Abstract
Objectives. We evaluated the efficacy of a motivational tobacco cessation treatment combined with nicotine replacement relative to usual care initiated in inpatient psychiatry.
Methods. We randomized participants (n = 224; 79% recruitment rate) recruited from a locked acute psychiatry unit with a 100% smoking ban to intervention or usual care. Prior to hospitalization, participants averaged 19 (SD = 12) cigarettes per day; only 16% intended to quit smoking in the next 30 days.
Results. Verified smoking 7-day point prevalence abstinence was significantly higher for intervention than usual care at month 3 (13.9% vs 3.2%), 6 (14.4% vs 6.5%), 12 (19.4% vs 10.9%), and 18 (20.0% vs 7.7%; odds ratio [OR] = 3.15; 95% confidence interval [CI] = 1.22, 8.14; P = .018; retention > 80%). Psychiatric measures did not predict abstinence; measures of motivation and tobacco dependence did. The usual care group had a significantly greater likelihood than the intervention group of psychiatric rehospitalization (adjusted OR = 1.92; 95% CI = 1.06, 3.49).
Conclusions. The findings support initiation of motivationally tailored tobacco cessation treatment during acute psychiatric hospitalization. Psychiatric severity did not moderate treatment efficacy, and cessation treatment appeared to decrease rehospitalization risk, perhaps by providing broader therapeutic benefit.
Tobacco use among persons with mental illness is 2 to 4 times as great as among the general US population, with costly and deadly consequences.1–3 Persons with serious mental illness have an average life expectancy 25 years shorter than in the general population; the chief causes of death are chronic tobacco-related diseases such as cardiovascular disease, lung disease, and cancer.4 Annually, 200 000 of the 435 000 deaths in the United States attributed to smoking are believed to be among individuals with mental illness or addictive disorders.5
Despite the significant health effects, smoking remains ignored or—even worse—encouraged in mental health settings.6,7 A minority of patients with mental illness report that a mental health provider has advised them to quit smoking, and some report active discouragement of quitting.8,9 Staff at some psychiatric hospitals still smoke with patients, rationalized as effective for building clinician–client rapport.10
Since 1993, US hospitals have banned tobacco use under mandate of the Joint Commission on the Accreditation of Healthcare Organizations.11 In response to outcries from patient advocacy groups, however, the commission permitted an exception for inpatient psychiatry; similar policy exemptions have been granted to psychiatric facilities in Europe and Australia.12–14 Nearly 20 years later, more than half of state inpatient psychiatry units in the United States permit smoking, and half sell cigarettes to patients.15 Even among hospitals that ban tobacco use, cessation advice and treatment are rare.15,16 Without intervention, almost all patients return to smoking after a smoke-free psychiatric hospitalization, most within minutes of hospital discharge.8 Integrated treatments are needed.
Nearly 8800 studies inform tobacco treatment clinical practice guidelines,17 and an extensive literature documents the efficacy of initiating treatment of tobacco dependence in hospital settings with general medical patients.18 Yet fewer than 2 dozen randomized clinical trials have treated smoking in persons with current mental illness,19 and the only published randomized trial examining inpatient psychiatry for initiating tobacco treatment was conducted with adolescents. The intervention group increased in motivation to quit, but the treatment effect on abstinence was not significant.20 The American Psychiatric Association identifies psychiatric hospitalizations as an ideal opportunity to treat tobacco dependence.21 Hospital-based tobacco treatment trials with the seriously mentally ill are needed to inform clinical practice guidelines.
An obstacle to tobacco treatment in mental health settings has been concern that termination of cigarette smoking will increase psychiatric symptoms. Many in the clinical, research, and public arenas believe that tobacco use serves as a form of self-medication for persons with psychiatric disorders.22,23 If this were true, psychiatric symptoms would be expected to worsen and mental health service use to increase following treatment of tobacco use. Tobacco treatment trials with smokers with clinical depression, posttraumatic stress disorder, and schizophrenia, however, have demonstrated no adverse effect of treating tobacco dependence or of quitting smoking on mental health recovery.24–29
Research has not examined the impact of treating tobacco dependence during an acute psychiatric hospitalization on mental health recovery. Patients for whom inpatient psychiatric care is deemed necessary typically present as suicidal, homicidal, or gravely disabled. The average length of inpatient psychiatric stay in the United States is about a week, and readmissions are common.8,16 Among patients hospitalized for mental illness in California in 2005 and 2006, 44% were rehospitalized within 12 months, reflecting the remitting and recurring natural course of many mental illnesses.30 In the literature, predictors of psychiatric hospitalization include psychosis, race/ethnicity (higher for African Americans), low socioeconomic status, and previous hospitalizations.24,31
We evaluated the efficacy of a tobacco cessation intervention initiated with adult smokers during an acute inpatient psychiatric hospitalization. The setting was a locked unit with a complete smoking ban that managed patients’ nicotine withdrawal with nicotine replacement therapy (NRT) during hospitalization but did not provide cessation services, discharge NRT, or treatment referrals. Hospitalization in the acute psychiatric setting tends to be brief and unrelated to smoking. Furthermore, few patients hospitalized for psychiatric illness intend to quit smoking in the next 30 days.8,32,33 For this reason, we focused on increasing motivation and engagement during a brief period of institutionalized abstinence and offered cessation treatment and access to 10 weeks of NRT up to 6 months following hospital discharge.
Our primary hypothesis was that participants randomized to the smoking cessation intervention would achieve greater 7-day point prevalence tobacco abstinence over 18 months after hospitalization than participants randomized to the usual care control condition. We examined psychiatric variables predictive of cessation success or failure. Our secondary aim was to assess the impact of the tobacco cessation intervention on mental health recovery and prediction of rehospitalization over the 18-month study follow-up, with adjustment for relevant clinical covariates.
