Abstract
We examined the relationship between serum 25-hydroxyvitamin D (25[OH]D) and all-cause mortality. We searched biomedical databases for articles that assessed 2 or more categories of 25(OH)D from January 1, 1966, to January 15, 2013. We identified 32 studies and pooled the data.
The hazard ratio for all-cause mortality comparing the lowest (0–9 nanograms per milliliter [ng/mL]) to the highest (> 30 ng/mL) category of 25(OH)D was 1.9 (95% confidence interval = 1.6, 2.2; P < .001). Serum 25(OH)D concentrations less than or equal to 30 ng/mL were associated with higher all-cause mortality than concentrations greater than 30 ng/mL (P < .01).
Our findings agree with a National Academy of Sciences report, except the cutoff point for all-cause mortality reduction in this analysis was greater than 30 ng/mL rather than greater than 20 ng/mL.
An inverse association was proposed between solar irradiance and incidence of colon and breast cancer, based on a mechanism involving insufficient vitamin D. Individuals with lower serum 25-hydroxyvitamin D (25[OH]D) have higher risk of breast1–3 and colon cancer,4–6 other specific cancers,7 all invasive cancers combined,8 and coronary heart disease.9,10 Physiological mechanisms for the inverse association of 25(OH)D with cancer have been reported.11
Despite research on the association between low vitamin D status and many diseases,12 no consensus has emerged on the optimal serum 25(OH)D concentration. The concern is whether it is safe to maintain serum 25(OH)D concentrations in the range high enough to prevent some types of cancers13–15 and coronary heart disease.9,10
We decided to analyze the strength and consistency of the inverse association between levels of serum 25(OH)D and age-adjusted mortality hazard ratios in a rapidly expanding field of public health. A previous meta-analysis summarized 12 studies,16 another summarized 14,17 and another summarized a broader range.18
We hypothesized that lower serum 25(OH)D was associated with higher all-cause mortality hazard ratios, and defined the age-adjusted hazard ratio for death from any cause as the outcome addressed by the meta-analysis. This analysis includes all studies of all-cause mortality hazard ratios by categories of serum 25(OH)D in healthy or general medical clinic cohorts that met the eligibility criteria. Twenty new studies of serum 25(OH)D and all-cause mortality entered the literature since the Zittermann et al. review,17 for a total of 32 in this review.19–50 Two studies in the review by Zittermann et al. did not meet the stringent inclusion criterion of the present study, and were not included.
METHODS
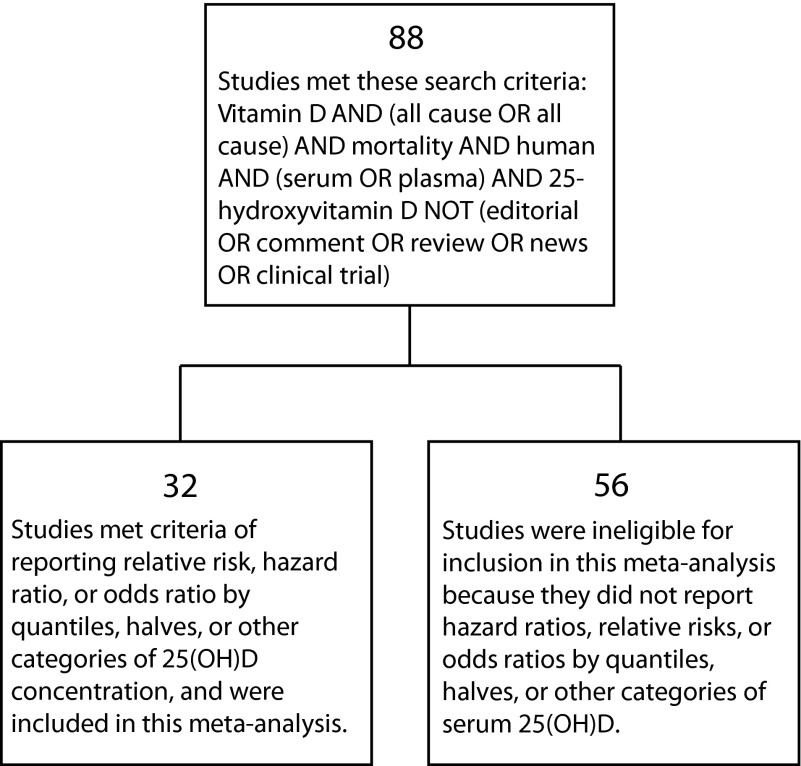
Two investigators (J. J. K. and S. B. M.) searched the biomedical literature for observational studies of serum 25(OH)D concentrations in association with age-adjusted all-cause mortality, published and indexed in databases between January 1, 1966, and January 15, 2013. The searchers were an experienced epidemiologist with a PhD in public health (epidemiology; S. B. M.) and a research scientist (J. K.). They found 88 studies (Figure 1). The inclusion criterion was any study reporting a measure of association according to 2 or more categories of serum 25(OH)D concentration. Of these, 32 studies19–50 met the inclusion criterion. The remaining 56 studies were ineligible for inclusion because they did not report an association by categories of serum 25(OH)D. We included studies of any design that met the inclusion criterion, although almost all (30 of 32) were cohort studies, whereas 2 were nested case–control studies within cohorts. Almost all were populations of volunteers recruited for follow-up studies or clinical trials, but a few were populations served by medical clinics (Table A, available as a supplement to this article at http://www.ajph.org). The investigators performed searches with PubMed, BIOSIS, Google Scholar, and Web of Science, and also performed hand searches of reference lists. All studies were published in medical journals, including 1 abstract.28 Because the analysis was limited to published research, we made no contacts with the authors of the articles included in the study.
