Abstract
Introduction
Glucocorticoid (GC) therapy in Duchenne muscular dystrophy (DMD) has altered disease progression, necessitating contemporary natural history studies.
Methods
The Cooperative Neuromuscular Research Group (CINRG) DMD Natural History Study (DMD-NHS) enrolled 340 DMD males, ages 2–28 years. A comprehensive battery of measures was obtained.
Results
A novel composite functional “milestone” scale scale showed clinically meaningful mobility and upper limb abilities were significantly preserved in GC-treated adolescents/young adults. Manual muscle test (MMT)-based calculations of global strength showed that those patients <10 years of age treated with steroids declined by 0.4±0.39 MMT unit/year, compared with −0.4±0.39 MMT unit/year in historical steroid-naive subjects. Pulmonary function tests (PFTs) were relatively preserved in steroid-treated adolescents. The linearity and magnitude of decline in measures were affected by maturational changes and functional status.
Conclusions
In DMD, long-term use of GCs showed reduced strength loss and preserved functional capabilities and PFTs compared with previous natural history studies performed prior to the widespread use of GC therapy.
Keywords: adolescent, adult, child/preschool, follow-up studies, health status, humans, locomotion, male, muscular dystrophies/classification, muscular dystrophies/Duchenne/physiopathology, muscular dystrophies/therapy, muscle strength/physiology, phenotype, quality of life/psychology, respiratory function tests
Duchenne muscular dystrophy (DMD) is an X-linked degenerative disorder of the dystrophin protein that causes progressive muscle weakness, usually leading to death in early adulthood.1 DMD is the most common neuromuscular disease of childhood and occurs with an incidence of about 30 per 100,000 live-born males across all ethnic groups.2 Although it is accepted that a majority of cases of DMD are inherited, studies have demonstrated that between 20% and 50% of DMD cases in various populations are the result of spontaneous mutations.3–6 The course of physical impairment is severe and inexorably progressive, and its description has varied little since Meryon’s and Duchenne’s early descriptions of the disease in the mid-19th century.7,8 In early childhood, motor developmental milestones are delayed and, by 4–5 years of age, these children rise from the floor in the classic adaptive standing pattern first described by Gowers,9 have increasing difficulty climbing stairs, and begin to have frequent falls. Muscles show a classic pattern of pseudohypertrophy, most notably in the calf muscles. With disease progression, boys begin to walk with a characteristic waddling gait with compensatory lumbar lordosis, shortened stride length, and widened base of support, which advances to a point where they require constant physical support and stabilization.10,11 Mean age to loss of ambulation in steroid-naive children is between ages 9 and 10 years.12–16 Over the ensuing years, patients typically develop worsening contractures, scoliosis, and progressive impairment of respiratory and cardiac function. From the earliest reports until the 1960s, death has typically occurred in the early to mid-teens due to respiratory complications or cardiac failure, but advances in preventive and supportive respiratory and cardiac therapies have led to a median survival in the middle to late twenties and growing chances of survival into the thirties for patients who receive aggressive care.13
Clinical trials have demonstrated that administering glucocorticoid (GC) therapy improves strength within weeks to a few months, and that these increases in strength can preserve ambulation for up to 2–3 years longer than for steroid-naive patients.17–22 However, few studies have assessed the long-term impact of GC-mediated improvements on maintaining strength, preserving function, and developing or preventing secondary health conditions. The aims of this study were to: (1) assess baseline levels of impairment and prevalence of secondary conditions from age 2 years to adulthood; and (2) evaluate the effect of chronic GCs in DMD on: (a) preservation of functional capabilities using a novel composite functional “milestone” scale scale showing clinically meaningful mobility and upper limb abilities; (b) progression of strength loss based on manual muscle testing versus historical reports of strength loss in steroid-naive patients; and (c) preservation of respiratory function based on pulmonary function tests (PFTs) across the age span.
METHODS
Participants and Schedule of Assessments
This prospective, multicenter, international study enrolled between 10 and 15 participants per year, ranging in age from 2 to <29 years. All participants were required to have a clinical picture consistent with typical DMD and family history and molecular diagnostic characterization of DMD-associated dystrophinopathy, as detailed in our companion study.23 Participants underwent assessments at baseline and months 3, 6, 9, and 12 (ambulatory) or months 6 and 12 (nonambulatory), which were timed to reproduce visit frequencies commonly employed in therapeutic clinical trials. One site conducted evaluations on an alternative schedule that was consistent with local care standards.
Protocol Approvals
The institutional review board or ethics review board at each participating institution approved the study protocol, consent, and assent documents. Informed consent/assent was obtained for each participant prior to conducting the study.
Assessment of Glucocorticoid Use
Historical and current use of GC therapy was documented, including medication used, age at onset of use, total duration of use, dose, and dose modification history. Patients were grouped as either: (1) GC-naive (not treated with GC ever, or treated <1 month total and not currently receiving GC); (2) current GC treatment recipients; or (3) past GC treatment recipients (treated in the past for ≥1 month with GC, but not currently receiving GC therapy).
Timed Function Testing, Functional Grades, and Functional Milestone Assessments
We measured timed function tests of standing from supine, climbing 4 standard stairs, and walking/running 10 meters. We did not test a small proportion of children <4 years of age due to their level of developmental ability. Standing from supine is defined as being able to stand without the use of furniture or assistance. We measured upper extremity function using the scale described by Brooke et al.24 and lower extremity function and mobility using the scale developed by Vignos et al.25 We created a 6-level composite of individual functional “milestone” tasks combining the results from the ability to perform the timed function tests and the Brooke and Vignos functional scales. The levels of this composite scale are: 0—able to complete all 3 timed tests; 1—unable to stand from supine, but performed the 4-step climb and the walk; 2—unable to climb 4 standard stairs, can walk 10 m, and with Vignos grade <5; 3—cannot rise from chair, but can walk 10 m, and with Vignos grade <7; 4—cannot walk 10 m, but can raise hand to mouth, and with Brooke grade <5; and 5—unable to raise a hand to the mouth, and with Brooke grade 5 or 6.
Health Status Assessment
Musculoskeletal and orthopedic history included spine or limb fractures, surgical tendon releases for contractures, spine stabilization surgery, and use of knee–ankle–foot orthoses (KAFOs) for ambulation and the dates of these events. Respiratory history included use of influenza and pneumococcal vaccinations, breathing exercises, cough assistance, and ventilatory assistance. History of gastrointestinal/nutritional issues included history of gastrostomy tube feeding for caloric supplementation.
Assessment of Anthropometrics
Anthropometric measures described by McDonald et al.23 included standing height (in centimeters) for participants who could stand unassisted, ulnar length (in millimeters; used to estimate standing height26), and weight (in kilograms or pounds).
