Abstract
Among the fundamental chemical transformations in organic synthesis, the [3,3]-sigmatropic rearrangement occupies a unique position as a powerful, reliable, and well-defined method for the stereoselective construction of carbon–carbon or carbon–heteroatom bonds. While many other reactions can unite two subunits and create a new bond, the strengths of sigmatropic rearrangements derive from their ability to enable structural reorganization with unmatched build-up of complexity. Recent applications that illustrate [3,3]-sigmatropic processes as a key concept in the synthesis of complex natural products are described in this tutorial review, covering literature from about 2001 through early 2009.
Introduction
In recent years, there have been numerous applications of [3,3]-sigmatropic rearrangements in increasingly complex settings, including the incorporation of the parent transformation into creatively designed tandem reaction sequences. Selected applications of such processes in the total synthesis of natural products are collected in this review, with a focus on completed total synthesis endeavors. One of the major lessons from this overview is that a diverse range of intricate structures can be prepared using sigmatropic rearrangements as a guiding concept in synthesis design, and these transformations are especially advantageous for the construction of congested stereocenters.
Not surprisingly, the Cope and Claisen variants are prevalent, but the use of other hetero-Cope rearrangements has also become more prominent. Based on the type of application, this article is divided into parts that include (1) a variety of transformations integrating [3,3]-sigmatropic rearrangements within one-pot cascade or tandem processes, (2) Cope rearrangements, (3) Claisen rearrangements, and (4) other hetero-Cope rearrangements.
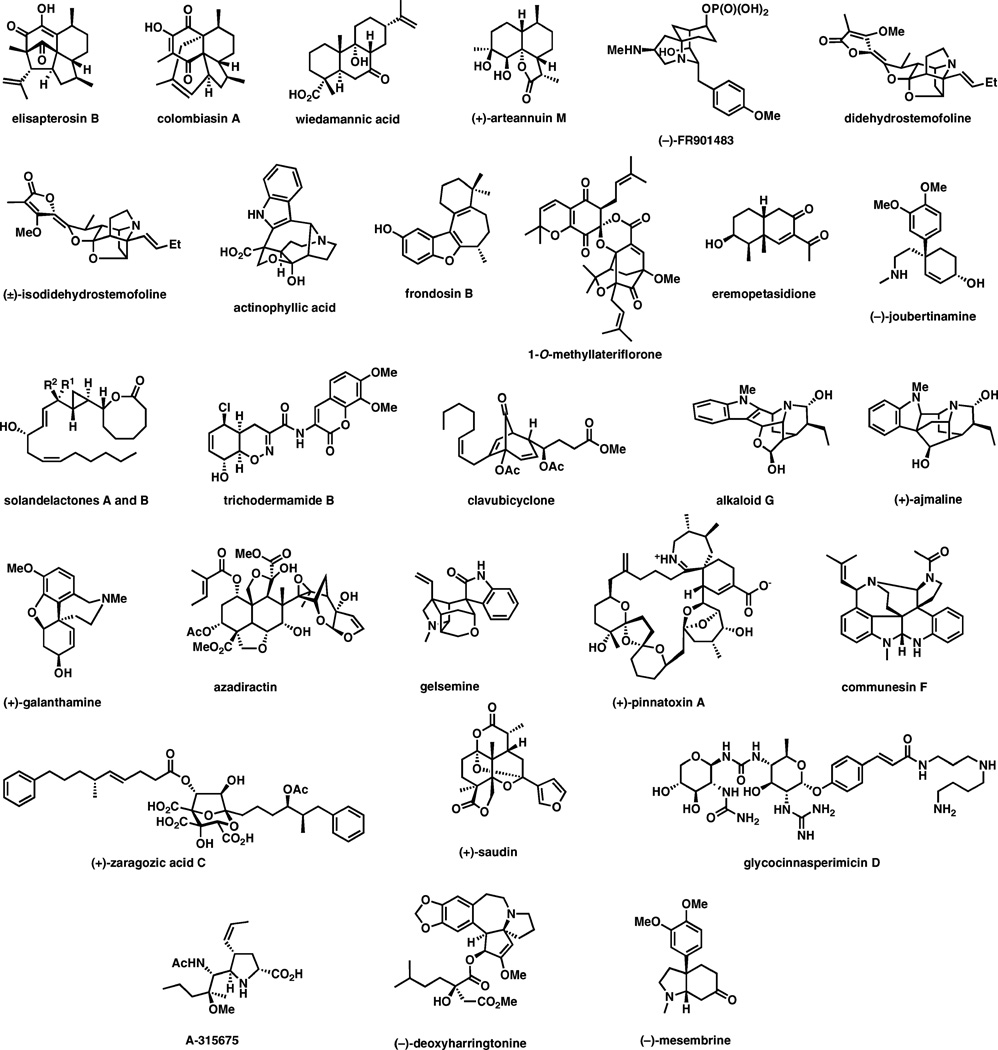
As evident from a summary of the natural products covered in this review (Fig. 1), the structural diversity of complex compounds prepared by strategies that incorporate sigmatropic rearrangements as a key step is truly astonishing. These include polyketides, terpenes, alkaloids, carbohydrates, and other types of molecules with a recurrent synthetic challenge: congested stereocenters.
Fig. 1.
A summary of natural products discussed in this review.
[3,3]-Sigmatropic rearrangements integrated into cascade/tandem processes
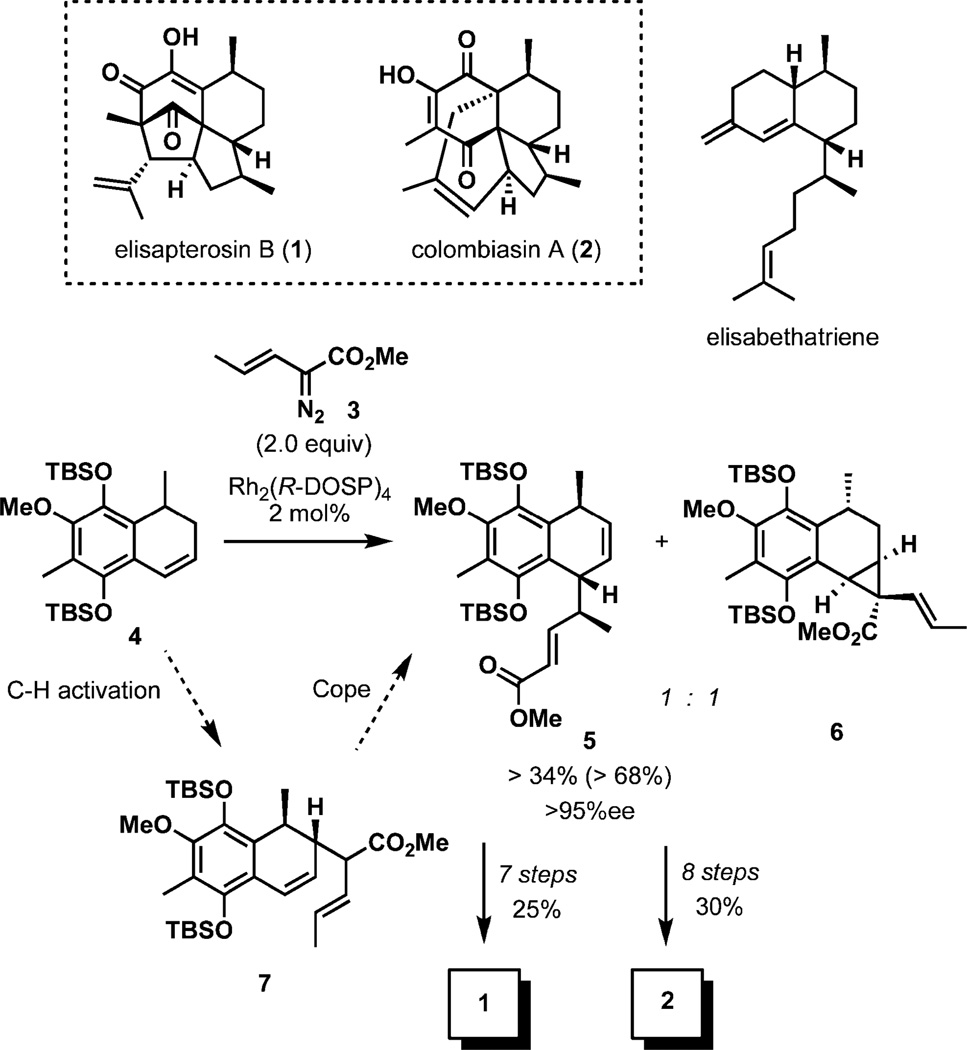
(−)-Colombiasin A and (−)-elisapterosin B
Gorgonian corals are the source of a family of polycyclic diterpines sharing a common biosynthetic origin from (+)-elisabethatriene.1 These natural products possess a broad spectrum of bioactivity, and present a significant challenge for synthesis due to distinctively arranged ring systems and stereocenters.
An elegant strategy that combines C–H activation and the Cope rearrangement has been recently implemented in the efficient total synthesis of two complex members of this family, (−)-elisapterosin B (1) and (−)-colombiasin A (2) (Scheme 1).2,3 During an impressive enantiodivergent reaction with vinyldiazoacetate 3, racemic dihydronaphthalene 4 is converted to two different enantioenriched products, one via C–H activation–Cope rearrangement (5) and the other by cyclopropanation (6). The initial C–H-insertion of 3 catalyzed by Rh2(R-DOSP)4 is the enantiodetermining step, furnishing intermediate 7, which undergoes a facile [3,3]-rearrangement at ambient temperature within 1.5 h to ester 5.
Scheme 1.
C-H activation-Cope rearrangement strategy in the synthesis of (−)-colombiasin A and (−)-elisapterosin B.
Ester 5, which is formed in high enantiopurity (> 95% ee), is a common substrate in the unified synthesis of 1 and 2. The synthesis of elisapterosin B (1) was completed in seven steps from 5, while for colombiasin (2) the completion of the synthesis required eight steps, underscoring the remarkable brevity of the approach.
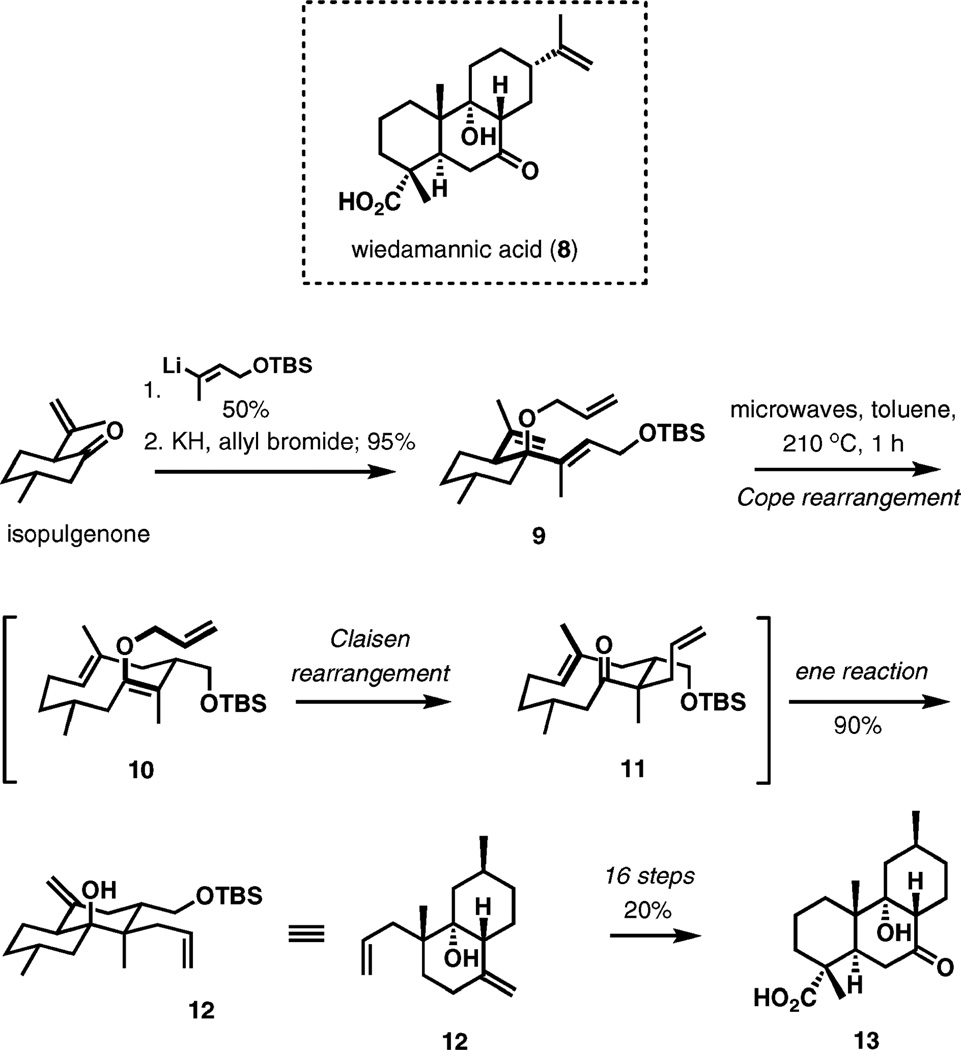
Wiedamannic acid and (+)-arteannuin M
A tandem oxy-Cope–Claisen–ene reaction is the keystone of a strategy for the syntheses of a number of diterpenes, including a plant natural product of the abietane family wiedamannic acid (8).4 In this illustrative case (Scheme 2), the substrate (9) is readily accessed in two steps from isopulegone.5 Upon microwave irradiation at 210 °C for 1 h, compound 9 undergoes structural reorganization to 12 in 90% yield. It is likely that the first step of the process, the oxy-Cope rearrangement (9 → 10), is the rate-determining step. The second [3,3]-shift (10 → 11) and the transannular ene reaction (11 → 12) take place under the same reaction conditions to complete the sequence. The effectiveness of chirality transfer is defined by macrocyclic conformational preferences of 10-membered cyclic intermediates such as 10 and 11, that is, during the Claisen rearrangement and the ene cyclization. It is understood that only one reasonable reactive conformation is available for the first [3,3]-sigmatropic transposition (as shown for 9 in Scheme 2). On the other hand, a careful analysis of the rearrangement with different substrates indicated that at least in some cases the ring inversion in the intermediate 10-membered rings is slow compared to the rate of the Claisen rearrangement, thereby making the oxy-Cope rearrangement a critical stereodetermining step as it defines the initial conformation of the macrocycles.
Scheme 2.
Tandem oxy-Cope–Claisen–ene reorganization of 10 in an approach to wiedamannic acid.
The rearrangement product (12) was then advanced to wiedamannic acid analog 13 in 16 efficient steps and 20% overall yield.