FIGURE 1—
Results of literature search for studies of serum 25-hydroxyvitamin D in association with all-cause mortality.
Note. 25(OH)D = 25-hydroxyvitamin D.
The assembled studies were all routine types of epidemiological research performed with standard, widely recognized study designs. All studies directly addressed the hypothesis that individuals with higher serum 25(OH)D concentrations have lower age-adjusted all-cause mortality hazard ratios.
We obtained Forest plots by using RevMan version 5 (Oxford University, Cochrane Collaboration, Oxford, England) along with age-adjusted all-cause mortality hazard ratios according to serum 25(OH)D concentration. We computed means of these hazard ratios for each stratum of 25(OH)D to obtain an overall dose–response curve. We did not include studies based solely on self-reported oral intake of vitamin D or solar exposure.51
We calculated age-adjusted hazard ratios comparing the lowest with the highest quantile of serum 25(OH)D concentration by using the O-E and variance method for combining studies, an application of the Peto method.52 We calculated the overall O-E and variance by using the individual hazard ratios from each study and their 95% confidence intervals.53
We calculated the P value for the overall summary odds ratio by using a z-score, where the numerator was the natural logarithm of the pooled hazard ratio and the denominator was the standard error of the natural logarithm of the pooled hazard ratio. This is a standard method for calculating the P value with Peto’s assumption-free method.54 Hazard ratios comparing the highest with the lowest quantiles for each study were displayed in a forest plot.55,56 We used the DerSimonian–Laird statistic with a random effects model to assess heterogeneity among studies.57
To provide a pooled estimate of the dose–response relationship between serum 25(OH)D and all-cause mortality, we determined the ratio of the age-adjusted hazard rates for each 10 nanograms per milliliter (ng/mL) stratum of 25(OH)D, compared with the lowest stratum of 25(OH)D in the study. We considered the highest stratum, typically 30 ng/mL or higher, the reference stratum. We plotted the mean of the hazard ratios for all studies for each stratum of 25(OH)D. We calculated 95% confidence intervals (CIs) for the pooled hazards ratios with standard procedures.58–60 The hazard ratios that were plotted were the age-adjusted hazard rate in each stratum divided by the age-adjusted hazard rate in individuals in the top stratum. We also calculated means weighted by the inverse of the variance of each study. This estimate was dominated by one study49 that was much larger than the others. Therefore, we used unweighted means of the hazard ratios, because they provided a combined dose–response curve not determined mainly by a single large study.49 We also fitted a standard decreasing exponential curve to the results, using the function f = y0+a*exp(-b*x). We determined the values of a and b by using multiple regression.
The rationale for selecting age-adjusted mortality hazard ratios as the data for this analysis was that these directly address the hypothesis of the study. It is routine to use age-adjusted mortality hazard ratios to take into account differences that may exist in the age distributions of the study populations. Two investigators independently extracted data from the articles, and there was a 100% cross-check between investigators of the accuracy of data extraction. All hazard ratios were age adjusted, reducing the chances of confounding by age.
This review was conducted according to recommendations of the Meta-Analysis of Observational Studies in Epidemiology Group,61 including providing subjective comments regarding the quality of each study (Table A, final column). Inclusion criteria were reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.62 Several journal articles became available after this study was completed. These are summarized in Table B (available as a supplement to this article at http://www.ajph.org). Their findings are consistent with the association found in the 32 studies that were analyzed. Their inclusion would not have substantially changed the conclusions of this meta-analysis.
RESULTS
Twenty-five studies identified a significant inverse relationship between 25(OH)D concentration and age-adjusted all-cause mortality hazard rates (Figure 2). In 5 studies,21,34,35,37,47 an inverse trend was present, but was not statistically significant. In 2 studies, 1 in the United States48 and 1 in Linxian, China,50 no association was seen. The studies are described and evaluated in Table A.
FIGURE 2—
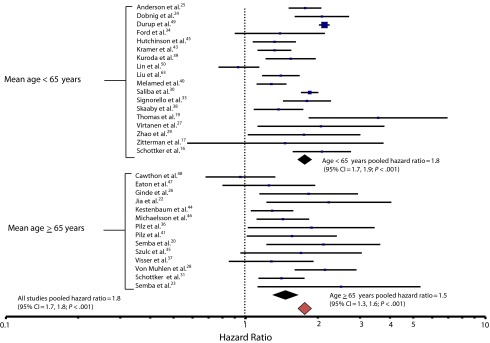
Age-adjusted all-cause mortality hazard ratios comparing lowest with highest categories of serum 25-hydroxyvitamin D, with 95% confidence intervals, in 32 studies of serum 25-hydroxyvitamin D in association with all-cause mortality, 1966–2013.
Note. CI = confidence interval
The overall age-adjusted hazard ratio for all-cause mortality comparing the lowest (0–9 ng/mL) to the highest (> 50 ng/mL) categories of 25(OH)D concentration was 1.9 (95% CI = 1.6, 2.2; P < .001; Figure 2). Serum concentrations less than or equal to 30 ng/mL also were associated with higher all-cause mortality when compared with those greater than 30 ng/mL (P < .01). The studies were not homogeneous (DerSimonian–Laird χ2 = 183.66; df = 28; P < .001). A funnel plot analysis (not shown) revealed no indication of publication bias.
