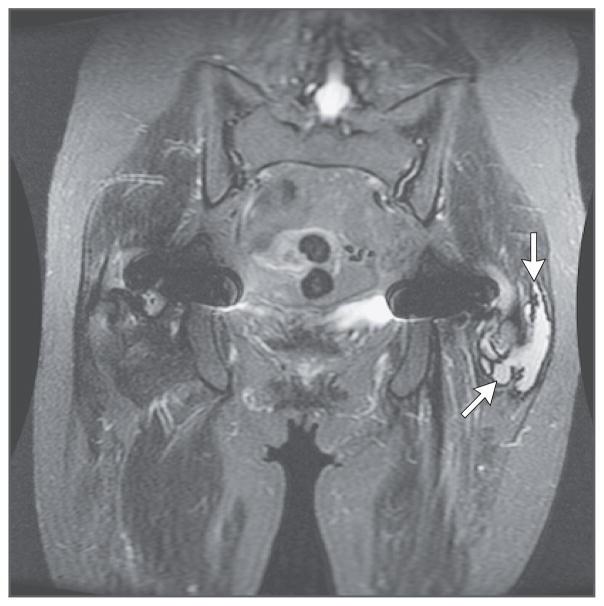
The patient had originally learned 13 months before transplantation that her bilateral DuPuy ASR metal-on-metal hip prostheses were recalled because of higher-than-expected failure rates. The manufacturer’s initial recommended course of action was plain radiography and a clinical examination every 6 months; these measures showed no evidence of abnormality in the prostheses. However, 7 months after transplantation, the patient was advised to undergo further hip imaging and measurement of serum cobalt levels owing to further safety concerns with her hip prostheses. Pelvic MRI showed bilateral thin-walled fluid collections that were consistent with pseudotumors caused by a reaction to metal implants (“metallosis”) (Fig. 3); the serum cobalt level was 287.6 μg per liter (reference value, <1.0).
Figure 3. Magnetic Resonance Image of the Pelvis 7 Months after Heart Transplantation.
A coronal sagittal short-tau inversion recovery image with arrows pointing to the left pseudotumor is shown. There was a symmetric decrease in muscle mass. A lobular, mildly septated fluid collection posterior to the left greater trochanter, measuring 6.6 cm by 4.2 cm by 1.9 cm, with a thin-walled, well-defined, low-signal rim and no extension into the muscles was suggestive of a metallosis pseudotumor.
The heart-transplantation team was contacted to help coordinate optimal timing for hip replacements. Cobalt levels and chromium levels were obtained from stored blood and LVAD apical core samples, all of which showed metallosis that was more than two orders of magnitude above the upper limit of the normal range (Table 1). Review of the electron-microscopical images of the left ventricular apical tissue from the patient’s native heart showed abnormal mitochondrial forms and electron-dense deposits that were consistent with cardiomyopathy related to cobalt–chromium toxicity (Fig. 2B). Since surveillance endomyocardial biopsies showed no evidence of rejection, the dose of prednisone was decreased rapidly to improve wound healing after arthroplasty.
Table 1.
Clinical Events, Results of Cardiac Imaging, and Laboratory Findings in the Patient.*
| Time of Assessment | Clinical Event | LVEF† | B-Type Natriuretic Peptide Level‡ | Cobalt Level in Serum§ | Cobalt Level in Heart Tissue¶ | Chromium Level in Serum |
|---|---|---|---|---|---|---|
| % | pg/ml | μg/liter | μg/g | μg/liter | ||
| 5 yr before transplantation | Right total hip replacement | |||||
|
| ||||||
| 4 yr before transplantation | Left total hip replacement | |||||
|
| ||||||
| 14 mo before transplantation | Onset of heart failure | 25 | ||||
|
| ||||||
| 6 mo before transplantation | 31 | 694 | 287.6|| | |||
|
| ||||||
| 3 mo after transplantation | LVAD implantation | 10 | >5000 | 8.32 | ||
|
| ||||||
| 7 mo after transplantation | 60 | 235 | 374.3 | |||
|
| ||||||
| 8 mo after transplantation | 54 | 298 | 398.6 | 248.9 | ||
|
| ||||||
| 11 mo after transplantation | Explantation of left hip prosthesis | 46 | 406 | 332.0 | ||
|
| ||||||
| 13 mo after transplantation | Explantation of right hip prosthesis | 51 | 391 | 106.0 | ||
|
| ||||||
| 14 mo after transplantation | 74 | 34.8 | ||||
|
| ||||||
| 16 mo after transplantation | 55 | 203 | 11.8 | |||
|
| ||||||
| 18 mo after transplantation | 58 | 199 | ||||
LVAD denotes left ventricular assist device.
The left ventricular ejection fraction (LVEF) was a quantitative estimation calculated with the use of Simpson’s biplane method, with qualitative visual review by a cardiologist to confirm accuracy.