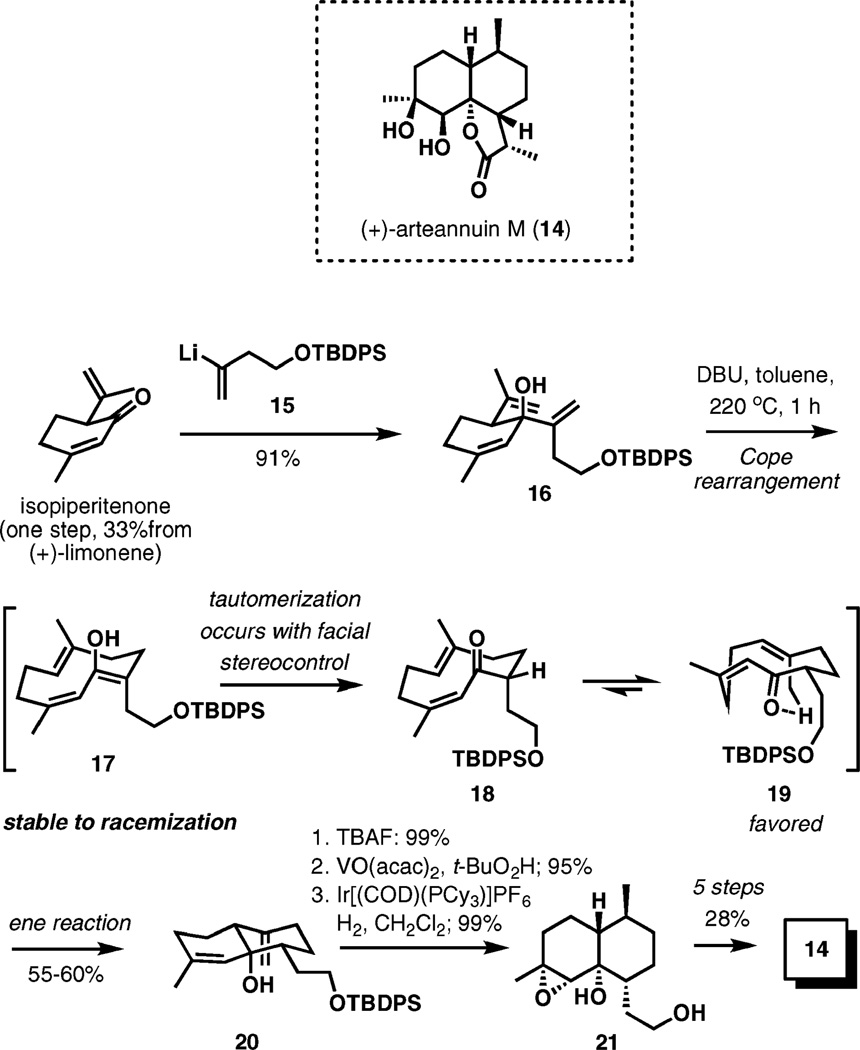
A similar tandem reaction sequence was used in a total synthesis of (+)-arteannuin M (14), an enantiomer of a sesquiterpene from Artemisia annua L. related to artemisin.6 Isopiperitenone obtained from (+)-limonene served as the starting material. Addition of organolithium reagent 15 provided the starting material to the key tandem oxy-Cope–ene reaction delivering decalin 20 in good yield (Scheme 3).
Scheme 3.
Tandem oxy-Cope–ene reaction in the total synthesis of (+)-arteannuin M.
The initial oxy-Cope rearrangement produces chiral 10-membered cyclic enol 17 that possesses no chiral centers. Remarkably, 17 appears to maintain its stereochemical integrity well under the reaction conditions because 20 is formed as an 89 : 11 mixture of enantiomers, as determined by Mosher ester analysis. The subsequent tautomerization presumably delivers a proton from the periphery of the macrocycle giving ketone 18, which, in contrast to 17, undergoes a conformational equilibration placing the silyloxyethyl substituent into a preferred pseudoequatorial position. The product then arises from the ene reaction of conformer 19.
After desilylation, directed epoxidation, and directed hydrogenation furnishing 21, the synthesis was completed in five steps and in a 28% overall yield.
(−)-FR901483
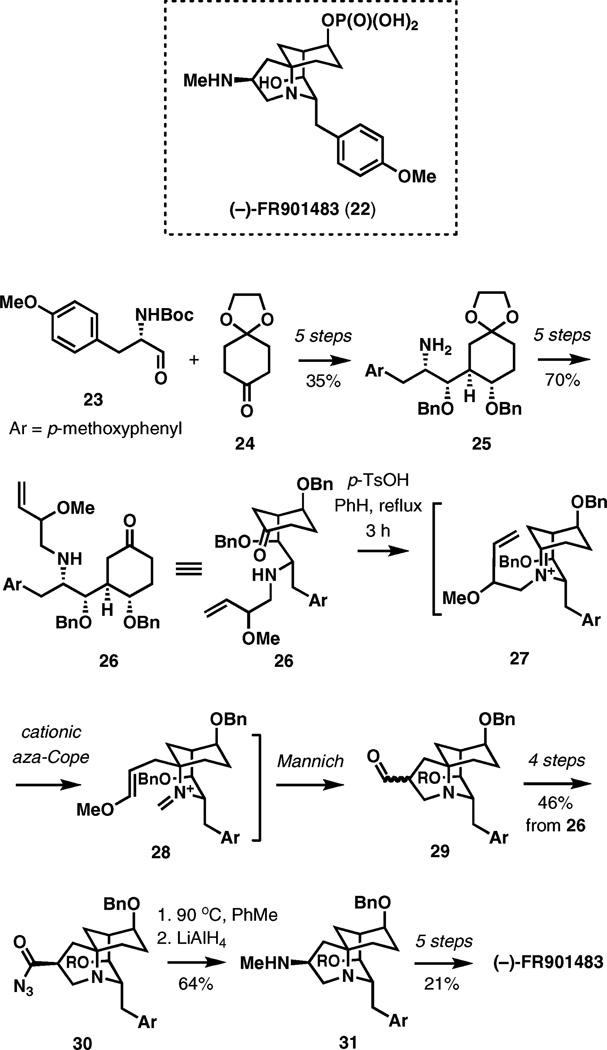
FR901483 (22) is an alkaloid discovered by researchers at Fujisawa Pharmaceutical Company Ltd as a part of a program to identify new immunosuppresant compounds that act by a different mechanism from cyclosporin A or FK506. Its recent formal total synthesis capitalizes on a tandem cationic aza-Cope rearrangement–Mannich cyclization strategy to assemble the tricyclic structure.7
As illustrated in Scheme 4, aldehyde 23 and ketone 24 were combined by an aldol reaction and advanced in four additional steps to amine 25. Another five steps were required for the attachment of the 2-methoxy-3-buten-1-yl substituent to the nitrogen atom and the unmasking the carbonyl group in 26, which served as the substrate for the key cascade transformation. In the event, exposure of amino ketone 26 to p-toluenesulfonic acid in benzene resulted in the formation of the bridgehead iminium ion 27, a [3,3]-sigmatropic shift, and Mannich cyclization of the new intermediate iminium ion 28. Aldehydes 29 are formed as a 2 : 1 mixture of diastereomers favoring the undesired endo-product, requiring subsequent equilibration.
Scheme 4.
Formal synthesis of (−)-FR901483 by aza-Cope–Mannich cyclization.
The formal synthesis is completed in six steps from 29 including the Curtius rearrangement of acyl azide 30 to install the remaining methylamino group, intercepting an advanced intermediate previously converted to the natural product by Sorensen and co-workers in two steps.8
(±)-Didehydrostemofoline and (±)-isodidehydrostemofoline
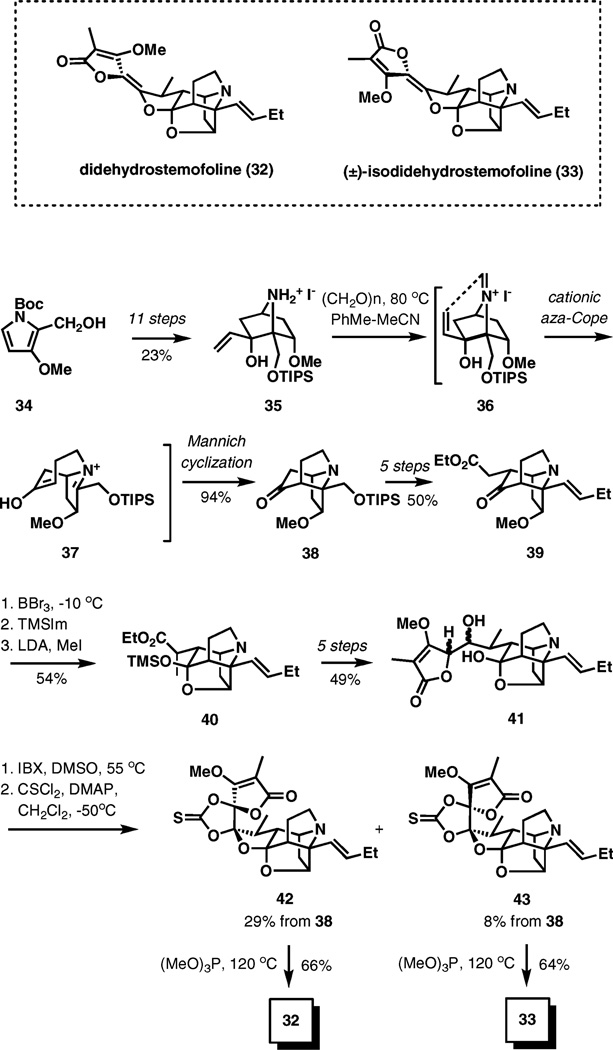
An aza-Cope–Mannich rearrangement strategy has also formed a foundation for the synthesis of (±)-didehydrostemofoline (asparagamine A, 32) and (±)-isodidehydrostemofoline (33) (Scheme 5).9 These hexacyclic alkaloids are found in plants of Stemona species which have been used in traditional Chinese, Japanese, and Thai medicine to treat respiratory disease, parasitic infestation, and as insecticides.
Scheme 5.
Aza-Cope–Mannich strategy in the synthesis of (±)-dide-hydrostemofoline and (±)-isodidehydrostemofoline.
The tandem reaction sequence has been applied relatively early in the synthesis for the construction of the characteristic 1-azatricyclo[5.3.0.04.10]decane nucleus of the natural products. Using a rare Diels–Alder reaction, pyrrole 34 was advanced to 7-azabicyclo[2.2.1]heptanol 35 by an 11-step sequence in a 23% overall yield.
One of the questions in this work was whether there is sufficient orbital overlap in iminium ion 36 that would allow the [3,3]-sigmatropic rearrangement. Under the indicated reaction conditions ((CH2O)n, PhMe–MeCN, 80 °C), ammonium salt 35 underwent a smooth aza-Cope–Mannich conversion to azatricyclodecanone 38 in 94% yield, thus validating the strategy. The requisite alkene and ethyl acetate side-chains were introduced in five steps, followed by the methyl ether cleavage, silyl ketal formation, and methylation of the ethyl ester at the α-position, delivering 40.
Attachment of the butenolide unit required an additional five transformations, followed by a formal C–H oxidation of 41 with IBX and the formation of thiocarbonates 42 and 43. The final double bond was established in a Corey–Winter process by thermolysis of the thiocarbonates in the presence of trimethyl phosphite at 120 °C, which delivered the natural products in good yields, completing the total synthesis endeavor.
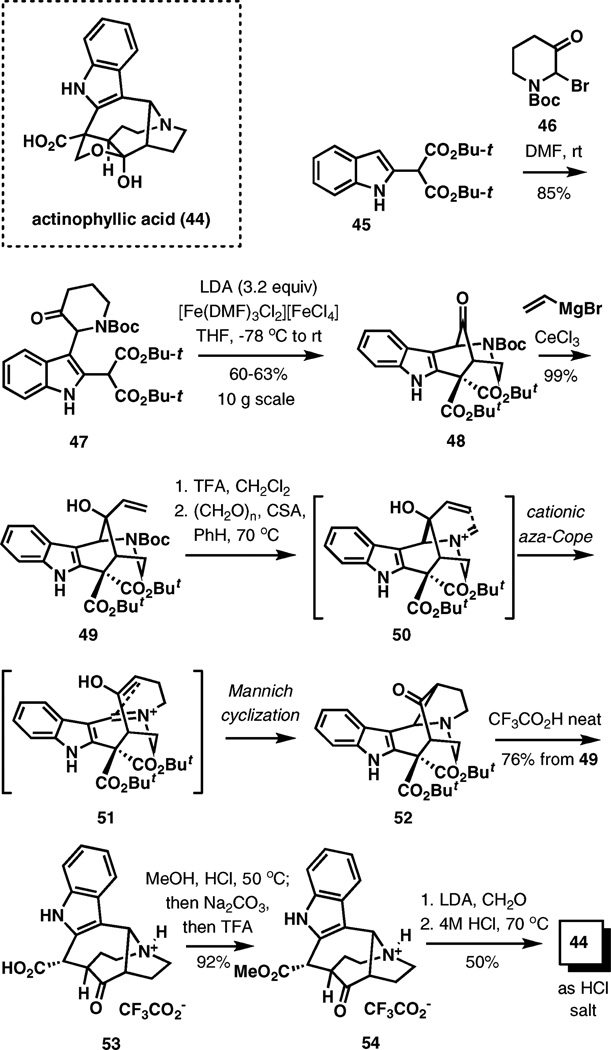
(±)-Actinophyllic acid
The third complex natural product discussed in this review that has been prepared by aza-Cope–Mannich rearrangement is actinophyllic acid (44, Scheme 6).10 Actinophyllic acid is a unique bioactive indole alkaloid extracted from the leaves of the tree Alstonia actinophylla collected on the Cape York Peninsula, Far North Queensland, Australia.
Scheme 6.
Aza-Cope–Mannich cascade in the synthesis of actinophyllic acid.
This concise synthesis begins with indole 45 prepared in one step from (o-nitrophenyl)acetyl chloride. Alkylation with bromide 46 gives keto malonate 47, which is lithiated with three equivalents of LDA, and the trianion is subjected to oxidative cyclization with an iron(III) complex. This scalable transformation provides 48 in good yields, and, after highly diastereoselective addition of vinylmagnesium bromide controlled by the bulky geminal tert-butyl esters, the setup for the aza-Cope–Mannich cascade is complete. Removal of the protecting group from the nitrogen atom in 49 and exposure of the product to paraform in the presence of camphorsulfonic acid in benzene resulted in a smooth transformation leading to amino ketone 52, which, after protonolysis of the tert-butyl esters, provided trifluoroacetate 53 in a 76% overall yield. Subsequently, the total synthesis was completed in three steps involving esterification, condensation of the lithium enolate generated from the resulting methyl ester 54 with formadehyde, and finally acidic hydrolysis.