METHODS
We recruited adult smokers between July 2006 and December 2008 from the locked inpatient psychiatry unit at the Langley Porter Psychiatric Institute, located on the University of California, San Francisco medical school campus. The institute implemented a 100% smoking ban in 1988 when the medical school campus went smoke-free. Similar to reports elsewhere,14 the conversion to a smoke-free unit was met with very little disruption to clinical care.34 Despite the ban, however, and consistent with other psychiatric facilities, few smokers treated at the hospital were advised or counseled to quit or provided with cessation medication or referrals at discharge.8,16
Procedures
Participants.
Research staff identified potentially eligible patients in the medical record and requested an introduction from unit staff. Patients interested in hearing about the study met with research staff to determine eligibility and complete informed consent procedures. After they completed the baseline assessment, we randomly assigned participants to the intervention or usual care condition through a computer-generated random assignment program stratified by cigarettes per day prior to hospitalization (> 15) and stage of change, variables predictive of quitting smoking and addressed by the intervention.35,36 Research staff were blinded to the randomization schedule.
Study inclusion criteria were smoking at least 5 cigarettes per day (because of provision of NRT in the intervention), being aged 18 years or older, and being fluent in English. Intention to quit smoking was not required. We required contact information for at least 2 collateral contacts for study tracking and confirming changes in smoking status. Exclusion criteria were contraindications for NRT use (e.g., recent myocardial infarction, pregnancy), high violence risk, and inability to consent because of hypersomnolence or severity of psychiatric symptoms. Patients who were aggressive or assaultive on the unit or unable to concentrate for 15 minutes because of their symptoms or sedation were reapproached later to assess eligibility.
Intervention.
Initiated during hospitalization by study staff, the intervention followed the Transtheoretical Model, which identifies 5 stages in quitting smoking: precontemplation (no intention to stop smoking), contemplation (intending to quit in the next 6 months), preparation (considering quitting in the next month with a 24-hour quit attempt in the past year), action (quit smoking for < 6 months), and maintenance (smoke-free for ≥ 6 months).37 Other key constructs of the model are temptations, decisional balance, and the processes of change. On-unit intervention components were access to NRT; completion of a computer-delivered, Transtheoretical Model–tailored intervention program with printed individualized report tailored to stage of change, temptations, decisional balance, and the processes of change; a stage-tailored print manual; a 15- to 30-minute cessation counseling session with a study counselor; and a letter mailed to the participant’s outpatient provider requesting cessation support. The individualized report directed participants to relevant exercises in the manual; materials were written at a sixth-grade reading level. Posthospitalization intervention contacts at months 3 and 6 repeated the computer intervention, which remembered participants’ earlier responses and provided ipsative feedback on how they changed over time, recommending next steps toward quitting smoking and maintaining abstinence. Study-provided nicotine patches were available for 10 weeks, initiated once the participant was ready to quit smoking and available up to the 6-month follow-up. To prevent loss or misuse, the patches were delivered in 2 installments (4- and 6-week supplies). We previously reported on the acceptability of the intervention components evaluated with the target population.38
Power.
We based power calculations on analysis of multiple time points and adjusted for projected rates of attrition.39 With 18-month 7-day point prevalence abstinence estimates of 7% and 14% in the control and intervention conditions, respectively, a required sample size of approximately 100 per group would result in minimum statistical power of 0.80 with type I error of 0.05. An increase of 7% abstinence would equate to nearly 6000 additional psychiatric inpatients going smoke-free annually in California alone.30
Measures
Descriptive measures.
We interviewed participants about their age, gender, race/ethnicity, completed education, annual income, housing stability, employment, marital status, and tobacco use history. We used the Fagerström Test of Nicotine Dependence,40 including time to first cigarette; smoking stages of change scale41; the Thoughts about Abstinence scale (desire, success, and difficulty, rated on 10-point scales)42; the Behavior and Symptom Identification Scale43; the Center for Epidemiologic Studies Depression Scale44; the 12-item Short Form45; the Alcohol Use Disorders Identification Test46; and the Drug Abuse Screening Test 10.47 We assessed Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition mood and schizophrenia spectrum disorders with the Computerized Diagnostic Interview Schedule.48 We also reviewed participants’ medical records and coded them for cause and duration of hospitalization.
Outcome measures.
The primary outcome was tobacco abstinence, assessed and verified at the 3-, 6-, 12-, and 18-month follow-ups as the number of cigarettes, even a puff, smoked in the past week (7-day abstinence) or use of any form of tobacco. Consensus guidelines recommend use of 7-day point prevalence abstinence in cessation induction studies with smokers unmotivated to quit, who will be quitting at different time points in the trial.49 Those reporting “no smoking, not even a puff” completed biochemical verification with an expired air carbon monoxide (CO) sample analyzed by a Bedfont Smokerlyzer. Carbon monoxide of 10 parts per million or less verified abstinence. For participants lost to follow-up or unable to return for biochemical verification, we called significant others listed on the study contact form to verify smoking status. We asked these informants whether the study participant had used any tobacco, even just a single puff on a cigarette, in the past 7 days.
The secondary outcome was rehospitalization for psychiatric illness. At each follow-up, we queried participants about overnight hospitalizations for psychiatric care.50 We assessed inpatient stays because hospitalization is the most costly type of care, reflects mental health decompensation, and is salient and hence easier for patients to recall than outpatient care. We documented rehospitalizations at Langley Porter Psychiatric Institute with the electronic billing system.
Analyses
Descriptive statistics summarized sample characteristics and the extent of intervention delivery. We examined treatment condition and baseline descriptive characteristics as predictors of attrition at trial end (month 18) and proposed a priori to control for predictors of attrition as a covariate in model testing.
To test the primary hypothesis, we ran a generalized estimating equation model with the logit link function (PROC GENMOD in SAS version 9.2; SAS Institute, Cary, NC), to examine abstinence versus smoking status at the 3- through 18-month follow-ups by condition. The model accounted for dependence of responses within individuals attributable to repeated measures and allowed us to derive effect estimates from all available data. The independent variables were intervention versus control condition plus variables that differed by condition at baseline or predicted attrition. We also used multiple imputation procedures to impute missing data, and for comparison with the literature, we examined abstinence rates with missing data coded as indicative of smoking.
