Abstract
Objectives. We assessed the contributions of the prevalence and disabling impact of specific diseases to educational disparities in the prevalence of disability.
Methods. We examined a large representative survey of the Dutch population, the Dutch Permanent Survey of Living Conditions (2001–2007; n = 24 883; ages 40–97 years). We attributed the prevalence of disability to chronic diseases by using their empirical associations and assuming independent competing causes of disability. We estimated contributions of prevalence and the disabling impact of diseases to disparities in disability using counterfactuals.
Results. We found that the prevalence of disability in individuals with only an elementary education was 19 to 20 percentage points higher than that in individuals with tertiary education. Sixty-five percent of this difference could be attributed to specific chronic diseases, but more so to their disabling impact (49%–51%) than to their prevalence (20%–29%). Back pain, neck or arm conditions, and peripheral vascular disease contributed most to the disparity in men, and arthritis, back pain, and chronic nonspecific lung disease contributed most to the disparity in women.
Conclusions. Educational disparities in the burden of disability were primarily caused by high disabling impacts of chronic diseases among low educated groups. Tackling disparities might require more effective treatment or rehabilitation of disability in lower socioeconomic groups.
Worldwide, health is strongly patterned along socioeconomic lines.1,2 Those with the lowest income or with the lowest level of education are consistently less healthy on numerous health indicators.1–3 For example, a recent study using data from the World Health Survey showed significant and substantial pro-rich inequality in the prevalence of disability in 43 of 49 countries worldwide.3 The disparities, also in disability, have not declined, but appear to have a strongly persistent nature.4–6 Reducing health disparities is a top priority of health policy agendas, and there is an urgent need to develop interventions that effectively reduce socioeconomic disparities in health.7 This requires that we know which diseases and determinants contribute most to health disparities, but studies assessing contributions of specific diseases to disparities in fatal and nonfatal health outcomes are limited in number.
With respect to fatal outcomes, several studies show that various diseases contribute to socioeconomic disparities in total mortality, but that a few conditions contribute most.1,8–13 For example, Huisman et al.8 found that among European men, cardiovascular diseases accounted for 39% of the difference between low and high educational groups in total mortality, cancer accounted for 24%, other diseases accounted for 32%, and external causes accounted for 5%. Among women, contributions were 60%, 11%, 30%, and 0%, respectively.8 A relative stagnation of cardiovascular mortality declines among low socioeconomic groups has contributed to the persistence and even widening of disparities in total mortality over the past few decades.5
With respect to nonfatal health outcomes, substantial disparities exist in terms of the occurrence of many chronic diseases and in generic health outcomes, such as disability.3,14 Only a handful of studies has investigated which diseases contribute most to the socioeconomic disparity in the burden of disability. In a study by Sainio et al.,15 diabetes contributed most to educational disparities in stair climbing limitations among men, and osteoarthritis of the knee and angina pectoris contributed most among women. In a study by Koster et al.,16 knee pain contributed most to an excess hazard for mobility limitations among low educated persons. In another study, Nusselder et al.17 found that in the Belgian population, back complaints and arthritis contributed most to educational disparities in years lived with functional mobility limitations among men, and that arthritis and chronic nonspecific lung disease contributed most among women.
The latter study clearly showed that substantial differences exist in the prevalence of and in the disabling impact of chronic diseases, and suggested that both contribute to the total disparity in disability. Because these 2 aspects may require an entirely different intervention approach, knowing their relative contribution to disparities in disability is crucial for policy development. To our knowledge, no study has investigated to what extent the total inequality in the burden of disability is explained by differences in the prevalence of diseases and by differences in the disabling impact. Our aim in this study was to assess contributions of differences in the prevalence and the disabling impact of specific diseases to educational disparities in the prevalence of disability.
METHODS
The study population consisted of participants from 7 successive years (2001–2007) of the Dutch Permanent Survey of Living Conditions, which is a repeated cross-sectional survey. This large survey aims to provide continuous information on various aspects of the living situation in a representative sample of the Dutch noninstitutionalized population. The individuals approached for the survey were identified through the population register of the Netherlands. To account for selective nonresponse and to ensure representativeness for the Dutch noninstitutionalized population, we used weights that were attached to the data. The Dutch Permanent Survey of Living Conditions data are available at http://www.dans.knaw.nl.
Information on education was collected through face-to-face interviews and information on disability, and the presence of chronic diseases through written questionnaires. From 2001 to 2007, 110 766 individuals were approached; the response rate was 62%. For our analysis, participants who were 40 years old and older (n = 32 233) were selected. Twenty-three percent of these individuals were not included in the study population because they lacked information on level of education (n = 186) or the presence of disability (n = 4761) and diseases (n = 6681). The number of persons in the study population, and the number of disabled and diseased individuals are presented as data available as a supplement to the online version of this article at http://www.ajph.org.