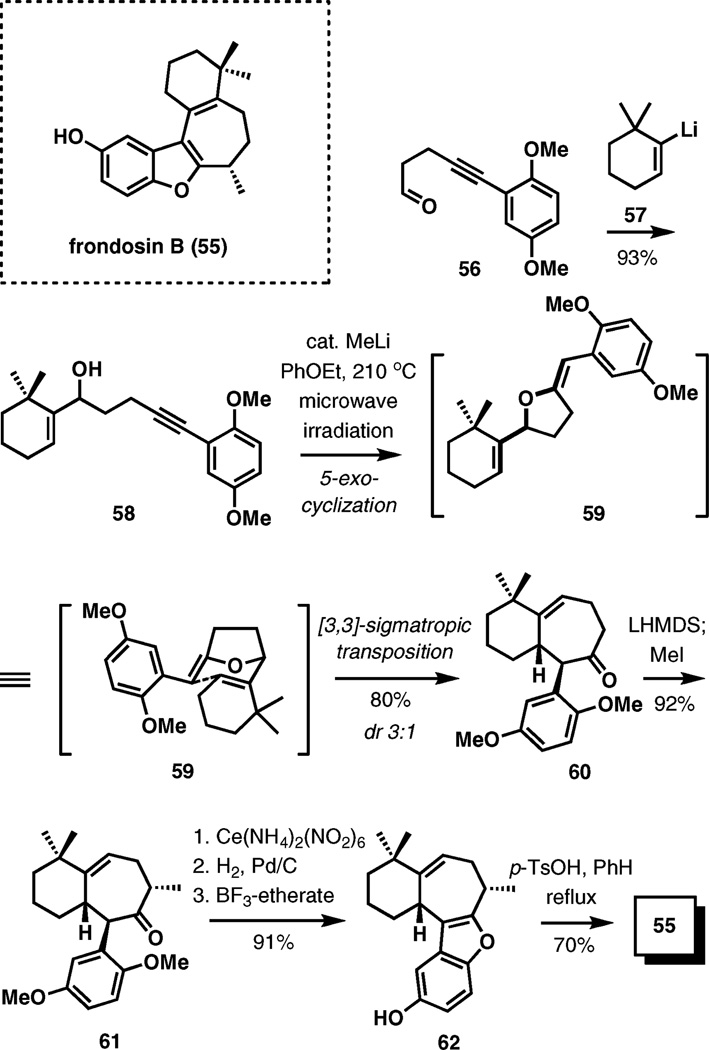
(±)-Frondosin B
A tandem anionic 5-exo-cyclization followed by Claisen rearrangement forms the basis of a strategy for the synthesis of frondosin B (Scheme 7).11 Frondosins are bioactive sesquiterpene hydroquinones isolated from the Micronesian marine sponge Dysidea frondosa. Their biological activity as antagonists of interleukin-8 holds the potential as a new platform for anticancer and antiviral therapy.
Scheme 7.
Frondosin B: anionic 5-exo-cyclization–Claisen rearrangement.
A direct transformation of alkyne 58 (prepared from aldehyde 56 in 93% yield) to tricycle 60 is the key event in a recent 8-step synthesis of (±)-frondosin B. Deprotonation of the hydroxyl group in 58 induces a 5-exo intramolecular addition to the triple bond, forming vinyl tetrahydrofuran intermediate 59, which is transformed to the product by the Claisen rearrangement occurring under the reaction conditions in 80% yield. A 3 : 1 mixture of diastereomers is formed, which is further enriched to 7 : 1 under basic conditions (NaOMe) in refluxing methanol. The minor diastereomer probably arises from a competing boat-like transition state in the [3,3]-sigmatropic rearrangement.
A highly stereoselective methylation of the kinetic lithium enolate produced from 60 afforded ketone 61. Oxidative– reductive demethylation of the hydroquinone fragment followed by boron trifluoride-etherate mediated benzofuran formation gave 62, and subsequent double bond migration under acidic conditions completes this concise synthesis.
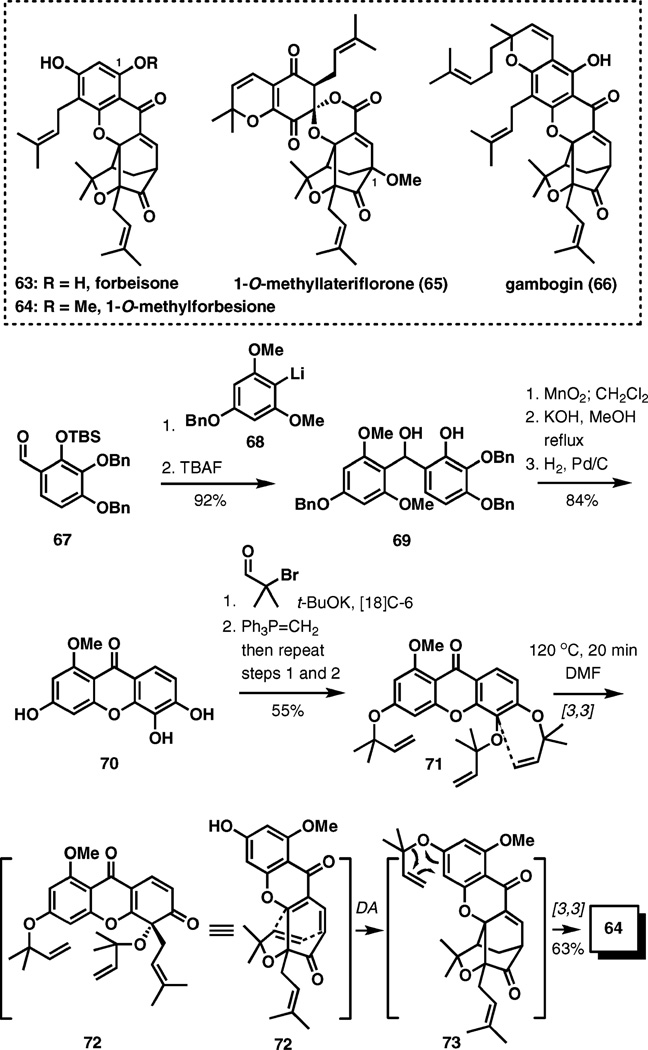
(±)-1-O-Methylforbesione, (±)-1-O-methyllateriflorone, and (±)-gambogin
The Guttiferae family of plants is a rich source of unusual polycyclic compounds that are potentially produced in nature according to an elegant biosynthetic hypothesis that involves successive Claisen rearrangement and intramolecular Diels–Alder reactions.12 This biosynthesis proposal inspired a general strategy for the synthesis of several related natural products (Scheme 8).
Scheme 8.
Synthesis of 1-O-methylforbesione.
Forbeisone (63) originates from the species Garcinia forbesii and possesses a characteristic 4-oxatricyclo[4.3.1.0]-decan-2-one ring system found in several other natural products within this group, some of which display cytotoxic properties. The recently reported synthesis of its 1-O-methylated analog (64) commenced with the addition of aryllithium reagent 68 to aldehyde 67.13 Removal of the silyl protecting group afforded 69 in a 92% overall yield. Oxidation of the benzylic hydroxyl group with manganese(IV) oxide, xanthone formation in basic methanol, and debenzylation with hydrogen in the presence of Pd/C led to 70 in an 84% overall yield. The requisite triple O-α,α-dimethylallylation of 70 was accomplished by repeated treatment with potassium tert-butoxide, α-bromo-isobutyraldehyde, and methylenetriphenylphosphorane furnishing xanthone 71 in a 55% overall yield.
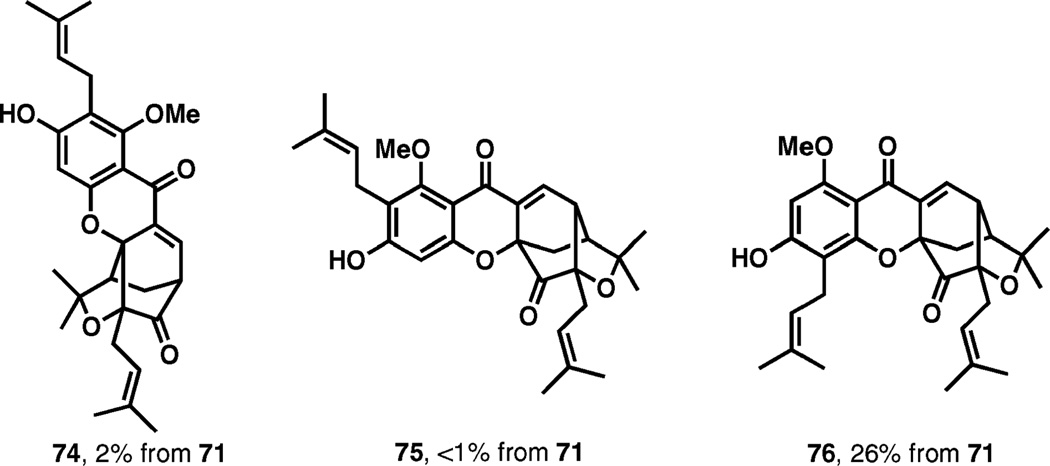
The pivotal cascade pericyclic reaction sequence started with the first [3,3]-sigmatropic rearrangement involving the α,α-dimethylallyl substituent at OH-6. Resulting quinone 72 is disposed for a Diels–Alder cyclization under the indicated reaction conditions (120 °C, DMF), and the final [3,3]-sigmatropic rearrangement delivers the product in 63% yield. The overall transformation is complete after only 20 min at 120 °C. Three other isomeric products, 74, 75, and 76 (Fig. 2), were also isolated in 2%, less than 1%, and 26% yields, respectively.
Fig. 2.
By-products in the isomerization of 71.
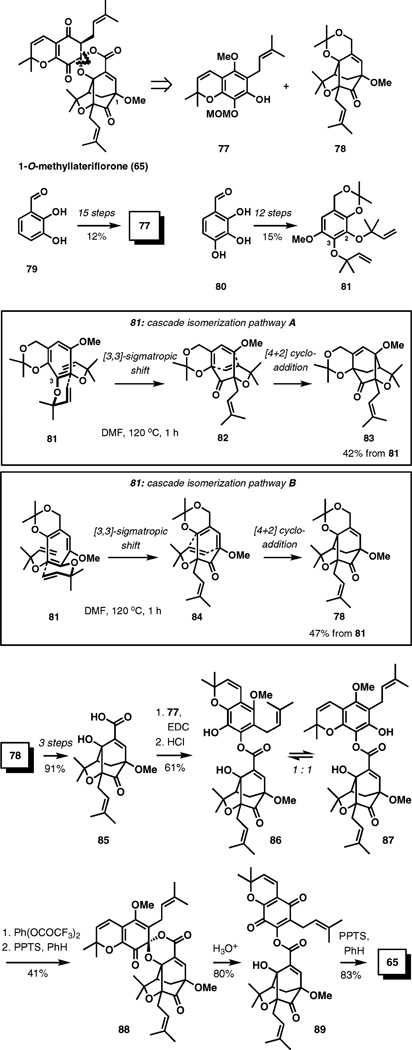
The spiroxalactone fragment in 1-O-methyllateriflorone (65) enhances the synthetic challenges presented by this class of natural products. Its unexpected stability is attributed in part to the poor orbital alignment prohibiting β-elimination of the spiroxo-acyl substituent. Focusing on this fragment, the synthesis of 65 reported in 2003 relies on a convergent disconnection across the spirolactone as shown in Scheme 9.14 Building blocks 77 and 78 are identified through this analysis. Coumarin derivative 77 is prepared by a 15-step process from 2,3-dihydroxybenzaldehyde (79) in a 12% overall yield. Pentasubstituted benzene 81, which served as the substrate for the central pericyclic isomerization event, was prepared from 2,3,4-trihydroxybenzaldehyde (80) in 12 steps and 15% overall yield.
Scheme 9.
Total synthesis of (±)-1-O-methyllateriflorone (65).
Upon heating a solution of 81 in DMF at 120 °C for 1 h, two products were cleanly produced arising from two competing pathways, both of which start with a [3,3]-sigmatropic rearrangement and terminate with a [4+2] cycloaddition. Pathway A (Scheme 9) engages the α,α-dimethylallyl substituent at OH-3 in 81 in the initial sigmatropic rearrangement, while in pathway B the substituent at OH-2 is reactive. The energy profile of both pathways must be highly similar because the two products are formed in comparable yields, with only a slight preference for the desired product (78).
The synthesis is completed by an initial three-step deprotection and oxidation of 78 to acid 85, followed by esterification (EDC, phenol 77) and removal of the MOM group under acidic conditions, which produced a rapidly equilibrating mixture of aryl esters 86 and 87. The mixture could be oxidized to spirolactone 88 following the treatment with pyridinium tosylate in refluxing benzene. Hydrolysis of the methyl enol ether in 88 resulted in lactone opening, forming ester 89, which upon exposure to pyridinium tosylate in refluxing benzene underwent spirolactonization to the final product 65. Remarkably, spirolactone 65 formed with high stereocontrol at the stereogenic spirocenter, suggesting high thermodynamic preference for the configuration observed in the natural product.
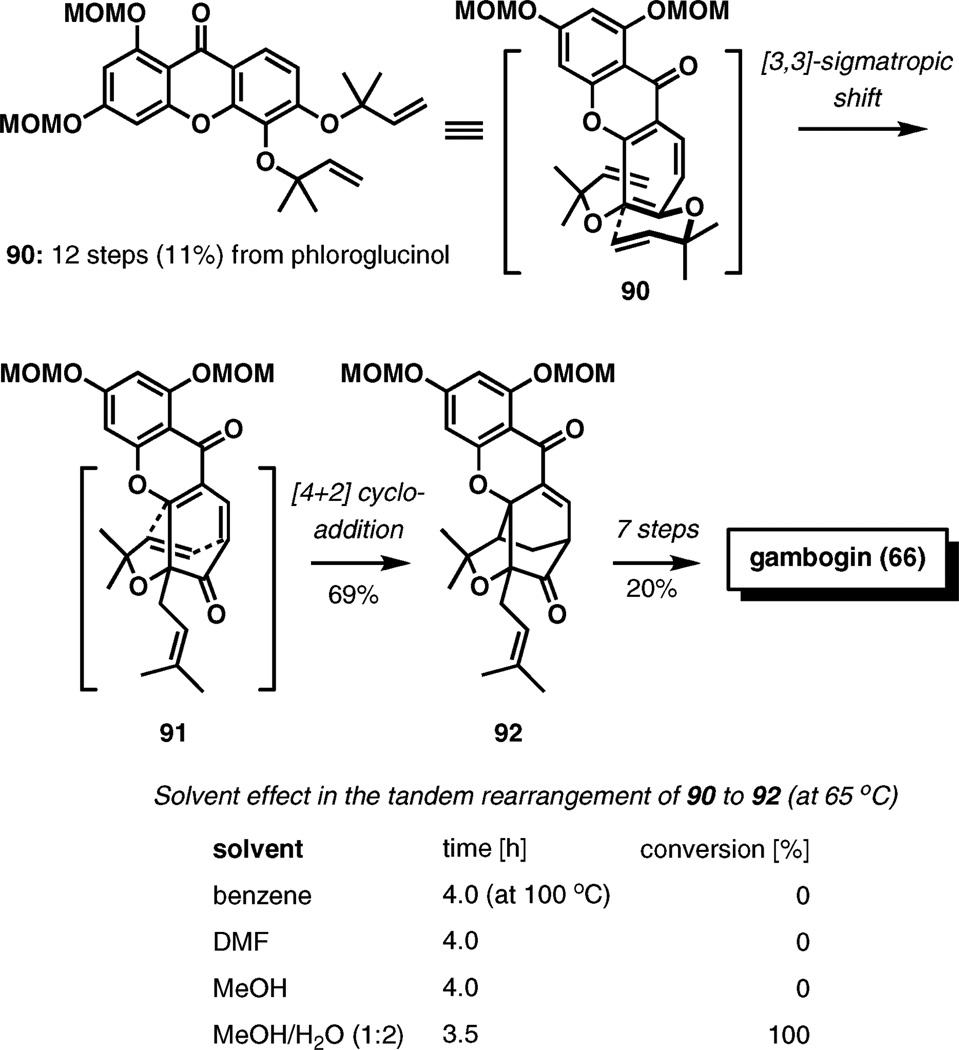
Gambogin (66) has been prepared by a similar tandem Claisen rearrangement–Diels–Alder cycloaddition process (Scheme 10).15 An attractive property of gambodin is its cytotoxicity against Hela and HEL cell lines with MIC of 6.25 and 3.13 mg mL−1, respectively. One of the most intriguing observations in the course of the synthesis is a significant acceleration of the key tandem reaction in water. The starting material, xanthone 90, was prepared from 1,3,5-trihydroxybenzene (phloroglucinol) in 12 steps and 11% overall yield.
