Abstract
Humans and genetically engineered mice with recessively inherited CPVT develop arrhythmia which may arise due to malfunction or degradation of calsequestrin (CASQ2). We investigated the relation between protein level and arrhythmia severity in CASQ2D307H/D307H (D307H), compared to CASQ2Δ/Δ (KO) and wild type (WT) mice. CASQ2 expression and Ca2+ transients were recorded in cardiomyocytes from neonatal or adult mice. Arrhythmia was studied in vivo using heart rhythm telemetry at rest, exercise and after epinephrine injection. CASQ2 protein was absent in KO heart. Neonatal D307H and WT hearts expressed significantly less CASQ2 protein than the level found in the adult WT. Adult D307H expressed only 20% of CASQ2 protein found in WT. Spontaneous Ca2+ release was more prevalent in neonatal KO cardiomyocytes (89%) compared to 33–36% of either WT or D307H, respectively, p < 0.001. Adult cardiomyocytes from both mutant mice had more Ca2+ abnormalities compared to control (KO: 82%, D307H 63%, WT 12%, p < 0.01). Calcium oscillations were most common in KO cardiomyocytes. We then treated mice with bortezomib to inhibit CASQ2D307H degradation. Bortezomib increased CASQ2 expression in D307H hearts by ~50% (p < 0.05). Bortezomib-treated D307H mice had lower CPVT prevalence and less premature ventricular beats during peak exercise. No benefit against arrhythmia was observed in bortezomib treated KO mice. These results indicate that the mutant CASQ2D307H protein retains some of its physiological function. Its expression decreases with age and is inversely related to arrhythmia severity. Preventing the degradation of mutant protein should be explored as a possible therapeutic strategy in appropriate CPVT2 patients.
Keywords: Arrhythmia, Calsequestrin, Mouse model, Calcium transients, Protein degradation, Bortezomib
1. Introduction
Catecholamine induced polymorphic ventricular tachycardia (CPVT) is an inherited arrhythmia caused by mutations in genes that regulate intracellular Ca2+ homeostasis. Patients suffering from CPVT are at risk of ventricular fibrillation and sudden death triggered by exercise or emotional stress [1,2]. Autosomal dominant CPVT1 is evoked by mutations in the cardiac ryanodine receptor (RyR2) gene [3], and autosomal recessive CPVT2 is caused by mutations in the cardiac calsequestrin (CASQ2) [4,5]. CASQ2 is a sarcoplasmic reticulum (SR) protein, that together with RyR2 triadin and junction are essential for the Ca2+ induced Ca2+ release mechanism in the cardiomyocyte [6]. Missense mutations are assumed to impair the conformational changes occurring in CASQ2 with Ca2+ binding and the ability to regulate RyR2 channel [2,4,7]. Other CPVT2 causing mutations introduce a null-allele [8]. The mouse models for recessively inherited CPVT, gene-targeted mice with homozygous D307H mutation (CASQ2D307H/D307H) or CASQ2 knock-out (CASQ2Δ/Δ), are viable and recapitulate the human phenotype. Polymorphic and bidirectional VT may be provoked by stress [9–11]. The phenotype of CASQ2D307H/D307H mice was attributed primarily to the degradation of the defective protein leading to a nearly complete protein deficiency despite preserved mRNA levels [10]. Yet, we have observed a difference in arrhythmia severity between adult CASQ2 D307H and knock-out mice and a decreased responsiveness to drug therapy in the later [9]. Because CASQ2 expression increases with developmental age [12] we investigated the relation between CASQ2 protein level and abnormal Ca2+ release in neonatal versus adult hearts, comparing wild type controls with CASQ2D307H/D307H and CASQ2Δ/Δ murine models.
2. Methods
The animal experiments conform to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication no. 85-23, revised 1996) and were approved by the institutional animal care and use committee of Tel Aviv University (no. 11-04-093). Experiments were carried out on gene targeted C57BL/6 mice modeling CPVT2, homozygous for CASQ2D307H/D307H mutation (D307H) or CASQ2Δ/Δ (KO), compared to age-matched wild type (WT). Of note, heterozygous mice have no phenotype and have CASQ2 expression comparable to wild type animals [10]. Mice were maintained and bred in a pathogen-free facility on regular rodent chow with free access to water and 12-h light and dark cycles.
2.1. Western blotting and immunohistochemistry
All reagents were purchased from Sigma Aldrich, Israel, unless otherwise indicated. CASQ2 expression analysis was conducted on extracts from heart ventricular homogenate samples (50 or 30 μg/lane) from neonatal (3 days old) and adult mice (4–8 month old). Protein levels were quantified by the Bradford reagent (Sigma, St. Louis, MO, USA), with bovine serum albumin as a standard. Heart ventricles homogenate samples were separated on 10% SDS-polyacrylamide gels under denaturing conditions and transferred to nitrocellulose membranes. The membranes were incubated for 1 h in blocking solution containing 5% milk powder in Tris Buffered Saline (TBS) at room temperature, and then was immunoblotted overnight at 4 °C with rabbit polyclonal antibodies against calsequestrin (ABCAM ab3516, Cambridge, MA, USA, 1:2500), and GAPDH (Santa Cruz Biotechnology, Dallas, Texas, USA, 1:200). Blots were then washed in TBS containing 0.1% Tween-20, and incubated for 1 h in TBS with FITC labeled gout polyclonal anti-rabbit IgG (Santa Cruz Biotechnology, Dallas, TX, USA, 1:500) at 25 °C. Immunoreactions were analyzed with enhanced chemiluminescence (ECL) and quantitated by densitometry.