The B-type natriuretic peptide level was measured by means of a UniCel DxI 800 immunoassay system (Beckman Coulter). The reference value is <100 pg per milliliter.
The reference value defined for persons in the general population without specific exposures to cobalt and chromium was <5.0 μg per liter.
Cardiac tissue samples obtained from persons without known cobalt exposure in an age-matched cohort to our patient showed a range of 0.1 to 0.2 μg per gram.
A cryopreserved sample initially drawn for panel-reactive antibody testing before transplantation was evaluated retrospectively. All other heavy-metal samples were collected in a certified metal-free tube.
Before the first hip surgery, the patient reported mild, progressive fatigue. At her preoperative evaluation 10 months after transplantation, the systolic blood pressure had decreased from 110 to 90 mm Hg, the level of BNP had increased from 235 to 406 pg per milliliter, and the LVEF in the cardiac graft had decreased from 60 to 46%. Repeat endomyocardial biopsy again showed no evidence of cellular or antibody-mediated rejection, and coronary angiography was normal.
Eleven months after heart transplantation, the patient’s original left DePuy ASR Summit hip-replacement system (size 1, cementless, high-offset DuoFix stem, 41-mm ball, and 46-mm one-piece cup) was replaced. At the time of surgery, there was gross metal staining along the entire anterior aspect of the abductor system, a pseudotumor (6 cm by 9 cm by 2 cm) was revealed on division of the iliotibial band, and metallosis was present on the hip joint. The patient recovered well postoperatively. Nine weeks after left hip surgery, the original right DePuy ASR Summit system (size 1 high-offset DuoFix stem, +2 43 ball, and size 48 one-piece cup) was also replaced, with operative findings similar to those for the contralateral hip. The postoperative course was complicated by deconditioning, a fall, and deep-vein thrombosis. After removal of both metal-on-metal hips, the serum cobalt levels rapidly decreased, the serum BNP level decreased, and the LVEF returned to the normal range (Table 1). With rehabilitation, the patient reported, “I feel stronger daily.”
COMMENTARY
Metal-on-metal hip implants replace traditional ceramic or polyethylene materials with a cobalt–chromium alloy for the lining of the head and cup of the prosthesis. Approximately 1 million patients around the world have received such hip prostheses, most of which were implanted between 2003 and 2010. Whereas these implants were intended to last longer than traditional implants, recent data suggest that they have increased failure rates.1 In addition, cobalt–chromium implants release metal ions into the surrounding tissue and bloodstream. Although the regulatory approval and safety surveillance processes for metal-on-metal hip prostheses have garnered recent attention in major medical journals,2–4 relatively little attention has been paid to the potential systemic effects of cobalt–chromium ion release — including the possibility of a potentially reversible cardiomyopathy — particularly in patients without symptomatic or objective findings of failure of implants used in arthroplasty.
Although the diagnosis of cardiomyopathy related to cobalt in such implants cannot be absolutely confirmed in this case, several features support cobalt cardiomyopathy as the likely diagnosis. These include the high serum and tissue cobalt levels in association with progressive dysfunction in the patient’s native heart, the recurrence of early clinical findings of dysfunction in the transplanted heart and apparent improvement after removal of the metal-on-metal devices, the absence of an alternative cause, the absence of ventricular dilatation typically seen in idiopathic dilated cardiomyopathy,5 and the mitochondrial abnormalities seen on micrographs of cardiac tissue. Mitochondrial injury is a nonspecific finding, but it has been reported in experimental models of cobalt cardiotoxicity. Most heavy metals can interfere with oxidative phosphorylation and damage the mitochondria. Noncardiac organ involvement, including thyroid, liver, neurologic, and bone marrow dysfunction, has also been described in association with systemic cobalt toxicity. This patient’s hypothyroidism and cataracts may also have been related to cobalt, although they are common conditions in the general population.
Cobalt cardiac toxicity was first characterized in the early 1960s as “Quebec beer-drinkers’ cardiomyopathy,” which was attributed to a cobalt-based foam-stabilizing agent. The clinical presentation during that epidemic was quite similar to the case presented here, with a rapidly progressive cardiomyopathy after the onset of symptoms in otherwise fairly young and healthy persons. 6 In more recent years, several cases of presumed cardiomyopathy related to cobalt implants used in arthroplasty have been described in the literature,7–11 but these reports have typically involved more overt prosthetic failure or resurfacing of the metal-on-metal prosthesis,12 and in all but one case, the cardiomyopathy was reversible with removal of the prothesis.11
Despite data from this case and other case reports documenting elevated levels of cobalt in serum and myocardium at the onset of heart failure, the threshold for serum and tissue cobalt levels that cause cardiomyopathy has not been defined. Whether T2-weighted MRI imaging can provide noninvasive risk stratification for cobalt cardiotoxicity among patients with high serum cobalt levels is unknown. It is also uncertain whether chelation therapy has a role in treatment.