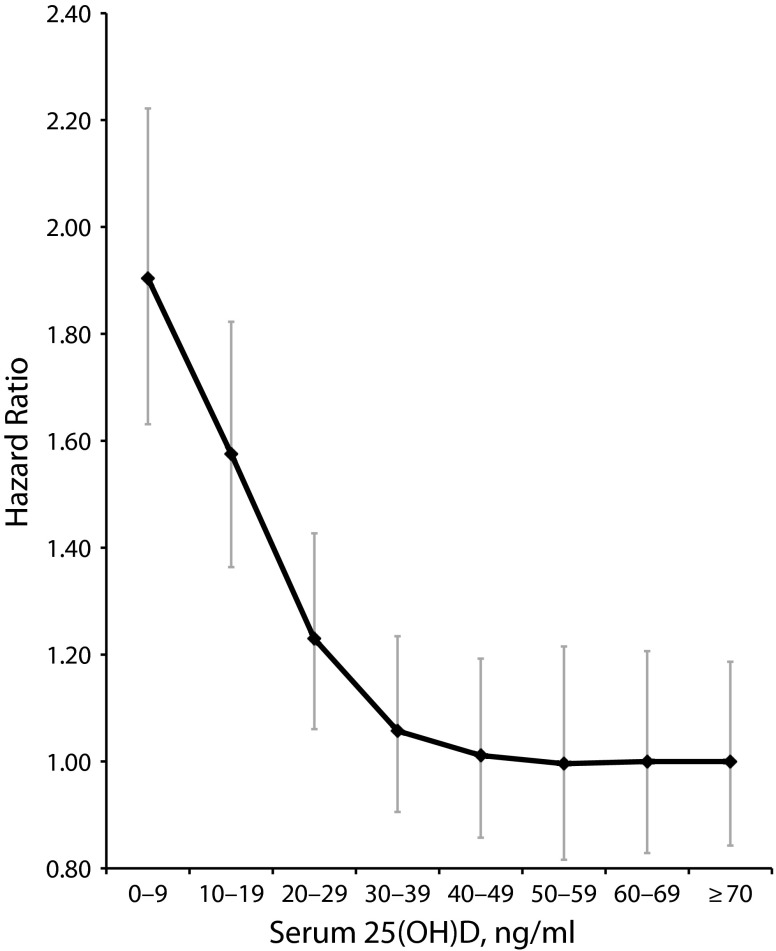
A pooled dose–response curve showed that the hazard ratios declined steeply between 0 ng/mL and 30 to 39 ng/mL, then appeared to plateau at serum 25(OH)D concentrations above 50 ng/mL (Figure 3). The curve was steep at lower concentrations through 30 to 39 ng/mL, with a slight trend toward lower risk at 40 to 49 ng/mL compared with 30 to 39 ng/mL. There was a reduction in the hazard ratio of 0.4 units from 0 to 9 ng/mL through 10 to 19 ng/mL, 0.3 units from 10 to 19 ng/mL through 20 to 29 ng/mL, and approximately 0.1 units from 20 to 29 ng/mL through 30 to 39 ng/mL. Overall, there was a mean drop of 0.1 units in the hazard ratio per 10 ng/mL of 25(OH)D.
FIGURE 3—
Overall age-adjusted hazard ratios for mortality, in 32 studies of serum 25-hydroxyvitamin D in association with all-cause mortality combined: 1966–2013.
Note. 25(OH)D = 25-hydroxyvitamin D.
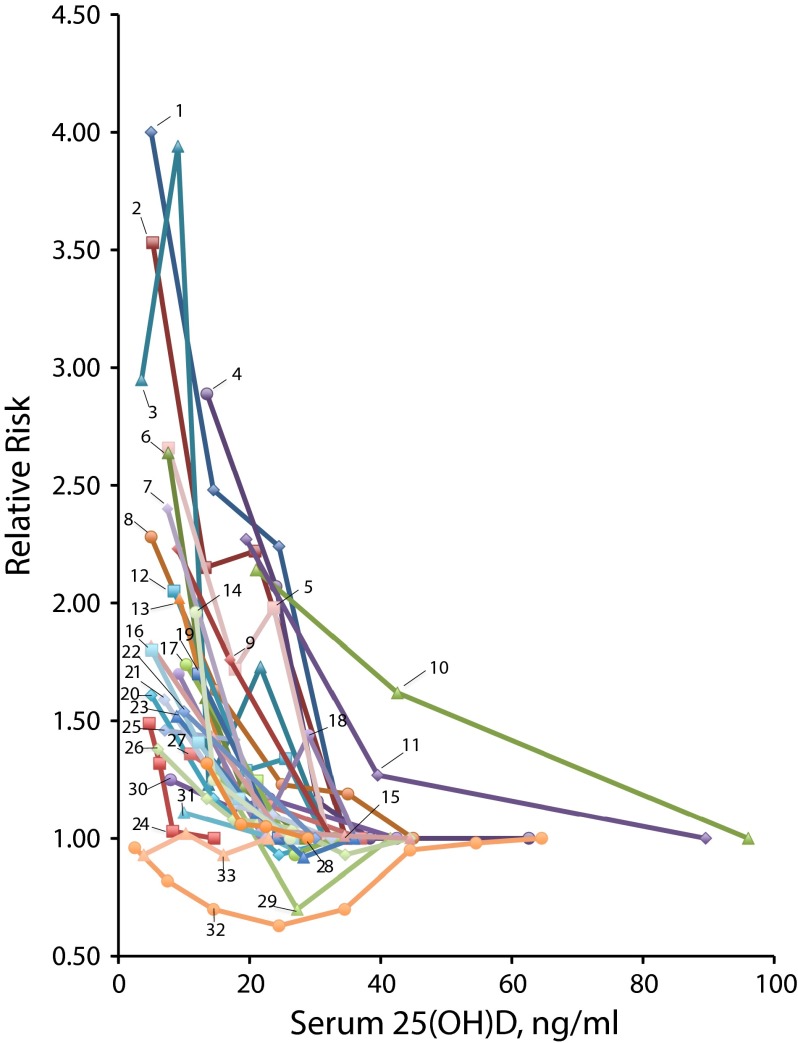
According to a decreasing exponential curve fit to the data from all studies combined, the point at which the estimated hazard ratio was no longer significantly different from 1.0 was a serum 25(OH)D level of 36 ng/mL. The relative hazard at that point was 1.09, and its 95% confidence limits were 1.0 and 1.2. The dose–response curve for each study is shown in Figure 4.