Formalin fixed cardiac tissues from 7 to 8 month old mice were dehydrated, embedded in paraffin and sectioned at 4 μm. Sections were de-waxed in xylene and rehydrated. After rinses in TBS, sections were blocked with 10% goat serum 30 min at room temperature and subsequently incubated with the primary antibody against calsequestrin (1:50) for 60 min at room temperature. Detection was performed with the Histostain SP Broad Spectrum kit (Zymed Laboratories, Invitrogen, NY, USA). Sections were incubated with a biotinylated second antibody for 30 min at room temperature, and subsequently were TBS rinsed with HRP-streptavidin. After TBS rinses, the antibody binding was visualized with the substrate-chromogen AEC (3-Amino-9-Ethyl-carbazole), counterstained with hematoxylin and coverslipped with an aqueous mounting fluid (glycergel).
2.2. Neonatal cardiomyocyte cultures
For neonatal cardiomyocyte culture preparations breeding was synchronized to obtain several litters of the same age. Hearts from newborn mice (2–3 days old, ~20 hearts per each preparation) were removed under sterile conditions and washed three times in phosphate buffered saline (PBS) to remove excess blood cells. The hearts were minced into small fragments and then agitated gently in RDB, a solution of proteolytic enzymes prepared from fig tree extract (Biological Institute, Ness-Ziona, Israel), as previously described [13]. The cell suspension was diluted to 1.2 × 106 cells/ml, and 1.5 ml of suspension was placed in 35-mm plastic culture dishes on collagen/gelatin-coated coverglasses. The cultures were incubated in a humidified atmosphere of 5% CO2, 95% air at 37 °C.
2.3. Sarcoplasmic reticulum Ca2+ uptake measurements
Accumulation of Ca2+ by the sarcoplasmic reticulum (SR) was measured in skinned neonatal cardiomyocytes as previously described [14]. Cultured neonatal cells, 7 days old, were incubated in 1 ml of skinning solution (140 mM KCl, 20 mM HEPES, 10 mM EGTA, 50 μg/ml saponin, 5 mM NaN3, 5 mM potassium oxalate, 1 μM ruthenium red, pH 7.4) at 25 °C for 30 min. The skinned cells attached to the dish were washed with Ca2+-uptake solution containing 140 mM KCl, 20 mM HEPES, 0.5 mM EGTA, 0.5 mM CaCl2, 5 mM NaN3, 5 mM potassium oxalate, 5 mM MgCl2, pH 7.0 at 37 °C. One ml of radioactive uptake solution (45Ca2+ 0.2 μCi/ml) was then added, and cells were incubated at 37 °C for 10 min, either in the presence or absence of 5 mM ATP. The uptake solution was removed, and the cells were rinsed five times with ice-cold fresh uptake solution. Cells were then lysed with 0.3 ml of 1% Triton X-100 and collected in counting vials containing 4 ml scintillation liquid (Hydrofluor, National Diagnostics, USA). Radioactivity was determined in DPM (disintegrations per minute) using a β-scintillation counter (Packard). Values for specific calcium uptake were derived by subtraction of counts obtained from calcium accumulation experiments performed in the absence of ATP. These values were converted to units of nmole calcium/min/mg protein by calibration with uptake solutions containing known amounts of calcium.
2.4. Intracellular Ca2+ measurements in mouse cardiomyocytes
Intracellular free calcium ([Ca2+]i) from individual neonatal cardiomyocytes was measured using the indicator indo-1-AM under a Zeiss fluorescent inverted microscope. The ratiometric methods for quantification of the results have been described previously [13,15]. Adult ventricular cardiomyocytes were isolated from 4 to 8 month old mice using a Langendorff isolated heart perfusion preparation and enzymatic digestion as previously described [10]. Cells were incubated at 37 °C in 2% CO2 until used. [Ca2+]i from individual adult cardiomyocytes was measured as above. Measurements were performed in electrically paced cells at 1 Hz via platinum electrodes, under baseline conditions and with isoproterenol (ISO) 10 μM.
2.5. Classification of Ca2+ abnormalities
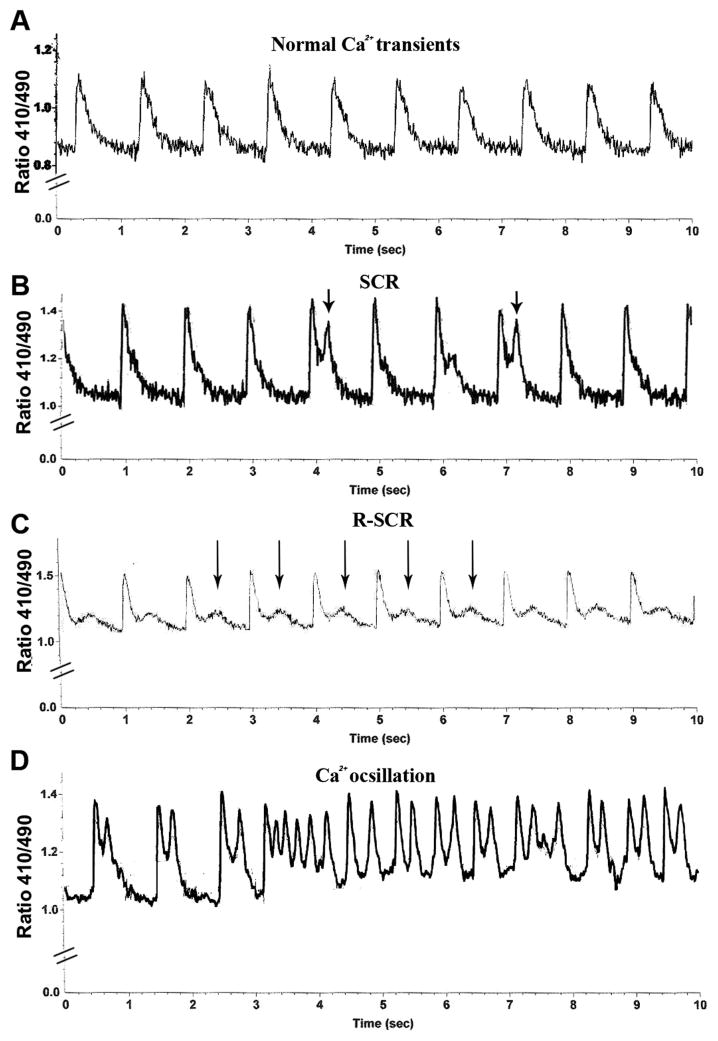
We studied Ca2+ irregularities at baseline and 5 min after exposure to isoproterenol [ISO, 10 μM] in adult and neonatal cardiomyocytes. We divided the observed Ca2+ irregularities into 3 categories (Fig. 1): (a) Spontaneous Ca2+ Release (SCR), ectopic Ca2+ release events that followed normal calcium transients. (b) Repetitive Spontaneous Ca2+ Release, R-SCR, spontaneous release of Ca2+ that occurred constantly after each normal Ca2+ transient. (c) Ca2+ oscillation, a series of more than three consecutive abnormal Ca2+ releases.