Disability and Disease Groups
Participants were asked if they were able to “follow a conversation in a group of 3 persons,” “have a conversation with one other person” (for both questions, using hearing aids if necessary), “read the small letters in the newspaper,” “recognize someone at 4 m distance” (for both questions, using eyeglasses if necessary), “carry a 5 kg object for 10 m,” “from an upward position, bend forward and take something from the ground,” and “walk 400 m without interruption.”18 Participants could answer “without difficulty,” with minor difficulty,” “with major difficulty,” and “no, I can’t,” and were considered disabled if 1 of the latter 2 answers were given at least once.
Using information from the questionnaire, dummy variables were created indicating the presence of diabetes mellitus, stroke, heart disease, peripheral vascular disease, cancer, chronic nonspecific lung disease, back pain, arthritis, neck or arm conditions, and other (data available as a supplement to the online version of this article at http://www.ajph.org). Skin cancer was not included because it is not associated with disability. According to the questions in the survey, peripheral vascular disease did not include vascular disease of the upper extremities. If participants experienced back pain or neck or arm conditions, but were currently free of complaints, the condition was regarded as no longer present. The presence of some diseases was assessed on the basis of 2 questions in the survey. If an answer on 1 question was missing, and the answer on the other did not indicate the presence of the disease, the information on this specific disease was considered “missing.”
Education was measured according to the highest completed level and was classified as elementary (1st to 6th grades), lower secondary (7th to 9th grades), upper secondary (10th to 12th grades or first 3 years of vocational education) and tertiary (community or junior colleges, vocational technical institutes, or university).
Statistical Analysis
We plotted the prevalence of disability for men and women by age and education. We derived the prevalence of specific chronic diseases among groups varying according to level of education from the empirical data. We adjusted for potential variation because of a different age composition by age standardization to the study populations of men and women.
The extent to which a specific disease contributes to the educational disparity in disability is dependent on educational differences in the prevalence and in the disabling impact of the disease. We quantified both aspects in our analysis. To estimate the disabling impact of diseases, we used a method that is described in detail elsewhere.17,19
In this method, empirical associations of disease and disability according to cross-sectional data are used to estimate the hazard of exposure to specific diseases for the occurrence of disability in the time preceding the survey. The hazard from exposure to each disease is assumed proportional to the distribution of diseases and disability in the cross-sectional data. Using additive regression, the hazard for disability is modeled dependent on specific diseases and on background. The coefficients estimated for diseases represent the disabling impact of the diseases. Background represents the hazard for disability in absence of a disease. The individual hazard for disability is obtained by summing the estimated regression coefficients for background and the diseases present in the individual. The individual probability for disability is equal to 1 − exp(− total individual hazard).
The regression model was specified as follows:
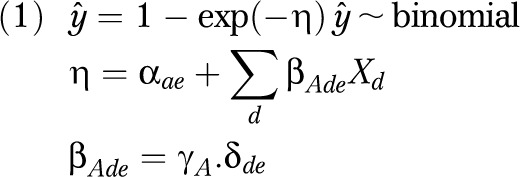 |
In the model,  is the estimated probability for disability in an individual, and
is the estimated probability for disability in an individual, and 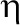 is the linear predictor. The linear predictor is equal to the sum of the background hazard
is the linear predictor. The linear predictor is equal to the sum of the background hazard 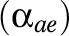 and the hazard of disability
and the hazard of disability 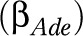 from the combination of diseases
from the combination of diseases 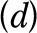 present in the respondent
present in the respondent  . The presence or absence of diseases was modeled using dummy variables with 0 or 1. Using likelihood ratio tests, it was decided to model background
. The presence or absence of diseases was modeled using dummy variables with 0 or 1. Using likelihood ratio tests, it was decided to model background 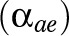 and disabling impacts
and disabling impacts 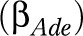 as age- and education-specific. To prevent an excessive number of parameters in the model, the age- and education-specific disabling impacts of each disease,
as age- and education-specific. To prevent an excessive number of parameters in the model, the age- and education-specific disabling impacts of each disease, 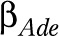 , were estimated as the product of an age pattern
, were estimated as the product of an age pattern 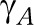 (3 age groups) equal for each disease and a disease effect
(3 age groups) equal for each disease and a disease effect 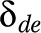 , which was allowed to vary by disease and education, but not by age.20,21 A same age pattern
, which was allowed to vary by disease and education, but not by age.20,21 A same age pattern 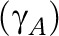 was used for each level of education, and separate models were fitted for men and women. Models were fitted using a quasi-Newton method programmed for the statistical package R, version 2.7.122 (Foundation for Statistical Computing, Vienna, Austria).
was used for each level of education, and separate models were fitted for men and women. Models were fitted using a quasi-Newton method programmed for the statistical package R, version 2.7.122 (Foundation for Statistical Computing, Vienna, Austria).