To better understand smoking behaviors among this clinical sample, we next examined demographic variables, tobacco use, psychiatric symptoms, and substance use as potential covariates of treatment effects. We tested associations first in univariate analyses and then entered significant variables into the final model.
For the secondary outcome of psychiatric rehospitalization, we ran a logistic regression with rehospitalization at any point during the 18-month trial as the dependent variable and independent variables of treatment condition, abstinence status, and covariates identified as relevant to psychiatric hospitalization in the literature.
RESULTS
During the 30-month recruitment period, the hospital had 1430 admissions. Patients smoking fewer than 5 cigarettes per day or living out of the area were not approached. Few patients were excluded for cognitive limitations (n = 8), agitation (n = 7), medical contraindications (n = 6), lack of contact information (n = 3), or being discharged from the hospital before full study enrollment (n = 3). Of 285 smokers identified as study eligible, 275 agreed to speak to study staff, and 224 provided informed consent, for a 79% recruitment rate (Figure A, available as a supplement to the online version of this article at http://www.ajph.org). The sample was demographically similar to the hospital’s patient population, and the 2 conditions did not differ on any of the baseline measured variables (for all comparisons, P > .1), indicating that the randomization was successful (Table 1).
TABLE 1—
Demographic, Clinical, and Tobacco Characteristics of Sample of Psychiatric Inpatients in a Randomized, Controlled Trial of a Smoking Cessation Intervention: San Francisco, CA, July 2006–December 2008
| Characteristic | Control Group (n = 111), Mean ±SD or No. (%) | Intervention Group (n = 113), Mean ±SD or No. (%) | Full Sample (n = 224), Mean ±SD or No. (%) |
| Demographic characteristics | |||
| Age, y | 39.9 ±14.1 | 39.9 ±13.5 | 39.9 ±13.8 |
| Gender | |||
| Male | 71 (64) | 63 (55.8) | 134 (59.8) |
| Female | 36 (32.4) | 48 (42.5) | 84 (37.5) |
| Transgender | 4 (3.6) | 2 (1.8) | 6 (2.7) |
| Race/ethnicity | |||
| White | 72 (64.9) | 74 (65.5) | 146 (65.2) |
| African American | 8 (7.2) | 12 (10.6) | 20 (8.9) |
| Hispanic/Latino | 7 (6.3) | 7 (6.2) | 14 (6.3) |
| Asian American/Pacific Islander | 10 (9.0) | 9 (8.0) | 19 (8.5) |
| Multiracial/other | 14 (12.6) | 11 (9.7) | 25 (11.2) |
| Marital status | |||
| Married/cohabiting | 24 (21.6) | 17 (15.0) | 41 (18.3) |
| Divorced, separated, widowed | 22 (19.8) | 25 (22.1) | 47 (21.0) |
| Single, never married | 65 (58.6) | 71 (62.8) | 136 (60.7) |
| Annual individual income, $ | |||
| < 10 000 | 35 (32.7) | 41 (37.3) | 76 (35.0) |
| 10 000–20 999 | 31 (29.0) | 24 (21.8) | 55 (25.3) |
| 21 000–40 999 | 20 (18.7) | 19 (17.3) | 39 (18.0) |
| ≥ 41 000 | 21 (19.6) | 26 (23.6) | 47 (21.0) |
| Unstably housed | 37 (33.3) | 36 (31.9) | 73 (32.6) |
| Education, y | 14.4 ±3.3 | 14.6 ±3.2 | 14.5 ±3.2 |
| Employment | |||
| Unemployed | 66 (59.5) | 59 (52.2) | 125 (55.8) |
| Employed | 25 (22.5) | 38 (33.6) | 63 (28.1) |
| Retired/student/homemaker | 20 (18.0) | 16 (14.2) | 36 (16.1) |
| Insurance coverage | |||
| Self-pay | 9 (8.1) | 11 (9.7) | 20 (8.9) |
| Private | 45 (40.5) | 58 (51.3) | 103 (46.0) |
| Medicare/MediCal | 57 (51.4) | 44 (38.9) | 101 (45.1) |
| Clinical characteristics | |||
| Reason for hospitalization | |||
| Voluntary admission | 13 (11.7) | 15 (13.3) | 28 (12.5) |
| Danger to self | 86 (77.5) | 83 (73.5) | 169 (75.4) |
| Danger to others | 1 (0.9) | 4 (3.5) | 5 (2.2) |
| Grave disabilitya | 11 (9.9) | 11 (9.7) | 22 (9.8) |
| Length of stay, d | 8.0 ±6.2 | 6.8 ±5.2 | 7.4 ±5.7 |
| Has psychiatric disability pension | 35 (32.7) | 27 (24.1) | 62 (27.7) |
| CESD-10 scoreb | 20.1 ±6.7 | 18.8 ±8.0 | 19.4 ±7.4 |
| Diagnosis | |||
| Unipolar disorder | 52 (46.8) | 53 (46.9) | 105 (46.9) |
| Bipolar disorder | 27 (24.3) | 29 (25.7) | 56 (25.0) |
| Schizophrenia spectrum disorder | 21 (18.9) | 13 (11.5) | 34 (15.2) |
| Other | 11 (9.9) | 18 (15.9) | 29 (12.9) |
| BASIS-24 summary scalec | 2.2 ±0.7 | 2.1 ±0.8 | 2.1 ±0.8 |
| AUDIT total scored | 10.7 ±9.9 | 9.2 ±9.8 | 10.0 ±9.9 |
| DAST-10 total scoree | 4.3 ±3.4 | 3.6 ±3.8 | 4.0 ±3.6 |
| SF-12f | |||
| Physical component summary | 49.3 ±12.4 | 48.0 ±12.6 | 48.7 ±12.5 |
| Mental component summary | 26.4 ±11.9 | 29.2 ±16.7 | 27.8 ±12.9 |
| Previous psychiatric hospitalization | |||
| None | 30 (27.0) | 33 (29.2) | 63 (28.1) |
| 1–2 | 23 (20.7) | 31 (27.4) | 54 (24.1) |
| 3–7 | 32 (28.8) | 23 (20.4) | 55 (24.6) |
| ≥ 8 | 26 (23.4) | 26 (23.0) | 52 (23.2) |
| Tobacco characteristics | |||
| Usual cigarettes/d | 19.0 ±14.7 | 18.9 ±11.2 | 19.0 ±13.0 |
| Cigarettes in 24 h prior to hospitalization | 20.3 ±14.5 | 19.7 ±13.4 | 20.0 ±13.9 |
| Cigarettes/d in wk prior to hospitalization | 19.0 ±16.3 | 19.1 ±12.9 | 19.0 ±14.7 |
| FTND total score | 4.7 ±2.6 | 4.8 ±2.5 | 4.7 ±2.5 |
| Time to first cigarette ≤ 30 min | 81 (73) | 88 (78) | 169 (75.4) |
| Age when first smoked a cigarette | 16.6 ±7.8 | 15.1 ±5.2 | 15.8 ±6.7 |