Fig. 1.
Representative traces of irregular Ca2+ release in murine cardiomyocytes. (A) Normal Ca2+ transients. (B) Spontaneous Ca2+ Release (SCR). (C) Repetitive Spontaneous Ca2+ Release (R-SCR). (D) Ca2+ oscillation. Arrows indicate abnormal calcium release events.
2.6. Experiments with bortezomib
Eight month old mice were implanted with a telemetry device (DSI St. Paul MM, device weight 3.8 g) as previously described [9]. After 2 days of recovery mice were treated with IV 1 μg/g bortezomib (Velcade, J-C Health Care Ltd., Kibbutz Shefayim, Israel) or 0.2 ml of 0.9% saline vehicle via tail vein according to Zhang et al. and van Hees et al. [16,17]. The treatment protocol lasted 11 days and included 4 injections on days 1, 4, 8 and 11. Provocation testing was performed at baseline, and on day 11, 1 h after the last injection [9]. Arrhythmia was studied with heart rhythm telemetry at rest, during treadmill exercise (Exer-6M, Columbus Instruments, OH, USA) and after IP injection of epinephrine 0.5 mg/kg. In brief, mice were forced to exercise on rodent treadmill gradually increasing the speed up to a maximum speed of 15 m/min. Then, after 5 min of rest, mice were injected with epinephrine followed by additional 5 min of telemetric recording. Ventricular tachycardia (VT) was defined as 4 or more consecutive ventricular beats. All other ventricular arrhythmias, i.e. premature beats, ventricular bigeminy, couplets and triplets were all defined as PVCs (premature ventricular contractions). The presence of CPVT in an animal was defined as any kind of VT (monomorphic, bidirectional, and polymorphic) either at rest or during exercise or pharmacological stress. “PVC count” was defined as a sum of all abnormal ventricular beats, and included premature beats, ventricular bigeminy, couplets and triplets. The “PVC count” was obtained for 1 min during peak exercise, i.e. the last 10 s of forced running and the subsequent 50 s of recovery. Thereafter, mice where anesthetized with isoflurane and hearts where removed and stored at −80 °C for protein analysis.
2.7. Statistical analysis
Results are presented as mean ± SD analyzed using Student’s t-test or chi square test, as appropriate. p < 0.05 was considered to be statistically significant.
3. Results
3.1. Protein analysis in CASQ2 mutant mice
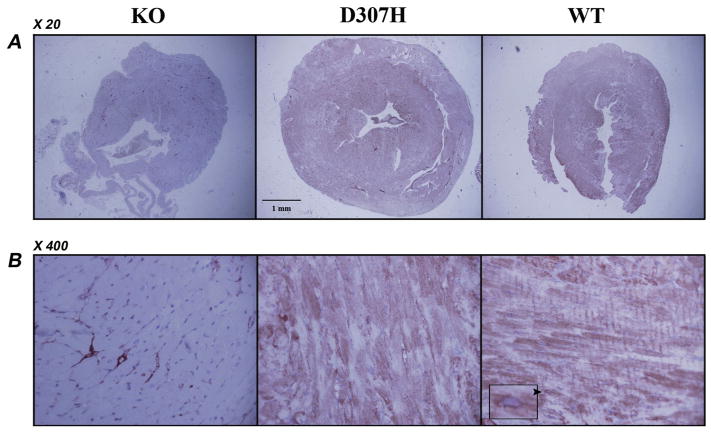
CASQ2 protein level was initially assessed in hearts of 4–8 month old mice. No protein was detected in KO hearts. CASQ2 D307H protein level was dramatically reduced to about 20% of the normal (Fig. 2A and B). Moreover, the previously described high-molecular-weight “calsequestrin-like proteins” [11,18], assumed to be CASQ2 polymers, were completely absent from D307H extracts (Fig. 2D). Immunohistochemistry with CASQ antibody on heart sections of 7–8 month old mice stained positive in WT and CASQ2 D307H hearts and was negative in the KO hearts (Fig. 3). In positively stained myocytes a perinuclear distribution and striation were observed, which were more prominent in WT. In KO a positive stain was observed only in the capillaries, which should be attributed to CASQ1 in the smooth muscle [19,20]. The D307H hearts exhibited a unique pattern characterized by variability in stain intensity between adjacent myocytes. Based on these data, we concluded that D307H mice express significantly less CASQ2 protein, while KO mice do not express CASQ2 at all. We further investigated whether these residual levels of CASQ2 in the D307H mice are responsible for the difference in arrhythmia susceptibility between D307H and KO mice as noted in our previous report [9]. The expression of CASQ2 was compared in adult versus neonatal mice. In normal mice CASQ2 expression increases with age [12] but in CASQ2 D307H mice, it markedly decreased with maturation (Fig. 2AC). In contrary to adults, there was no difference between CASQ2 protein expression levels in WT and D307H neonates.