Outcome Measures Commonly Used in Clinical Trials
We measured timed function tests (TFTs, in seconds) for standing from supine, 10-m walk/run, and timed stair climbing (for 4 stairs), as described by McDonald et al.23 We measured passive range of motion for knee extension, ankle dorsiflexion, elbow extension, and wrist extension to the nearest 5°.24,27,28 We measured skeletal muscle strength23 in all participants who were able to follow 1-step directions and who were strong enough to perform a 1-person assisted stand–pivot transfer to the examination table. Measurements included the modified Medical Research Council (MRC) manual muscle test (MMT)29–31 and quantitative isometric strength of hand grip, elbow flexors and extensors, and knee flexors.32,33 We measured forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), peak expiratory flow rate (PEFR), maximal inspiratory pressure (MIP), and maximal expiratory pressure (MEP).23
Statistical Methods
Analyses were conducted primarily using cross-sectional data from the baseline visits except for the 1-year longitudinal analysis of MMT scores, which used data from baseline and 3-, 6-, 9-, and 12-month visits. Frequencies of events or functional levels were shown as percentages and compared within age groups by GC status using exact chi-square tests due to the small numbers in some groups. Measurements of strength and range of motion were summarized using mean and standard deviation. Due to skewness (see below), TFTs and PFTs were summarized using box-and-whisker plots. PFTs were compared using Kruskal–Wallis exact tests. For the Brooke and Vignos functional tests, stacked bar graphs of the distribution of grade levels within each 1-year age group showed trends of loss of function with increasing age. Percentages of participants accomplishing each of the 6 levels in the combined scale described in timed function testing, functional grades, and functional milestone assessments (above) within each age group between the 3 GC-use groups were compared using an exact chi-square test with P = 0.01 for significance to account for 5 outcomes. Ordinal regression models were fit to both the Brooke and Vignos scales and the combined “milestone” scale as outcomes using age and GC grouping as predictors.
For the timed tests, if a participant was unable to perform a task due to weakness and was 4–18 years of age, we assigned an imputed value of zero velocity for that assessment in order to prevent a bias in the assessment of median velocity due to exclusion of those who could not perform the test. The resulting data are skewed due to having multiple zero-velocity observations, and therefore values were summarized using box-and-whisker plots.
The MMT was the only strength test assessed in a previous natural history cohort.29 To compare the current natural history cohort with the previous Clinical Investigation of Duchenne Dystrophy (CIDD) cohort, we duplicated their methodology, calculating longitudinal rates of change from baseline to the 12-month visit. Thus, we calculated the mean and SD of the slopes based on each individual’s regression line of change in MMT over 12 months, including all MMT assessments performed within the period. Thus, all participants with at least 2 MMT assessments and up to 5 assessments were included. Because the majority of our study population was GC treated and plots of MMT data versus age showed an apparent inflection point around 10 years of age, we further subdivided our analysis into 2 age groups of <10 years and ≥10 years, due to the observed increased rate of decline in the older age group.
RESULTS
Population Characteristics
Between May 2005 and July 2009, we enrolled 340 individuals with DMD (age range 2–28 years) and their primary caregiver(s) at 20 participating study centers. The median site enrollment was 14 participants (range 3–49 participants per site). At baseline, 210 of 340 (62%) participants were receiving GC therapy, 48 of 340 (14%) were past GC users, and 82 of 340 (24%) were GC-naive. At baseline, 194 of 340 (57%) participants were ambulatory.
Performance by Functional Grades and “Milestones.”
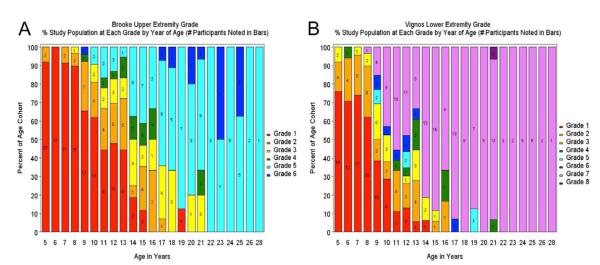
We obtained functional grades using the Brooke and Vignos scales in 80% of participants who were 4–6 years of age, and nearly 100% of participants who were ≥7 years of age. The percentage and number of participants at the Brooke upper extremity and Vignos lower extremity functional grades by year of age are shown in Figure 1. Examination of upper extremity grades (Fig. 1A) shows that there was little phenotypic variability in arm function at <9 years of age, with scores typically of grades 1 or 2. In participants between the ages 9 and 18 years, there was wider variability, with scores covering the entire range. Most participants >18 years of age scored at grade 5 or 6. Examination of lower extremity grades by age (Fig. 1B) showed a similar pattern of disease progression, with notable differences between the ages of 9 and 13 years, coinciding with the typical pattern of transition from ambulatory to nonambulatory status. In the ordinal logistic regression models for the Brooke and Vignos scales, current GC-use participants predicted better upper and lower extremity function [odds ratios (ORs) 0.22 and 0.23 for 1 function level lower, confidence intervals (CIs) 0.1–0.42 and 0.47, respectively, P < 0.001 in both models] than GC-naive participants after controlling for age (P < 0.001). There were no significant differences between the GC-naive users and past GC users in the upper extermity, but past users had worse (OR for 1 function level lower = 4.5, CI 1.1–18.5, P = 0.04) lower extremity function than GC-naive participants.
FIGURE 1.
(A) Brooke upper extremity grade. (B) Vignos lower extremity grade. Percent study population at each grade by year of age (number of participants noted in bars).
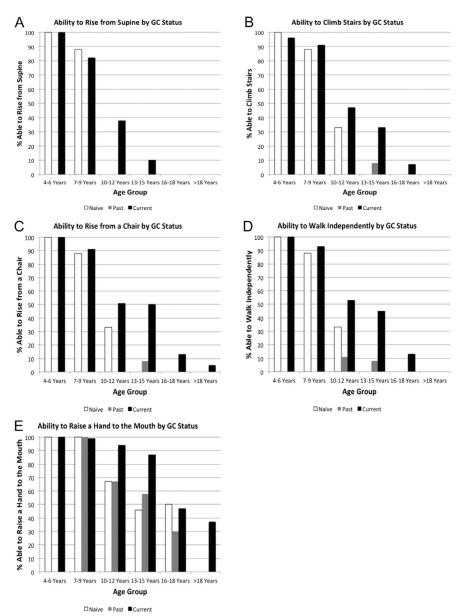
Ability to perform functional “milestone” tasks by age group and GC treatment status is shown in Figure 2. Regardless of GC-treatment status, loss of functional abilities occurred in a predictable order beginning with loss of the ability to stand from supine and subsequent loss of stair climbing, loss of the ability to rise from a chair followed by loss of ability to walk, and finally loss of the ability to raise a hand to the mouth. However, across all ages >6 years, GC-treated subjects displayed preservation of function relative to previously GC-treated and GC-naive peers (exact chi-square P-values 0.0024–0.043 within each age group function level subgroup that had at least 5 participants able to achieve the function level, comparing the GC distribution between those who did and did not achieve the function level). In the ordinal regression model using the “milestone” combined Brooke and Vignos and timed function as the outcome variable, GC users had significantly better functional milestones than GC-naive patients [1-level worse milestone level OR = 0.34 (CI 0.17–0.67), P = 0.0022]. Past GC users had significantly worse functional milestone levels than GC-naive patients [1-level difference OR = 3.1 (CI 1.2–8.1), P = 0.022]. In those DMD patients aged ≥18 years treated long term with GCs, 37% maintained the ability to get the hand to the mouth and feed independently as compared with 0% of those who were steroid-naive and 0% of past steroid users.
FIGURE 2.
Ability to perform functional milestones by glucocorticoid status for: (A) standing from supine; (B) climbing stairs; (C) rising from a chair; (D) walking independently; and (E) raising a hand to the mouth. Numbers of participants (denominator) by age cohort in (A)–(E) who were GC-naive, current GC users, and past GC users (respectively) are as follows: 4–6 years (N = 26, 27, 0); 7–9 years (N = 8, 68, 2); 10–12 years (N = 6, 47, 9); 13–15 years (N = 9, 30, 12); 16–18 years (N = 4, 15, 10); >18 years (N = 16, 19, 15).