FIGURE 4—
Dose–response relationships in each study between serum 25-hydroxyvitamin D and age-adjusted hazard ratios for all-cause mortality, in 32 studies of serum 25-hydroxyvitamin D in association with all-cause mortality: 1966–2013
Note. 25(OH)D = 25-hydroxyvitamin D. 1. Thomas et al.19; 2. Semba et al. 201020; 3. Zittermann et al.17; 4. Jia et al.22; 5. Semba et al. 200923; 6. Dobnig et al.24; 7. Anderson et al.25; 8. Ginde et al.26; 9. Virtanen et al.27; 10. Von Muhlen et al. (men)28; 11. Von Muhlen et al. (women)28; 12. Zhao et al.29; 13. Saliba et al.30; 14. Schöttker et al.31; 15. Johansson et al.32; 16. Signorello et al.33; 17. Ford et al.34; 18. Szulc et al.35; 19. Pilz et al. (2009)36; 20. Visser et al.37; 21. Skaaby et al.38; 22. Kuroda et al.39; 23. Melamed et al.40; 24. Pilz et al. (2012)41; 25. La Croix et al.42; 26. Kramer et al.43; 27. Kestenbaum et al.44; 28. Hutchinson et al.45; 29. Michaëlsson et al.46; 30. Eaton et al.47; 31. Cawthon et al.48; 32. Durup et al.49; 33. Lin et al.50
Because one possible source of heterogeneity among studies was differences in the length of follow-up, we analyzed whether length of follow-up was related to the association between 25(OH)D and all-cause mortality. We sorted the studies into 2 categories of length of follow-up with respect to the median length of follow up of 7 years. The mean hazard ratio for studies of less than 7 years duration was 2.2, whereas it was 1.6 for studies of greater than or equal to 7 years duration (P < .05).
DISCUSSION
This study confirmed an inverse association between serum 25(OH)D concentrations and age-adjusted all-cause mortality rates. Overall, individuals whose 25(OH)D concentrations were in the lowest quantile (0–9 ng/mL) had nearly twice the age-adjusted death rate as those in the highest quantile (> 35 ng/mL).
Ingestion of some minerals, such as boron, may reduce the rate of catabolism of 25(OH)D, potentially resulting in accumulation of 25(OH)D that could create an appearance of higher mortality rates at higher 25(OH)D concentrations, because of the presence in the population of sick individuals who may be unintentionally consuming such minerals in supplements.64 By contrast, in healthy populations, such as the Rancho Bernardo cohort,28 higher hazard ratios were not present at higher 25(OH)D concentrations. Overall, there was no harm associated with being in the top quantile of 25(OH)D, which was generally 35 to 40 ng/mL or higher. Some degree of caution may be reasonable at higher 25(OH)D concentrations until more data pertinent to higher concentrations are available.
Adjustments
All studies but 1 adjusted for age, and that study stratified by age.28 Many studies adjusted for other covariates. These adjustments revealed that the hazard ratios were not much affected by adjustment. For example, 7 of the 32 studies adjusted for body mass index,21,24,33,35,43,47,50 but body mass index did not appear to play a major role in the association. Six studies adjusted for physical activity,19,24,33,35,46,47 but these adjustments also did not play a major role in the association. Seven studies adjusted for race,26,30,40,42–44,47 without major changes in their conclusions. Of the 23 studies that did not adjust for race, 20 were in areas with racially and ethnically homogenous populations. A total of 8 studies adjusted for smoking,19,21,33,35,43,46,47,50 but smoking also did not influence the association between serum 25(OH)D and all-cause mortality. One study adjusted for 17 covariates, yet the association persisted.47
Fifteen studies used volunteers from the general population enrolled in cohort studies20,23,27,28,32,35–38,42,44–46,48,50 or registered as patients of a medical practice,22,31 2 others used electronic medical record systems of health maintenance or insurance organizations,25,30 and 3 used data from the Third National Health and Nutrition Examination Survey population26,40,43 or other National Health and Nutrition Examination Survey data.29,34 Two studies21,26 analyzed coronary angiography patients, but any patient who was acutely ill was excluded. Several other studies used clinic patients of various types.19,41,49 Zittermann et al. used a mixture of ill and healthy individuals.21 One study used patients from a randomized controlled trial of lifestyle intervention on cardiovascular disease38 and another47 used data from the Women’s Health Initiative. The association that was found between high serum 25(OH)D and lower mortality rates persisted despite the diversity of types of studies and geographic sites.
