Abstract
Objective
To assess the frequency and prognostic utility of small, short duration left ventricular (LV) myocardial perfusion defects during dobutamine cardiovascular magnetic resonance (DCMR) stress imaging.
Methods
We performed first-pass contrast-enhanced DCMR at peak stress in 331 consecutively recruited individuals (aged 68±8 years, 50% men) at intermediate risk of a future cardiac event. Size, location and persistence of low signal intensity perfusion defects were recorded. Cardiac events were assessed by personnel blinded to imaging results for a median of 24 months after DCMR.
Results
Among the fifty-five individuals (16.6%) who exhibited small (<25% myocardial thickness) and short duration (<5 frames in persistence) perfusion defects, diabetes was more prevalent (p=0.019) and no cardiac events were observed. Large, persistent perfusion defects were associated with coronary artery disease (CAD), prior myocardial infarction, and decreased LV function (p<0.001 for all) and increased 2-year risk of cardiac event (HR 10.3, p<0.001, CI 3.3–33.0).
Conclusion
In individuals with diabetes, hypertension, or CAD at intermediate risk for a future cardiac event, small, short duration DCMR perfusion defects are not associated with an increased 2-year risk of subsequent cardiac event.
Keywords: coronary artery disease, dobutamine cardiac magnetic resonance, prognosis
INTRODUCTION
Large (≥25% of the myocardial transmural thickness), persistent (≥3 to 10 frames in duration) regions of low signal intensity observed during first pass gadolinium enhanced dobutamine cardiovascular magnetic resonance (DCMR) stress tests identify regions of inducible ischemia that are associated with an adverse cardiac prognosis.1,2 To date however, the prognostic utility of regions of small (low signal intensity that are <25% of the transmural thickness) or short duration (<5 frames after the appearance of contrast in the left ventricular [LV] myocardium) defects that in some cases represent dark rim artifacts are unknown.3,4 Moreover, the frequency with which these small or short duration defects occur in patients with an intermediate risk of a future cardiac event (e.g., those with diabetes, hypertension or coronary artery disease [CAD]) is unknown. Accordingly, we performed this study to determine the frequency with which small and/or short perfusion defects (that sometimes may in fact represent artifacts) occur in patients with diabetes, hypertension or CAD. Also, we sought to determine the prognostic utility of these defects for forecasting future cardiac events.
MATERIALS AND METHODS
Study Population
This study was performed in accordance with the National Institutes of Health grant R01HL076438 titled the “Pulmonary Edema and Stiffness of the Vascular System” or PREDICT. The purpose of PREDICT is to identify abnormalities of the cardiovascular (CV) system that forecast a first episode of congestive heart failure (CHF) in middle aged and elderly individuals at risk for, but yet to experience, CHF. The study was approved by the Institutional Review Board of the Wake Forest University School of Medicine, is registered with clinicaltrials.gov (NCT00542503), and each participant provided witnessed informed consent. The PREDICT study enrolled participants ages 55 to 85 years with known prior CAD, diabetes or hypertension and who had a left ventricular ejection fraction (LVEF) >25%, no myocardial infarction (MI) within 4 months prior to enrollment, and did not exhibit severe chronic obstructive airway disease (forced expiratory lung volume >0.5 L).
Between 2007 and 2010, 331 consecutive participants underwent DCMR stress perfusion testing on a 1.5T (Siemens Avanto) whole-body imaging system using a phased-array cardiac surface coil according to previously published techniques.5,6 Individuals with a contraindication to DCMR (noncompatible biometallic implants or claustrophobia), or for the administration of intravenous dobutamine (severe hypertension, unstable angina, cardiac arrhythmias) or gadolinium (glomerular filtration rate <60 mL/min/1.73 m2, allergy), or moderate to severe aortic stenosis were excluded from participating.
Cardiac Magnetic Resonance Imaging Protocol
Cine white blood images 8mm thick with 2mm gap were acquired from multiple short axis planes of the LV spanning the cardiac apex to the base at rest and after low and high doses of intravenous dobutamine.5,6 During the scans, dobutamine was infused incrementally from a low dose (7.5 mcg/kg/min) to a high dose (20 to 40 mcg/kg/min) with or without atropine (up to 1.5 mg) to achieve 80% of the maximum predicted heart rate response for age. Resting LVEF was measured using a modified Simpson’s rule technique according to previously published methods.6
Throughout stress, LV wall motion was continuously monitored and recorded to identify evidence of inducible LV wall motion abnormalities indicative of ischemia that would necessitate termination of the pharmacologic stress infusion. Left ventricular wall motion was assessed with a visual scoring system in which 1 was equivalent to normal wall motion, 2 equaled hypokinesis, 3 equaled akinesis, and 4 was associated with dyskinesis. An inducible wall motion abnormality was defined as an incremental increase in the score of ≥1 in any given segment.