Longitudinal Assessment of Modified MRC Manual Muscle Test Scores
The average MMT score at baseline was 6.7 (SD = 1.1) in those participants who could be assessed for this test. Analysis of averages of individuals’ slopes of strength change in all ambulatory participants ≥5 years of age who were able to perform MMT evaluations at least twice during the 5 potential evaluations from baseline to month 12 (n = 163), including all evaluations done during the 12 months, showed an overall decrease of 0.22 (1.07) MMT unit/year. For those age <10 years, the decrease was 0.14 (0.96) MMT unit/year (n = 115), which is less than half the previously described rate of −0.4 (0.39) MMT unit/year,29 albeit more variable. Of those individuals, 32% had a slope increase of >0.1 MMT unit/year vs. 15% as reported by Brooke et al.,29 and 14% had a slope decrease of ≥1.0 MMT unit/year. For participants ≥10 years of age, the decrease was 0.42 (1.29) MMT unit/year (n = 48), which is consistent with previous reports.29 Of these individuals, 17% had a slope increase of >0.1 MMT unit/year, and 13% had a slope decrease of ≥1.0 MMT unit/year. Approximately 75% of these participants were on GC therapy throughout the 12 months. Their results are comparable to the whole group, except for a somewhat decreased variability, and a slower decrease among participants ≥10 years of age.
Feasibility and Cross-Sectional Characteristics of Common Clinical Trial Outcome Measures
Prevalence of Important Clinical Events
For both GC-treated and GC-naive study participants at the baseline evaluation, we assessed lifetime prevalence of events that often raise concern among families and health-care professionals when discussing the use of GC therapy and for interventions commonly used to maintain mobility and function. Table 1 shows information on the development of clinical events. Prevalence for all measures increased with age, as expected, but the number of individuals with events is small for all types of events. There were no significant differences in rates except for surgical stabilization in the 13–15-year-old group, where only 1 patient required this in the GC group (n = 30) compared with 3 patients in the naive group (n = 9) and 4 patients in the past-user group (n = 12) (P = 0.013).
Table 1.
Number of individuals with DMD with major clinical events by age group and glucocorticoid treatment status.
| Age group (years) |
GC status | n | Fractures | Surgical contracture release |
Surgical spine stabilization |
KAFO for ambulation |
Nutrition with PEG |
Invasive ventilation |
Noninvasive ventilation |
|---|---|---|---|---|---|---|---|---|---|
| 4–6 | Naive | 26 | 2 (8) | 0 | 0 | 0 | 0 | 0 | 0 |
| Current | 27 | 3 (11) | 1 (4) | 0 | 0 | 0 | 0 | 0 | |
| 7–9 | Naive | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Current | 68 | 8 (12) | 2 (3) | 1 (1) | 0 | 1 (1) | 0 | 3 (4) | |
| Past | 2 | 1 (50) | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10–12 | Naive | 6 | 1 (17) | 3 (50) | 0 | 1 (17) | 0 | 0 | 0 |
| Current | 47 | 7 (15) | 9 (19) | 0 | 5 (11) | 0 | 0 | 0 | |
| Past | 9 | 4 (44) | 1 (11) | 0 | 0 | 0 | 0 | 0 | |
| 13–15 | Naive | 9 | 2 (22) | 1 (11) | 3 (33) | 0 | 1 (11) | 0 | 0 |
| Current | 30 | 10 (33) | 5 (17) | 1 (3) | 2 (7) | 0 | 0 | 0 | |
| Past | 12 | 5 (42) | 3 (25) | 4 (33) | 1 (9) | 1 (8) | 0 | 1 (8) | |
| 16–18 | Naive | 4 | 3 (75) | 0 | 2 (50) | 1 (25) | 1 (25) | 0 | 2 (50) |
| Current | 15 | 8 (53) | 5 (33) | 5 (33) | 5 (33) | 1 (7) | 0 | 3 (20) | |
| Past | 10 | 5 (50) | 6 (60) | 5 (50) | 1 (11) | 1 (10) | 0 | 3 (30) | |
| >18 | Naive | 16 | 3 (19) | 5 (31) | 9 (56) | 4 (25) | 3 (20) | 2 (13) | 9 (56) |
| Current | 19 | 8 (42) | 11 (58) | 7 (37) | 8 (42) | 1 (5) | 0 | 6 (32) | |
| Past | 15 | 7 (47) | 7 (47) | 10 (67) | 5 (33) | 1 (7) | 1 (7) | 4 (47) |
Data show number (%) of individuals. GC, glucocorticoid; KAFO, knee–ankle–foot orthoses; PEG, percutaneous endoscopic gastrostomy.
Anthropometric Characteristics
Anthropometric characteristics by age group are shown in Table 2. Collection of weight data and ulnar length was feasible in the entire study cohort regardless of age. Standing height was reported only for those participants who could stand appropriately, and the decline in numbers with increasing age group was apparent (Table 2). No adult participant (>18 years old) was able to stand.
Table 2.
Anthropometric measures: mean, standard deviation (SD), and number (%) who performed each study by age cohort.
| Standing height (cm) |
Ulnar length (cm) |
Weight (kg) |
||||
|---|---|---|---|---|---|---|
| Age group (years) |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
| 4–6 | 107.9 (6.2) | 53 (100) | 16.4 (1.1) | 51 (96) | 20.2 (3.7) | 53 (100) |
| 7–9 | 123.1 (6.5) | 68 (87) | 19.1 (1.3) | 78 (100) | 27.6 (8.0) | 78 (100) |
| 10–12 | 132.4 (10.8) | 27 (44) | 21.3 (2.1) | 62 (100) | 41.9 (14.6) | 62 (100) |
| 13–15 | 136.7 (12.1) | 15 (29) | 23.3 (2.7) | 51 (100) | 51.6 (18.1) | 51 (100) |
| 16–18 | 145.0 (7.1) | 2 (7) | 25.5 (1.9) | 29 (100) | 62.9 (20.7) | 29 (100) |
| >18 | – | – | 25.0 (2.0) | 50 (100) | 62.4 (27.3) | 50 (100) |
Timed Function Testing
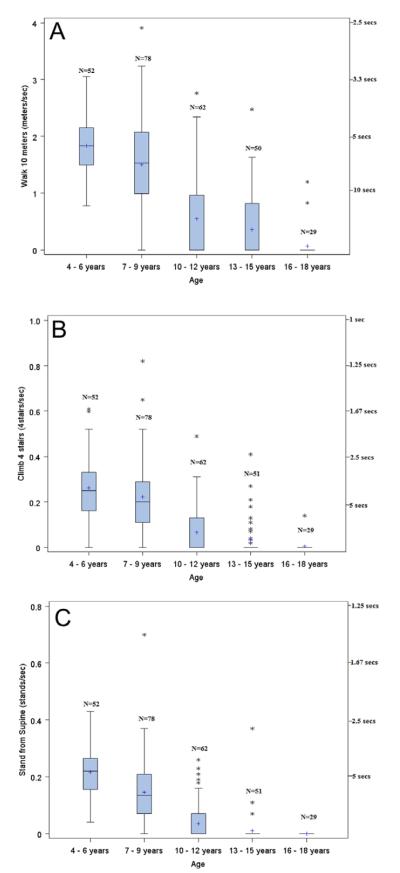
Timed function test characteristics by age group are shown in Figure 3. Calculating TFT evaluation results as a distance over time velocity enabled the use of 0 as an attainable speed in all 3 measures, even in instances where the participant could not be assessed due to disease progression and was thus assigned an imputed velocity of 0. Zero velocities were imputed for 0–1 (0–2%) subject in the 4–6-year-old group, 8–15 (10–19%) subjects in the 7–9-year-old group, 34–44 (55–71%) subjects in the 10–12-year-old group, 37–48 (73–94%) subjects in the 13–15-year-old group, and 27–29 (93–100%) subjects in the 16–18-year-old group. As noted earlier, no participants could perform the tests in the >18-year-old group. Figure 3 also shows the consistent decrease in velocity with increasing age for all 3 tasks. After 9 years of age, very few participants were able to stand from supine without the aid of furniture or a person. In addition, very few were able to climb 4 stairs at that time. However, many were still able to walk 10 meters. Although the median pace (including the non-walkers as 0 velocity) in the 10–12-year-old age group was 0.0 m/s, 25% of participants were still walking at a pace of ≥1.0 m/s at that age.