Fig. 2.
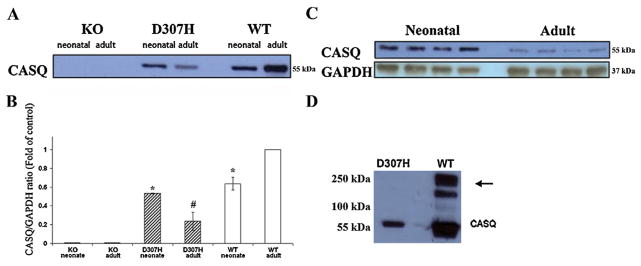
Expression of CASQ2 protein in adult and neonatal mouse hearts. (A) Age dependent expression of CASQ2 in WT, D307H and KO Ventricular homogenates (50 μg protein/lane, n = 3). (B) Quantitative comparison of age-dependent CASQ2 expression in WT and mutant heart ventricles. Data is presented as CASQ2/GAPDH ratio relative to WT (Each neonatal homogenate sample comprised 4–5 hearts, n = 3, mean ± SD, p < 0.05 *; p < 0.01 #). (C) Representative blot showing the decrease in CASQ2 expression in adult D307H compared to neonates. (D) Representative blot WT hearts showing the high molecular weight “CASQ-like proteins”, assumed to be CASQ2 polymers, which are not detectable in the mutant (arrow). D307H, CASQ2D307H/D307H; KO, CASQ2Δ/Δ; WT; wild type; CASQ, calsequestrin.
Fig. 3.
Immunohistochemistry in hearts of CASQ2 mutant mice. Representative sections of mouse hearts stained with anti-calsequestrin antibody X20 (A) and X400 (B). Diffuse staining for CASQ2 and striations are prominent in WT heart. A predominantly perinuclear distribution may be noted (arrowhead and insert). KO hearts has no staining apart from the capillary network, which is attributable to cross reactivity with CASQ1 in the smooth muscle. D307H had a non-homogenous positive staining in the myocardium. D307H, CASQ2D307H/D307H; KO, CASQ2Δ/Δ; WT, wild type; CASQ, calsequestrin. n = 3 hearts/group, 2 sections/heart, animal age 7–8 month.
3.2. Sarcoplasmic reticulum Ca2+ ATPase (SERCA) activity
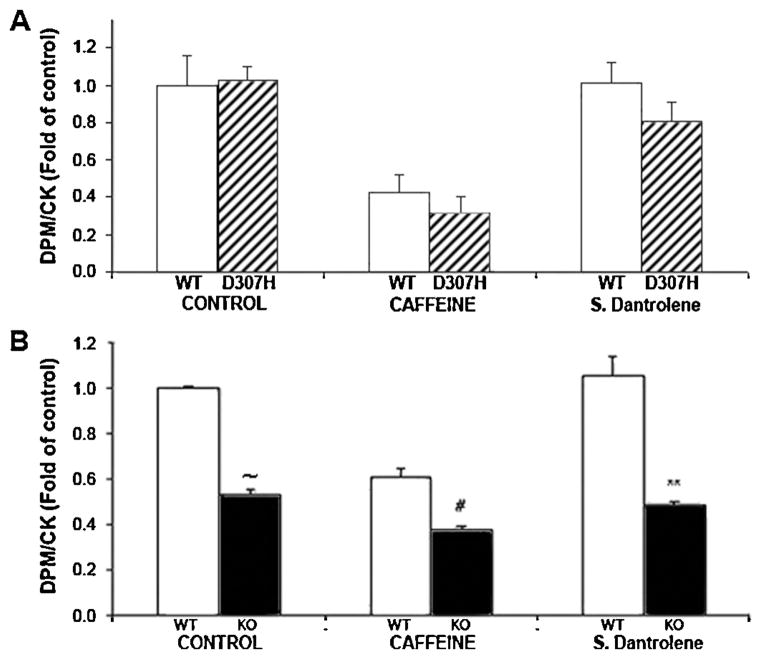
To assess the SERCA activity we measured SR Ca2+ uptake in skinned neonatal cardiomyocytes. The comparison of Ca2+ uptake was evaluated relatively to creatine kinase (CK) activity in order to eliminate differences in cardiomyocyte proliferation per culture dish. No significant difference was found in the Ca2+ uptake into the SR between D307H and WT myocytes. However, a decrease was found in the uptake of Ca2+ into the SR of KO myocytes (Fig. 4). Because RyR2 expression is increased in mutant myocytes [10], we measured Ca2+ uptake in the presence of RyR2 blocker, sodium dantrolene [40 μM] or caffeine [10 mM], which induces Ca2+ release from the SR, to evaluate SERCA activity independent of changes in RyR2 function. Again, there was a decreased uptake to SR of KO compared with WT but no difference between D307H and WT (Fig. 4).
Fig. 4.
SR Ca2+ uptake in neonatal cardiomyocytes. (A) SR Ca2+ uptake in D307H neonatal compared to WT. (B) SR Ca2+ uptake in KO cardiomyocytes vs. WT. Results are presented as DPM/CK ratio, mean ± SD, n = 3 experiments/group. All data were corrected to control values in WT which were taken as 1. Comparisons are made to WT under similar conditions: Caffeine (10 mM); Sodium dantrolene (40 μM). DPM, disintegrations per minute; CK, creatine kinase; SR, sarcoplasmic reticulum, D307H, CASQ2D307H/D307H; WT, wild type. p < 0.01 #; p < 0.001 **; p < 0.00001 ~.