Scheme 10.
Total synthesis of (±)-gambogin.
After initial encouraging results from the cascade rearrangement of 90 to 92 in DMF at 100 °C that furnished the desired product in 69% yield along with an isomer in 23% yield, the effect of the solvent on the rate of the reaction was investigated. As can be seen from the table in Scheme 10, no reaction was observed at a lower temperature (65 °C) unless water is a part of the solvent mixture. In methanol–water (1 : 2), the reaction is complete within 3.5 h at 65 °C, reflecting a dramatic rate enhancement in the presence of water. The ratio of the two previously observed isomeric products remained unaffected.
The synthesis was completed in seven additional steps in a 20% overall yield from 92.
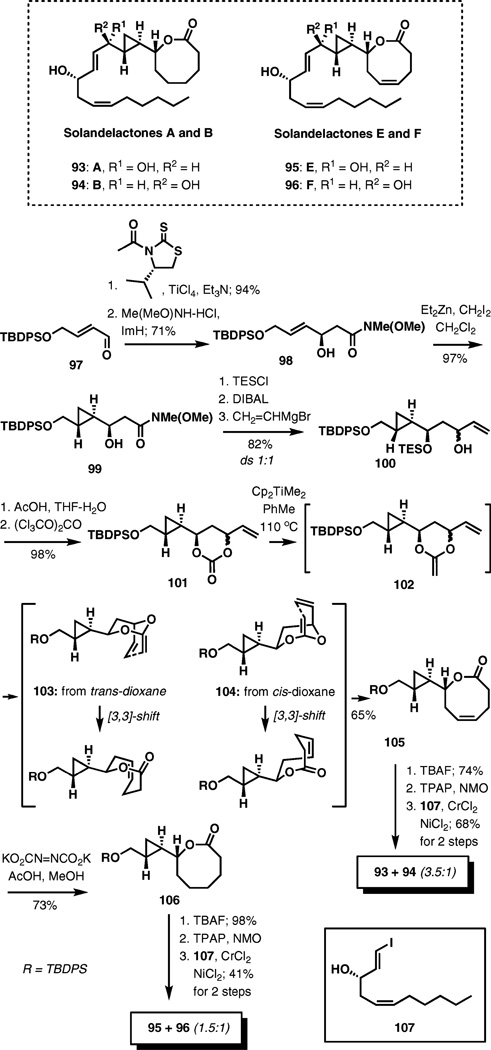
Solandelactones A, B, E, and F
Conversion of an ester carbonyl group into a methylene fragment is an attractive approach to incorporating Claisen rearrangements into tandem reaction sequences for a rapid build-up of complexity. The recently described synthesis of solandelactones is an illustration of this idea.16
As outlined in Scheme 11, the synthesis starts with a stereocontrolled acetate aldol addition to aldehyde 97. After replacing the chiral auxiliary with an N-methyl-N-methoxy-amide, the resulting allylic alcohol was subjected to cyclopropanation, delivering 99 as the sole diastereomer in 97% yield. The next three steps were required to produce a 1 : 1 mixture of allylic alcohols 100, which were converted to cyclic carbonates 101 in two steps.
Scheme 11.
Total synthesis of solandelactones.
Treatment of 101 with a solution of Petasis reagent in toluene at 110 °C resulted in the formation of a mixture of cis- and trans-2-methylene-1,3-dioxanes 102 followed by in situ [3,3]-sigmatropic rearrangement to 105 in 65% yield. Formation of a single lactone from a mixture of stereoisomers can be easily understood by considering transition structures 103 and 104, noticing that no new stereocenters are formed and the configuration of the double bond in the forming ring is dictated by conformational preferences in the transition structures. Thus, a chair-like arrangement of the allyl vinyl ether system experiencing bond reorganization should lead to the trans-configuration of the double bond.
From 8-membered lactone 105, the synthesis of solandelactones A and B was completed in three steps: (1) deprotection of the primary hydroxyl group, (2) oxidation to the unstable cyclopropane carboxaldehyde, and (3) side-chain attachment by a Nozaki–Hiyama–Kishi reaction using vinyl iodide 107. Compounds 93 and 94 were obtained as a 3.5 : 1 mixture of stereoisomers.
Preparation of solandelactones E and F required an initial hydrogenation of 105. Diimide proved to be the optimal reagent for this transformation as other methods failed due to sensitivity of the cyclopropane fragment. From 106, the synthesis was completed in an identical three steps as used previously for the synthesis of solandelactones A and B, with diastereoselection of 1.5 : 1 in the Nozaki–Hiyama–Kishi coupling step.
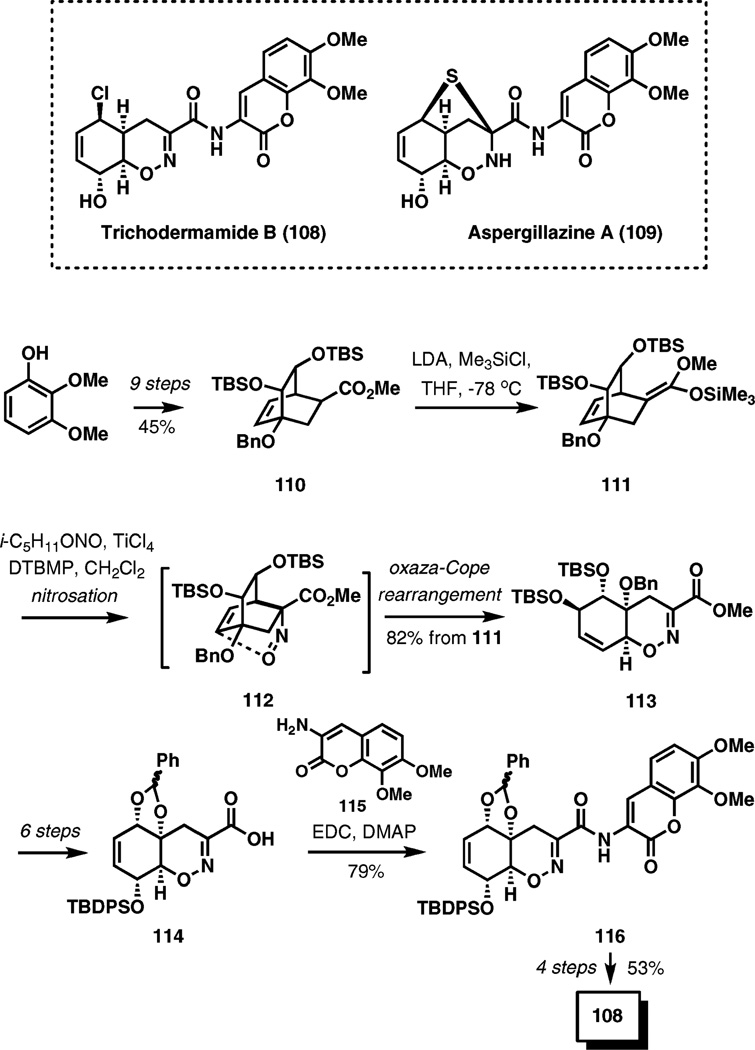
(±)-Trichodermamide B
The synthesis of trichodermamide B highlights the utility of a rare hetero-Cope rearrangement that incorporates two heteroatoms. Trichodermamides are a family of unusual highly modified dipeptides isolated from marine and terrestrial fungi. Aspergillazine A is another intriguing compound in this class. A distinguishing feature in their structure is the oxazine ring.
A tandem nitrosation–oxaza-Cope rearrangement has been developed as a new method for the synthesis of bicyclic oxazines17 and then applied in the recent total synthesis of trichodermamide B (Scheme 12).18 The substrate for the key transformation, bridged bicyclic ester 110, was prepared from 2,3-dimethoxyphenol by a 9-step sequence in a 45% overall yield. Enolization with LDA and trapping the lithium enolate intermediate with chlorotrimethylsilane afforded silyl ketene acetal 111. Electrophilic nitrosation of 111 with nitrosyl chloride, generated in situ by a reaction of titanium tetrachloride and isoamyl nitrite in dichloromethane, produced α-nitrosoester 112, which under reaction conditions undergoes a [3,3]-sigmatropic transposition to furnish the final product, bicyclic oxazine 113 in an 82% overall yield. The sigmatropic rearrangement requires Lewis acid activation and is complete within an hour at 0 °C under these reaction conditions.
Scheme 12.
Tandem nitrosation–oxaza-Cope rearrangement in the total synthesis of trichodermamide B.
In the following six steps, compound 113 was advanced to the functionalized α-oximino carboxylic acid 114 in preparation for the convergent attachment of the amino coumarine fragment. In the event, the desired amide was readily formed employing amine 115 in the presence of EDC and DMAP in 79% yield. The next four straightforward transformations completed the first total synthesis of trichodermamide B.
Cope rearrangement
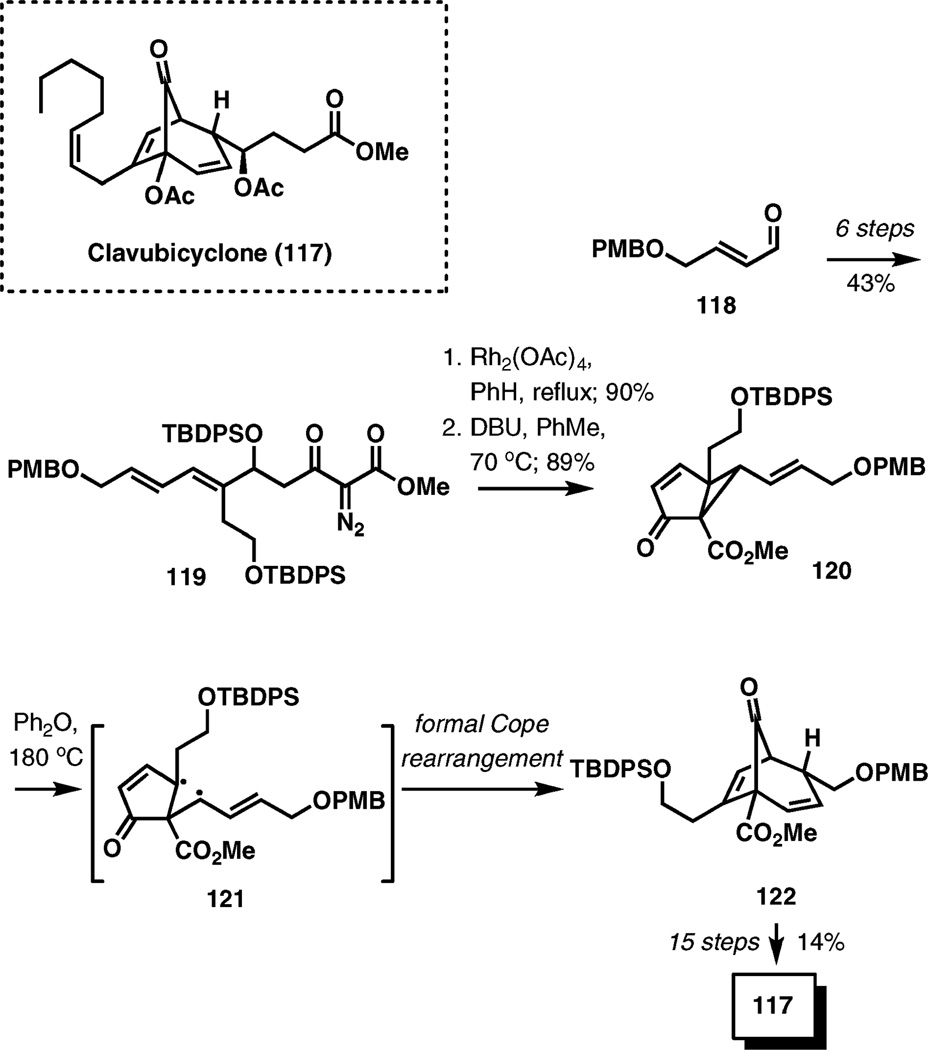
(±)-Clavubicyclone
A Cope rearrangement is the key reaction in the construction of the bicyclo[3.2.1]octane skeleton of clavubicyclone, a prostanoid compound isolated from Okinawan soft coral Clavularia viridis.
The starting divinylcyclopropane 120 was prepared in 8 steps (34% overall yield) from 4-(p-methoxybenzyloxy)-2-butenal (118) via a rhodium-catalyzed intramolecular cyclopropanation of 119 (Scheme 13). Thermolysis of a solution of 120 in diphenyl ether at 180 °C during 0.5 h provided the desired Cope rearrangement product 122 in 58% yield. The yield is much lower if the reaction is carried out at lower temperatures.
Scheme 13.
The synthesis of (±)-clavubicyclone by a Cope rearrangement.
Clearly, the trans arrangement of the vinyl substituents on the cyclopropane ring in 120 prevents a concerted [3,3]-sigmatropic process, therefore the Cope rearrangement must proceed through a diradical mechanism.
The synthesis is completed in 15 steps from 122 in a 14% overall yield.