For each disabled person from the study population, the disability was partitioned to the diseases present in the individual and to background.19,23 In this approach, the contribution of each disease was expressed as a proportion, and was dependent on the combination of the diseases present in the individual and on the hazards estimated. Among groups of similar ages, gender and level of education, and the individual proportions of disability attributed to each disease were summed (total sum) to estimate the number of persons disabled because of each disease. The number disabled because of each disease divided by the number of persons in a group of a specific age, gender, and level of education provided the contribution of each disease to the prevalence of disability in that group. The sum of the contributions of diseases and background was equal to the prevalence of disability in this group. The contribution of each disease to the educational disparity in the prevalence of disability was calculated as the contribution of the disease among persons with an elementary education minus the contribution among those with a tertiary education.
We calculated the contribution of differences in disease prevalence and disabling impact to the disparity in the prevalence of disability using counterfactual scenarios. In these scenarios, we quantified the contribution of differences in the prevalence of diseases as the reduction in the prevalence of disability using the prevalence of diseases of persons with a tertiary education for persons with an elementary education. That is, the disabling impacts and background estimated among low educated individuals were applied to a population with a distribution of diseases as observed among high educated individuals. Similarly, we quantified the contribution of the disabling impact using the disabling impact of diseases of persons with a tertiary education for persons with an elementary education.
The software for additive regression and for calculation of the prevalence of disability by cause is available from the authors on request.
RESULTS
There was a clear educational gradient in the prevalence of disability, with a stepwise increase of disability for each step down the educational ladder. Compared with those who had a tertiary education, men and women with only an elementary education had a 20-percentage-points higher prevalence of disability (Figure 1).
FIGURE 1—
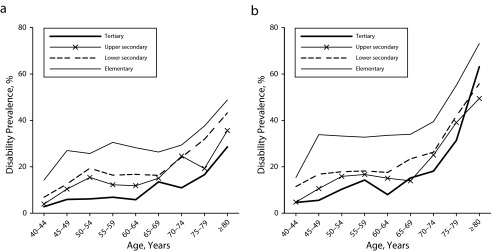
Prevalence of disability according to age groups and level of education for (a) men and (b) women: pooled from the Dutch Permanent Survey of Living Conditions, 2001–2007.
Note. The sample size was n = 24 883.
The prevalence of most diseases was also patterned according to an educational gradient, with a higher prevalence for persons with a lower level of education (Table 1). For men, the difference in the prevalence between persons with an elementary and tertiary education was largest for neck or arm conditions (9.2 percentage points), back pain (7.6 percentage points), and arthritis (7.4 percentage points). For women, the difference was largest for neck or arm conditions (6.3 percentage points), arthritis (6.1 percentage points), and diabetes (5.8% percentage points).
TABLE 1—
Prevalence of Chronic Diseases by Level of Education and Gender: Pooled From the Dutch Permanent Survey of Living Conditions, 2001–2007
| Disease/Condition | Tertiary, % (95% CI) | Upper Secondary, % (95% CI) | Lower Secondary, % (95% CI) | Elementary, % (95% CI) | P (Overall Difference) |
| Men | |||||
| DM | 5.1 (4.4, 5.9) | 6.6 (5.9, 7.4) | 6.5 (5.6, 7.5) | 9.0 (7.9, 10.3) | < .001 |