Intravenous gadoteridol (MultiHance) was infused at peak stress at a dose of 0.1 mmol/kg to obtain stress perfusion imaging. Perfusion imaging was obtained from the short axis orientation in the middle and distal regions (2 slice positions) with consecutive R-R interval analysis. Ten minutes after the administration of contrast, gradient-echo inversion recovery images were collected in the same short axis planes used to assess LV volumes for the purpose of detecting late gadolinium enhancement (LGE). A fast gradient-echo (FGRE) sequence was used to achieve multislice coverage
Image Analysis
In accordance with the American Heart Association Scientific Statement,7 the 10 middle and distal LV myocardial segments were assessed for perfusion, LGE and inducible wall motion abnormalities. For each LV myocardial segment, regions of first-pass hypoperfusion and LGE were measured utilizing the greatest linear distance of the hypoperfusion or LGE defect along a radial spoke traversing the LV endocardial to epicardial surfaces within each LV myocardial segment (Figure 1). The radial length of the perfusion or LGE defect was therein expressed as a percentage of the total wall thickness. The number of frames for each perfusion defect present was calculated from peak LV myocardial enhancement until complete resolution of the defect.
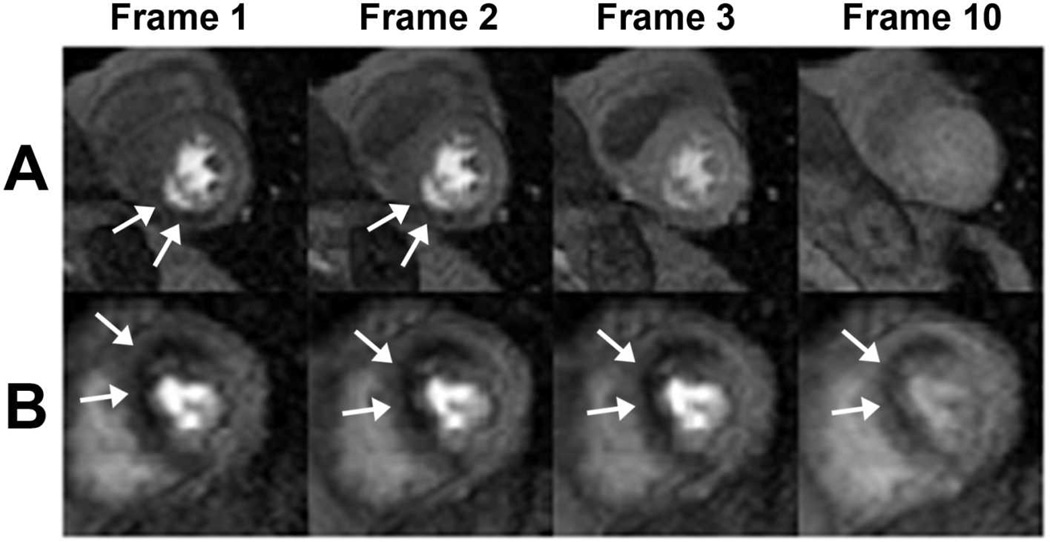
Figure 1. Dobutamine cardiac magnetic resonance perfusion imaging.
Dobutamine perfusion imaging as observed in the axial mid myocardial plane. A small and short duration inferior perfusion defect is demonstrated in Panel (A) whereas Panel (B) shows an example of a large and persistent anteroseptal defect.
Follow-Up
Personnel unaware of the study design or stress testing results contacted each subject (or, if deceased, an immediate family member); the date of this contact was used for calculating follow-up times. The subjects were contacted routinely every 4 to 5 months. Any change in physical state, medical condition, or medication use was confirmed by review of the participant’s medical records. Consistent with previously published techniques, hard events were defined as nonfatal MI (angina of ≥30 min duration, and either ≥2 mm ST-segment elevation in 2 consecutive electrocardiogram leads or a rise in serum troponin I or creatine kinase level and its MB fraction 2 times the upper limit of normal),8 cardiac death (death in the presence of acute coronary syndrome, significant cardiac arrhythmia, or refractory CHF),9 or admission to the hospital for unstable angina with subsequent coronary artery revascularization.6,10 When available, electrocardiogram, enzymatic, or autopsy data were used to substantiate cardiac mortality. In the case of 2 simultaneous cardiac events, the first event was selected for the purpose of analysis. Time to any event was defined as time to first event. Median follow-up time was calculated according to the method proposed by Schemper and Smith.11
Statistical Analysis
Patient characteristics were summarized by the mean and standard deviation for continuous measures and then number and percentage for dichotomous measures. Perfusion defect size and persistence (no defect, small perfusion defect [<25% of the LV myocardial thickness], short duration perfusion defect [<5 frames in duration], large perfusion defect [≥25% of the LV myocardial thickness], or long duration perfusion defect [≥5 frames in duration]) were defined in agreement with previously published techniques.1,2
The equality of the means of continuous measures between subjects grouped as no defect, small or short duration defect, or large and long duration defect was tested using analysis of variance. The equality of the percentages of dichotomous measures was compared between these three groups and was tested using the Chi-squared test. The fraction of patients predicted to be cardiac event free was estimated by Kaplan-Meier plots and pair-wise comparisons were made using the log-rank test. The increased risk predicted by a clinical risk measure was estimated by the proportional hazards model. All tests were made at the 5% two-sided level of significance.