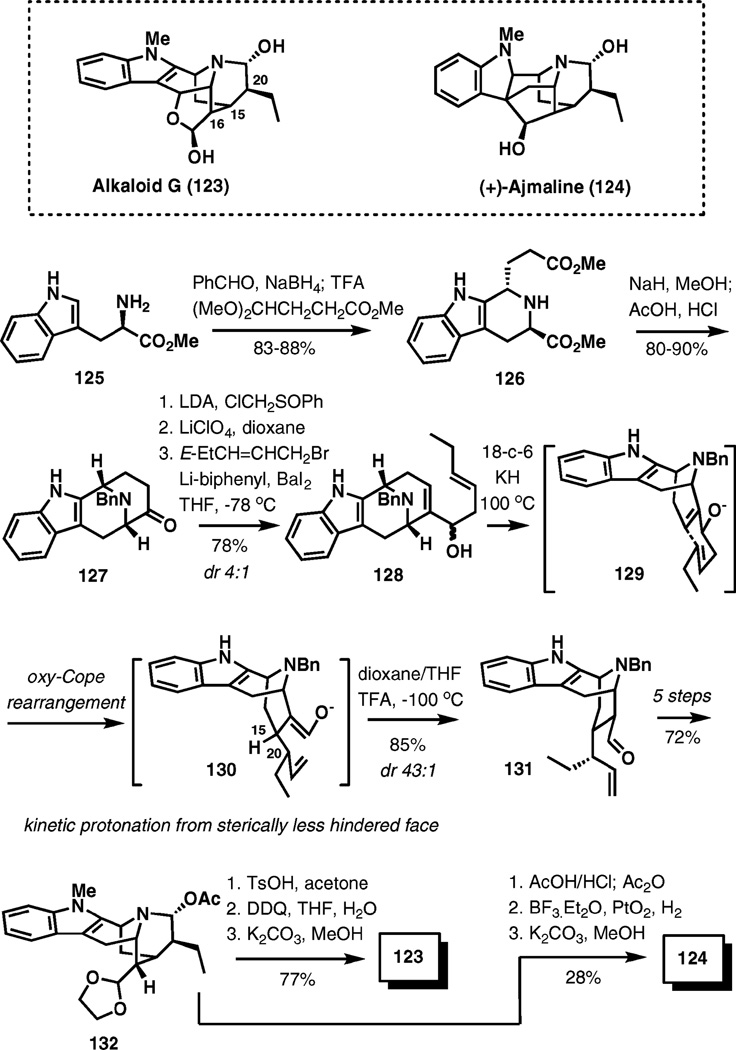
(+)-Ajmaline and alkaloid G
(+)-Ajmaline is a clinically important alkaloid with historical significance that was isolated from the roots of Rauwolfia serpentina in 1931 (Scheme 14). Alkaloid G was obtained from cell cultures of R. serpentina after feeding experiments with (+)-ajmaline. These structurally related alkaloids are attractive synthetic targets due to the presence of an unusual polycyclic framework with an appended indole.
Scheme 14.
Oxy-Cope rearrangement in the enantioselective synthesis of alkaloid G and (+)-ajmaline.
An oxy-Cope rearrangement was used to install the three key stereogenic centers of these molecules at C15, C16, and C20 in a highly stereocontrolled manner.19 The synthesis begins with d-(+)-triptophane methyl ester (125), which is advanced to ketone 127 by a highly efficient two-pot process. The next three steps include spirooxiranophenylsulfoxide formation, its fragmentation to α,β-unsaturated aldehyde in the presence of lithium perchlorate, and a Barbier–Grignard addition of the allylbarium reagent delivering homoallylic alcohols 128 in a 78% overall yield as a 4 : 1 mixture of diastereomers.
Upon deprotonation with potassium hydride in the presence of 18-crown-6, thermolysis of the resultant alkoxide 129 led to enolate 130 via a [3,3]-sigmatropic transposition apparently through a chair-like transition state for both stereoisomers of the starting material. This process established the C15 and C20 stereocenters. After extensive experimentation, it was found that a kinetic quench of 130 from the less hindered face can be achieved with trifluoroacetic acid in a mixture of THF and dioxane at −100 °C. In this manner, aldehyde 131 was isolated in an 85% yield with 43 : 1 diastereoselectivity. It has been demonstrated that 131 is the thermodynamically less stable isomer and can be epimerized at the C16 position under basic conditions, which favor the C16 epimer with up to 86% selectivity.
A subsequent five steps were required to prepare intermediate 132 in a 72% overall yield. This compound is a divergence point in the synthesis of alkaloid G and (+)-ajmaline, each of which was reached in three steps from 132, in 77 and 28% overall yields, respectively.
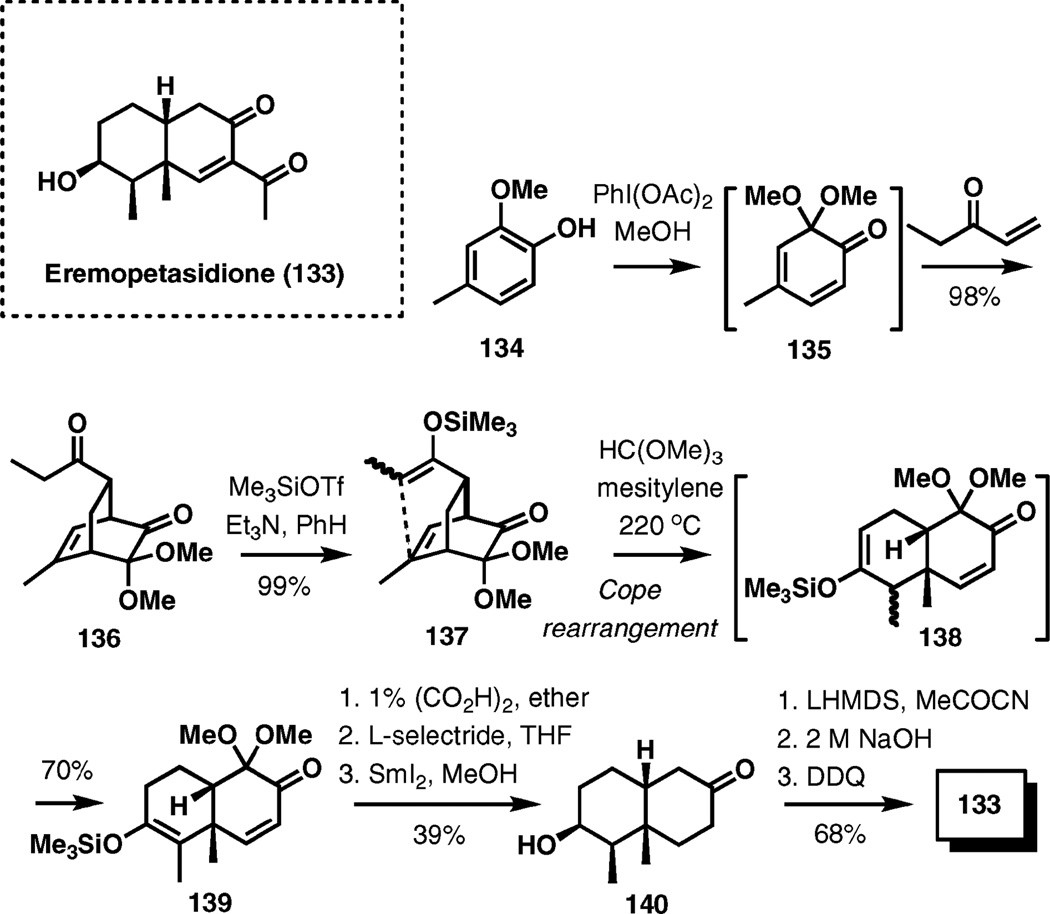
(±)-Eremopetasidione
Another recent application of Cope rearrangement was highlighted in the construction of the decalin ring system of eremopetasidione (Scheme 15).20 (−)-Eremopetasidione was isolated from rhizomes of Petsites japonicus MAXIM., which have been used in the treatment of tonsillitis, contusions, and poisonous snake bites in Chinese medicine.
Scheme 15.
Synthesis of (±)-eremopetasidione.
Oxidation of 2-methoxy-4-methylphenol (134) with iodo-benzene diacetate in methanol and trapping the intermediate o-benzoquinone dimethylketal (135) with ethyl vinyl ketone in a Diels–Alder cycloaddition provided bicyclo[2,2,2]octenone 136 in nearly quantitative yield. Enolization of 136 under electrophilic conditions led to a 1 : 1 mixture of E- and Z-silyl enol ethers 137. The Cope rearrangement of 137 took place at 220 °C, forming the decalin intermediate 138 initially, and subsequent double bond migration occurring under the reaction conditions provided the thermodynamically more stable tetrasubstituted enol ether 139 in 70% yield.
Hydrolysis of the enol ether gave a 1 : 1 separable mixture of ketones, and the desired diastereomer was reduced first with l-selectride and then with samarium iodide to hydroxy ketone 140. Enolization of the ketone with LHMDS and acylation using Mander’s reagent, hydrolysis of the resulting O-acetate, and oxidation completed the synthesis of (±)-eremopetasidione.
This strategy was also exploited in the synthesis of related natural products (±)-3β-angeloyloxyfuranoeremophilane and (±)-3β-methacryloyloxyfuranoeremophilane with related decalin ring systems.
Claisen rearrangement
The Claisen rearrangement is probably the most extensively utilized [3,3]-sigmatropic process, and only a few recent applications in total synthesis where this reaction features prominently are presented in this section.
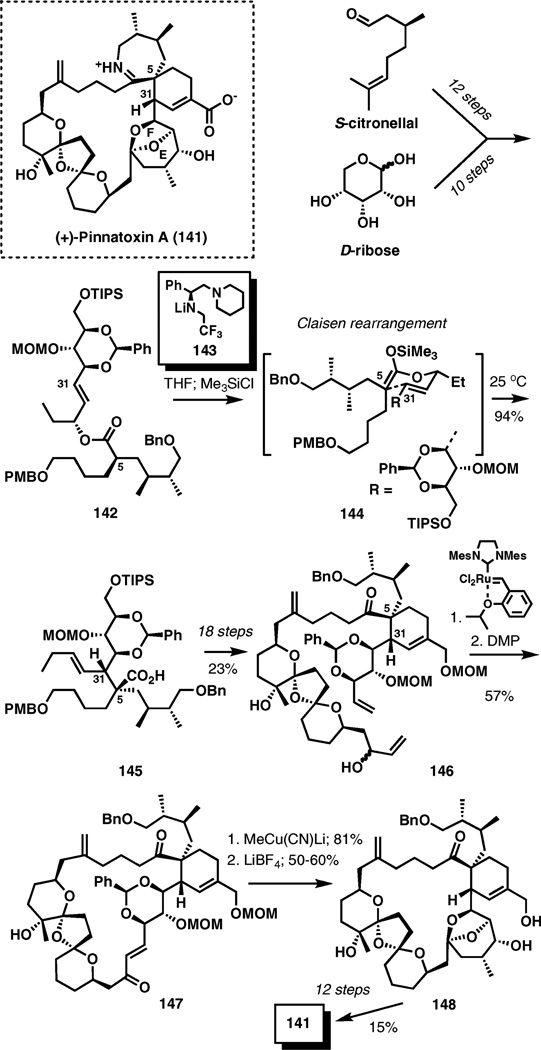
(+)-Pinnatoxin A
Pinnatoxin A is a potent marine toxin isolated from edible mussels Pinna muricata, however, the producing organism has not been identified. Its complex structure comprises several medium size rings incorporated into a 27-membered carbo-cycle. The spiroimine fragment with a quaternary center at C5 is among the most characteristic structural attributes of the molecule.
The Claisen rearrangement is a central part of the strategy for the introduction of the quaternary stereocenter at C5 in a recent enantioselective total synthesis of pinnatoxin A (Scheme 16).21 The complex starting material for the reaction, ester 142, was prepared by a short convergent approach from (S)-citronellic acid and d-ribose.
Scheme 16.
The Claisen rearrangement of ester 142 in the synthesis of (+)-pinnatoxin A.
Enolization of 142 with chiral lithium amide base 143 was required for smooth and stereoselective formation of the Z-enolate which is trapped as silyl ketene acetal 144.22 Upon warming to room temperature, the Claisen rearrangement takes place and delivers carboxylic acid 145 in a 94% yield, completing the highly stereoselective installation of the congested stereocenters at C5 and C31.
The product of the Claisen rearrangement was advanced to intermediate 146 for the macrocycle formation by ring-closing metathesis. Using the Hoveyda–Grubbs II catalyst followed by oxidation, enone 147 was accessed in a 57% overall yield. Methylcuprate addition and hydrolysis of the acetal protecting groups in the presence of LiBF4 and accompanying EF-ketal formation delivered 148. Another 12 steps were required to complete the total synthesis of (+)-pinnatoxin A.
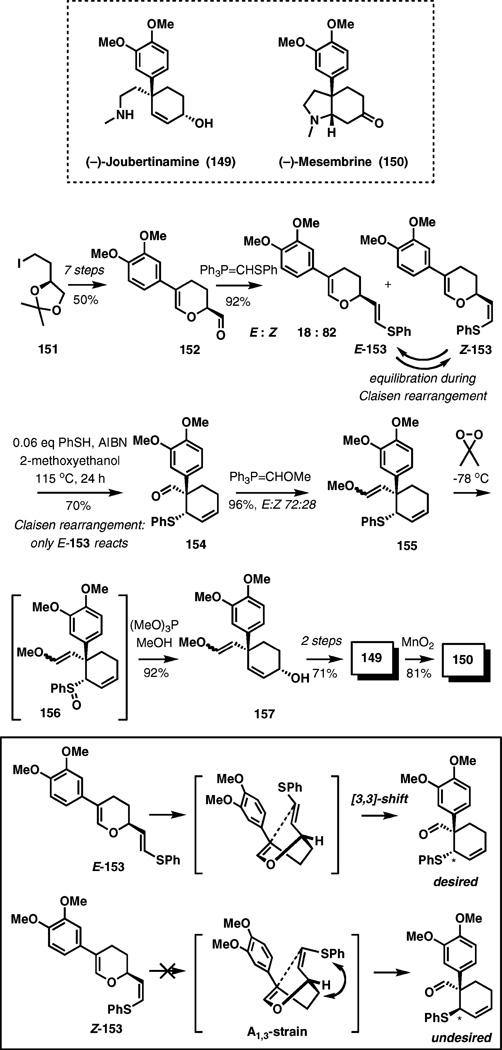
(−)-Joubertinamine and (−)-mesembrine
Joubertinamine and mesembrine are alkaloids found in the Sceletium plants, which have been used for centuries by the indigenous populations of southern Africa as mood enhancers and appetite suppressants. In a recent unified synthesis of these alkaloids that also constitutes the first enantioselective synthesis of joubertinamine, two successive sigmatropic rearrangements have been employed to introduce the benzylic quaternary center and the allylic hydroxyl group of joubertinamine (Scheme 17).23
Scheme 17.
A unified total synthesis of (−)-joubertinamine and (−)-mesembrine.
A mixture of vinyl sulfides E-153 and Z-153 was subjected to thermal Claisen rearrangement at 115 °C. As can be seen from the transition structures in Scheme 17, A1,3-allylic strain prevents access to the reactive conformer for the Z-isomer, and only the E-isomer undergoes the rearrangement. Although the desired E-153 is the minor component of the starting mixture, equilibration of E- and Z-153 under radical reaction conditions (cat. PhSH, AIBN) allowed the direction of both isomers to cyclohexene 154 stereoselectively in a 70% yield.
After olefination of the aldehyde (154 → 155), the second sigmatropic process was carried out upon oxidation of the vinyl sulfide to the corresponding allylic sulfoxide (156) with dimethyldioxirane at −78 °C. Exposure of 156 to trimethyl phosphite and the ensuing Mislow–Evans rearrangement cleanly delivered allylic alcohol 157, and the enantioselective synthesis of joubertinamine is completed in two steps and a 71% yield from this compound. Known oxidation of joubertinamine to mesembrine was achieved with manganese(IV) oxide in an 81% yield.