| Age when started smoking regularly | 19.3 ±8.5 | 19.6 ±7.8 | 19.4 ±8.1 |
| Smoking, y | 20.2 ±13.7 | 19.6 ±13.6 | 19.9 ±13.6 |
| Thoughts about abstinence | |||
| Desire to stop | 5.4 ±3.0 | 5.8 ±3.1 | 5.6 ±3.0 |
| Expectancy of success with quitting | 5.0 ±2.9 | 5.0 ±3.2 | 5.0 ±3.1 |
| Difficulty staying abstinent | 7.4 ±2.5 | 7.5 ±2.6 | 7.4 ±2.6 |
| Stage of Change | |||
| Precontemplation | 44 (39.6) | 42 (37.2) | 86 (38.4) |
| Contemplation | 49 (44.1) | 54 (47.8) | 103 (46.0) |
| Preparation | 18 (16.2) | 17 (15.0) | 35 (15.6) |
| Quit attempt in past y (≥ 24 h) | 42 (38.2) | 54 (47.8) | 96 (42.9) |
| Quit attempt in lifetime (≥ 24 h) | 88 (81.5) | 97 (85.8) | 185 (82.6) |
| Advised to quit in past y by mental health provider | 54 (48.6) | 53 (47.3) | 107 (47.8) |
| Advised to quit in past y by other medical provider | 69 (62.2) | 63 (55.8) | 132 (58.9) |
| Ever received cessation counseling from health care provider | 5 (4.5) | 4 (3.5) | 9 (4.0) |
Note. AUDIT = Alcohol Use Disorders Identification Test; BASIS = Behavior and Symptom Identification Scale; CESD = Center for Epidemiologic Studies Depression Scale; DAST = Drug Abuse Screening Test; FTND = Fagerström Test of Nicotine Dependence; SF = Short Form. All group comparisons for intervention vs control, P > .1. For comparison, 572 unique patients were hospitalized at Langley Porter Psychiatric Institute from 2006 to 2007 with mean age of 44.3 years (SD = 16.4); 51% were men; 66% were White, 11% Asian American/Pacific Islander, 8% African American, 4% Latino, and 11% other ethnic/racial group. Clinical diagnoses were 47% unipolar depression, 16% bipolar, and 26% psychotic disorders, including schizophrenia spectrum disorders or mood disorders with psychotic symptoms. A majority (75%) were privately insured, and length of stay averaged 10 days with a median of 6.
Mental disorder that renders a person unable to take care of basic needs or obtain food, clothing, and shelter.
Range = 0–30; ≥ 11 indicates significant depressive symptoms.
Range = 0–4; higher indicates worse mental health.
Range = 0–40; ≥ 8 indicates hazardous and harmful drinking.
Range = 0–10; ≥ 3 indicates moderate drug problems.
Range = 0–100, national norm = 50, SD = 10; lower scores indicate worse functioning.
The mean age of the sample (n = 224) was 40 years (SD = 14 years); 60% of participants were male, and 65% were White. Major psychiatric diagnosis groups were unipolar depression (47%), bipolar depression (25%), and schizophrenia spectrum disorders (15%). A minority (46%) were privately insured. Hospital stays averaged 7.4 days (SD = 5.7; median = 6.0; mode = 5).
On the Behavior and Symptom Identification Scale 24, participants’ severity scores averaged 1.2 (SD = 1.2) on the psychosis subscale and 2.1 (SD = 0.8) for the summary score, slightly higher (more severe) than published values for an inpatient sample.51 A sample majority (69%) reported problematic alcohol or illicit drug use on the Alcohol Use Disorders Identification Test and Drug Abuse Screening Test. Reasons for hospitalization were danger to self (75%), grave disability (10%), and danger to others (2%); 13% were voluntary admissions. Most participants (72%) had a previous psychiatric hospitalization.
Participants averaged around a pack of cigarettes per day, and the amount that they smoked in the day and week prior to hospitalization did not differ from their reported usual daily number of cigarettes (Table 1). The sample was moderately nicotine dependent (Fagerström Test of Nicotine Dependence score: mean = 4.7; SD = 2.5); 75% reported smoking their first cigarette within 30 minutes of waking. Most (83%) quit for 24 hours in their lifetime and 43% in the past year. Smoking stages of change were precontemplation (38%), contemplation (46%), and preparation (16%). In the past year, 48% of participants were advised to quit smoking by a mental health provider and 59% by a general health provider. Only 4% reported ever receiving cessation counseling from a medical provider.
Intervention Delivery and Retention
Among intervention participants, 97% completed the initial computer and counseling session during their acute stay, 2% completed it after leaving the hospital, and 1 participant left the hospital abruptly and was unreachable. After hospitalization, 72% and 50% completed their second and third computer intervention contacts, respectively. About half of intervention participants (49%) accessed study-provided NRT, with a mean of 7 weeks requested (SD = 3 weeks).
More than 80% of follow-up assessments were completed at all time points, with no difference by treatment condition; retention was 80% for the intervention group and 82% for the usual care group at the 18-month follow-up (Figure A). Of the measured variables, only stage of change predicted 18-month completion; retention for participants in precontemplation was 72%; in contemplation, 89%; and in preparation, 80% (P = .018). Four participants died during their involvement in the study, with 2 deaths in each condition. Causes of death were suicide and homicide (intervention condition) and lung disease52 and drug overdose (usual care).