FIGURE 3.
Timed function tests by age group for: (A) 10-m walk/run; (B) climb 4 stairs, and (C) stand from supine. The boxand-whisker figures show velocity; a velocity of 0 is imputed for all participants who could not perform the test. The limits of the box are the 25th and 75th percentile. The median (middle line) and mean (“1”) are shown within the box. The whiskers are 1.5 times the interquartile length, starting from the edge of the boxes; asterisks indicate data values of outliers beyond the whiskers. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Range of Motion
Passive range of motion (ROM) characteristics by age group are shown in Tables 3 and 4. Larger numbers in the positive direction indicate better ROM, whereas larger numbers in the negative direction indicate restricted ROM. Upper extremity ROM measures were obtained in 77% of study participants <7 years of age, in 100% of participants 7–9 years of age, and in 96–100% of participants ≥10 years of age (Table 3). Lower extremiy ROM measures were obtained in 91% of participants <7 years of age and in 100% of participants 7–9 years of age (Table 4). In ambulatory participants, ROM progressed toward 0 or negative values with increasing age.
Table 3.
Upper extremity range of motion: mean, standard deviation (SD), and number (%) who performed each study by age cohort.
| Wrist extension (°) |
Elbow extension (°) |
|||
|---|---|---|---|---|
| Age group (years) |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
| 4–6 | 78.4 (23.2) | 41 (77) | 4.0 (5.9) | 41 (77) |
| 7–9 | 70.8 (26.1) | 78 (100) | 1.0 (5.9) | 78 (100) |
| 10–12 | 65.5 (27.1) | 62 (100) | −3.8 (13.4) | 62 (100) |
| 13–15 | 57.7 (33.7) | 49 (96) | −16.0 (22.5) | 49 (96) |
| 16–18 | 28.8 (43.3) | 28 (97) | −28.6 (22.8) | 29 (100) |
| >18 | 17.3 (41.8) | 48 (96) | −42.9 (29.4) | 50 (100) |
Table 4.
Lower extremity range of motion: mean, standard deviation (SD), and number (%) who performed each study by age cohort.
| Knee extension (°) |
Ankle dorsiflexion (°) |
|||
|---|---|---|---|---|
| Age group (years) |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
| 4–6 | 2.9 (5.8) | 48 (91) | 6.5 (7.1) | 48 (91) |
| 7–9 | −1.8 (9.4) | 78 (100) | −0.1 (10.7) | 78 (100) |
Quantitative Muscle Strength
Skeletal muscle strength characteristics by age group are shown in Tables 5, 6, and 7. Participants >6 years of age were reliably able to perform quantitative grip strength testing (Table 5). Knee and elbow flexor and extensor strength data collection by quantitative isometric testing was possible in 72–81% of participants 4–6 years of age, and in 88–100% of those 7–9 years of age, depending on the muscle group assessed (Table 6). A smaller subset of older participants (52% of the 10–12-year cohort, 37% of the 13–15-year cohort, and 0% of those ≥16 years of age) were able to perform an assisted ≥stand–pivot transfer utilizing the study-defined testing safety criteria and were thus able to provide strength testing data (Table 7).
Table 5.
Quantitative hand grip strength: mean, standard deviation (SD), and number (%) who performed each study by age cohort.
| QMT hand grip (lbs.) |
||
|---|---|---|
| Age group (years) | Mean (SD) | n (%) studied |
| 4–6 | 10.2 (3.6) | 43 (81) |
| 7–9 | 12.4 (5.5) | 78 (100) |
| 10–12 | 13.5 (7.3) | 62 (100) |
| 13–15 | 12.0 (8.1) | 51 (100) |
| 16–18 | 9.8 (8.4) | 29 (100) |
| >18 | 5.7 (5.7) | 50 (100) |
Table 6.
Quantitative muscle strength testing in children: mean, standard deviation (SD), and number (%) who performed each study by age cohort.
| QMT elbow extensor (lbs.) |
QMT elbow flexor (lbs.) |
QMT knee extensor (lbs.) |
QMT knee flexor (lbs.) |
|||||
|---|---|---|---|---|---|---|---|---|
| Age group (years) |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
| 4–6 | 7.0 (2.8) | 38 (72) | 7.6 (2.3) | 41 (77) | 15.5 (7.7) | 41 (77) | 9.7 (3.3) | 40 (75) |
| 7–9 | 6.0 (3.0) | 68 (97) | 8.0 (3.2) | 68 (97) | 10.9 (7.3) | 68 (97) | 12.1 (3.7) | 68 (97) |
Table 7.
Quantitative muscle strength testing in preteens and teens able to perform assisted self-transfer: mean, standard deviation (SD), and number (%) who performed each study by age cohort.
| QMT elbow extensor (lbs.) |
QMT elbow flexor (lbs.) |
QMT knee extensor (lbs.) |
QMT knee flexor (lbs.) |
|||||
|---|---|---|---|---|---|---|---|---|
| Age group (years) |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
| 10–12 | 6.3 (3.7) | 32 (100) | 8.1 (3.8) | 32 (100) | 8.3 (6.4) | 32 (100) | 11.4 (7.0) | 32 (100) |
| 13–15 | 6.7 (3.8) | 19 (100) | 7.5 (3.8) | 19 (100) | 9.0 (4.5) | 19 (100) | 10.3 (6.8) | 19 (100) |
Pulmonary Function
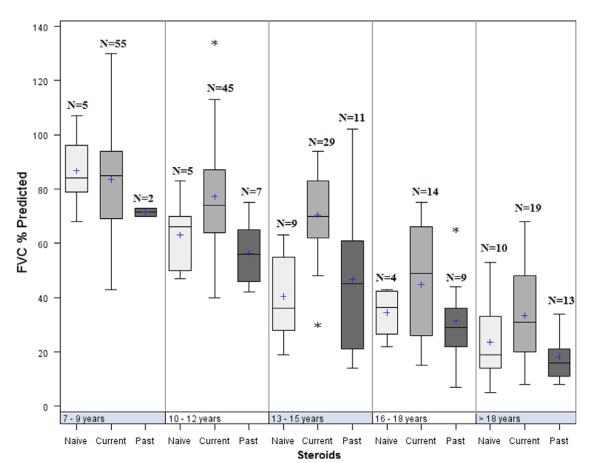
Pulmonary function characteristics by age group are shown in Table 8. Pulmonary function measures were feasible and performed in most participants aged ≥7 years (commonly appreciated as a lower age limit for reliability testing). FVC, FEV1, and PEFR were obtained in 79–95% of study participants ≥7 years of age. MIP and MEP were obtained in 95–100% of study participants in the same age groups. Results show that MIP and MEP were already very compromised in the 7–9-year-old participants (mean 63% and 47% predicted, respectively), whereas FEV1 and FVC were more modestly impacted. Percent predicted values for FVC by age and GC treatment status demonstrated higher overall function for GC-treated boys across the age groups 10–12 and 13–15 years and older, as shown in Figure 4 (P-values 0.0001–0.039 for all 5 PFTs in this age group, with the exception of MEP in the 10–12-year group, P = 0.08). By 16 years of age, all parameters were <50% of predicted values of healthy children and, by adulthood, all parameters were at approximately 25% predicted values of healthy adults. There was large variability in the pulmonary function results in the younger participants, which declined with advancing participant age. The range of results decreased; however, even among the adult participants there was substantial variability in the results of the pulmonary function evaluations.