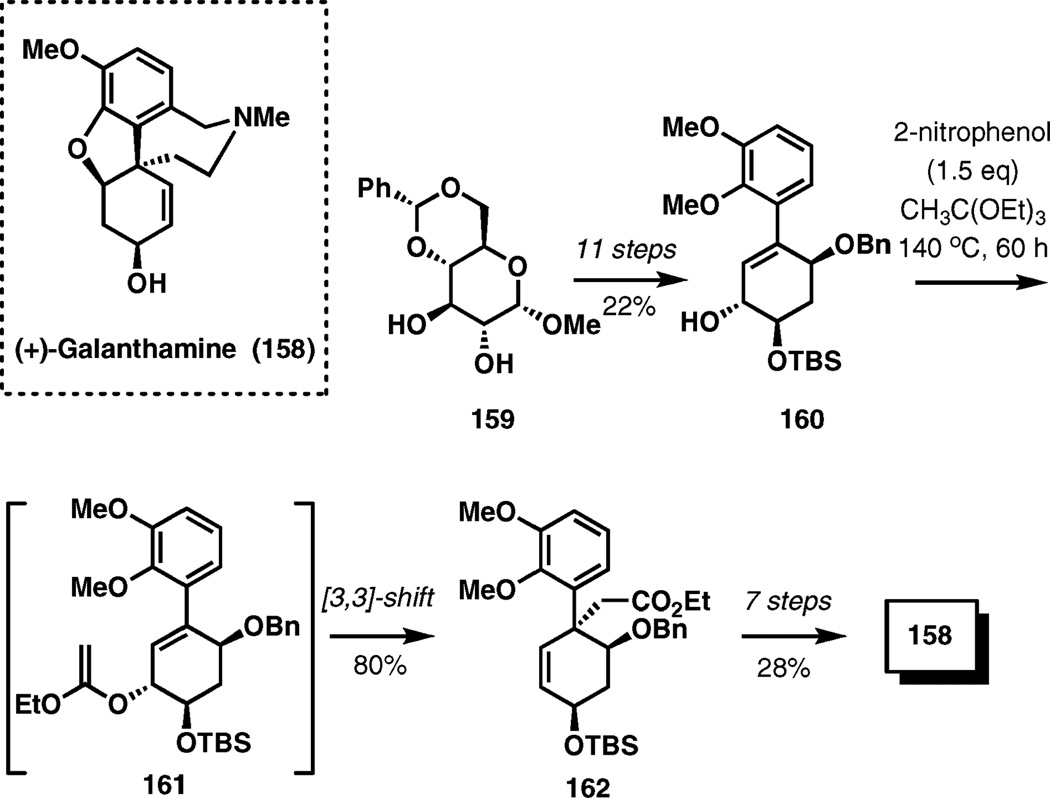
(+)-Galanthamine
Benzylic quaternary stereocenters of a number of alkaloids have been established by Claisen rearrangement, and the synthesis of (+)-galanthamine summarized in this section is an illustration.
(−)-Galanthamine is a typical alkaloid of the Amaryllidaceae family that has been reported to be an acetylcholinesterase inhibitor and an allosteric modulator of the neuronal nicotinic receptor for acetylcholine. Its enantiomer has been recently prepared from methyl 4,6-di-O-benzylidene-α-d-glucose as shown in Scheme 18.24 Allylic alcohol 160 derived in 11 steps from 159 was subjected to a modified Johnson–Claisen rearrangement using 2-nitrophenol as a mild acid catalyst at 140 °C for 60 h. Under these conditions, desired product 162 incorporating the requisite quaternary stereocenter was isolated in 80% yield. The synthesis reached completion in 7 additional steps (28% overall yield).
Scheme 18.
Claisen rearrangement in the synthesis of (+)-galanthamine.
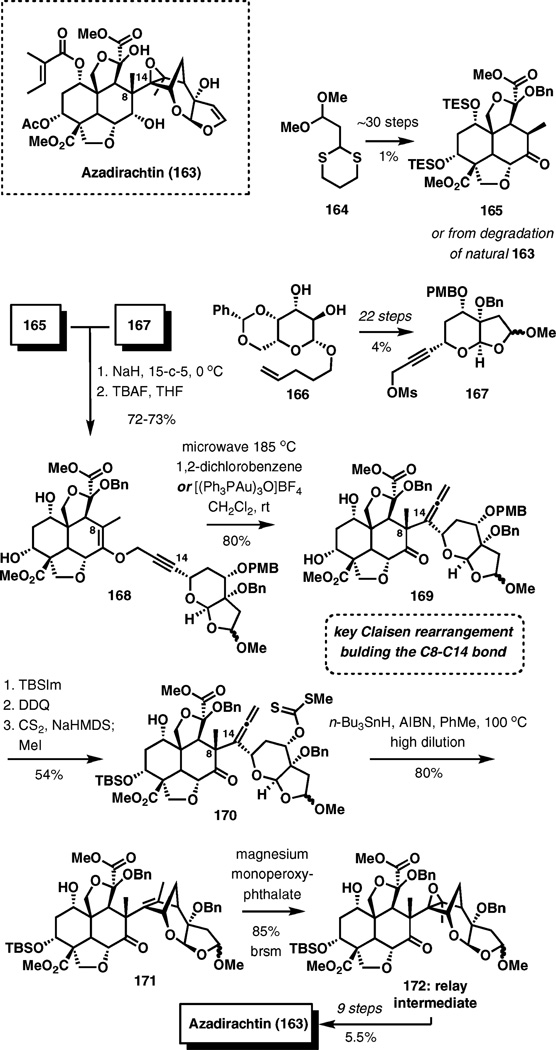
Azadirachtin
Azadirachtin is a potent antifeedant first isolated from the Indian neem tree Azadiracta indica in 1968. Its impressive structure stimulated significant interest as a target for chemical synthesis, and many efforts revealed that forging the congested C8–C14 bond presents a particular challenge. Ultimately, the Claisen rearrangement was found to be uniquely successful for this purpose, allowing for the completion of the total synthesis of azadirachtin (Scheme 19).25
Scheme 19.
Claisen rearrangement as a key step in the total synthesis of azadirachtin.
Building blocks 165 and 167 were prepared from dithiane 164 and d-galactose derivative 166 in 30 and 22 steps, respectively. A convergent coupling of these intermediates was accomplished by O-alkylation of the sodium enolate generated from ketone 165 with sodium hydride in the presence of 15-crown-5 in THF with propargylic mesilate 167 in good yield. Subsequent removal of the silyl protecting groups gave 168.
The key Claisen rearrangement of 168 to 169 could be carried out under thermal (185 °C) or gold(i)-catalyzed conditions with equal efficiency (80% yield), creating the C8–C14 bond and at the same time forming the allene for the radical cyclization of methyl xanthate 170 (3 steps, 54% yield from 169). The radical cyclization of 170 gave 171 in an 80% yield, and subsequent epoxidation of the tetrasubstituted olefin, one of the greatest challenges in this synthesis, could be accomplished with magnesium monoperoxyphthalate under carefully controlled reaction conditions in the presence of a radical inhibitor. The epoxidation led to relay intermediate 172, which was obtained previously from degradation studies and converted to azadirachtin in 9 steps, completing the long total synthesis campaign.
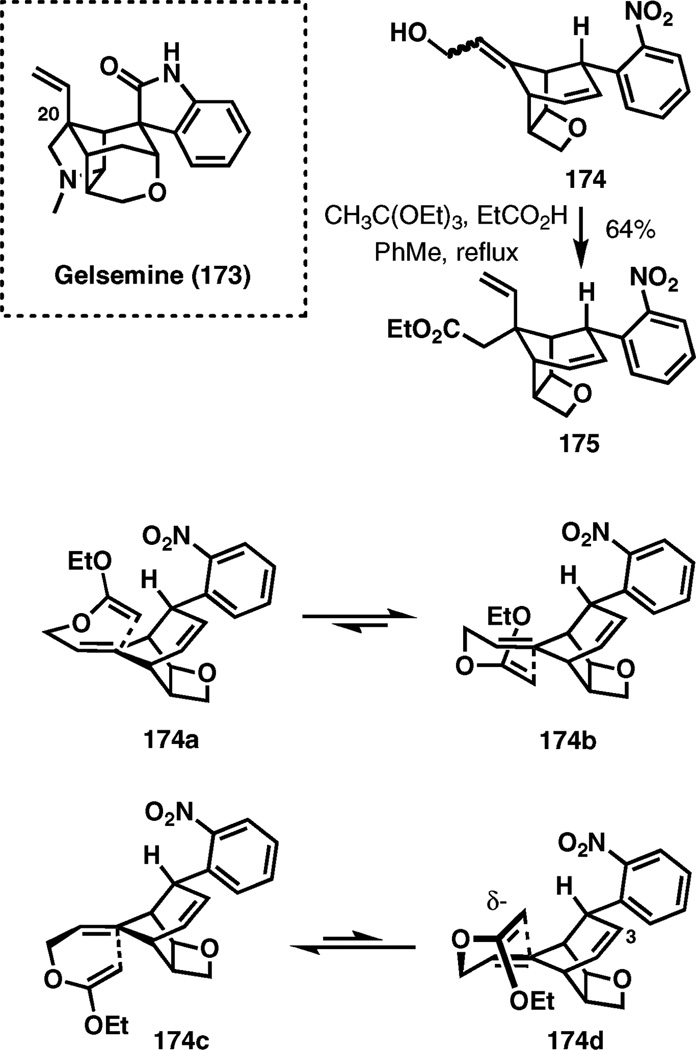
(±)-Gelsemine
A Claisen rearrangement was a useful method for the construction of the C20 quaternary stereocenter in a recent synthesis of gelsemine.26 In the event, the Johnson–Claisen variant of the reaction was employed. Each stereoisomer of 174 led to a single and identical product, ester 175. The stereochemical convergence was explained by steric repulsion between the ethyl enolate and the axial benzylic hydrogen in 174a, and by an unfavorable electrostatic interaction between the π-system of the C3–C14 double bond and the enolate in 174d. Thus, transition structures 174b and 174c are preferred, both leading to 175 (Scheme 20).
Scheme 20.
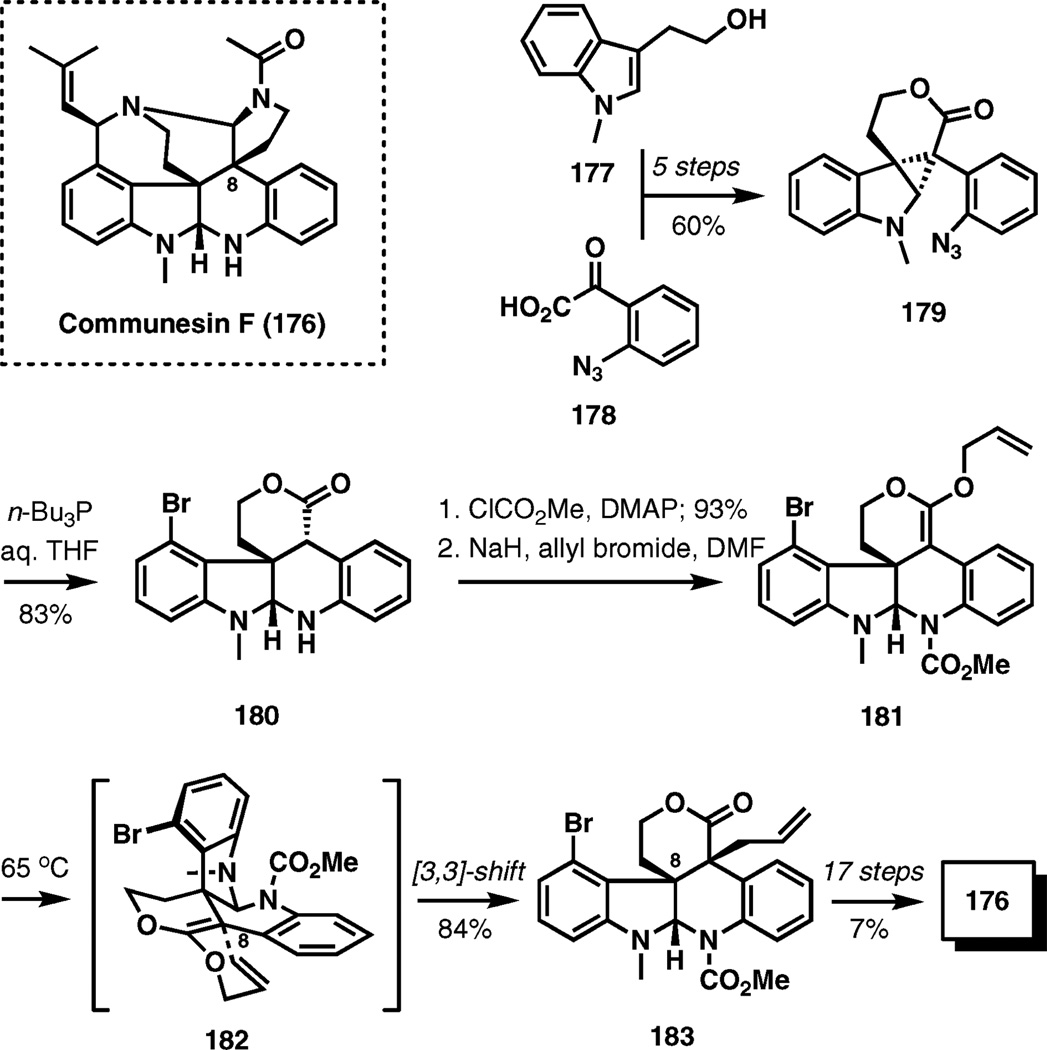
(±)-Communesin F
Indole alkaloid communesin F belongs to a family of compounds isolated from a marine fungal strain of Penicillium species that now includes eight members (A–H). The first completed synthesis in this area reaches communesin F via a Claisen rearrangement that crafts the quaternary stereocenter at C8,27 again highlighting the power of sigmatropic reactions in creating congested chemical bonds.28
Indole 177 and keto acid 178 were combined by esterification and efficiently advanced to cyclopropane 179 in a 60% overall yield. Staudinger reduction of the azide with tri-n-butyl-phosphine was accompanied by cyclopropane ring-opening to give pentacyclic lactone 180. After protection of the nitrogen as methyl carbamate, O-allylation of the sodium enolate generated from the resulting lactone with allyl bromide in dimethylformamide with ensuing Claisen rearrangement at elevated temperatures (65 °C) provided α-allylation product 183 in a very good yield.
Successful isolation of the O-allyl intermediate (181) confirmed that the α-allylation occurs via a sigmatropic rearrangement rather than by a direct C-allylation of the sodium enolate. The chair-like transition structure 182 explains the sense of stereocontrol during the formation of the C8 stereocenter. Thus, the top face of the vinyl ether π-system is blocked by the bromoarene substituent, leading to the shown arrangement during the [3,3]-sigmatropic transposition.