3.3. Abnormal Ca2+ release in neonatal and adult cardiomyocytes
Spontaneous Ca2+ irregularities (Fig. 1) are an indicator of abnormal calcium release during diastole and susceptibility to arrhythmia in the whole heart. Neonatal and adult cardiac cells were tested for Ca2+ irregularities and the generation of SCR and oscillations with isoproterenol (ISO). Unlike adult cardiomyocytes, neonatal cells had some Ca2+ irregularities at baseline but KO had a higher prevalence compared to both neonatal D307H or WT (67% vs. 16 and 15%, respectively, p < 0.01). With ISO, neonatal KO myocytes had more Ca2+ irregularities compared to either WT or D307H (Fig. 5). No difference in Ca2+ irregularities was observed between WT and D307H neonatal myocytes. Adult cardiomyocytes had normal Ca2+ transients at baseline. After ISO, both D307H and KO myocytes, but not WT, suffered from frequent Ca2+ irregularities (Fig. 5). Although Ca2+ irregularities were as common in the adult D307H as in KO cardiomyocytes, the most severe form, Ca2+ oscillations, was detected primarily in KO (27% compared to 6% of D307H, p = 0.08). Considering the apparent association between the level of CASQ2 D307H protein and the prevalence of abnormal Ca2+ release in D307H cardiomyocytes, we assumed that the D307H CASQ2 protein preserves at least some of its function.
Fig. 5.
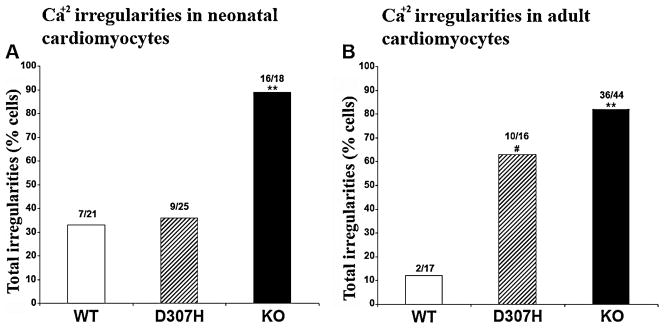
Abnormal Ca2+ release in isoproterenol-treated neonatal and adult cardiomyocytes. Columns represent the cells that exhibited any Ca2+ irregularities after isoproterenol exposure. Ca2+ irregularities in cardiomyocytes from neonatal (A) and adult mice (B). D307H, CASQ2D307H/D307H; CASQ2Δ/Δ, KO; wild type, WT. Chi-Test. p < 0.01 #; p < 0.001 **. Number of adult cells: WT n = 17 cells from 4 mice. D307H: n = 16 cells from 4 mice. KO n = 44 cells from 13 mice. Each neonatal cardiomyocyte culture was prepared by pooling 15–20 neonatal hearts; n = 4 different preparations/group.
3.4. Attenuating CASQ2 D307H protein degradation to treat CPVT
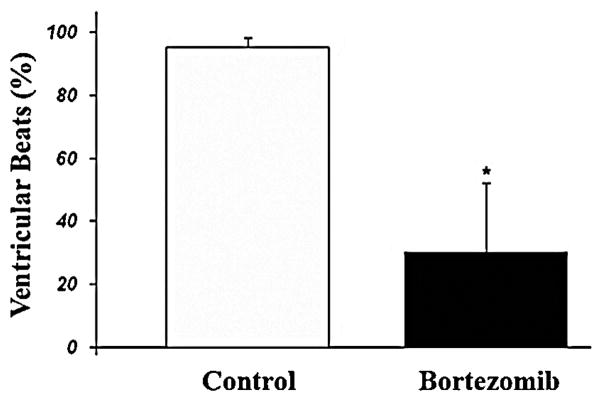
Given the inverse association between CASQ2 protein expression and calcium irregularities as well as lower arrhythmia susceptibility in D307H mice [9] we hypothesized that inhibiting D307H CASQ2 degradation might protect against arrhythmias. Bortezomib (Velcade) is a specific and potent proteasome inhibitor [17] which reversibly inhibits the activity of the 26S proteasome in mammalian cells. Adult D307H mice, which received an 11 day course of bortezomib were compared with saline-treated CASQ2 D307H mice and CASQ2 KO mice treated with bortezomib. Bortezomib treatment produced a trend for increase in the sinus heart rate in both groups of animals (p = NS). All mice suffered from the severe arrhythmia including VT on baseline. Bortezomib-treated D307H mice had a ~50% increase in the D307H CASQ2 protein expression (Fig. 6) and a decrease in VT prevalence at rest and during exercise stress (Table 1). The amount of abnormal ventricular beats on peak effort markedly decreased in CASQ2 D307H mice following bortezomib treatment (Fig. 7). However, bortezomib therapy did not affect the arrhythmia induced by epinephrine. We also found no change in severity of arrhythmia in bortezomib-treated CASQ2 KO mice (Table 1) and in saline treated D307H mice (n = 3, data not shown).
Fig. 6.
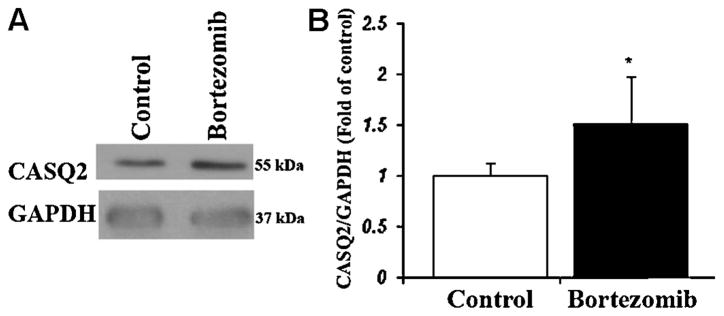
CASQ2 protein expression in D307H mouse hearts after bortezomib treatment. (A) Representative blot of heart homogenates (50 μg/lane) shows CASQ2 protein expression in D307H mice treated by 4 doses of bortezomib or saline-vehicle. (B) Densitometry confirming a significant increase in CASQ2 expression levels. Data is presented as a ratio of CASQ2/GAPDH, mean ± SD compared to saline treated mice (referred to as 1); D307H, CASQ2D307H/D307H; CASQ, calsequestrin. n = 3 hearts/group, p < 0.05 *.