Table 8.
Pulmonary function testing: mean, standard deviation (SD), and number (%) who performed each study by age cohort.
| FVC % predicted |
FEV1 % predicted |
PEFR % predicted |
MIP % predicted |
MEP % predicted |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age group (years) |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
Mean (SD) |
n (%) studied |
| 7–9 | 83.5 (17.3) | 62 (79) | 85.1 (19.9) | 62 (79) | 73.2 (21.7) | 62 (79) | 62.8 (21.5) | 74 (95) | 46.6 (19.6) | 76 (97) |
| 10–12 | 73.4 (18.8) | 57 (92) | 73.3 (16.9) | 57 (92) | 70.0 (18.5) | 57 (92) | 51.0 (15.8) | 61 (98) | 36.7 (16.4) | 60 (97) |
| 13–15 | 59.7 (21.8) | 49 (96) | 60.0 (21.5) | 49 (96) | 59.9 (18.1) | 49 (96) | 42.7 (16.3) | 51 (100) | 30.3 (13.0) | 51 (100) |
| 16–18 | 38.9 (18.8) | 27 (93) | 40.5 (19.7) | 27 (93) | 39.7 (18.8) | 27 (93) | 28.3 (11.6) | 29 (100) | 18.8 (10.2) | 28 (97) |
| >18 | 26.4 (15.2) | 42 (84) | 28.1 (15.6) | 42 (84) | 29.6 (16.9) | 41 (82) | 23.7 (14.7) | 48 (96) | 16.3 (10.8) | 48 (96) |
FIGURE 4.
Percent predicted forced vital capacity by age and GC treatment groups. The limits of the box are the 25th and 75th percentile. The median (middle line) and mean (1) are shown within the box. The whiskers are 1.5 times the interquartile length starting from the edge of the boxes; the asterisks are data values of outliers beyond the whiskers. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Functional “Milestones” Preserved in GC-Treated Adolescents and Young Adults
The data demonstrate that our cohort exhibited significantly preserved functional “milestones” in the currently GC-treated participants, including the ability to stand from supine, climb stairs, rise from a chair, walk independently, and raise the hands to the mouth. This issue is especially compelling when considering the use of GC therapy in older and less functional nonambulatory patients, in whom there is a relative lack of efficacy and safety data that can be obtained from the literature. This point was highlighted by Bushby and colleagues in the recently published DMD care guidelines, which identify continued use of GC therapy in individuals who are nonambulatory with limited upper extremity function as an area in need of further research.34 Although clinical trials have demonstrated increases in strength due to GC use in early and middle childhood, our observational data suggest that there may be significant long-term functional benefits associated with continued GC treatment during the preteen and teenage years, and even during adulthood. Prolonged ambulation, improved ability to perform positional transfers, and maintenance of self-feeding abilities are all expected to have a significant positive impact on functional activities of daily living and, subsequently, on health-related quality of life. Our data suggest that those who continue to receive GC therapy through their teenage years and even into adulthood may be more likely to preserve lower and upper extremity function compared with those who remained untreated. The interesting observation of significant functional deficits demonstrated between the past GC users relative to GC-naive patients is an area that will require further study using future longitudinal data.
Fractures in Cohort Rare and Unrelated to Steroid Use
There are lingering concerns among parents and practitioners about increased fracture risk due to GC use. Although reported instances of fractures were few, our cohort did not show substantive differences in fracture prevalence percentages between the GC-treated and GC-naive groups, even in those with continued GC therapy into their third decade. The prevalence of fractures in those <13 years of age is generally low, and in those >13 years it is comparable, regardless of GC status. A detailed description of other major risks of GC treatment, such as weight gain, cataracts, and behavioral changes, experienced by our cohort is beyond the scope of this investigation. These topics are addressed comprehensively in our companion study focusing on GCs in DMD.
One-Year Change in Manual Muscle Test Scores Decreased Compared with Previous Reports in Younger Steroid-Naive Patients
We compared our MMT score data over 12 months with findings by Brooke et al. in 1983.29 Comparison with the CIDD cohort is not without challenges. Evaluation of that cohort occurred prior to the advent of molecular diagnostic criteria, and it is possible that even the very thorough clinical diagnostic criteria may have failed to exclude some individuals with milder phenotypes, such as those with intermediate or Becker-type dystrophinopathies, limb-girdle muscular dystrophies, or sarcoglycanopathies. Still, individuals with those diagnoses frequently have a slightly less aggressive clinical course. Therefore, had those individuals been excluded from the study, the reported clinical course could have been even more severe, which would have increased the magnitude of difference in our observations. Alternately, given the lack of molecular diagnostics, individuals in the CIDD cohort may have been identified later in the course of their disease, or they could have been more severe and identified earlier. Thus, the younger individuals in the CIDD cohort could have displayed a more severe course, thus creating a recruiting bias that would emphasize differences with our cohort. Regardless of the impact, those possibilities underscore the need for study of a cohort defined using more contemporary diagnostic standards.
Despite the challenges in interpretation, we propose that our study cohort demonstrates that individuals with DMD today who are <10 years of age are declining in strength and function at a rate that is less than half of that previously described. The increased variability of disease progression can be explained in part by the mix of GC-treated and GC-naive individuals in our sample, as well as by the greater proportion of children who demonstrated increased MMT scores. The functional significance of the reduced rate of progression we attribute to widespread GC use in early and mid-childhood is that children with DMD today are likely to have a much higher degree of functional ability and participation during critical periods of childhood development. Maintaining a higher degree of strength and function when entering the adolescent growth spurt may be associated with improved spine stability and a reduced need for spinal instrumentation and fusion, improved pulmonary function capacity due to preservation of thoracic musculature and structure, prolonged preservation of self-care abilities, and overall reductions in developing secondary medical conditions. These alterations in the natural history of DMD would be expected to ultimately lead to improved survival characteristics. Individuals in our cohort who were ≥10 years of age demonstrated an overall decrease in strength consistent with, but more variable than data from Brooke and colleagues.29 This suggests that early GC treatment maximizes functional preservation and raises the baseline level of strength and function through the adolescent transition, even if the overall rate of progression of weakness during the second decade of life is similar to that seen in previous reports.