There was substantial heterogeneity among the studies. One possible reason is that the hazard ratio was higher for studies with shorter length of follow-up, specifically less than 7 years compared with those with greater than or equal to 7 years. This may have occurred because less time on the average had elapsed between measurement of 25(OH)D and death in the studies whose follow-up was shorter. If a participant’s 25(OH)D changed since study measurement, that would cause misclassification of the exposure. If the change were more or less random, it would tend to make the observed hazard ratio come out closer to 1.0 than is true. If it were not random, it could influence the hazard ratio in either direction. In this analysis, it appeared that longer follow-up was associated with hazard ratios closer to 1.0. This source of heterogeneity could be addressed in future studies by taking multiple measurements of 25(OH)D over time.
Differences in the amount of adjustment may account for some of the heterogeneity that was present among studies. All studies adjusted for age, and most adjusted for other covariates. Even with extensive adjustment, residual confounding is always possible. Confounding is also possible by factors that were not measured, such as genetic predisposition. It is inherently difficult to completely exclude any possibility of confounding in observational studies, so caution in interpretation of the findings is appropriate.
Supplements of 2000 to 4000 international units (IU) per day of vitamin D3 would produce an approximately 20 to 40 ng/mL increase in serum 25(OH)D.65,66 Two randomized controlled clinical trials using 2000 IU per day of vitamin D3 are under way in the United States, a study designed to replicate the study by Lappe et al.,8 and a new randomized controlled trial by Manson et al.67 These studies will not have results available for several years.
Limitations
The validity of a meta-analysis depends upon the validity of the studies that were analyzed. Meta-analyses may include studies with different designs and varied populations. An argument could be made against combining studies with heterogeneous results. However, the benefits of a meta-analysis that is inclusive probably outweigh concerns about heterogeneity. Some meta-analyses may exclude studies that increase the heterogeneity of findings, and make estimates of combined results from an analysis limited to studies with similar findings. In the present study, this would have required limiting the studies with similar findings, and excluding those that were dissimilar. A case can be made for performing a meta-analysis limited to studies that are homogeneous, and there is reasonable logic to including only studies with similar results. Although excluding studies to ensure homogeneity is a reasonable concept, it has a countervailing disadvantage of losing some of the information available from existing eligible studies when the combined estimate is calculated. This analysis included all studies that met the inclusion criteria. This choice makes the findings as comprehensive as possible because all relevant studies are included. Including all studies is arguably a conservative approach, because if studies that caused heterogeneity had been dropped, the results would more strongly support the existence of an association of higher serum 25(OH)D with lower all-cause mortality rates.
We obtained the pooled dose–response gradient for the present study (Figure 3) by calculating hazard ratios for each of the 32 studies for each of 8 strata of 25(OH)D and calculating the mean hazard ratio in all studies for each stratum. We also calculated weighted means of hazard ratios, using the inverse of the variance of the hazard ratio of each study as its weight. The weighted approach resulted in the findings of one study49 largely determining the shape of the dose–response gradient, with only minor contributions from other studies. A few studies did not report hazard ratios for the highest few strata, so they did not contribute to the mean hazard ratios for those strata. The curve fit that most closely approximated the observed findings was a decreasing exponential.
A weakness inherent in observational studies is that they may be subject to confounding. For example, people who were ill may not have received as much solar exposure outdoors as those who were healthy. As a result, serum 25(OH)D could be lower to begin with in ill people, who were likely to be at higher risk of death than healthy people. This could have created a spurious association between low serum 25(OH)D and high risk of death. This is known as reverse causation. Therefore, any study detecting an inverse association of serum 25(OH)D with mortality rates must be regarded as tentative because of the possibility that healthier people may have spent more time outdoors than those who had illnesses that may have predisposed them to premature death. We observed an inverse association even in studies with longer follow-up periods (≥ 7 years), which partly mitigates concern about reverse causation. We did not include studies consisting of solely urgently, critically ill patients. This is because a critical illness may have influenced 25(OH)D concentration. Excluding studies of such patients may have reduced the risk of reverse causation, although not necessarily definitively so, and it would have reduced the generalizability of the findings with regard to critically ill patients.
Studies that did not report mortality by categories of serum 25(OH)D were also not eligible for this study, because such data are needed to calculate dose–response or to compare risk of death. All studies were published in biomedical journals and met the usual standard for design of epidemiological studies. Almost all (30 of 32) were cohort studies, which is the strongest observational study design; the other 2 were nested case–control studies, which are of similar capability. The endpoint of mortality hazard ratios does not require a complicated procedure for diagnosis, and it unlikely that there were substantial differences among the studies in accounting for mortality. Determination of vital status is a strong point of virtually all cohort studies. The only substantial differences in quality among studies would be attributable to laboratory procedures used for determining the concentration of serum 25(OH)D. Regarding laboratory methods, there are some data showing that high-performance liquid chromatography (either with ultraviolet detection or coupled with liquid chromatography–mass spectrometry) is usually considered as a gold standard. The problem of determining circulating 25(OH)D is not confined just to the method itself as the precision of different commercial kits could be very different.68 The validity of this analysis is dependent on the validity of the laboratory procedures that were used in each study. All studies used standard, well-recognized testing procedures. A brief assessment of the quality of the design and analysis of each study is provided in the last column of Table A.