RESULTS
Data regarding the 331 study participants are displayed in Table 1. One hundred seventy-five (52.9%) participants exhibited no perfusion defects, 156 (47.1%) had perfusion defects in at least one cardiac segment: 67 (20.2%) exhibited small (<25% myocardial thickness) perfusion defects, 62 (18.7%) exhibited short duration (<5 frames duration) perfusion defects, 89 (26.9%) exhibited large (≥25% myocardial thickness) perfusion defects and 94 (28.4%) exhibited long duration (≥5 frames in duration) perfusion defects. The perfusion defects of <5 frames in duration were found primarily in the inferior LV myocardial segments (55% inferiorly compared to 45% in all other myocardial territories). Perfusion defects were more likely to be isolated to a single AHA cardiac segment (18.1% of participants), compared to perfusion defects of two (10.9%), three to five (13.5%) or six to ten AHA cardiac segments (4.5%).
Table 1.
Patient Characteristics, Medical History, and Cardiac Measures
| No defect (n = 175) |
Small or short duration defect (<5 frames or <25% myocardial thickness) (n = 74) |
Large and persistent defect (≥5 frames and ≥25% myocardial thickness) (n = 82) |
p-value | |
|---|---|---|---|---|
| Age (yrs) | 67.5 [8.1] | 67.4 [7.1] | 68.8 [7.9] | 0.40 |
| Systolic blood pressure (mmHg) | 142 [16] | 144 [18.9] | 142 [19.0] | 0.59 |
| Diastolic blood pressure (mmHg) | 77 [10.0] | 75 [10.0] | 75 [10.0] | 0.31 |
| Heart rate (bpm) | 66 [11] | 68 [10] | 66 [11] | 0.41 |
| Creatinine (mg/dL) | 0.87 [0.16] | 0.86 [0.18] | 0.91 [0.16] | 0.14 |
| Glomerular filtration rate (ml/min) | 60.0 [0] | 60.0 [0.1] | 60.0 [0] | 0.17 |
| Left ventricular ejection fraction (%) | 58.5 [7.1] | 59.6 [5.9] | 54.2 [9.8] | < 0.001 |
| Men | 86 (49.7) | 28 (37.8) | 51 (48.8) | 0.010 |
| Tobacco use | 82 (47.1) | 27 (36.5) | 40 (49.4) | 0.21 |
| Hypertension | 153 (87.4) | 67 (90.5) | 76 (92.7) | 0.42 |
| Diabetes mellitus | 59 (33.7) | 39 (52.7) | 38 (42.7) | 0.019 |
| Coronary artery disease | 42 (24.0) | 16 (21.6) | 40 (49.8) | < 0.001 |
| Myocardial infarction | 19 (10.9) | 4 (5.4) | 20 (24.4) | 0.001 |
| Hypercholesterol | 122 (69.7) | 55 (64.3) | 67 (81.7) | 0.12 |
| Beta blocker | 63 (36.2) | 22 (30.1) | 40 (48.0) | 0.045 |
| Angiotensin-converting enzyme inhibitors | 70 (40.2) | 30 (40.5) | 42 (51.2) | 0.22 |
| Diuretics | 92 (52.9) | 42 (56.8) | 36 (43.9) | 0.24 |
| Angiotensin receptor blocker | 46 (26.4) | 24 (32.4) | 17 (20.7) | 0.25 |
| Digitalis | 2 (1.2) | 1 (1.4) | 1 (1.2) | 0.99 |
| Lipid lowering agents | 126 (72.4) | 60 (81.1) | 64 (78.0) | 0.30 |
| Left ventricular ejection fraction <55% | 24 (12.2) | 5 (8.2) | 23 (31.9) | < 0.001 |
| Wall motion score ≥2 | 8 (5.3) | 1 (1.5) | 8 (10.5) | .062 |
Total number of patients shown with either mean value demonstrated with [standard deviation] from mean noted, or percentage (of total). P-value demonstrates similarity among all three subgroups.