Due to the previously documented increased sensitivity of other methods compared with MMT32,33 and the challenges of maintaining consistency of MMT in international multicenter clinical trials, the trend has been to power clinical trials on the basis of other clinical endpoints. The changing natural history of DMD due to GC therapy appears, at least from the perspective of MMT, to have resulted in smaller-than-expected 1-year changes in the younger patients and increased variability due to differing rates of disease progression that could significantly impact clinical trial results using the measure. Future data from this natural history study characterizing QMT and TFT and recently added endpoints, such as the 6-minute walk test (6MWT),35,36 the North Star Ambulatory Assessment,37–39 and the 9-Hole Peg Test,40,41 will thus be critical for the design of future clinical trials and will supplement the emerging literature on comparative endpoints from smaller populations, which has been showing a high degree of agreement between outcome measures.38,39
Clinical Trial Outcome Measures are Feasible and Continuous across a Wide Range of Ages and Functions
Current natural history data pertaining to clinically meaningful endpoints are critical when considering the design of clinical trials for individuals with DMD. This investigation has presented a cross-sectional overview of our study cohort at their baseline evaluations. Examination of the data in Tables 2 through 8 show that means and standard deviations of commonly used measures varied across age groups, as did the proportion of the population who were able to complete those measures. From a feasibility standpoint, we found that commonly used clinical trial endpoints measuring overall physical and pulmonary function can be implemented across most ages and stages of disease severity. However, with the exception of quantitative grip testing, we observed limitations due to contractures and positioning in current testing methods for both MMT and QMT, which restricted evaluations to ambulatory and mildly to moderately affected nonambulatory patients.
Our data further suggest that some outcome measures should be expected to show longitudinal changes that are increasing, stable, or decreasing, depending on the age of the participant. This is illustrated by comparing quantitative muscle strength tests in the 4–6-year and 7–9-year age groups, where stable elbow flexor strength and increasing knee flexor and hand grip strength were consistent with the concept that, in younger children, deterioration of strength can be outpaced by normal growth and motor development. The implication is that consideration should be given to the design of trials with respect to stratification factors, wherein different groups are expected to progress at different rates depending on their age, developmental level, or stage of disease, thus leading to differing clinically important effect sizes. Also of note are those adolescents described in Table 7, who retained a higher degree of function. They are capable of testing using the current manual and quantitative strength testing methods. They do not, however, represent the entire population at those ages. Current strength testing methods could remain a useful tool in the context of a clinical trial directed specifically at such a group of individuals. Our data also reveal that there were gaps in current assessment techniques, which limits their utility in boys who are not able to transfer to an examination table. Modifying existing strength testing protocols will allow for inclusion of a broader sample of nonambulatory DMD participants in future studies. As we explore new endpoints for use in clinical trials, we should strive to develop assessments that maintain continuity of measures across the entire population whenever possible.
The underlying causes of variability in DMD disease progression, outcome measure performance, and degrees of response to GC therapy across the current survivable lifespan are still not well understood. In patients with similar diagnostic and clinical characteristics, DMD progresses at different rates, and there are few reliable ways to predict its clinical course. As a result, researchers have seen such variability reduce the statistical power to detect treatment differences in clinical trials. Because DMD is a rare disease with a limited patient population, increasing planned sample sizes is not often the optimal choice. Consistent with providing input to a personalized medicine approach, future analyses of longitudinal data sets will facilitate identification of factors that explain variability in progression and treatment response and optimization of selection of study cohorts and clinical trial outcome measures. One such way to reduce heterogeneity in study populations in clinical trials is to include metrics of functionality as eligibility criteria, and to consider stratification by age or function-related groups. Specific measures for a trial should be chosen based on their ability to reveal a “decline phase” within a specified age range or level of function.42
In conclusion, for the individuals in our cohort, use of GCs into adolescence confers a higher level of function—one that is likely to have a significant, positive, long-term impact on functional ability, independence, health-related quality of life, and survival. Through an analysis of the first complete year of data on 340 boys and young men with DMD using the same techniques as the widely cited CIDD natural history study,29 we found that rates of progression in our cohort in early and middle childhood decreased to less than half the rate described in the late 1980s prior to routine use of GC therapy. These results, based on analyses of currently used clinical trial outcome measures, show that GC treatment contributes to a “new natural history” that alters the characteristic progression of DMD. This decreased rate of strength loss and improved function creates a wider age range where existing validated clinical trial outcome measures are feasible, and also underscores the need for development of additional clinical trial measures that can be applied continuously across ages and stages of disease.
Acknowledgments
This study was funded by grants from the U.S. Department of Education/NIDRR (H133B031118, H133B090001), the U.S. Department of Defense (W81XWH-09-1-0592), the NIH (UL1RR031988, U54HD053177, UL1RR 024992, U54RR026139, 2U54HD053177, G12RR003051, 1R01AR061875, RO1AR062380), and Parent Project Muscular Dystrophy.
Abbreviations
- ANOVA
analysis of variance
- CDC
U.S. Centers for Disease Control and Prevention
- CIDD
Clinical Investigation of Duchenne Dystrophy
- CINRG
Cooperative International Neuromuscular Research Group
- CK
creatine kinase
- DMD
Duchenne muscular dystrophy
- DMD-NHS
CINRG Duchenne Natural History Study
- FEV1
forced expiratory volume 1 second
- FVC
forced vital capacity
- KAFO
knee–ankle–foot orthosis
- LSI
Life Satisfaction Index
- LVEF
Left ventricular ejection fraction
- MEP
maximal expiratory pressure
- MIP
maximal inspiratory pressure
- MMT
MRC modified manual muscle test
- MRC
Medical Research Council
- NG
no prior glucocorticoids
- PedsQL
Pediatric Quality of Life Inventory
- PEFR
peak expiratory flow rate
- POSNA
Pediatric Orthopedic Society of North America Pediatric Musculoskeletal Health Questionnaire
- PSQI
Pittsburgh Sleep Quality Index
- QMT
quantitative isometric muscle strength testing
- SF
left ventricular shortening fraction
- TFT
timed function testing
- WHO
World Health Organization
APPENDIX.
Study Collaborators (CINRG Investigators)
Sunaram Medical Foundation and Apollo Children’s Hospital: V. Vishwanathan, MD, S. Chidambaranathan, MD; Holland Bloorview Kids Rehabilitation Hospital: W. Douglas Biggar, MD; Alberta Children’s Hospital: Jean K. Mah, MD; Queen Sylvia Children’s Hospital: Mar Tulinius, MD; Children’s National Medical Center: Robert Leshner, MD, Carolina Tesi-Rocha, MD; Royal Children’s Hospital: Andrew Kornberg, MD, Monique Ryan, MD; Hadassah Hebrew University Hospital: Yoram Nevo, MD; Instituto de Neurosciencias Fundacion Favaloro: Alberto Dubrovsky, MD; Mayo Clinic: Nancy Kuntz, MD, Sherilyn Driscoll, MD; Washington University, St. Louis: Anne Connolly, MD, Alan Pestronk, MD; Children’s Hospital of Virginia: Jean Teasley, MD; University of Tennessee, Memphis: Tulio Bertorini, MD; Children’s Hospital of Westmead: Kathryn North, MD; University of Alberta: Hanna Kolski, MD; University of Puerto Rico: Jose Carlo, MD; University of Pavia and Niguarda Ca’ Granda Hospital: Ksenija Gorni, MD; Texas Children’s Hospital: Timothy Lotze, MD; University of Minnesota: John Day, MD.
These findings were presented in part at the Proceedings of the American Academy of Neurology, April 2009 and April 2010, and the International Congress of Neuromuscular Disorders, July 2010.