The reader should be cautioned that age-adjusted hazard ratios do not automatically guarantee fair comparisons of mortality risks between study populations that are as different as those analyzed with regard to the distribution of risk factors for death other than the serum 25(OH)D level. Also, the objective of this study was to determine the overall long-term appropriateness and safety of various concentrations of serum 25(OH)D, rather than its associations with specific causes of death, such as cardiovascular disease. It is not possible to draw conclusions regarding specific causes of death from the data provided here.
Strengths
A meta-analysis has the advantage of larger numbers and, therefore, higher precision than any individual study. It also provides an analysis procedure for assessing the possibility of publication bias, the funnel plot. In the present study, the plot was funnel-shaped and symmetrical around the overall relative risk of 1.9 for lowest compared with highest category of serum 25(OH)D. In general, studies with the highest precision tended to be closest in magnitude to the overall point estimate of the relative risk, with some exceptions. The overall shape of the funnel plot argues against the possibility of substantial publication bias.
Another strength of this study is that 30 of the 32 studies were favorable with respect to the inverse association of 25(OH)D with all-cause mortality. These 30 studies differed only in degree of association, not direction, increasing confidence that there is an inverse association between 25(OH)D and all-cause mortality rates. Randomized controlled trials would provide a higher level of assurance that this association was not attributable to confounding or reverse causation. It would not take a large number of randomized controlled trials to provide this assurance, but it would take 5 to 10 years to conduct them.
The findings of this study may be generalized to populations similar to persons who volunteer to participate in medical research, enroll in clinical programs, or are Medicare recipients. Generalization to other demographic groups may not be appropriate. For example, almost all studies in this analysis were based on predominantly White populations, so the findings cannot necessarily be generalized to persons of other races. Studies of the type we encountered overrepresent older individuals, so the results may not apply to younger people. Similar studies should be performed of younger and non-White populations.
Conclusions
There was a downward slope in hazard ratios of mortality according to the serum 25(OH)D concentration. This confirms observations from the National Academy of Sciences–Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium69 that concentrations less than 20 ng/mL are too low for safety. The present analysis also suggests that serum 25(OH)D concentrations of less than 30 ng/mL may be too low for safety.
A target range of 25(OH)D of greater than 30 ng/mL could be achieved in most individuals by intake of approximately 1000 IU per day of vitamin D3,12 which is one quarter the National Academy of Sciences–Institute of Medicine tolerable upper level of intake of 4000 IU per day at ages 9 years and older.69 Although it is above the National Academy of Sciences–Institute of Medicine–recommended daily allowance of 600 to 800 IU per day, intake of 1000 IU per day has been reported as safe for daily use for almost all adults, according to the recent Endocrine Society clinical guidelines.12 Still, some authors have expressed concern about the efficacy and absolute safety of doses greater than 1000 IU per day, so caution is reasonable.69,70 The Endocrine Society has established a tolerable upper-limit intake of 10 000 IU per day at ages 19 years and older.12 Doses of vitamin D3 below 10 000 IU per day in adults have not been associated with toxicity, and serum 25(OH)D concentrations less than 200 ng/mL are generally not considered toxic.12 This leaves a considerable margin of safety for efforts to raise the population concentration of 25(OH)D to 40 ng/mL.
Well-monitored randomized controlled clinical trials using larger doses of vitamin D3 would be desirable. While such studies are being considered, it would be reasonable to try to improve the vitamin D3 status of the population, because of its known associations with lower risk of several diseases. The tolerability of vitamin D3 intake of 1000 IU per day at ages 9 years and older has been confirmed by a committee report monograph of the National Academy of Sciences–Institute of Medicine.69
Acknowledgments
The authors would like to express their deep gratitude and appreciation to CAPT Gregory Utz, MD, US Navy Medical Corps, for his many contributions and steadfast support of this research program and his outstanding leadership as the commanding officer of the Naval Health Research Center from 2011 to 2013.
Note. The positions expressed in this article are solely those of the authors, and do not represent official positions of the Bureau of Medicine and Surgery, Department of the Navy, Department of Defense, or the US Government. The authors have no conflicts of interest except for W. B. Grant, who receives funding from the Vitamin D Council (San Luis Obispo, CA), UV Foundation (McLean, VA), Bio-Tech Pharmacal (Fayetteville, AR), and the Vitamin D Society (Canada).
Human Participant Protection
This article was a systematic review and analysis of published studies of aggregated data. No personally identifiable information was used. This type of research is exempt from human participants review (45 CFR 46.101[b]).