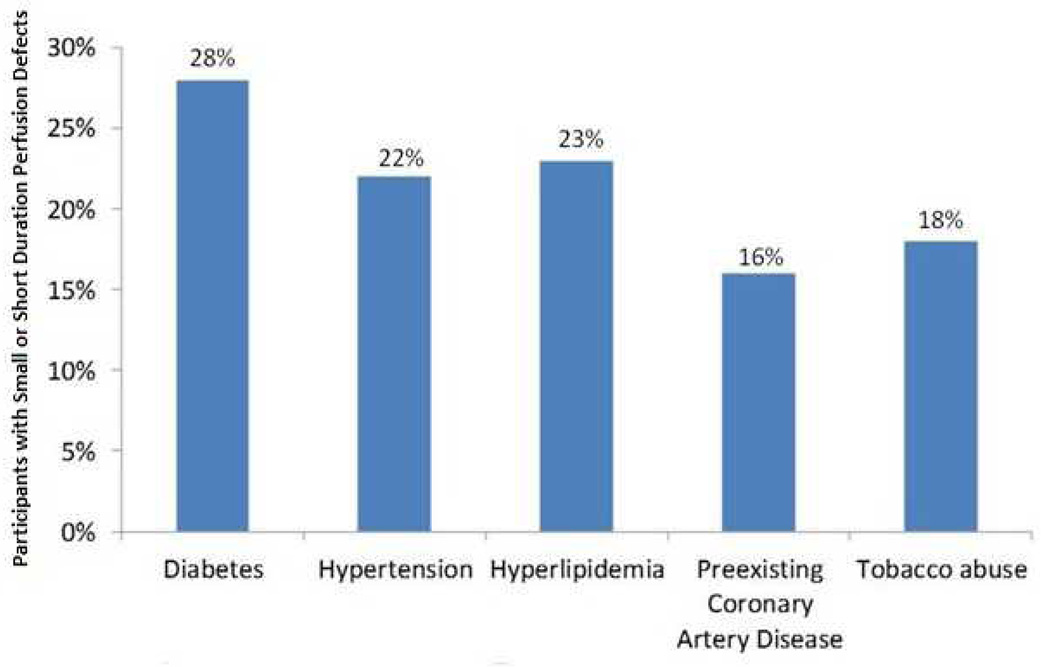
The clinical cardiac risk factors among participants identified in Table 1 were similar with three exceptions. First, CAD, prior MI and decreased LVEF were more prevalent in those patients with large and persistent (≥5 frames) perfusion defects (p<0.001). Second, participants with large and persistent perfusion defects exhibited a higher incidence of beta-blocker usage (p=0.05); otherwise, the medication profiles of the participants were similar. Third, among participants with small or short duration perfusion defects, there was an increased incidence of diabetes (p=0.02) compared to those patients with no perfusion defects or larger defects. As Figure 2 demonstrates, the occurrence of these small or short duration perfusion defects was common among those individuals with various co-morbidities often associated with future cardiac events.
Figure 2. Occurrence rate of small or short duration perfusion defects among cardiac risk factors.
Graph displaying the percentage of small or short duration perfusion defects among study participants with various cardiovascular co-morbidities.
The median follow-up time for identifying CV events was 24.4 (range 0.3 to 40.3) months. Cardiac events were observed among 16 participants in the cohort, including one cardiac death, 3 MIs and 12 hospital admissions for unstable angina and coronary artery revascularization. Four of these cardiac events occurred in patients without perfusion defects, none in those with small or short duration defects, and 12 in those with large, persistent perfusion defects. The overall cardiac event rate was found to be 2.5% per year which is consistent with an intermediate cardiac risk population.
No cardiac events occurred in subjects who exhibited a perfusion defect that persisted from 1 to 4 frames in duration regardless of the percentage of myocardium involved (Table 2). Likewise, there were no cardiac events experienced by individuals who exhibited a perfusion defect of <25% of a single cardiac segment regardless of frame duration (Table 2). In comparison to participants with perfusion defects of <5 frames in duration and <25% LV myocardial thickness (Table 2, upper left), those with perfusion defects of ≥5 frames in duration and ≥25% LV myocardial thickness (Table 2, bottom right) experienced an increased risk of a future cardiac event (p=.004)
Table 2.