The authors thank the patients and families who volunteered to take part in this project. We also thank Dr. Josh Benditt, Dr. Louis Boitano, Dr. David Birnkrant, Dr. David Connuck, Dr. Jonathan Finder, Dr. Veronica Hinton, Dr. Katherine Mathews, and Dr. Richard Moxley for their expert advice during study development. We thank Dr. Susan Sparks and Erynn Gordon for their expert review of all DMD diagnostic test results. We also thank the dedicated CINRG members who continue to commit countless hours to this effort. The CINRG group is comprised of the following institutions and individuals: University of California, Davis: Michelle Cregan, Erica Goude, Merete Glick, Linda Johnson, Nanette Joyce, Bethany Lipa, Alina Nicorici, Andrew Skalsky, Amanda Witt; Sundaram Medical Foundation and Apollo Children’s Hospital, Chennai: Suresh Kumar, Holland Bloorview Kids Rehabilitation Hospital: Laila Eliasoph, Elizabeth Hosaki, Angela Gonzales, Vivien Harris; Alberta Children’s Hospital: Angela Chiu, Edit Goia, Jennifer Thannhauser, Lori Walker, Caitlin Wright, Mehrnaz Yousefi; Queen Sylvia Children’s Hospital: Ann-Christine Alhander, Lisa Berglund, Ann-Berit Ekstrom, Anna-Karin Kroksmark, Ulrika Sterky; Children’s National Medical Center: Marissa Birkmeier, Sarah Kaminski; Royal Children’s Hospital: Kate Carroll, Katy DeValle, Rachel Kennedy, Dani Villano; Hadassah Hebrew University Hospital: Adina Bar Leve, Itai Shurr, Elana Wisband, Debbie Yaffe; Instituto de Neurosciencias Fundacion Favaloro: Luz Andreone, Jose Corderi, Lilia Mesa, Lorena Levi; Mayo Clinic: Krista Coleman-Wood, Ann Hoffman, Wendy Korn-Petersen, Duygu Selcen; University of Pittsburgh: Hoda Abdel-Hamid, Christopher Bise, Ann Craig, Sarah Hughes, Casey Nguyen, Jason Weimer; Washington University, St. Louis: Paul Golumbak, Glenn Lopate, Justin Malane, Betsy Malkus, Kenkicki Nozaki, Renee Renna, Jeanine Schierbacker, Catherine Seiner, Charlie Wulf; Children’s Hospital of Virginia: Susan Blair, Barbara Grillo, Karen Jones, Eugenio Monasterio; University of Tennessee, Memphis: Judy Clift, Cassandra Feliciano, Masanori Igarashi, Rachel Young; Children’s Hospital of Westmead: Kristy Rose, Richard Webster, Stephanie Wicks; University of Alberta: Lucia Chen, Cameron Kennedy; University of Puerto Rico: Brenda Deliz, Sheila Espada, Pura Fuste, Carlos Luciano; University of Pavia: Luca Capone, Niguarda Ca’ Granda Hospital: Maria Beneggi, Valentina Morettini, Texas Children’s Hospital: Anjali Gupta, Robert McNeil; University of Minnesota: Amy Erickson, Marcia Margolis, Cameron Naughton, Gareth Parry, David Walk; The CINRG Coordinating Center: Naomi Bartley, Paola Canelos, Robert Casper, Lauren Hache, Mohammad Ahmed, Angela Zimmerman.
Footnotes
Disclosures: The authors take full responsibility for the contents of this work, which do not represent the views of the U.S. Department of Education, the National Institutes of Health (NIH), the Department of Veterans Affairs, or the United States Government. R.T.A. has served as a consultant for PTC Therapeutics, Inc. A.C. serves as a consultant for GlaxoSmithKline. D.M.E. serves on the speakers bureau for and has received funding for travel and speaker honoraria from Athena Diagnostics, Inc.; she also serves as a consultant for Acceleron Pharma, HALO Therapeutics, AVI Biopharma, the Gerson Lehman Group, and Medacorp. J.M.F. serves on a scientific advisory board for Prosensa, serves on the editorial board of Neuromuscular Disorders, and serves/has served as a member of the CINRG Executive Committee and as a consultant for Prosensa, GlaxoSmithKline, Genzyme Corporation, PTC Therapeutics, Inc., and Acceleron Pharma. E.K.H. is a member of the CINRG Executive Committee, has served as a consultant for Genzyme Corporation and PTC Therapeutics, Inc., and has received travel assistance from Parent Project Muscular Dystrophy. E.P.H. has served on advisory committees for AVI BioPharma, Inc., as a consultant with the Gerson Lehman Group, Medacorp, and Lazard Capital, and is cofounder, board member, and shareholder of ReveraGen Biopharma. C.M.M. has served on advisory committees for PTC Therapeutics, Inc., Sarepta Therapeutics, Inc., GlaxoSmithKline, Prosensa, HALO Therapeutics, Shire HGT, and Novartis AG. The remaining authors have no conflicts of interest to disclose.
C.M.M. is the study’s principal investigator; E.K.H., R.T.A., A.C., and C.M.M. are study chairs.
REFERENCES
- 1.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Emery AE. Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul Disord. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 3.Haldane JB. Mutation in the sex-linked recessive type of muscular dystrophy; a possible sex difference. Ann Hum Genet. 1956;20:344–347. doi: 10.1111/j.1469-1809.1955.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 4.Bucher K, Ionasescu V, Hanson J. Frequency of new mutants among boys with Duchenne muscular dystrophy. Am J Med Genet. 1980;7:27–34. doi: 10.1002/ajmg.1320070107. [DOI] [PubMed] [Google Scholar]
- 5.Caskey CT, Nussbaum RL, Cohan LC, Pollack L. Sporadic occurrence of Duchenne muscular dystrophy: evidence for new mutation. Clin Genet. 1980;18:329–341. doi: 10.1111/j.1399-0004.1980.tb02293.x. [DOI] [PubMed] [Google Scholar]
- 6.Danieli GA, Mostacciuolo ML, Pilotto G, Angelini C, Bonfante A. Duchenne muscular dystrophy: data from family studies. Hum Genet. 1980;54:63–68. doi: 10.1007/BF00279050. [DOI] [PubMed] [Google Scholar]
- 7.Meryon E. On fatty degeneration of the voluntary muscles. Lancet. 1851;2:588–589. [Google Scholar]
- 8.Duchenne GBA. Album de photographes pathologiques. Balliere; Paris: 1862. [Google Scholar]
- 9.Gowers W. Pseudo-hypertrophic muscular paralysis. Churchill; London: 1879. [Google Scholar]
- 10.Sutherland DH, Olshen R, Cooper L, Wyatt M, Leach J, Mubarak S, et al. The pathomechanics of gait in Duchenne muscular dystrophy. Dev Med Child Neurol. 1981;23:3–22. doi: 10.1111/j.1469-8749.1981.tb08442.x. [DOI] [PubMed] [Google Scholar]
- 11.D’Angelo MG, Berti M, Piccinini L, Romei M, Guglieri M, Bonato S, et al. Gait pattern in Duchenne muscular dystrophy. Gait Posture. 2009;29:36–41. doi: 10.1016/j.gaitpost.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 12.McDonald CM, Abresch RT, Carter GT, Fowler WM, Jr, Johnson ER, Kilmer DD, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74(suppl):S70–92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- 13.Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12:926–929. doi: 10.1016/s0960-8966(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 14.Mendell JR, Province MA, Moxley RT, III, Griggs RC, Brooke MH, Fenichel GM, et al. Clinical investigation of Duchenne muscular dystrophy. A methodology for therapeutic trials based on natural history controls. Arch Neurol. 1987;44:808–811. doi: 10.1001/archneur.1987.00520200012009. [DOI] [PubMed] [Google Scholar]