Abstinence Status
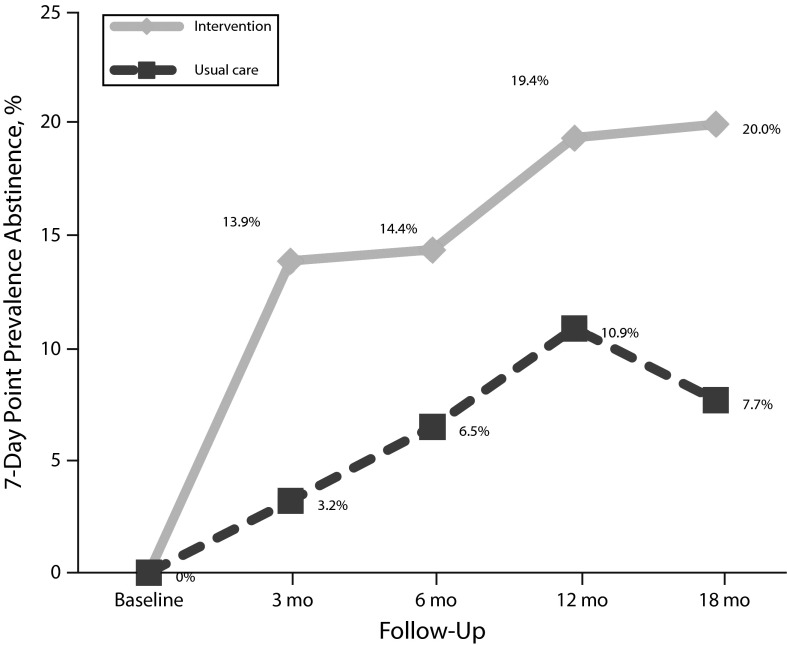
To test the primary hypothesis concerning treatment differences in tobacco abstinence, we ran a generalized estimating equation linear model comparing the verified 7-day point prevalence abstinence rates across the postbaseline assessments, with adjustment for stage of change, which was related to attrition. Verified 7-day point prevalence abstinence rates were 3.2% for control and 13.9% for treatment participants at the 3-month follow-up, 6.5% and 14.4% at 6 months, 10.9% and 19.4% at 12 months, and 7.7% and 20.0% at 18 months, respectively. We modeled the abstinence rates over 18 months and found that the treatment conditions were significantly different (generalized estimating equation model: odds ratio [OR] = 3.15; 95% confidence interval [CI] = 1.22, 8.14; P = .018; Figure 1). We confirmed abstinence by CO testing (33%) and collateral reports (67%), with no difference by group for type of verification (χ2 = 0.03; df = 1; P = .873). The lack of condition × time interaction effect was attributable to our not including the baseline assessment (all smokers) in the model. The greatest separation between conditions occurred at the first assessment. The results indicated that, summed across all time points, the treatment condition resulted in a greater percentage of abstinent participants than the usual care condition, which was a direct test of our primary hypothesis.
FIGURE 1—
Verified point prevalence abstinence rates by treatment condition and time in a randomized controlled trial of a smoking cessation intervention among psychiatric inpatients: San Francisco, CA, July 2006–December 2008
Next, we examined potential covariates of treatment effects to better understand quitting smoking in this clinical population. Demographic variables, psychiatric diagnoses, and baseline measures of mood (Center for Epidemiologic Studies Depression Scale, Behavior and Symptom Identification Scale 24, Short Form 12) and substance use (Alcohol Use Disorders Identification Test, Drug Abuse Screening Test) did not predict abstinence at any follow-up time point (all, P ≥ .156). Baseline measures of motivation (stages of change, thoughts about abstinence scales) and dependence (time to first cigarette) predicted abstinence status significantly (P < .05), and we entered them into a multivariate model (Table 2). Significant terms in the final model were treatment condition, smoking within 30 minutes of waking, expectation of success with quitting, and perceived difficulty with avoiding smoking relapse.
TABLE 2—
Model Predicting Tobacco Abstinence Over an 18-Month Study Period in a Randomized Controlled Trial of a Smoking Cessation Intervention Among Psychiatric Inpatients: San Francisco, CA, July 2006–December 2008
| Variable | OR (95% CI) | P |
| Intercept | 0.34 (0.05, 2.45) | .281 |
| Condition (usual care) | 0.26 (0.09, 0.72) | .01 |
| Time | 1.03 (0.99, 1.08) | .181 |
| Condition × time | 1.01 (0.94, 1.09) | .752 |
| Desire to quit | 1.02 (0.90, 1.15) | .785 |
| Anticipated success with quitting | 1.17 (1.06, 1.31) | .003 |
| Perceived difficulty with staying quit | 0.86 (0.76, 0.97) | .017 |
| Time to first cigarette < 30 min | 0.51 (0.26, 0.96) | .039 |
| Stage of change | ||
| Precontemplation | 0.52 (0.38, 2.10) | .227 |
| Contemplation | 0.90 (0.18, 1.51) | .803 |
| Preparation (Ref) | 1.00 |
Note. CI = confidence interval; OR = odds ratio. The sample size was n = 224.
To check whether the missing data biased our findings, we used multiple imputation.53,54 We used PROC MI and PROC MIANALYZE in SAS version 9.3 with 20 imputations and a fully conditional specification approach for an arbitrary missing data pattern of a categorical variable (i.e., smoking status).55 The final resulting P value for treatment condition was .013, supporting our main finding.
For comparison with the literature, we coded participants lost to follow-up as smoking, excluding deceased participants. Verified 7-day point prevalence abstinence rates were 2.8% for control and 12.4% for treatment participants at the 3-month follow-up, 5.5% and 12.5% at 6 months, 9.2% and 16.1% at 12 months, and 6.4% and 16.2% at 18 months, respectively. We modeled the abstinence rates over 18 months and found significant differences for the treatment condition (generalized estimating equation model: OR = 3.39; 95% CI = 1.32, 8.72; P = .011).