Table 1.
Arrhythmia prevalence in bortezomib-treated CASQ2 mutant mice.
| Genotype | N (mice) | Sinus rate (beats/min, mean ± SD) | Rest PVC |
Rest VT |
Stress PVC |
Stress VT |
Epi PVC |
Epi VT |
|---|---|---|---|---|---|---|---|---|
| KO Baseline | 4 | 448 ± 3 | 4 | 4 | 4 | 4 | 4 | 4 |
| KO Bortezomib | 4 | 507 ± 31 | 4 | 4 | 4 | 4 | 4 | 4 |
| D307H Baseline | 4 | 495 ± 144 | 4 | 3 | 4 | 4 | 4 | 4 |
| D307H Bortezomib | 4 | 557 ± 51 | 1 | 0* | 4 | 1* | 4 | 4 |
Mutant mice (D307H and KO) were treated with 4 injections of bortezomib [1 μg/g] IV for 11 days. Then, Mice were subjected to telemetric heart rhythm monitoring and provocation testing on day 11, 1 h after the last injection (see Section 2). Results were compared to arrhythmia incidence in the same mice subjected to the same provocation testing prior to bortezomib therapy. A decrease in arrhythmia incidence was observed in D307H mice following bortezomib treatment. However, there was no protection of bortezomib against arrhythmia after epinephrine [0.5 μg/g] stimulation. There was no effect on arrhythmia in the KO group.
Numbers represent number of mice having a given type of arrhythmia. Abbreviations: Epi: epinephrine; PVC: premature ventricular contraction, VT, ventricular tachycardia; D307H, CASQ2D307H/D307H; CASQ2Δ/Δ, KO; wild type, WT. p <0.05 *
Fig. 7.
Premature ventricular beat incidence in D307H mice during peak exercise on baseline and after bortezomib treatment. The ventricular beats (%) represent the proportion of ventricular beats to total beats in a 1 min recording during the maximal effort on treadmill. D307H, CASQ2D307H/D307H. p < 0.05 *; n = 4 mice.
4. Discussion
CPVT2 is caused by homozygous null-allele or point mutations in CASQ2. The current study compared two mouse models of recessively inherited human CPVT: CASQ2 protein knock-out or D307H point mutation. Our results point to a quantitative difference in disease severity between null-allele and missense models which appear to be determined by the level of the residual CASQ2 protein. Homozygous CASQ2 knockout hearts have neither RNA nor protein. D307H CASQ2 hearts have a normal amount of RNA but a very low protein, suggesting protein deficiency as a unifying mechanism of various CASQ2 mutations causing recessive CPVT [10,21]. We demonstrate that despite a severe decrease in the cardiac CASQ2 expression, the presence of residual mutant protein (Figs. 2 and 3), affects the arrhythmic phenotype. We have previously shown a lower severity of arrhythmia and a better response to therapy in D307H compared to KO mice [9]. In contrary to normal CASQ2 expression in D307H mice after birth [12], the expression of mutant protein decreases with age. Adult D307H mice expressed only ~20% of the normal level of CASQ2 protein (Fig. 2). An age-related decrease in D307H CASQ2 protein expression (Fig. 2) correlated with abnormal Ca2+ release. There was no difference between neonatal D307H and WT cells irrespective of the mutation but KO cardiomyocytes (lacking the protein) have more Ca2+ irregularities (Fig. 5). Among adult cardiomyocytes, both mutants differed from the WT, but there was slight difference between D307H and KO. KO had more severe disturbances in Ca2+ release following ISO. Given the normal CASQ2D307H mRNA level in adulthood (10), the decreased protein level should be explained by proteolysis rather than transcriptional alterations. Missense mediated protein decay should be prompted by abnormal structure of a mutant protein. The disappearance of the high-molecular-weight “calsequestrin-like proteins” in the adult D307H mice (Fig. 2D) may indicate a problem of the mutant CASQ2 monomer to polymerize [11,18] suggesting a structural/functional abnormality. Additionally, the mutation may also altered the calcium affinity or altered the interaction with RyR2 [21]. However, Ca2+ uptake experiments to the SR suggest that D307H CASQ2 protein has a residual function. A decrease in Ca2+ uptake to the SR was found only in KO but not in D307H cardiomyocytes (Fig. 4). Ca2+ stores in D307H as in WT could be increased by inhibiting ryanodine channel with dantrolene and depleted with caffeine. Abnormal Ca2+ uptake could be caused by decreased SERCA activity. According to Knollmann et al. [11], increased SR volume but no change in SERCA expression in KO could lead to a decrease in relative SERCA activity. We did not measure SR volume. The fact that most of Ca2+ uptake to SR is preserved in the KO implies that mechanisms unrelated to CASQ2 are involved in SR Ca2+ binding. These mechanisms are very important, allowing CASQ2 KO mice (and patients) to survive and preserve a near-normal cardiac function at rest. These compensations obviously fail during augmented Ca2+ cycling, occurring during exercise or sympathetic stimulation.