The Medicines and Healthcare Products Regulatory Agency in the United Kingdom released updated guidelines in June 2012, which include the recommendation that any patient with a DePuy ASR prosthesis should undergo annual follow-up for the lifetime of the implant, including both imaging and measurement of blood cobalt and chromium levels.13 The recommendations of the Food and Drug Administration are much less specific and less rigorous. 14 Testing for heavy metals is not part of the standard evaluation for otherwise unexplained cardiomyopathy in patients without obvious exposure.15 This case suggests that a variety of clinical specialists in orthopedics, cardiology, cardiac surgery, endocrinology, rheumatology, ophthalmology, and pathology and their patients may benefit from improved awareness and multidisciplinary communication regarding the potential risks associated with these devices.
Acknowledgments
We thank the members of the care team, including Jeffrey Rubinstein, M.D., JoAnn Lindenfeld, M.D., and Randy Boer, for assisting with clinical information; David Murray, M.D., Ph.D., Department of Pathology, Mayo Clinic, for his expertise in heavy metals and for testing specimens; Brian Petersen, M.D., Section of Musculoskeletal Radiology, University of Colorado, for interpretation of imaging studies; Gary Mierau, Ph.D., and Eric Wartchow, Department of Pathology, Children’s Hospital Colorado, for providing expertise and images from ultrastructural examination of cardiac tissue obtained from the patient; and Brahmajee K. Nallamothu, M.D., M.P.H., University of Michigan, for assistance in preparing an earlier version of the manuscript.
Footnotes
In this Journal feature, information about a real patient is presented in stages (boldface type) to an expert clinician, who responds to the information, sharing his or her reasoning with the reader (regular type). The authors’ commentary follows.
Dr. Allen reports receiving consulting fees from Johnson & Johnson, Novartis, Janssen, and Amgen; Dr. Wolfel, lecture fees from Medical Educational Resources; and Dr. Ambardekar, consulting fees from Cytokinetics. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Graves SE, Rothwell A, Tucker K, Jacobs JJ, Sedrakyan A. A multinational assessment of metal-on-metal bearings in hip replacement. J Bone Joint Surg Am. 2011;93(Suppl 3):43–7. doi: 10.2106/JBJS.K.01220. [DOI] [PubMed] [Google Scholar]
- 2.Ardaugh BM, Graves SE, Redberg RF. The 510(k) ancestry of a metal-on-metal hip implant. N Engl J Med. 2013;368:97–100. doi: 10.1056/NEJMp1211581. [DOI] [PubMed] [Google Scholar]
- 3.Rising JP, Reynolds IS, Sedrakyan A. Delays and difficulties in assessing metal-on-metal hip implants. N Engl J Med. 2012;367(1):e1. doi: 10.1056/NEJMp1206794. [DOI] [PubMed] [Google Scholar]
- 4.Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410. doi: 10.1136/bmj.e1410. [DOI] [PubMed] [Google Scholar]
- 5.Centeno JA, Pestaner JP, Mullick FG, Virmani R. An analytical comparison of cobalt cardiomyopathy and idiopathic dilated cardiomyopathy. Biol Trace Elem Res. 1996;55:21–30. doi: 10.1007/BF02784165. [DOI] [PubMed] [Google Scholar]
- 6.Mercier G, Patry G. Quebec beer-drinkers’ cardiomyopathy: clinical signs and symptoms. Can Med Assoc J. 1967;97:884–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Oldenburg M, Wegner R, Baur X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J Arthroplasty. 2009;24(5):825.e15–825.e20. doi: 10.1016/j.arth.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92:2847–51. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- 9.Machado C, Appelbe A, Wood R. Arthroprosthetic cobaltism and cardiomyopathy. Heart Lung Circ. 2012;21:759–60. doi: 10.1016/j.hlc.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Pelclova D, Sklensky M, Janicek P, Lach K. Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clin Toxicol (Phila) 2012;50:262–5. doi: 10.3109/15563650.2012.670244. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert CJ, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639.e1–639.e2. doi: 10.1016/j.cjca.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–51. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 13.Medical device alert: all metal-on-metal (MoM) hip replacements (MDA/2012/036) London: Medicines and Healthcare products Regulatory Agency; Jun 25, 2012. ( http://www.mhra.gov.uk/home/groups/dts-bs/documents/medicaldevicealert/con155767.pdf) [Google Scholar]
- 14.General recommendations for orthopaedic surgeons AFTER metal-on-metal hip replacement surgery (follow-up) Silver Spring, MD: Food and Drug Administration; ( http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241667.htm) [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):e240–e319. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]