From 183, the synthesis is completed in 17 steps and in a 7% overall yield (Scheme 21).
Scheme 21.
The total synthesis of communesin F.
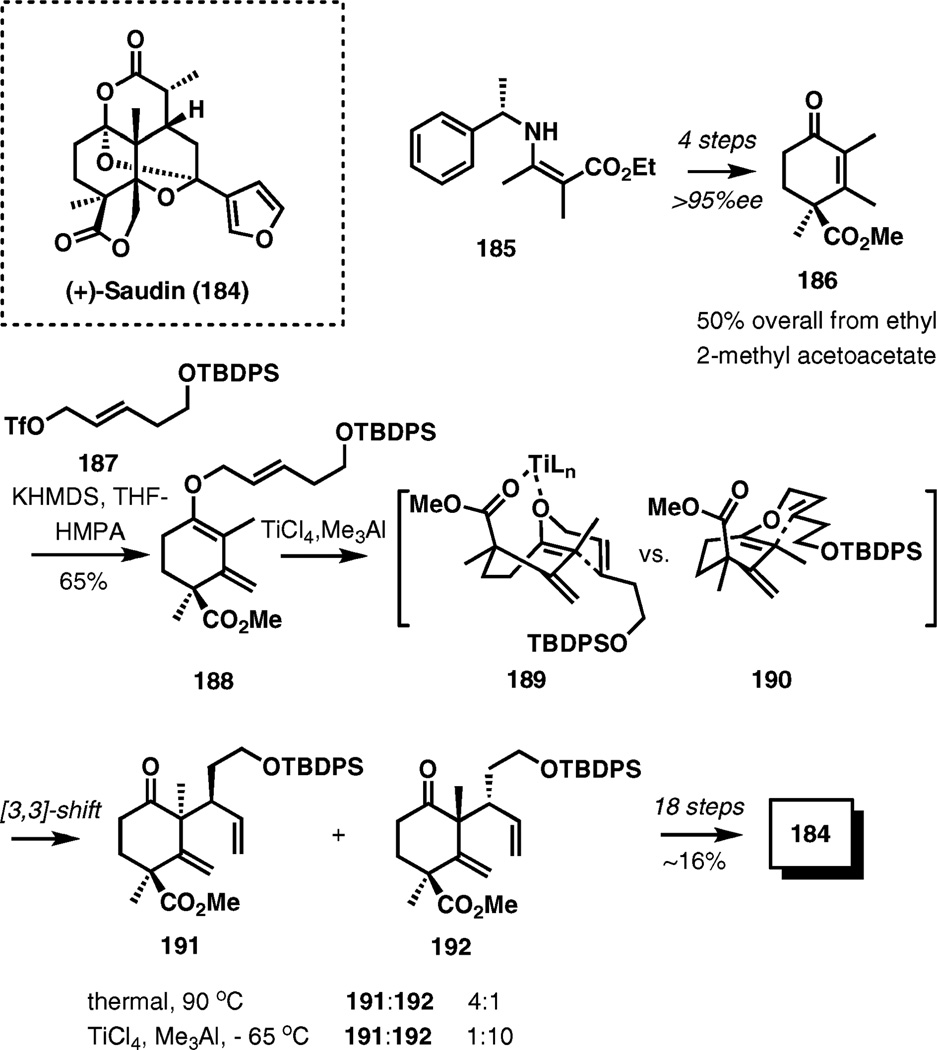
(+)- and (−)-Saudin
An unusual Lewis acid mediated Claisen rearrangement was employed in an enantioselective total synthesis of saudin.29 In this case, the Lewis acid served to induce a conformational change in the substrate to favor the desired stereochemical outcome of the process (Scheme 22).
Scheme 22.
Stereocontrol through complexation in the Claisen rearrangement en route to saudin.
The potassium dienolate of 186 accessed from enamine 185 (4 steps, 50% overall yield from ethyl 2-methyl acetoacetate, 495% ee) was O-allylated with triflate 187 in 65% yield. The resulting vinyl allyl ether served as the substrate for the Claisen rearrangement which under thermal conditions gives a mixture of transposition products 191 and 192 in a 4 : 1 ratio favoring undesired 191. On the other hand, the transposition in the presence of titanium tetrachloride and trimethylaluminium selectively provides 191 with a 10 : 1 ratio. Importantly, this rearrangement occurs at −65 °C, suggesting acceleration of the [3,3]-sigmatropic process in the presence of Lewis acids.
The stereochemical outcomes were rationalized by considering transition structure 190 for the thermal rearrangement and structure 189 for the reaction mediated by TiCl4 and Me3Al. (+)-Saudin was obtained in 18 steps and ~ 16% overall yield from 192. Using the enantiomer of 185, an identical sequence of reactions provided the natural enantiomer, (−)-saudin.
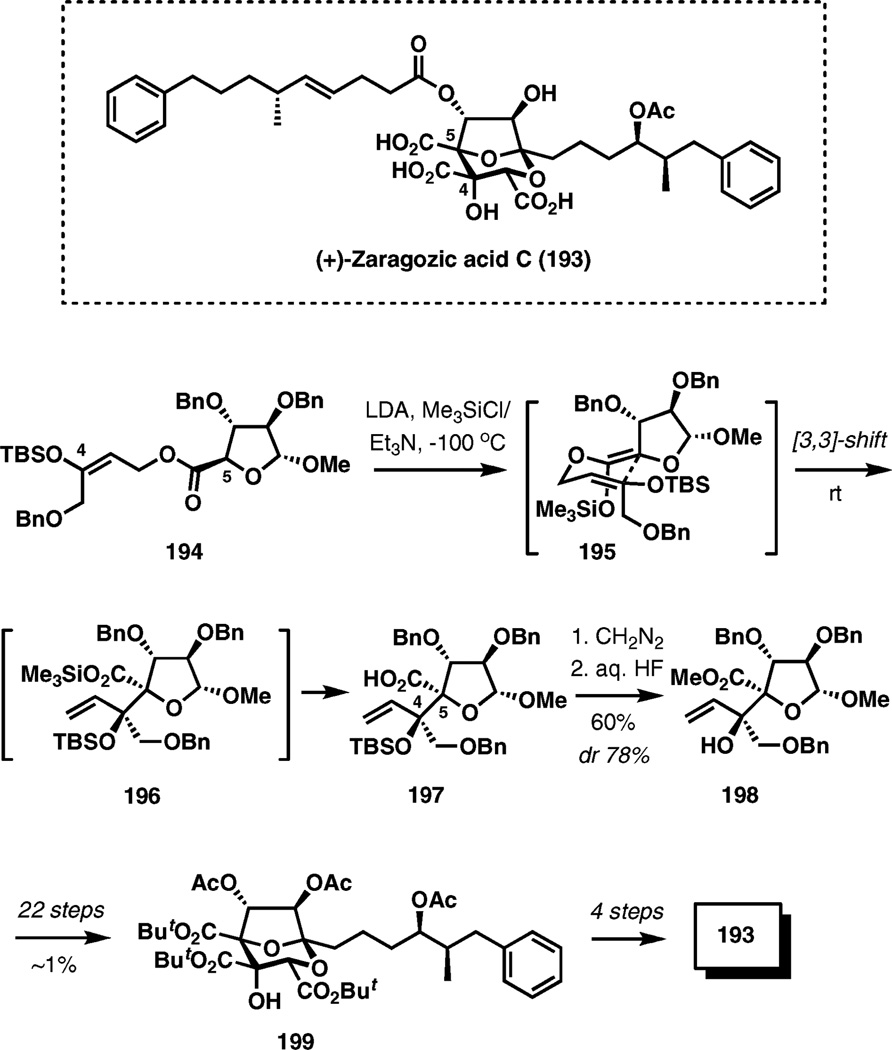
(+)-Zaragozic acid C
The Ireland–Claisen rearrangement was an important element in the strategy for a recent formal synthesis of (+)-zaragozic acid C.30 Zaragozic acids were identified as potent inhibitors of squalene synthase and promising cholesterol-lowering compounds, and their unusual structures inspired several total synthesis endeavors (Scheme 23).
Scheme 23.
Zaragozic acid C.
l-Arabinose-derived ester 194 was stereoselectively enolized with LDA and the resulting Z-enolate, formed via chelation control, was trapped as the silyl ketene acetal 195. The rearrangement occurred at room temperature preferentially through the expected chair-like transition structure to give the desired product with established C4 and C5 stereocenters of the target compound with 78% diastereoselectivity. Treatment of the crude product with diazomethane followed by desilylation with aqueous hydrofluoric acid gave 198 in 60% yield from ester 194.
The formal synthesis was then completed by a 22-step preparation of intermediate 199, which had been converted to zaragozic acid C in four steps in a previously reported synthesis.
Applications of other hetero-Cope rearrangements
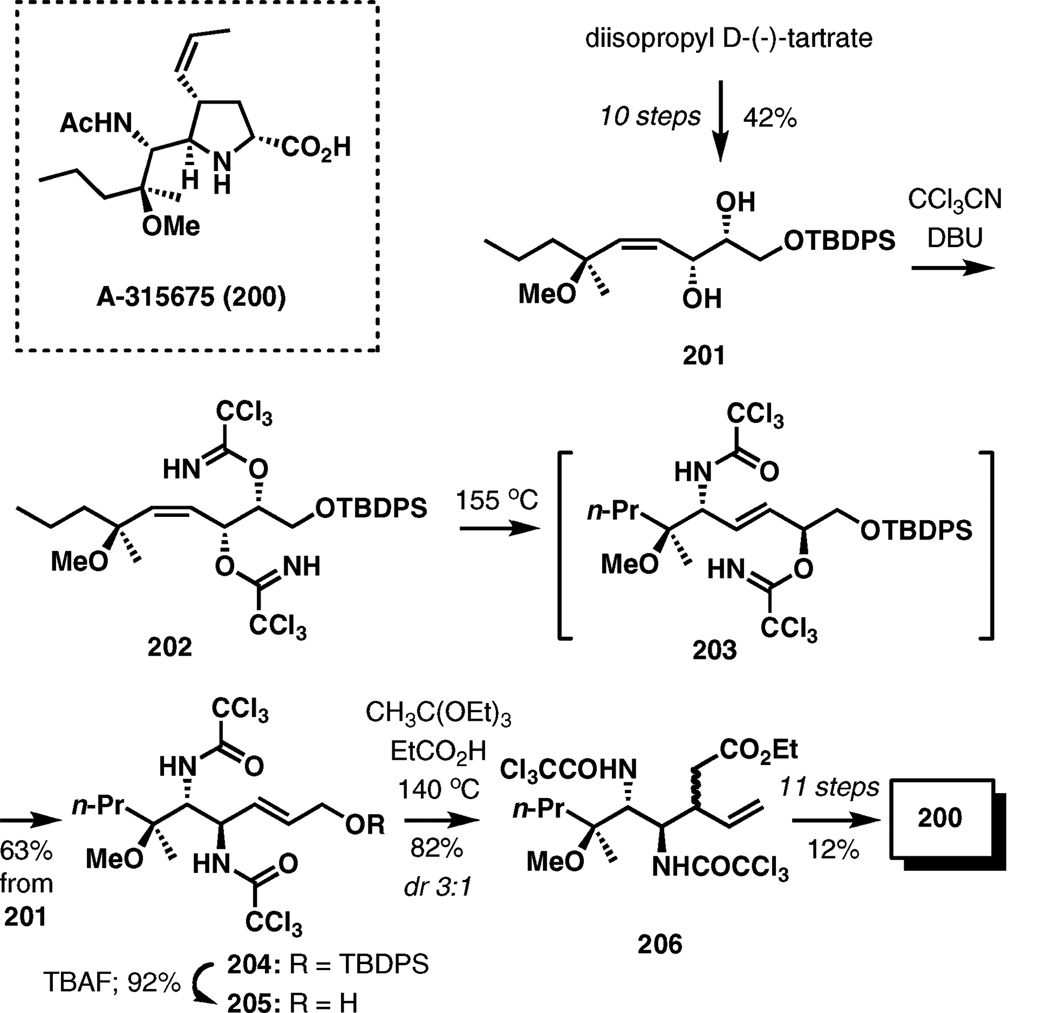
A-315675
An extensive use of sigmatropic rearrangements, including the trichloroacetimidate rearrangement (the Overman rearrangement) characterizes the recent synthesis of the antiinfluenza agent A-315675 developed by the Abbott scientists (Scheme 24).31
Scheme 24.
Consecutive sigmatropic rearrangements in the synthesis of A-315675.
Allylic diol 201 was prepared from diisopropyl tartrate by a straightforward 10-step sequence. Treatment with trichloro-acetonitrile in the presence of DBU afforded bis-trichloro-acetimidate 202. When heated at 155 °C, intermediate 202 undergoes a double [3,3]-sigmatropic rearrangement through classic chair-like transition structures, first forming 203, and then the ultimate product bis-trichloroacetamide 204 in a 63% yield from diol 201. After deprotection of the allylic hydroxyl group in 204, the resulting alcohol was subjected to the Johnson–Claisen rearrangement to give ester 206 in 82% yield as a 3 : 1 mixture of diastereoisomers favoring the undesired isomer. An equilibration at a later stage corrected the stereochemistry, and the synthesis of 200 was completed in 11 steps (12% overall yield) from 206.
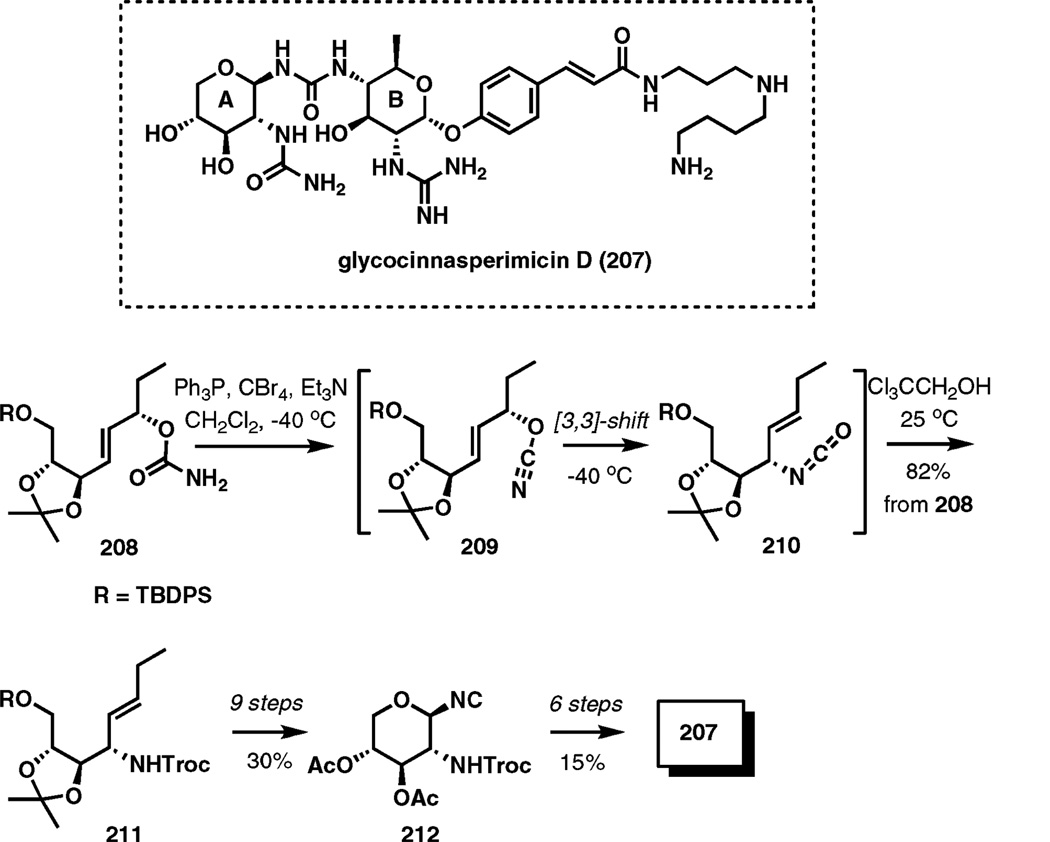
Glycocinnasperimicin D
Glycocinnasperimicin D is an amino glycoside antibiotic isolated from the fermentation broth of Nocardia. The amino sugar antibiotic is important due to its broad spectrum activity against Gram-negative bacteria, yet it is no longer available from the natural source as a result of mutation in the producing organism.