We then hypothesized that attenuating mutant protein degradation in D307H CASQ2 mice might help to prevent arrhythmia. Abnormal proteins accruing following inherited or acquired mutation are degraded by an intracellular quality control mechanism, the Ubiquitin–Proteasome System (UPS) [22,23]. The UPS is the major mechanism used by the cell to achieve chaperone-dependent protein degradation. It eliminates and prevents the aggregation of defective proteins which might damage the cell [24]. Because 80–90% of cellular proteins are degraded via UPS, degrading mutant proteins may contribute to cellular damage by delaying the degradation of other damaged proteins which accrue due to oxidative stress and aging [25,26]. UPS abnormalities have been associated with initiation and progression of cardiac diseases [27–29]. Endoplasmic reticulum associated degradation (ERAD), is another process which removes misfolded proteins from the endoplasmic reticulum, polyubiquitinates and then degrades them through UPS [30–32]. Cellular organelles such as SR, mitochondria are poorly developed before birth and their maturation continues from neonatal till adult phase [33,34]. We assume that mutant CASQ2 is not degraded in the neonatal myocytes because the UPS or the ERAD are also immature in this age group. Bortezomib is a highly specific and potent proteasome inhibitor used as an anti-neoplastic agent for plasma cell dyscrasia [17]. In our study, bortezomib attenuated arrhythmia at rest and during exercise stress in D307H mice (Table 1) concomitant with increasing the level of D307H CASQ2 protein (Fig. 6). Bortezomib may be cardiotoxic and even cause arrhythmia [35–37]. In other models bortezomib had antiarrhythmic activity by inhibiting degradation of other proteins [38]. One such candidate is the G protein coupled Receptor Kinase 2 (GRK2) that regulates the β-AR sensitivity [39]. Downregulation of GRK2 has been related to increased sensitivity of β-AR to its agonists in canine border-zone post myocardial infarction, leading to increased vulnerability to ventricular tachycardia [40,41]. Bortezomib also causes peripheral neuropathy [42], which might attenuate CPVT through sympathetic denervation [43]. Nonetheless, the absence of treatment benefit in CASQ2 KO mice suggests that a non-specific antiarrhythmic effect is unlikely. Bortezomib failed to prevent arrhythmia following epinephrine injection. This may be attributed to insufficient increase in mutant CASQ2 expression compared to the normal level.
Collectively, our results prove the concept that increasing mutant CASQ2 expression might serve as a complementary therapy in CPVT in appropriate patients. Novel agents to inhibit the endoplasmic reticulum associated degradation (ERAD) may further increase the efficacy of this approach.
Acknowledgments
Funding sources
This work was funded by Israel Science Foundation grants 876/2005 and 763/10.
We are grateful for the technical assistance by Elena Chepurko DVM and for editorial help by Ms. Elaine Finkelstein.
Footnotes
Disclosures
None.
References
- 1.Katz G, Arad M, Eldar M. Catecholaminergic polymorphic ventricular tachycardia from bedside to bench and beyond. Curr Probl Cardiol. 2009;34:9–43. doi: 10.1016/j.cpcardiol.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–83. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 4.Eldar M, Pras E, Lahat H. A missense mutation in the CASQ2 gene is associated with autosomal-recessive catecholamine-induced polymorphic ventricular tachycardia. Trends Cardiovascul Med. 2003;13:148–51. doi: 10.1016/s1050-1738(03)00025-2. [DOI] [PubMed] [Google Scholar]
- 5.Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–84. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 7.Lahat H, Eldar M, Levy-Nissenbaum E, Bahan T, Friedman E, Khoury A, et al. Autosomal recessive catecholamine- or exercise-induced polymorphic ventricular tachycardia: clinical features and assignment of the disease gene to chromosome 1p 13–21. Circulation. 2001;103:2822–7. doi: 10.1161/01.cir.103.23.2822. [DOI] [PubMed] [Google Scholar]
- 8.Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, et al. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:21–6. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- 9.Katz G, Khoury A, Kurtzwald E, Hochhauser E, Porat E, Shainberg A, et al. Optimizing catecholaminergic polymorphic ventricular tachycardia therapy in calsequestrin-mutant mice. Heart Rhythms. 2010;7:1676–82. doi: 10.1016/j.hrthm.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, et al. Increased calreticulin and ryanodine receptors by calsequestrin-2 (CASQ2) mutations cause catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2007;117:1814–23. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–20. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langdown ML, Holness MJ, Sugden MC. Effects of prenatal glucocorticoid exposure on cardiac calreticulin and calsequestrin protein expression during early development and in adulthood. Biochem J. 2003;371:61–9. doi: 10.1042/BJ20021771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shneyvays V, Mamedova L, Zinman T, Jacobson K, Shainberg A. Activation of A(3)adenosine receptor protects against doxorubicin-induced cardiotoxicity. J Mol Cell Cardiol. 2001;33:1249–61. doi: 10.1006/jmcc.2001.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halili-Rutman I, Hershko C, Link G, Rutman AJ, Shainberg A. Inhibition of calcium accumulation by the sarcoplasmic reticulum: a putative mechanism for the cardiotoxicity of adriamycin. Biochem Pharmacol. 1997;54:211–4. doi: 10.1016/s0006-2952(97)00108-1. [DOI] [PubMed] [Google Scholar]
- 15.Fixler D, Tirosh R, Zinman T, Shainberg A, Deutsch M. Differential aspects in ratio measurements of [Ca2+]i relaxation in cardiomyocyte contraction following various drug treatments. Cell Calcium. 2002;31:279–87. doi: 10.1016/s0143-4160(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Ahsan MH, Zhu L, Sambucetti LC, Purchio AF, West DB. NF-kappaB and not the MAPK signaling pathway regulates GADD45beta expression during acute inflammation. J Biol Chem. 2005;280:21400–08. doi: 10.1074/jbc.M411952200. [DOI] [PubMed] [Google Scholar]