References
- 1.Lowe LC, Guy M, Mansi J et al. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41(8):1164–1169. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Bertone-Johnson ER, Chen W, Holick MF et al. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1991–1997. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 3.Abbas S, Linseisen J, Slanger T et al. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer—results of a large case–control study. Carcinogenesis. 2008;29(1):93–99. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- 4.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2(8673):1176–1178. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 5.Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1(8424):307–309. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- 6.Kearney J, Giovannucci E, Rimm EB et al. Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men. Am J Epidemiol. 1996;143(9):907–917. doi: 10.1093/oxfordjournals.aje.a008834. [DOI] [PubMed] [Google Scholar]
- 7.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94(6):1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 8.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Song Y, Manson JE et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5(6):819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 2009;19(7):468–483. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF, Binkley NC, Bischoff-Ferrari HA et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 13.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30(2):113–125. doi: 10.1111/j.1365-2036.2009.04022.x. [DOI] [PubMed] [Google Scholar]
- 14.Mohr SB, Gorham ED, Alcaraz JE et al. Serum 25-hydroxyvitamin D and prevention of breast cancer: pooled analysis. Anticancer Res. 2011;31(9):2939–2948. [PubMed] [Google Scholar]
- 15.Gandini S, Boniol M, Haukka J et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128(6):1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 16.Schöttker B, Ball D, Gellert C, Brenner H. Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res Rev. 2013;12(2):708–718. doi: 10.1016/j.arr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95(1):91–100. doi: 10.3945/ajcn.111.014779. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury R, Kunutsor S, Vitezova A et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014 doi: 10.1136/bmj.g1903. Epub ahead of print April 1, 2014:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas GN, O’Hartaigh B, Bosch JA et al. Vitamin D levels predict all-cause and cardiovascular disease mortality in subjects with the metabolic syndrome: the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Diabetes Care. 2012;35(5):1158–1164. doi: 10.2337/dc11-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semba RD, Houston DK, Bandinelli S et al. Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur J Clin Nutr. 2010;64(2):203–209. doi: 10.1038/ejcn.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zittermann A, Schleithoff SS, Frisch S et al. Circulating calcitriol concentrations and total mortality. Clin Chem. 2009;55(6):1163–1170. doi: 10.1373/clinchem.2008.120006. [DOI] [PubMed] [Google Scholar]
- 22.Jia X, Aucott LS, McNeill G. Nutritional status and subsequent all-cause mortality in men and women aged 75 years or over living in the community. Br J Nutr. 2007;98(3):593–599. doi: 10.1017/S0007114507725163. [DOI] [PubMed] [Google Scholar]
- 23.Semba RD, Houston DK, Ferrucci L et al. Low serum 25-hydroxyvitamin D concentrations are associated with greater all-cause mortality in older community-dwelling women. Nutr Res. 2009;29(8):525–530. doi: 10.1016/j.nutres.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobnig H, Pilz S, Scharnagl H et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JL, May HT, Horne BD et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106(7):963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57(9):1595–1603. doi: 10.1111/j.1532-5415.2009.02359.x. [DOI] [PubMed] [Google Scholar]
- 27.Virtanen JK, Nurmi T, Voutilainen S, Mursu J, Tuomainen TP. Association of serum 25-hydroxyvitamin D with the risk of death in a general older population in Finland. Eur J Nutr. 2011;50(5):305–312. doi: 10.1007/s00394-010-0138-3. [DOI] [PubMed] [Google Scholar]
- 28.Von Muhlen D, Garland C, Bettencourt R, Barrett-Connor E. Are serum levels of 25-hydroxyvitamin D or 1,25-dihydroxyvitamin D associated with longevity? Circulation. 2009;119(10):e318. [Google Scholar]
- 29.Zhao G, Ford ES, Li C, Croft JB. Serum 25-hydroxyvitamin D levels and all-cause and cardiovascular disease mortality among US adults with hypertension: the NHANES linked mortality study. J Hypertens. 2012;30(2):284–289. doi: 10.1097/HJH.0b013e32834e1f0a. [DOI] [PubMed] [Google Scholar]
- 30.Saliba W, Barnett O, Rennert HS, Rennert G. The risk of all-cause mortality is inversely related to serum 25(OH)D levels. J Clin Endocrinol Metab. 2012;97(8):2792–2798. doi: 10.1210/jc.2012-1747. [DOI] [PubMed] [Google Scholar]
- 31.Schöttker B, Haug U, Schomburg L et al. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr. 2013;97(4):782–793. doi: 10.3945/ajcn.112.047712. [DOI] [PubMed] [Google Scholar]
- 32.Johansson H, Oden A, Kanis J et al. Low serum vitamin D is associated with increased mortality in elderly men: MrOS Sweden. Osteoporos Int. 2012;23(3):991–999. doi: 10.1007/s00198-011-1809-5. [DOI] [PubMed] [Google Scholar]
- 33.Signorello LB, Han X, Cai Q et al. A prospective study of serum 25-hydroxyvitamin D levels and mortality among African Americans and non-African Americans. Am J Epidemiol. 2013;177(2):171–179. doi: 10.1093/aje/kws348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford ES, Zhao G, Tsai J, Li C. Vitamin D and all-cause mortality among adults in USA: findings from the National Health and Nutrition Examination Survey Linked Mortality Study. Int J Epidemiol. 2011;40(4):998–1005. doi: 10.1093/ije/dyq264. [DOI] [PubMed] [Google Scholar]
- 35.Szulc P, Claustrat B, Delmas PD. Serum concentrations of 17beta-E2 and 25-hydroxycholecalciferol (25OHD) in relation to all-cause mortality in older men—the MINOS study. Clin Endocrinol (Oxf) 2009;71(4):594–602. doi: 10.1111/j.1365-2265.2009.03530.x. [DOI] [PubMed] [Google Scholar]