- 15.Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Florence J, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39:475–481. doi: 10.1212/wnl.39.4.475. [DOI] [PubMed] [Google Scholar]
- 16.Angelini C, Perini F, Turella E, Intino M, Pini A, Ottolini A, et al. A trial with a new steroid in Duchenne muscular dystrophy. In: Angel-ini C, Danieli GA, Fontanari D, editors. Muscular dystrophy research. Excerpta Medica; Amsterdam: 1991. pp. 173–179. [Google Scholar]
- 17.Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, et al. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med. 1989;320:1592–1597. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 18.Fenichel GM, Florence JM, Pestronk A, Mendell JR, Moxley RT, III, Griggs RC, et al. Long-term benefit from prednisone therapy in Duchenne muscular dystrophy. Neurology. 1991;41:1874–1877. doi: 10.1212/wnl.41.12.1874. [DOI] [PubMed] [Google Scholar]
- 19.Fenichel GM, Mendell JR, Moxley RT, III, Griggs RC, Brooke MH, Miller JP, et al. A comparison of daily and alternate-day prednisone therapy in the treatment of Duchenne muscular dystrophy. Arch Neurol. 1991;48:575–579. doi: 10.1001/archneur.1991.00530180027012. [DOI] [PubMed] [Google Scholar]
- 20.Griggs RC, Moxley RT, III, Mendell JR, Fenichel GM, Brooke MH, Pestronk A, et al. Prednisone in Duchenne dystrophy. A randomized, controlled trial defining the time course and dose response. Arch Neurol. 1991;48:383–388. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
- 21.Connolly AM, Schierbecker J, Renna R, Florence J. High dose weekly oral prednisone improves strength in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12:917–925. doi: 10.1016/s0960-8966(02)00180-3. [DOI] [PubMed] [Google Scholar]
- 22.Escolar DM, Hache LP, Clemens PR, Cnaan A, McDonald CM, Viswanathan V, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. 2011;77:444–452. doi: 10.1212/WNL.0b013e318227b164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald CM, Henricson E, Abresch R, Han JJ, Escolar DM, Florence J, et al. The Cooperative International Neuromuscular Research Group Duchenne Natural History Study—a longitudinal investigation in the era of glucocorticoid therapy: design of protocol and the methods used. Muscle Nerve. 2013;XX:XXX–XXX. doi: 10.1002/mus.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981;4:186–197. doi: 10.1002/mus.880040304. [DOI] [PubMed] [Google Scholar]
- 25.Vignos PJJ, Spencer GEJ, Archibald KC. Management of progressive muscular dystrophy of childhood. JAMA. 1963;184:89–96. doi: 10.1001/jama.1963.03700150043007. [DOI] [PubMed] [Google Scholar]
- 26.Gauld LM, Kappers J, Carlin JB, Robertson CF. Height prediction from ulna length. Devel Med Child Neurol. 2004;46:475–480. doi: 10.1017/s0012162204000787. [DOI] [PubMed] [Google Scholar]
- 27.Pandya S, Florence JM, King WM, Robison JD, Oxman M, Province MA. Reliability of goniometric measurements in patients with Duchenne muscular dystrophy. Phys Ther. 1985;65:1339–1342. doi: 10.1093/ptj/65.9.1339. [DOI] [PubMed] [Google Scholar]
- 28.Fowler WM, Jr, Abresch RT, Aitkens S, Carter GT, Johnson ER, Kilmer DD, et al. Profiles of neuromuscular diseases. Design of the protocol. Am J Phys Med Rehabil. 1995;74(suppl):S62–69. doi: 10.1097/00002060-199509001-00002. [DOI] [PubMed] [Google Scholar]
- 29.Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Miller JP, et al. Clinical investigation in Duchenne dystrophy: 2. Determination of the “power” of therapeutic trials based on the natural history. Muscle Nerve. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 30.Florence JM, Pandya S, King WM, Robison JD, Signore LC, Wentzell M, et al. Clinical trials in Duchenne dystrophy. Standardization and reliability of evaluation procedures. Phys Ther. 1984;64:41–45. doi: 10.1093/ptj/64.1.41. [DOI] [PubMed] [Google Scholar]
- 31.Florence JM, Pandya S, King WM, Robison JD, Baty J, Miller JP, et al. Intrarater reliability of manual muscle test (Medical Research Council scale) grades in Duchenne’s muscular dystrophy. Phys Ther. 1992;72:115–122. doi: 10.1093/ptj/72.2.115. [DOI] [PubMed] [Google Scholar]
- 32.Escolar DM, Henricson EK, Mayhew J, Florence J, Leshner R, Patel KM, et al. Clinical evaluator reliability for quantitative and manual muscle testing measures of strength in children. Muscle Nerve. 2001;24:787–793. doi: 10.1002/mus.1070. [DOI] [PubMed] [Google Scholar]
- 33.Mayhew JE, Florence JM, Mayhew TP, Henricson EK, Leshner RT, McCarter RJ, et al. Reliable surrogate outcome measures in multicenter clinical trials of Duchenne muscular dystrophy. Muscle Nerve. 2007;35:36–42. doi: 10.1002/mus.20654. [DOI] [PubMed] [Google Scholar]
- 34.Bushby K, Finkel R, Birnkrant D, Case L, Clemens P, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 35.McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Elfring GL, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve. 2010;41:500–510. doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- 36.McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Atkinson L, et al. The 6-minute walk test in Duchenne/Becker muscular dystrophy: Longitudinal observations. Muscle Nerve. 2010;42:966–974. doi: 10.1002/mus.21808. [DOI] [PubMed] [Google Scholar]
- 37.Mayhew A, Cano S, Scott E, Eagle M, Bushby K, Muntoni F. Moving towards meaningful measurement: Rasch analysis of the North Star Ambulatory Assessment in Duchenne muscular dystrophy. Devel Med Child Neurol. 2011;53:535–542. doi: 10.1111/j.1469-8749.2011.03939.x. [DOI] [PubMed] [Google Scholar]
- 38.Mazzone E, Martinelli D, Berardinelli A, Messina S, D’Amico A, Vasco G, et al. North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2010;20:712–716. doi: 10.1016/j.nmd.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Mazzone ES, Messina S, Vasco G, Main M, Eagle M, D’Amico A, et al. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscul Disord. 2009;19:458–461. doi: 10.1016/j.nmd.2009.06.368. [DOI] [PubMed] [Google Scholar]
- 40.Poole JL, Burtner PA, Torres TA, McMullen CK, Markham A, Marcum ML, et al. Measuring dexterity in children using the Nine-hole Peg Test. J Hand Ther. 2005;18:348–351. doi: 10.1197/j.jht.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Smith YA, Hong E, Presson C. Normative and validation studies of the Nine-hole Peg Test with children. Percept Mot Skills. 2000;90:823–843. doi: 10.2466/pms.2000.90.3.823. [DOI] [PubMed] [Google Scholar]
- 42.Henricson E, Abresch R, Han JJ, Nicorici A, Goude Keller E, Elfring G, et al. Percent-predicted 6-minute walk distance in Duchenne muscular dystrophy to account for maturational influences. PLoS Curr. 2012;4:RRN1297. doi: 10.1371/currents.RRN1297. [DOI] [PMC free article] [PubMed] [Google Scholar]