In a recent total synthesis of glycocinnasperimicin D,32 an unusual hetero-Cope rearrangement has been used to install an amino-group of one of the carbohydrate building blocks. As illustrated in Scheme 25, cyanate 209 obtained by dehydration of carbamate 208 underwent an efficient low-temperature sigmatropic transposition to isocyanate 210, which was trapped with trichloroethyl alcohol to give protected allylic amine 211 in an 82% overall yield. The next 9 steps completed the assembly of 212, the precursor to the A-ring of the target compound. After attachment of the fully elaborated B-ring fragment, the total synthesis of 207 was accomplished in six additional steps.
Scheme 25.
Allyic cyanate to isocyanate rearrangement in the synthesis of glycocinnasperimicin D.
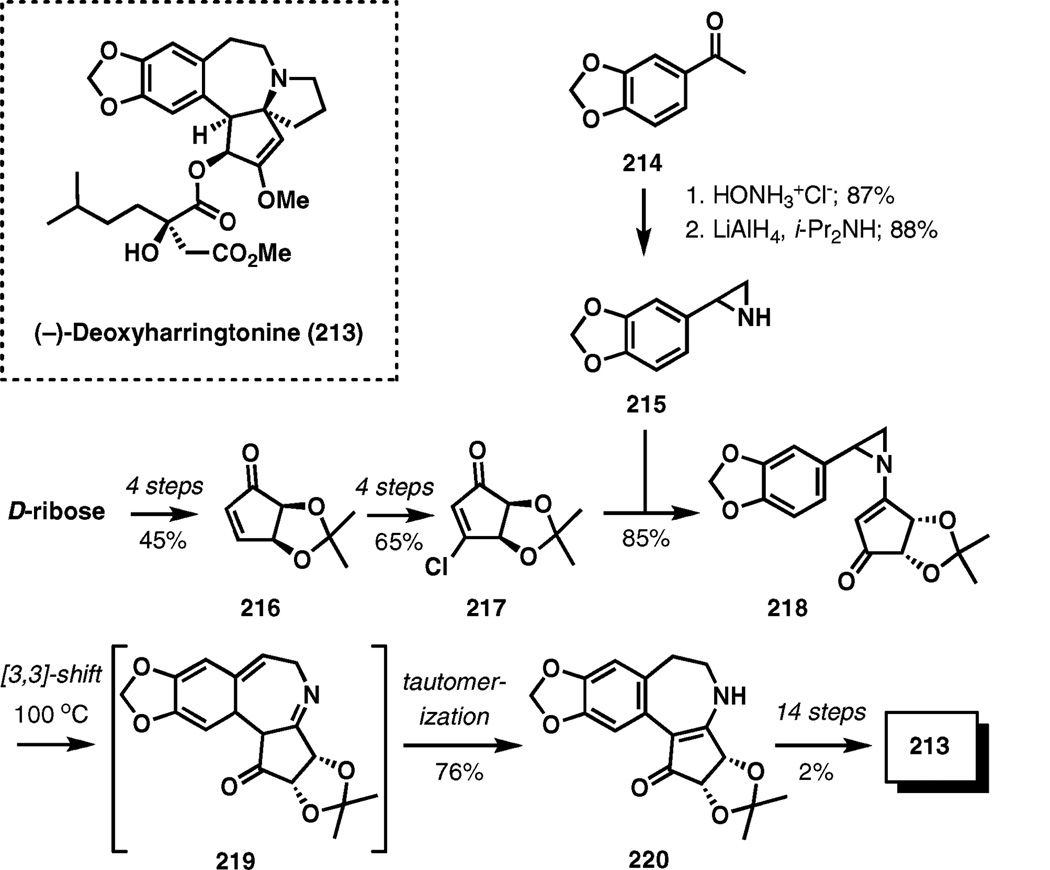
(−)-Deoxyharringtonine
Strain release was the driving force for the aza-Cope rearrangement employed in the synthesis of (−)-deoxyharringtonine, an anti-leukemia alkaloid isolated from a plant of the Cephalotaxus genus (Scheme 26).33
Scheme 26.
[3,3]-Sigmatropic transposition of N-vinyl-2-arylaziridine in the total synthesis of (−)-deoxyharringtonine.
The substrate was assembled by coupling of 3-chloro-2-cyclopentenone 217 and aziridine 215 in 85% yield. For this purpose, chloride 217 was obtained in four steps from 216 readily available from d-ribose, while aziridine 215 was prepared in two steps from 3,4-methylenedioxyacetophenone (214).
The [3,3]-sigmatropic transposition of N-vinyl-2-arylaziridine 218 required heating to 100 °C. The initial rearrangement product 219 undergoes a facile tautomerization to final product 220 isolated in 76% yield. A stepwise ionic process proceeding via a p-quinone methide cation followed by a 7-exo-cyclization is also possible for the conversion of 218 to 220.
The synthesis of 213 was accomplished in an additional 14 steps (2% overall yield from 220).
Conclusions
As can be clearly appreciated from the selection of total syntheses described in this review, the classic [3,3]-sigmatropic rearrangement continues to inspire new methods and strategies for the preparation of a diverse group of complex and densely functionalized molecules. These reactions are especially effective in the construction of sterically congested chiral centers. Furthermore, incorporation of [3,3]-sigmatropic transpositions into cascade reaction sequences is a powerful approach to rapid build-up of complexity in organic synthesis.
Biographies

Elizabeth A. Ilardi
Elizabeth A. Ilardi received her BS in Chemistry from The College of William and Mary in Virginia, in 2006. She is currently working on her PhD in organic chemistry under the supervision of Dr Armen Zakarian at the University of California at Santa Barbara. The focus of her studies includes the development of new methodologies to be applied towards the total syntheses of natural products.

Craig E. Stivala
Craig E. Stivala received his BS in chemistry at Syracuse University, in New York. In 2006, he joined the research group under the direction of Professor Armen Zakarian, currently at the University of California at Santa Barbara. His current research focuses on acyclic stereocontrol in the Ireland–Claisen rearrangement of α-branched esters and its application toward the synthesis of spiroimine natural products. He is the recent recipient of the TRDRP (Tobacco-Related Disease Research Program) Dissertation Award.

Armen Zakarian
Armen Zakarian completed his undergraduate studies at Moscow State University in 1994. His Diploma was carried out at the Zelinsky Institute of Organic Chemistry with Dr Vladimir Borodkin. He received his PhD under the direction of Professor Robert A. Holton at Florida State University in 2001, and spent two years with Professor Larry E. Overman (University of California, Irvine) as a postdoctoral research associate. He began his independent academic appointment in August 2004, and he is currently at the Department of Chemistry and Biochemistry, University of California at Santa Barbara. His research interests include the total synthesis of natural products, bioorganic chemistry, and the development of synthetic methodology.
Footnotes
Part of the rapid formation of molecular complexity in organic synthesis themed issue.
Notes and references
- 1.For a review, see: Heckrodt TJ, Mulzer J. Top. Curr. Chem. 2005;244:1–42.
- 2.Davies HML, Dai X, Long MS. J. Am. Chem. Soc. 2006;128:2485–2490. doi: 10.1021/ja056877l. [DOI] [PubMed] [Google Scholar]
- 3.For a related synthesis of (+)-erogorgiaene, see: Davies HWL, Walji AM. Angew. Chem., Int. Ed. 2005;44:1733–1735. doi: 10.1002/anie.200462227.
- 4.Sauer ELO, Barriault L. J. Am. Chem. Soc. 2004;126:8569–8575. doi: 10.1021/ja048301m. [DOI] [PubMed] [Google Scholar]
- 5.Sauer ELO, Barriault L. Org. Lett. 2004;6:3329–3332. doi: 10.1021/ol0487635. [DOI] [PubMed] [Google Scholar]
- 6.Barriault L, Deon DH. Org. Lett. 2001;3:1925–1927. doi: 10.1021/ol015970l. [DOI] [PubMed] [Google Scholar]
- 7.Brummond KM, Hong S-p. J. Org. Chem. 2005;70:907–916. doi: 10.1021/jo0483567. [DOI] [PubMed] [Google Scholar]
- 8.Scheffler G, Seike H, Sorensen EJ. Angew. Chem., Int. Ed. 2000;39:4593–4596. doi: 10.1002/1521-3773(20001215)39:24<4593::aid-anie4593>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Brüggemann M, McDonald AI, Overman LE, Rosen MD, Schwink L, Scott JP. J. Am. Chem. Soc. 2003;125:15284–15285. doi: 10.1021/ja0388820. [DOI] [PubMed] [Google Scholar]
- 10.Martin CL, Overman LE, Rohde JM. J. Am. Chem. Soc. 2008;130:7568–7569. doi: 10.1021/ja803158y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li X, Ovasaka TV. Org. Lett. 2007;9:3837–3840. doi: 10.1021/ol701633z. see also: Li X, Kyne RE, Ovasaka TV. Tetrahedron. 2007;63:1899–1906.
- 12.Quillinan AJ, Scheinmann F. J. Chem. Soc. D. 1971:966. [Google Scholar]
- 13.Nicolaou KC, Li J. Angew. Chem., Int. Ed. 2001;40:4264–4268. doi: 10.1002/1521-3773(20011119)40:22<4264::AID-ANIE4264>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Nicolaou KC, Sasmal PK, Xu H, Namoto K, Ritzen A. Angew. Chem., Int. Ed. 2003;42:4225–4229. doi: 10.1002/anie.200351805. [DOI] [PubMed] [Google Scholar]
- 15.Nicolaou KC, Xu H, Wartmann M. Angew. Chem., Int. Ed. 2005;44:756–761. doi: 10.1002/anie.200462211. [DOI] [PubMed] [Google Scholar]
- 16.White JD, Lincoln CM, Yang J, Martin WHC, Chan DB. J. Org. Chem. 2008;73:4139–4150. doi: 10.1021/jo800335g. [DOI] [PubMed] [Google Scholar]
- 17.Zakarian A, Lu C-D. J. Am. Chem. Soc. 2006;128:5356–5357. doi: 10.1021/ja0609710. [DOI] [PubMed] [Google Scholar]
- 18.Lu C-D, Zakarian A. Angew. Chem., Int. Ed. 2008;47:6829–6831. doi: 10.1002/anie.200801652. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Xu Q, Yu P, Liu X, Cook JM. Org. Lett. 2001;3:345–348. doi: 10.1021/ol000331g. [DOI] [PubMed] [Google Scholar]
- 20.Hsu D-S, Hsu P-Y, Lee Y-C, Liao C-C. J. Org. Chem. 2008;73:2554–2563. doi: 10.1021/jo702115x. [DOI] [PubMed] [Google Scholar]; Hsu D-S, Hsu P-Y, Liao C-C. Org. Lett. 2001;3:263–265. doi: 10.1021/ol000355n. [DOI] [PubMed] [Google Scholar]
- 21.Stivala CE, Zakarian A. J. Am. Chem. Soc. 2008;130:3774–3776. doi: 10.1021/ja800435j. [DOI] [PubMed] [Google Scholar]
- 22.Qin Y, Stivala CE, Zakarian A. Angew. Chem., Int. Ed. 2007;46:7466–7469. doi: 10.1002/anie.200702142. [DOI] [PubMed] [Google Scholar]
- 23.Ilardi EA, Isaacman MJ, Qin Y, Shelly SA, Zakarian A. Tetrahedron. 2009;65:3261–3269. [Google Scholar]
- 24.Tanimoto H, Kato T, Chida N. Tetrahedron Lett. 2007;48:6267–6270. [Google Scholar]
- 25.Veitch GM, Beckmann E, Burke BJ, Boyer A, Maslen SL, Ley SV. Angew. Chem., Int. Ed. 2007;46:7629–7632. doi: 10.1002/anie.200703027. [DOI] [PubMed] [Google Scholar]; Veitch GM, Beckmann E, Burke BJ, Boyer A, Ayats C, Ley SV. Angew. Chem., Int. Ed. 2007;46:7633–7635. doi: 10.1002/anie.200703028. [DOI] [PubMed] [Google Scholar]
- 26.Ng FW, Lin H, Danishefsky SJ. J. Am. Chem. Soc. 2002;124:9812–9824. doi: 10.1021/ja0204675. [DOI] [PubMed] [Google Scholar]
- 27.Seo JH, Artman III GD, Weinreb SM. J. Org. Chem. 2006;71:8891–8900. doi: 10.1021/jo061660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Wu H, Shen L, Qin Y. J. Am. Chem. Soc. 2007;129:13794–13795. doi: 10.1021/ja075705g. [DOI] [PubMed] [Google Scholar]
- 29.Boeckman RK, Jr, Ferreira MRR, Mitchell LH, Shao P. J. Am. Chem. Soc. 2002;124:190–191. doi: 10.1021/ja017194i. [DOI] [PubMed] [Google Scholar]
- 30.Bunte JO, Cuzzupe AN, Daly AM, Rizzacasa MA. Angew. Chem., Int. Ed. 2006;45:6376–6380. doi: 10.1002/anie.200602507. [DOI] [PubMed] [Google Scholar]
- 31.Momose T, Hama N, Higashino C, Sato H, Chida N. Tetrahedron Lett. 2008;49:1376–1379. [Google Scholar]
- 32.Nishiyama T, Isobe M, Ichikawa Y. Angew. Chem., Int. Ed. 2005;44:4372–4375. doi: 10.1002/anie.200500892. [DOI] [PubMed] [Google Scholar]
- 33.Eckerbarger JD, Wilmot JT, Gin DY. J. Am. Chem. Soc. 2006;128:10370–10371. doi: 10.1021/ja063304f. [DOI] [PMC free article] [PubMed] [Google Scholar]