Number (Percent) of CV Events by Degree of Perfusion Defect
| Maximum % perfusion defect |
||||
|---|---|---|---|---|
| <25% | ≥25% | Total | ||
| Maximum | 1−4 frames | 0/55 (0%) | 0/7 (0%) | 0/62 (0%) |
| Frame Duration | ≥5 frames | 0/12 (0%) | 12/82 (15%) | 12/94 (14%) |
| Total | 0/67 (0%) | 12/89 (14%) | 12/156 (8%) | |
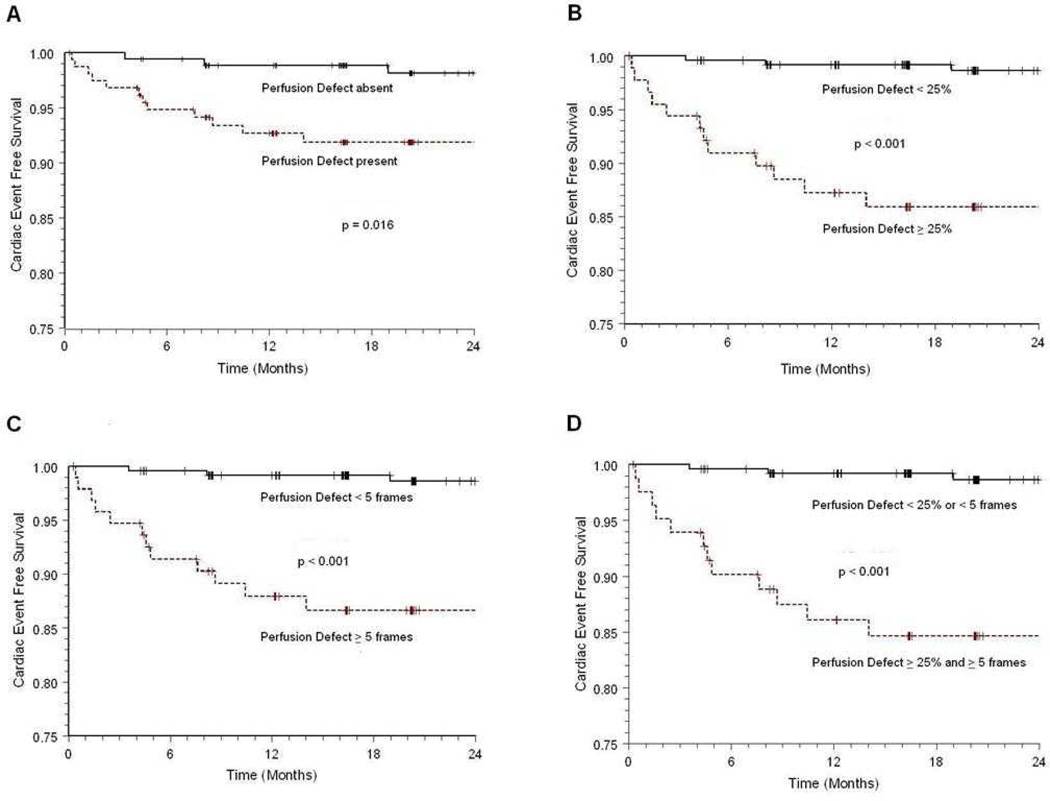
The cardiac event rate experienced by participants increased with perfusion defect persistence and size (Figure 3). The presence of both a large (≥25% LV myocardium) and persistent (≥5 frames in duration) defect conveyed an increased incidence of future cardiac events compared to small or short duration perfusion defects. When analyzed together (as shown in Figure 3, Panel D), both defect size and duration yielded the best prognostic tool for forecasting future cardiac events.
Figure 3. Kaplan-Meier curves demonstrating prognostic risk based on perfusion defect frame duration and defect size.
Kaplan-Meier curve analysis showing the difference in cumulative cardiac event rate when patients are stratified according to the (A) absence or presence of any perfusion defect, (B) absence of a perfusion defect or presence of perfusion defects <25% of the myocardium versus at least one perfusion defect ≥25% of the myocardium, (C) absence of a perfusion defect or presence of perfusion defects <5 frames in duration versus at least one perfusion defect ≥5 frames in duration, and (D) absence of a perfusion defect or presence of perfusion defects <25% of the myocardium or <5 frames in duration versus at least one perfusion defect ≥25% of myocardium and ≥5 frames in duration. Cardiac events were defined as cardiac death, myocardial infarction and unstable angina warranting hospitalization and revascularization.
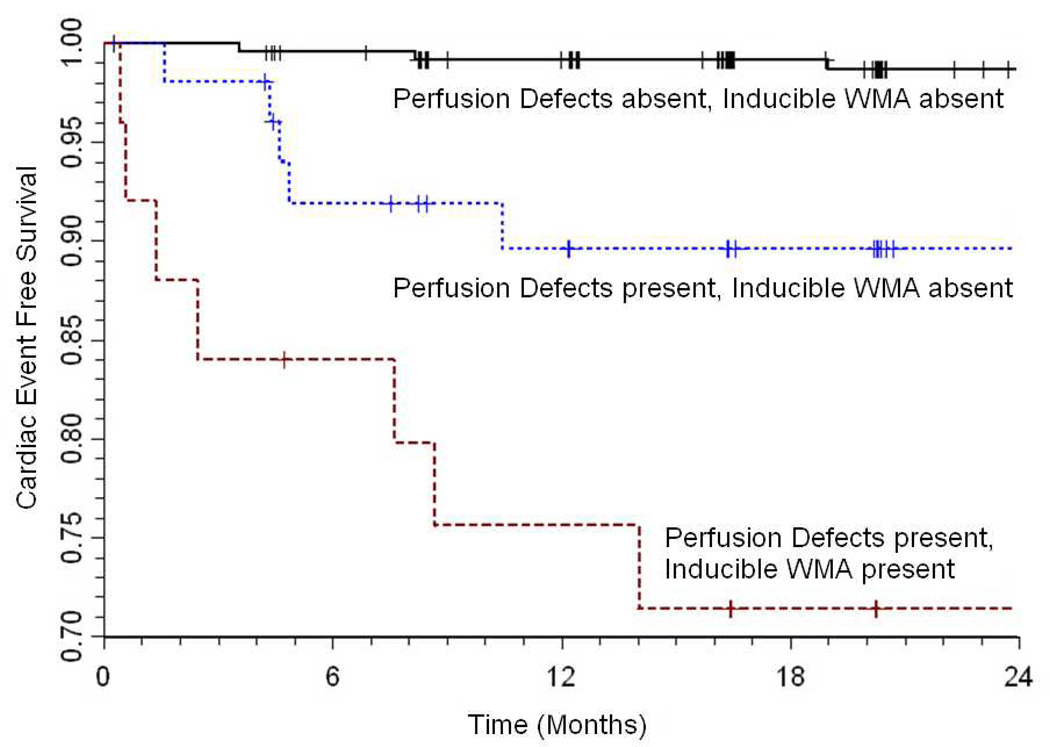
Individuals with both large and persistent perfusion defects exhibited more inducible wall motion abnormalities (Table 1). Inducible wall motion abnormalities in the presence of both large and persistent perfusion defects were associated with future cardiac events (hazard ratio [HR] 39.9, p<0.001, confidence interval [CI] 7.9–201.4) compared to individuals without perfusion defects or inducible wall motion abnormalities (Figure 4). Among patients with both a large and persistent perfusion defect, the presence of an inducible wall motion abnormality conveyed an increased risk of a future cardiac event (HR 4.0, p=0.04, CI 1.1–14.9) compared to those patients without an inducible wall motion abnormality (Figure 4).