Psychiatric Rehospitalization
The sample had 234 psychiatric rehospitalizations: 140 among control and 94 among intervention participants (t (223) = 2.10; P = .036). Over 18 months of follow-up, 47% of participants were rehospitalized, comparable to California statewide data (44% in 12 months).30 Because of the large proportion of zeros in the distribution, we dichotomized the data as rehospitalized versus not: 56% in usual care versus 44% in intervention. Abstinence status was not associated with rehospitalization at any follow-up point (P > .282). For inclusion in model testing, a single calculated variable indicated tobacco abstinence at any follow-up point. A logistic regression with rehospitalization as the dependent variable tested independent variables of treatment condition, abstinence status, and covariates identified as relevant to psychiatric hospitalization in the literature. In the final model, treatment condition, previous hospitalization, baseline psychotic symptoms, and unstable housing were significant predictors of psychiatric rehospitalization, and years of education and tobacco abstinence were not (Table 3). An association between rehospitalization and African American race, although it did not reach the traditional cut-off for statistical significance, had a P value very close to .05. For treatment condition, the significant OR indicated a 92% greater likelihood of rehospitalization among usual care than intervention participants.
TABLE 3—
Model Predicting Rehospitalization Over an 18-Month Study Period in a Randomized Controlled Trial of a Smoking Cessation Intervention Among Psychiatric Inpatients: San Francisco, CA, July 2006–December 2008
| Variable | OR (95% CI) | P |
| Condition (usual care) | 1.92 (1.06, 3.49) | .031 |
| Race (African American) | 3.04 (0.97, 9.58) | .057 |
| Psychotic symptoms (BASIS-24) | 1.43 (1.09, 1.89) | .01 |
| Education, y | 1.06 (0.97, 1.16) | .219 |
| Unstably housed | 2.09 (1.12, 3.92) | .021 |
| Quit during 18-mo trial | 0.56 (0.28, 1.14) | .108 |
| Previous psychiatric hospitalization | ||
| None (Ref) | 1.00 | |
| 1–2 | 1.60 (0.70, 3.63) | .266 |
| 3–7 | 2.13 (0.95, 4.77) | .067 |
| ≥ 8 | 3.21 (1.37, 7.54) | .008 |
Note. BASIS = Behavior and Symptom Identification Scale; CI = confidence interval; OR = odds ratio. The sample size was n = 224.
DISCUSSION
Despite the high prevalence of smoking among individuals with mental illness, the low rates of quitting, and the devastating health effects, clinical treatment research with comorbid smokers remains limited. Ours was the first study with a substantial sample size to prospectively track the smoking behaviors and rehospitalizations of psychiatric patients over 18 months. Our findings indicated that smokers hospitalized with psychiatric disorders will enter into treatment and can successfully quit smoking and that tobacco cessation treatment does not increase, and may even decrease, rehospitalization risk, perhaps by providing broader therapeutic benefit.
These data are encouraging, build upon existing evidence of tobacco treatment success with smokers with mental illness,24–29 and support the feasibility and efficacy of initiating tobacco treatment services in inpatient psychiatry. Demographic variables, psychiatric diagnosis, and measures of mental health and nonnicotine substance use severity did not predict differences in quitting tobacco, but measures of cessation motivation and tobacco dependence did. This sample of smokers with serious acute mental illness appeared similar to the general population in response to treatment, with an 18-month quit rate of 20% (vs 25% in the general population),35 and in predictors of treatment outcome.
Our findings confirm the efficacy of an innovative, acceptable, and feasible multicomponent intervention for initiating tobacco treatment in inpatient psychiatry. Although few participants were ready and intending to quit smoking at the time of study enrollment, smoking abstinence increased over the study period, indicating engagement in the quitting process. This pattern of increasing abstinence over time is characteristic of stage-tailored interventions: the proportion of individuals reaching the action or maintenance stages of change increases over time35 and contrasts with the characteristic relapse curves of action-oriented cessation interventions initiated with individuals highly motivated to quit smoking.
Hospitalization, lasting a median of 6 days, offered an opportunity for initiating tobacco treatment, with nearly all participants completing the on-unit intervention components prior to hospital discharge. Smoke-free psychiatric hospitalizations afford a unique window of opportunity for initiating tobacco treatment that is not currently provided in other settings. At baseline, fewer than half of participants reported being advised by a mental health provider to quit smoking in the past year, and only 4% reported ever receiving cessation counseling from a medical provider.
Strengths and Limitations
Our study, with a 79% recruitment rate, diagnostically mixed sample, and more than 80% retention, had high internal and external validity and supports the broad delivery of cessation intervention services in the inpatient psychiatric setting. Another study strength was the consistency in findings under different strategies of handling and modeling missing data. Outcomes comprised both tobacco abstinence and mental health recovery. The finding of fewer hospitalizations over time in the treatment group is novel and warrants further investigation to determine replicability and to identify the causal mechanisms, such as possible generalization of greater therapeutic contact in the intervention group to mental health outcomes.
Limitations were implementation at a single site and a sample size not powered to detect differences by diagnostic group, although patterns suggested little variability in treatment effects. Although we have no compelling reason to expect these results not to generalize to other populations of smokers hospitalized for psychiatric disorders, additional studies are needed, especially with samples with a larger proportion of poor, non-White, less educated, uninsured smokers and smokers with schizophrenia spectrum disorders. Notably, the quit rate for participants with schizophrenia was 20% in the treatment group and 7% in the control group, similar to the sample’s overall abstinence rates.