- 17.van Hees HW, Li YP, Ottenheijm CA, Jin B, Pigmans CJ, Linkels M, et al. Proteasome inhibition improves diaphragm function in congestive heart failure rats. Am J Physiol Lung Cell Mol Physiol. 2008;294:260–1268. doi: 10.1152/ajplung.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cala SE, Scott BT, Jones LR. Intralumenal sarcoplasmic reticulum Ca(2+)-binding proteins. Semin Cell Biol. 1990;1:265–75. [PubMed] [Google Scholar]
- 19.Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004;85:33–69. doi: 10.1016/j.pbiomolbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Volpe P, Martini A, Furlan S, Meldolesi J. Calsequestrin is a component of smooth muscles: the skeletal- and cardiac-muscle isoforms are both present, although in highly variable amounts and ratios. Biochem J. 1994;301:465–9. doi: 10.1042/bj3010465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, et al. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008;103:298–306. doi: 10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- 22.Kelly SM, Vanslyke JK, Musil LS. Regulation of ubiquitin–proteasome system mediated degradation by cytosolic stress. Mol Biol Cell. 2007;18:4279–91. doi: 10.1091/mbc.E07-05-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutations Wetzel R. Off-pathway aggregation of proteins. Trends Biotechnol. 1994;12:193–8. doi: 10.1016/0167-7799(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 24.Grune T, Merker K, Sandig G, Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun. 2003;305:709–18. doi: 10.1016/s0006-291x(03)00809-x. [DOI] [PubMed] [Google Scholar]
- 25.Sarikas A, Carrier L, Schenke C, Doll D, Flavigny J, Lindenberg KS, et al. Impairment of the ubiquitin–proteasome system by truncated cardiac myosin binding protein C mutants. Cardiovasc Res. 2005;66:33–44. doi: 10.1016/j.cardiores.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–30. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 27.Powell SR. The ubiquitin–proteasome system in cardiac physiology and pathology. Am J Physiol Heart Circ Physiol. 2006;291:H1–9. doi: 10.1152/ajpheart.00062.2006. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Su H, Ranek MJ. Protein quality control and degradation in cardiomyocytes. J Mol Cell Cardiol. 2008;45:11–27. doi: 10.1016/j.yjmcc.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson C, Ike C, Willis PWt, Stouffer GA, Willis MS. The bitter end: the ubiquitin–proteasome system and cardiac dysfunction. Circulation. 2007;115:1456–63. doi: 10.1161/CIRCULATIONAHA.106.649863. [DOI] [PubMed] [Google Scholar]
- 30.Fu HY, Minamino T, Tsukamoto O, Sawada T, Asai M, Kato H, et al. Over-expression of endoplasmic reticulum-resident chaperone attenuates cardiomyocyte death induced by proteasome inhibition. Cardiovasc Res. 2008;79:600–10. doi: 10.1093/cvr/cvn128. [DOI] [PubMed] [Google Scholar]
- 31.Powell SR. The cardiac 26S proteasome: regulating the regulator. Circ Res. 2006;99:342–5. doi: 10.1161/01.RES.0000239412.40685.61. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch C, Gauss R, Horn SC, Neuber O, Sommer T. The ubiquitylation machinery of the endoplasmic reticulum. Nature. 2009;458:453–60. doi: 10.1038/nature07962. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Yasui K, Opthof T, Ishiki R, Lee JK, Kamiya K, et al. Developmental changes of Ca2+ handling in mouse ventricular cells from early embryo to adulthood. Life Sci. 2002;71:1279–92. doi: 10.1016/s0024-3205(02)01826-x. [DOI] [PubMed] [Google Scholar]
- 34.Piquereau J, Novotova M, Fortin D, Garnier A, Ventura-Clapier R, Veksler V, et al. Postnatal development of mouse heart: formation of energetic microdomains. J Physiol. 2010;588:2443–54. doi: 10.1113/jphysiol.2010.189670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voortman J, Giaccone G. Severe reversible cardiac failure after bortezomib treatment combined with chemotherapy in a non-small cell lung cancer patient: a case report. BMC Cancer. 2006;6:129. doi: 10.1186/1471-2407-6-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enrico O, Gabriele B, Nadia C, Sara G, Daniele V, Giulia C, et al. Unexpected cardiotoxicity in haematological bortezomib treated patients. Br J Haematol. 2007;138:396–7. doi: 10.1111/j.1365-2141.2007.06659.x. [DOI] [PubMed] [Google Scholar]
- 37.Hacihanefioglu A, Tarkun P, Gonullu E. Acute severe cardiac failure in a myeloma patient due to proteasome inhibitor bortezomib. Int J Hematol. 2008;88:219–22. doi: 10.1007/s12185-008-0139-7. [DOI] [PubMed] [Google Scholar]
- 38.Yu X, Huang S, Patterson E, Garrett MW, Kaufman KM, Metcalf JP, et al. Proteasome degradation of GRK2 during ischemia and ventricular tachyarrhythmias in a canine model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;289:1960–7. doi: 10.1152/ajpheart.00328.2005. [DOI] [PubMed] [Google Scholar]
- 39.Bunemann M, Hosey MM. G-protein coupled receptor kinases as modulators of G-protein signalling. J Physiol. 1999;517:5–23. doi: 10.1111/j.1469-7793.1999.0005z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu X, Patterson E, Huang S, Garrett MW, Kem DC. Tumor necrosis factor alpha, rapid ventricular tachyarrhythmias, and infarct size in canine models of myocardial infarction. J Cardiovasc Pharmacol. 2005;45:153–9. doi: 10.1097/01.fjc.0000151930.12026.b7. [DOI] [PubMed] [Google Scholar]
- 41.Yu X, Zhang M, Kyker K, Patterson E, Benovic JL, Kem DC. Ischemic inactivation of G protein-coupled receptor kinase and altered desensitization of canine cardiac beta-adrenergic receptors. Circulation. 2000;102:2535–40. doi: 10.1161/01.cir.102.20.2535. [DOI] [PubMed] [Google Scholar]
- 42.Utecht KN, Kolesar J. Bortezomib: a novel chemotherapeutic agent for hematologic malignancies. Am J Health Syst Pharm. 2008;65:1221–31. doi: 10.2146/ajhp070272. [DOI] [PubMed] [Google Scholar]
- 43.Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–9. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]