Figure 4. Kaplan-Meier curves demonstrating prognostic risk based on presence of inducible wall motion abnormalities with or without perfusion defects.
Kaplan-Meier curve analysis showing the difference in cumulative cardiac event rate when patients with no perfusion defects and those with large and long duration perfusion defects (defined as ≥25% in size with ≥5 frame duration) are stratified according to the presence or absence of inducible ischemia (defined as a wall motion abnormality score ≥1). Cardiac events were defined as cardiac death, myocardial infarction and unstable angina warranting hospitalization and revascularization.
Late gadolinium enhancement images were obtained in 330 individuals (99.7%) in our patient cohort with 1 participant experiencing swallowing difficulty and dry mouth after administration of intravenous gadoteridol and expressing desire to terminate their examination prior to the acquisition of LGE images. The 2-year incidence of cardiac events in participants without evidence of both perfusion defect and LGE was 1.5%; the cardiac event rate in participants without a perfusion defect but with some LGE was 0%; the cardiac event rate in participants with any perfusion defect but without LGE was 11.1%; and the cardiac event rate in participants with both any perfusion defect and any LGE was 20.2%. Only 4 participants with short duration perfusion defects of <5 frames in duration had the same corresponding AHA segment of LGE noted (6.6%) compared with 32 participants who had persistent perfusion defects ≥5 frames in duration and the same corresponding AHA segment of LGE (34.7%).
DISCUSSION
The results of this study indicate several important findings. First, both small (20.2%) and short duration (18.7%) perfusion defects occur relatively frequently in a patient population at intermediate risk for future cardiac events who are often referred for DCMR utilizing first-pass gadolinium enhanced perfusion techniques. Second, small and short duration perfusion defects are more likely to occur in diabetics, may or may not be associated with LGE, and were not associated with an increased risk of future cardiac events during a follow-up period of 2 years. Third, the presence of inducible wall motion abnormalities in the setting of large and persistent perfusion defects conveyed an increased risk of future cardiac events. Finally, as prior studies have shown, the presence of large, persistent perfusion defects and LGE are associated with an increased risk of future cardiac events in those at intermediate risk of a future cardiac event.
In 2008, Gebker et al., demonstrated that the addition of gadolinium first pass perfusion imaging to wall motion assessments improved the overall sensitivity (91% compared to 85%) of DCMR in identifying patients with >70% coronary artery stenosis by coronary angiography.2 In addition, in a study of 1,493 patients, Korosoglou et al., identified individuals at high risk of future cardiac events based on DCMR perfusion analysis.1 Importantly, studies to date have not specified whether small perfusion defects that occur during DCMR are frequent or whether they confer an increased risk of a future cardiac event.1,2,12
The frame duration of a clinically significant DCMR induced perfusion defect has varied from 3 to 5 in prior reports.1,2 At present, frame duration is defined as the number of frames for which a perfusion defect is present after peak LV myocardial enhancement.1 Defects present <5 frames have previously constituted “short duration” defects. Assuming a frame duration of 4, reliable triggering (no skipped beats), and an R-R interval of 500 to 800 msec, this translates to approximately 2000 to 3200 msec (or 2 to 3 seconds) after appearance of contrast within the LV myocardium. As shown in Figure 1 (row A), small and short duration defects are concerning for artifacts and may lead to false diagnoses. These artifacts may arise from partial volume effects at the interface of myocardium and LV blood pool,13 cardiac motion during acquisition,14 non-uniform weighting of raw k-space data in the phase-encode direction,13 magnetic susceptibility from contrast bolus,15–17 or Gibb’s ringing from k-space truncation.4 Strategies have been developed to ascertain artifacts from small and short duration defects using rest and stress images with delayed enhancement,3 however, this approach may be less helpful in those with ischemia and prior infarction.13
The incidence of both small and short duration perfusion defects proved to be quite common, occurring in approximately 20% of our patient population. As shown in Figure 2, these defects occurred frequently among patients with co-morbidities associated with cardiac events. The patient population enrolled in this study demonstrated a high prevalence of well-established cardiac risk factors, such as CAD, diabetes mellitus, and hypertension with a large majority (88.8%) exhibiting a preserved LVEF (≥55%). The presence of CAD and previous MI were associated with an increased incidence of large, persistent perfusion defects.