To verify abstinence status, we used a combination of CO and collateral contact reports. We used this hybrid form of verification because of the anticipated complexity of getting participants to travel to the research offices to complete verification, the tailoring of treatment to readiness to quit (i.e., quitting was not a set expectation), and the greater acceptance of tobacco use among psychiatric populations; hence, demand characteristics of the trial were low, providing little incentive for participants to falsify their abstinence status. Collateral contact reports also had the advantage of covering a longer period of exposure (7 days) relative to CO (24 hours). Previous analytic reviews have refuted the necessity of bioconfirmation of tobacco abstinence,56,57 and a randomized controlled tobacco treatment study by Patten et al. with 256 smokers with alcohol use disorders found that collateral contact reports contradicted self-reported abstinence as often as or more than CO testing and showed high concordance (92%) with CO levels.58 In our study, collateral contacts were approached if participants could not be located or, among participants reporting abstinence, to confirm nonsmoking status. We found that collateral contacts were twice as likely to report participants to be smoking as nonsmoking. Among participants reporting abstinence, verifications corroborated self-report in all but 3 cases.
Conclusions
Our findings demonstrate that supporting people with serious mental illness in smoking cessation efforts during their hospital stay and beyond is feasible and worthwhile. It is possible to provide effective support across the hospital and community continuum, with little burden on services, with significant effects on cessation, and without harm to mental health recovery.
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse (K23 DA018691 to J. J. P. and K05 DA016752 and P50 DA09253 to S. M. H.). The computerized tailored tobacco cessation program and print workbook based on the Transtheoretical Model were developed by Pro-Change Behavior Systems, Inc.
We acknowledge the expert advice and consultation provided by Neal Benowitz, MD, Victor Reus, MD, and C. Barr Taylor, MD, and the contributions of the fellows and research staff who worked on this trial over the 5 years of investigation: Janine Cataldo, PhD, RN, Jessica Clifton, MA, Jennifer Dukes, Kathleen Gali, MPH, Norval Hickman, PhD, MPH, Desiree Leek, MD, Anayansi Lombadero, Maryam Najafi, MD, and Amanda Schweizer.
Human Participant Protection
The institutional review board of the University of California, San Francisco approved the study procedures, and all participants provided informed consent.
References
- 1.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 2.McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010;100(12):2464–2472. doi: 10.2105/AJPH.2009.188136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg ML, Williams JM, Ziedonis DM. Financial implications of cigarette smoking among individuals with schizophrenia. Tob Control. 2004;13(2):206. [PMC free article] [PubMed] [Google Scholar]
- 4.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder SA. A 51-year-old woman with bipolar disorder who wants to quit smoking. JAMA. 2009;301(5):522–531. doi: 10.1001/jama.2008.982. [DOI] [PubMed] [Google Scholar]
- 6.Crockford D, Kerfoot K, Currie S. The impact of opening a smoking room on psychiatric inpatient behavior following implementation of a hospital-wide smoking ban. J Am Psychiatr Nurses Assoc. 2009;15(6):393–400. doi: 10.1177/1078390309353347. [DOI] [PubMed] [Google Scholar]
- 7.Robertson ER. I am a psychiatrist at the Hawaii State Hospital. Bates no. 522700931–522700932. Tobacco Documents Online. Available at: http://tobaccodocuments.org/landman/522700931-0932.html. Accessed March 8, 2010.
- 8.Prochaska JJ, Fletcher L, Hall SE, Hall SM. Return to smoking following a smoke-free psychiatric hospitalization. Am J Addict. 2006;15(1):15–22. doi: 10.1080/10550490500419011. [DOI] [PubMed] [Google Scholar]
- 9.Prochaska JJ, Reyes RS, Schroeder SA, Daniels AS, Doederlein A, Bergeson B. An online survey of tobacco use, intentions to quit, and cessation strategies among people living with bipolar disorder. Bipolar Disord. 2011;13(5–6):466–473. doi: 10.1111/j.1399-5618.2011.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickens GL, Stubbs JH, Haw CM. Smoking and mental health nurses: a survey of clinical staff in a psychiatric hospital. J Psychiatr Ment Health Nurs. 2004;11(4):445–451. doi: 10.1111/j.1365-2850.2004.00741.x. [DOI] [PubMed] [Google Scholar]
- 11.Accreditation Manual for Hospitals. Oakbrook Terrace, IL: Joint Commission on Accreditation of Healthcare Organizations; 1992. [PubMed] [Google Scholar]
- 12.Foderaro LW. Battling demons, and nicotine hospitals’ smoking bans are new anxiety for mentally ill. New York Times. February 19, 1995 [Google Scholar]
- 13.House of Commons Health Committee. Smoking in Public Places: First Report of Session 2005–06. Vol HC 485-I. London, UK: Stationery Office Limited; 2005. [Google Scholar]
- 14.Lawn S, Pols R. Smoking bans in psychiatric inpatient settings? A review of the research. Aust N Z J Psychiatry. 2005;39(10):866–885. doi: 10.1080/j.1440-1614.2005.01697.x. [DOI] [PubMed] [Google Scholar]
- 15.Lane JGM, Werdel MB, Schacht L, Ortiz G, Parks J. Smoking policies and practices in state psychiatric facilities: survey results from 2008. Alexandria, VA: National Assocation of State Mental Health Program Directors Research Institute, Inc; 2009. [Google Scholar]
- 16.Prochaska JJ, Gill P, Hall SM. Treatment of tobacco use in an inpatient psychiatric setting. Psychiatr Serv. 2004;55(11):1265–1270. doi: 10.1176/appi.ps.55.11.1265. [DOI] [PubMed] [Google Scholar]
- 17.Fiore MC, Jaen CR, Baker TB . Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- 18.Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD001837.pub2. 2007(3):CD001837. [DOI] [PubMed] [Google Scholar]
- 19.Banham L, Gilbody S. Smoking cessation in severe mental illness: what works? Addiction. 2010;105(7):1176–1189. doi: 10.1111/j.1360-0443.2010.02946.x. [DOI] [PubMed] [Google Scholar]
- 20.Brown RA, Ramsey SE, Strong DR et al. Effects of motivational interviewing on smoking cessation in adolescents with psychiatric disorders. Tob Control. 2003;12(suppl 4):iv3–iv10. doi: 10.1136/tc.12.suppl_4.iv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Practice Guideline for the Treatment of Patients with Substance Use Disorders. 2nd ed. Arlington, VA: American Psychiatric Association; 2006. [Google Scholar]