- 36.Pilz S, Dobnig H, Nijpels G et al. Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf) 2009;71(5):666–672. doi: 10.1111/j.1365-2265.2009.03548.x. [DOI] [PubMed] [Google Scholar]
- 37.Visser M, Deeg DJ, Puts MT, Seidell JC, Lips P. Low serum concentrations of 25-hydroxyvitamin D in older persons and the risk of nursing home admission. Am J Clin Nutr. 2006;84(3):616–622. doi: 10.1093/ajcn/84.3.616. quiz 671–672. [DOI] [PubMed] [Google Scholar]
- 38.Skaaby T, Husemoen LL, Pisinger C et al. Vitamin D status and incident cardiovascular disease and all-cause mortality: a general population study. PLoS ONE. 2012;7(12):e52423. doi: 10.1371/journal.pone.0052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuroda T, Shiraki M, Tanaka S, Ohta H. Contributions of 25-hydroxyvitamin D, co-morbidities and bone mass to mortality in Japanese postmenopausal women. Bone. 2009;44(1):168–172. doi: 10.1016/j.bone.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilz S, Dobnig H, Tomaschitz A et al. Low 25-hydroxyvitamin D is associated with increased mortality in female nursing home residents. J Clin Endocrinol Metab. 2012;97(4):E653–E657. doi: 10.1210/jc.2011-3043. [DOI] [PubMed] [Google Scholar]
- 42.LaCroix AZ, Kotchen J, Anderson G et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64(5):559–567. doi: 10.1093/gerona/glp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramer H, Sempos C, Cao G et al. Mortality rates across 25-hydroxyvitamin D (25[OH]D) levels among adults with and without estimated glomerular filtration rate <60 ml/min/1.73 m2: the Third National Health and Nutrition Examination Survey. PLoS ONE. 2012;7(10):e47458. doi: 10.1371/journal.pone.0047458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kestenbaum B, Katz R, de Boer I et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58(14):1433–1441. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutchinson MS, Grimnes G, Joakimsen RM, Figenschau Y, Jorde R. Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population: the Tromso study. Eur J Endocrinol. 2010;162(5):935–942. doi: 10.1530/EJE-09-1041. [DOI] [PubMed] [Google Scholar]
- 46.Michaëlsson K, Baron JA, Snellman G et al. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr. 2010;92(4):841–848. doi: 10.3945/ajcn.2010.29749. [DOI] [PubMed] [Google Scholar]
- 47.Eaton CB, Young A, Allison MA et al. Prospective association of vitamin D concentrations with mortality in postmenopausal women: results from the Women’s Health Initiative (WHI) Am J Clin Nutr. 2011;94(6):1471–1478. doi: 10.3945/ajcn.111.017715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cawthon PM, Parimi N, Barrett-Connor E et al. Serum 25-hydroxyvitamin D, parathyroid hormone, and mortality in older men. J Clin Endocrinol Metab. 2010;95(10):4625–4634. doi: 10.1210/jc.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durup D, Jorgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: the CopD study. J Clin Endocrinol Metab. 2012;97(8):2644–2652. doi: 10.1210/jc.2012-1176. [DOI] [PubMed] [Google Scholar]
- 50.Lin SW, Chen W, Fan JH et al. Prospective study of serum 25-hydroxyvitamin D concentration and mortality in a Chinese population. Am J Epidemiol. 2012;176(11):1043–1050. doi: 10.1093/aje/kws285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, Lof M, Veierod MB, Sandin S, Adami HO, Weiderpass E. Ultraviolet exposure and mortality among women in Sweden. Cancer Epidemiol Biomark Prev. 2011;20(4):683–690. doi: 10.1158/1055-9965.EPI-10-0982. [DOI] [PubMed] [Google Scholar]
- 52.Deeks J, Higgins J. Statistical Algorithms in Review Manager 5. Oxford, England: The Cochrane Collaboration; 2010. [Google Scholar]
- 53.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deeks J, Altman DG, Bradburn M. Statistical Methods for Examining Heterogeneity and Combining Results From Several Studies in a Meta-analysis. London, England: BMJ Publications; 2002. [Google Scholar]
- 55.Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322(7300):1479–1480. doi: 10.1136/bmj.322.7300.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeh J, D’Amico F. Forest plots: data summaries at a glance. J Fam Pract. 2004;53(12):1007. [PubMed] [Google Scholar]
- 57.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 58.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19(4):251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 59.Katz D, Baptista J, Azen S, Pike M. Obtaining confidence intervals for the risk ratio in cohort studies. Biometrics. 1978;34:469–474. [Google Scholar]
- 60.Kirkwood B, Sterne A. Essential Medical Statistics. 2nd ed. Malden, MA: Blackwell Publishing Ltd; 2003. Comparing rates; pp. 240–248. [Google Scholar]
- 61.Stroup DF, Berlin J, Morton S et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 62.Moher D, Liberati A, Altman D The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu L, Chen M, Hankins SR et al. Drexel Cardiovascular Health Collaborative Education, Research, and Evaluation Group. Serum 25-hydroxyvitamin D concentration and mortality from heart failure and cardiovascular disease, and premature mortality from all-cause in United States adults. Am J Cardiol. 2012;110(6):834–839. doi: 10.1016/j.amjcard.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Miljkovic D, Miljkovic N, McCarty M. Up-regulatory impact of boron on vitamin D function—does it reflect inhibition of 24-hydroxylase? Med Hypotheses. 2004;63(6):1054–1056. doi: 10.1016/j.mehy.2003.12.053. [DOI] [PubMed] [Google Scholar]
- 65.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8(3):222–230. doi: 10.1007/s001980050058. [DOI] [PubMed] [Google Scholar]
- 66.Tripkovic L, Lambert H, Hart K et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1357–1364. doi: 10.3945/ajcn.111.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manson JE, Bassuk S, Lee I et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ross A, Taylor C, Yaktine A, Del Valle H Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 70.Rosen CJ, Abrams S, Aloia J et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97(4):1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]