Interestingly, the prevalence of diabetes mellitus was higher among participants with small or short duration perfusion defects compared to those without perfusion defects or those with both large and persistent perfusion defects. Prior studies using adenosine cardiac magnetic resonance stress have identified individuals with perfusion defects of less than 1/3 of the LV myocardium that persist for <5 frames and cross two coronary artery territories that were found to have impaired coronary blood flow by angiography.18 This suggests possible underlying small vessel disease; however, the definitive cause of small and short duration DCMR perfusion defects remains uncertain.
Prior studies have shown that DCMR stress perfusion imaging is a reliable tool in the detection of underlying CAD 19 and predicting future adverse cardiac events.1,2 Our study results in individuals at intermediate risk of future CV events suggested that the presence of a perfusion defect increased the risk of sustaining a future cardiac event (Figure 3, Panel A). On further analysis, however, this finding was driven by the strong association of large, persistent perfusion defects with future cardiac events. In those with perfusion defects ≥25% of the thickness of the LV endocardium at end diastole and ≥5 frames in duration, we found a 15% risk of future cardiac events during our follow-up period of 24.4 months (Table 2). Importantly however, there were no cardiac events observed in our participant population with small or short duration perfusion defects (Figure 3).
There was a statistically significant increase in future cardiac events observed in participants with both large and persistent perfusion defects compared to those with small and short duration defects. Participants with large and short duration defects or small and persistent defects occurred in a minority of our study population (5.7%). While no cardiac events were observed in either of these groups, given the small number of participants in which these perfusion defects occurred, future studies are required to determine whether small but persistent, or large but short duration defects are of clinical significance.
The prognostic role and safety of DCMR for identifying those at intermediate risk of a future cardiac event using dobutamine induced changes in LV wall motion has been well established.3,6,20–23 Our findings demonstrating an association between large and persistent perfusion defects and the occurrence of inducible wall motion abnormalities supports these previous findings (Figure 3).
The presence of any LGE has been clinically shown to predict adverse cardiac events independent of segmental wall motion abnormalities24 or inducible perfusion defects observed during stress testing.25 Our cohort demonstrated that perfusion defects of any size in the presence of LGE were associated with a 20.2% cardiac event rate over 2 years. Short duration perfusion defects were not as closely associated with LGE as were persistent perfusion defects. Our study demonstrated that short duration perfusion defects occur with concomitant LGE in the same AHA myocardial segment at a frequency approximately 1/5 the rate that was found in association with large, persistent perfusion defects.
Study Limitations
Our study exhibits the following limitations. First, our overall event rate was low. This may have been due to the fact that our patient population, although at intermediate to high risk for CV disease, was recruited through hospital in- and out-patient clinic referrals to participate in this research study. As a result, they may not exhibit the same severity of disease as seen in an overtly symptomatic population admitted for evaluation of acute chest pain. In addition, many participants with evidence of ischemia during testing received near immediate catheterization and revascularization. Thus, it is possible that this more aggressive medical or invasive cardiac therapy upon awareness of individual patients risk profiles based on their perfusion study could also have contributed to our low overall cardiac event rate.
Second, our study assessed the presence of DCMR stress induced perfusion defects and delayed enhancement images without resting perfusion imaging. Therefore, previously described dark rim artifacts may have accounted for many of our small, short duration perfusion defects.3,4 The findings of our study, however, underscore the clinical utility of large, persistant perfusion defects and confirm that the presence of small, short duration perfusion defects are not associated with future cardiac events in an intermediate risk population.
In a large cohort of patients at intermediate risk for future cardiac events, dobutamine induced perfusion defects of <5 frames duration or <25% of total myocardial thickness are associated with a low 2-year risk of sustaining future cardiac events. The results of this study indicate a highly favorable prognosis for men and women with underlying CAD, hypertension, and diabetes (a patient population commonly referred for stress testing) who do not exhibit new inducible wall motion abnormalities or large, persistent perfusion defects during DCMR.
Acknowledgments
Sources of Funding: Research supported in part by National Institutes of Health grants R01HL076438, T32HL091824, and P30 AG21332.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Craig Hamilton, PhD, discloses past consultancy to and current stock/stock options with MRI Cardiac Services. All other authors state no conflicts of interest to disclose.