- 22.Prochaska JJ. Ten critical reasons for treating tobacco dependence in inpatient psychiatry. J Am Psychiatr Nurses Assoc. 2009;15(6):404–409. doi: 10.1177/1078390309355318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prochaska JJ, Hall SM, Bero LA. Tobacco use among individuals with schizophrenia: what role has the tobacco industry played? Schizophr Bull. 2008;34(3):555–567. doi: 10.1093/schbul/sbm117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall SM, Prochaska JJ. Treatment of smokers with co-occurring disorders: emphasis on integration in mental health and addiction treatment settings. Annu Rev Clin Psychol. 2009;5:409–431. doi: 10.1146/annurev.clinpsy.032408.153614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evins AE. Review: bupropion increases abstinence from smoking without affecting mental state in people with schizophrenia. Evid Based Ment Health. 2010;13(4):120. doi: 10.1136/ebmh.13.4.120. [DOI] [PubMed] [Google Scholar]
- 26.Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD007253.pub2. 2010(6):CD007253. [DOI] [PubMed] [Google Scholar]
- 27.Williams JM, Anthenelli RM, Morris CD et al. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(5):654–660. doi: 10.4088/JCP.11m07522. [DOI] [PubMed] [Google Scholar]
- 28.George TP, Vessicchio JC, Sacco KA et al. A placebo-controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia. Biol Psychiatry. 2008;63(11):1092–1096. doi: 10.1016/j.biopsych.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu BS, Weinberger AH, Mancuso E et al. A preliminary feasibility study of varenicline for smoking cessation in bipolar disorder. J Dual Diagn. 2012;8(2):131–132. doi: 10.1080/15504263.2012.671067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Readmissions to California Hospitals, 2005 to 2006. Sacramento, CA: California Office of Statewide Health Planning and Development; 2010. [Google Scholar]
- 31.Martinez JM, Marangell LB, Simon NM et al. Baseline predictors of serious adverse events at one year among patients with bipolar disorder in STEP-BD. Psychiatr Serv. 2005;56(12):1541–1548. doi: 10.1176/appi.ps.56.12.1541. [DOI] [PubMed] [Google Scholar]
- 32.Reichler H, Baker A, Lewin T, Carr V. Smoking among in-patients with drug-related problems in an Australian psychiatric hospital. Drug Alcohol Rev. 2001;20(2):231–237. [Google Scholar]
- 33.Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction. 2009;104(5):719–733. doi: 10.1111/j.1360-0443.2009.02545.x. [DOI] [PubMed] [Google Scholar]
- 34.Haller E, McNiel DE, Binder RL. Impact of a smoking ban on a locked psychiatric unit. J Clin Psychiatry. 1996;57(8):329–332. [PubMed] [Google Scholar]
- 35.Prochaska JJ, Velicer WF, Prochaska JO, Delucchi K, Hall SM. Comparing intervention outcomes in smokers treated for single versus multiple behavioral risks. Health Psychol. 2006;25(3):380–388. doi: 10.1037/0278-6133.25.3.380. [DOI] [PubMed] [Google Scholar]
- 36.Gritz ER, Carr CR, Rapkin D et al. Predictors of long-term smoking cessation in head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 1993;2(3):261–270. [PubMed] [Google Scholar]
- 37.Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9):1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 38.Prochaska JJ, Hall SE, Hall SM. Stage-tailored tobacco cessation treatment in inpatient psychiatry. Psychiatr Serv. 2009;60(6):848. doi: 10.1176/appi.ps.60.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med. 1998;17(14):1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 40.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 41.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 42.Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. J Consult Clin Psychol. 1990;58(2):175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- 43.Eisen SV, Normand SL, Belanger AJ, Spiro A, 3rd, Esch D. The Revised Behavior and Symptom Identification Scale (BASIS-R): reliability and validity. Med Care. 2004;42(12):1230–1241. doi: 10.1097/00005650-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 45.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 47.Cocco KM, Carey KB. Psychometric properties of the Drug Abuse Screening Test in psychiatric outpatients. Psychol Assess. 1998;10(4):408–414. [Google Scholar]
- 48.Blouin AG, Perez EL, Blouin JH. Computerized administration of the Diagnostic Interview Schedule. Psychiatry Res. 1988;23(3):335–344. doi: 10.1016/0165-1781(88)90024-8. [DOI] [PubMed] [Google Scholar]
- 49.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 50.Barnett PG, Wong W, Hall S. The cost-effectiveness of a smoking cessation program for out-patients in treatment for depression. Addiction. 2008;103(5):834–840. doi: 10.1111/j.1360-0443.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 51.Dawood N, Vaccarino V, Reid KJ, Spertus JA, Hamid N, Parashar S. Predictors of smoking cessation after a myocardial infarction: the role of institutional smoking cessation programs in improving success. Arch Intern Med. 2008;168(18):1961–1967. doi: 10.1001/archinte.168.18.1961. [DOI] [PubMed] [Google Scholar]
- 52.Prochaska JJ, Schane R, Leek D, Hall SE, Hall SM. Investigation into the cause of death of a 56-year-old man with serious mental illness. Am J Psychiatry. 2008;165(4):453–456. doi: 10.1176/appi.ajp.2007.07091455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schafer JL. Analysis of Incomplete Multivariate Data. New York, NY: Chapman and Hall; 1997. [Google Scholar]
- 54.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- 55.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 56.Glasgow RE, Mullooly JP, Vogt TM et al. Biochemical validation of smoking status: pros, cons, and data from four low-intensity intervention trials. Addict Behav. 1993;18(5):511–527. doi: 10.1016/0306-4603(93)90068-k. [DOI] [PubMed] [Google Scholar]
- 57.Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;111(1):23–41. doi: 10.1037/0033-2909.111.1.23. [DOI] [PubMed] [Google Scholar]
- 58.Patten CA, Martin JE, Filter KJ, Wolter TD. Utility and accuracy of collateral reports of smoking status among 256 abstinent alcoholic smokers treated for smoking cessation. Addict Behav. 2002;27(5):687–696. doi: 10.1016/s0306-4603(01)00202-7. [DOI] [PubMed] [Google Scholar]