REFERENCES
- 1.Korosoglou G, Elhmidi Y, Steen H, et al. Prognostic value of high-dose dobutamine stress magnetic resonance imaging in 1,493 consecutive patients: Assessment of myocardial wall motion and perfusion. J Am Coll Cardiol. 2010;56:1225–1234. doi: 10.1016/j.jacc.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Gebker R, Jahnke C, Manka R, et al. Additional value of myocardial perfusion imaging during dobutamine stress magnetic resonance for the assessment of coronary artery disease. Circ Cardiovasc Imaging. 2008;1:122–130. doi: 10.1161/CIRCIMAGING.108.779108. [DOI] [PubMed] [Google Scholar]
- 3.Klem I, Heitner JF, Shah DJ, et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006;47:1630–1638. doi: 10.1016/j.jacc.2005.10.074. [DOI] [PubMed] [Google Scholar]
- 4.Di Bella EV, Parker DL, Sinusas AJ. On the dark rim artifact in dynamic contrast-enhanced MRI myocardial perfusion studies. Magn Reson Med. 2005;54:1295–1299. doi: 10.1002/mrm.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton CA, Link KM, Salido TB, et al. Is imaging at intermediate doses necessary during dobutamine stress magnetic resonance imaging? J Cardiovasc Magn Reson. 2001;3:297–302. doi: 10.1081/jcmr-100108582. [DOI] [PubMed] [Google Scholar]
- 6.Hundley WG, Morgan TM, Neagle CM, et al. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–2233. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 7.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 8.Alpert JS, Thygesen K, Antman E, et al. Myocardial infarction redefined--A consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 9.Mangano DT, Browner WS, Hollenberg M, et al. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery: The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323:1781–1788. doi: 10.1056/NEJM199012273232601. [DOI] [PubMed] [Google Scholar]
- 10.Dall'armellina E, Morgan TM, Mandapaka S, et al. Prediction of cardiac events in patients with reduced left ventricular ejection fraction with dobutamine cardiovascular magnetic resonance assessment of wall motion score index. J Am Coll Cardiol. 2008;52:279–286. doi: 10.1016/j.jacc.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 12.Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: Adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 13.Kellman P, Arai AE. Imaging sequences for first pass perfusion - A review. J Cardiovasc Magn R. 2007;9:525–537. doi: 10.1080/10976640601187604. [DOI] [PubMed] [Google Scholar]
- 14.Storey P, Chen Q, Li W, et al. Band artifacts due to bulk motion. Magnet Reson Med. 2002;48:1028–1036. doi: 10.1002/mrm.10314. [DOI] [PubMed] [Google Scholar]
- 15.Arai AE. Magnetic resonance first-pass myocardial perfusion imaging. Top Magn Reson Imaging. 2000;11:383–398. doi: 10.1097/00002142-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber WG, Schmitt M, Kalden P, et al. Dynamic contrast-enhanced myocardial perfusion imaging using saturation-prepared TrueFISP. J Magn Reson Imaging. 2002;16:641–652. doi: 10.1002/jmri.10209. [DOI] [PubMed] [Google Scholar]
- 17.Fenchel M, Helber U, Simonetti OP, et al. Multislice first-pass myocardial perfusion imaging: Comparison of saturation recovery (SR)-TrueFISP-two-dimensional (2D) and SR-TurboFLASH-2D pulse sequences. J Magn Reson Imaging. 2004;19:555–563. doi: 10.1002/jmri.20050. [DOI] [PubMed] [Google Scholar]
- 18.Pilz G, Klos M, Ali E, et al. Angiographic correlations of patients with small vessel disease diagnosed by adenosine-stress cardiac magnetic resonance imaging. J Cardiovasc Magn Reson. 2008;10:8. doi: 10.1186/1532-429X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelle S, Chiribiri A, Vierecke J, et al. Long-term prognostic value of dobutamine stress CMR. JACC Cardiovasc Imaging. 2011;4:161–172. doi: 10.1016/j.jcmg.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Wahl A, Paetsch I, Gollesch A, et al. Safety and feasibility of high-dose dobutamine-atropine stress cardiovascular magnetic resonance for diagnosis of myocardial ischaemia: Experience in 1000 consecutive cases. Eur Heart J. 2004;25:1230–1236. doi: 10.1016/j.ehj.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99:763–770. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 22.Paetsch I, Jahnke C, Wahl A, et al. Comparison of dobutamine stress magnetic resonance, adenosine stress magnetic resonance, and adenosine stress magnetic resonance perfusion. Circulation. 2004;110:835–842. doi: 10.1161/01.CIR.0000138927.00357.FB. [DOI] [PubMed] [Google Scholar]
- 23.Hundley WG, Hamilton CA, Thomas MS, et al. Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation. 1999;100:1697–1702. doi: 10.1161/01.cir.100.16.1697. [DOI] [PubMed] [Google Scholar]
- 24.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 25.Steel K, Broderick R, Gandla V, et al. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation. 2009;120:1390–1400. doi: 10.1161/CIRCULATIONAHA.108.812503. [DOI] [PMC free article] [PubMed] [Google Scholar]