| Stroke | 2.4 (2.0, 3.0) | 3.7 (3.1, 4.3) | 4.2 (3.5, 5.1) | 4.8 (4.0, 5.7) | < .001 |
| Heart disease | 7.4 (6.5, 8.4) | 6.7 (5.9, 7.6) | 9.6 (8.6, 10.7) | 11.2 (10.0, 12.5) | < .001 |
| PVD | 1.8 (1.4, 2.3) | 2.7 (2.3, 3.3) | 3.7 (3.0, 4.5) | 5.5 (4.7, 6.5) | < .001 |
| Cancer | 4.2 (3.5, 4.9) | 4.4 (3.8, 5.1) | 4.1 (3.4, 4.8) | 3.9 (3.2, 4.7) | .78 |
| CNSLD | 5.4 (4.7, 6.3) | 6.7 (5.9, 7.4) | 7.5 (6.5, 8.6) | 10.3 (9.1, 11.7) | < .001 |
| Back pain | 6.5 (5.7, 7.4) | 10.2 (9.3, 11.1) | 11.0 (9.8, 12.3) | 14.1 (12.6, 15.7) | < .001 |
| Arthritis | 10.4 (9.4, 11.5) | 14.3 (13.3, 15.4) | 16.8 (15.4, 18.3) | 17.8 (16.2, 19.4) | < .001 |
| Neck/arm conditions | 7.4 (6.5, 8.3) | 10.9 (10.0, 11.8) | 13.3 (12.0, 14.7) | 16.6 (15.0, 18.4) | < .001 |
| Other | 17.3 (16.0, 18.6) | 18.5 (17.4, 19.7) | 19.3 (17.8, 20.9) | 21.1 (19.3, 23.0) | .009 |
| Women | |||||
| DM | 2.3 (1.7, 3.2) | 4.4 (3.7, 5.2) | 5.4 (4.7, 6.1) | 8.1 (7.3, 9.0) | < .001 |
| Stroke | 1.9 (1.3, 2.6) | 2.3 (1.8, 2.9) | 2.7 (2.3, 3.3) | 3.7 (3.1, 4.3) | .001 |
| Heart disease | 2.9 (2.2, 3.8) | 4.4 (3.7, 5.3) | 4.2 (3.6, 4.8) | 5.3 (4.6, 6.0) | .001 |
| PVD | 1.7 (1.2, 2.5) | 2.3 (1.8, 3.0) | 3.1 (2.6, 3.7) | 3.8 (3.2, 4.4) | < .001 |
| Cancer | 7.5 (6.4, 8.8) | 7.0 (6.2, 8.0) | 6.4 (5.7, 7.2) | 6.7 (5.9, 7.6) | .428 |
| CNSLD | 7.0 (6.0, 8.2) | 7.4 (6.6, 8.4) | 7.4 (6.6, 8.2) | 10.7 (9.6, 11.9) | < .001 |
| Back pain | 9.7 (8.5, 11.1) | 10.9 (9.9, 12.0) | 10.8 (9.9, 11.8) | 13.5 (12.4, 14.8) | < .001 |
| Arthritis | 22.2 (20.4, 24.1) | 24.6 (23.1, 26.1) | 25.3 (24.0, 26.6) | 28.3 (26.7, 29.8) | < .001 |
| Neck/arm conditions | 14.7 (13.3, 16.3) | 19.5 (18.2, 20.9) | 18.4 (17.2, 19.6) | 21.0 (19.6, 22.5) | < .001 |
| Other | 29.6 (27.7, 31.5) | 32.8 (31.3, 34.4) | 31.9 (30.5, 33.4) | 37.1 (35.3, 38.9) | < .001 |
Note. CI = confidence interval; CNSLD = chronic nonspecific lung disease; DM = diabetes mellitus; PVD = peripheral vascular disease. The prevalence of diseases in each education group is calculated as the number of persons who indicated that they had the disease, divided by the total number of persons in that group. Estimates were weighted and age-standardized to the study population of men and women. The sample size was n = 24 883.
For most diseases, the disabling impact was highest among persons with the lowest level of education, but not for all (Table 2). For example, in men, the highest disabling impact of arthritis was in those with upper secondary education. In men, the difference in the disabling impact according to level of education was significant for cancer, back pain, and neck or arm conditions. In women, the disabling impact differed significantly for back pain and arthritis.
TABLE 2—
Estimates of the Disabling Impact of Chronic Diseases (Hazard), by Level of Education and Gender: Pooled From the Dutch Permanent Survey of Living Conditions, 2001–2007
| Disease/Condition | Tertiary, b (95% CI) | Upper Secondary, b (95% CI) | Lower Secondary, b (95% CI) | Elementary, b (95% CI) | P (Overall Difference) |
| Men | |||||
| DM | 0.00 (0.00, 0.00) | 0.05 (−0.01, 0.11) | 0.03 (−0.05, 0.10) | 0.07 (−0.03, 0.18) | .091 |
| Stroke | 0.14 (0.02, 0.26) | 0.22 (0.10, 0.33) | 0.23 (0.08, 0.39) | 0.31 (0.13, 0.50) | .503 |
| Heart disease | 0.06 (0.00, 0.12) | 0.03 (−0.02, 0.07) | 0.15 (0.06, 0.23) | 0.12 (0.02, 0.22) | .086 |
| PVD | 0.08 (−0.04, 0.20) | 0.23 (0.08, 0.38) | 0.24 (0.06, 0.42) | 0.38 (0.18, 0.57) | .139 |
| Cancer | 0.01 (−0.05, 0.06) | 0.13 (0.04, 0.22) | 0.00 (0.00, 0.00) | 0.03 (−0.08, 0.14) | .011 |
| CNSLD | 0.12 (0.04, 0.19) | 0.07 (0.01, 0.13) | 0.14 (0.04, 0.24) | 0.16 (0.05, 0.28) | .473 |
| Back pain | 0.05 (−0.01, 0.11) | 0.20 (0.13, 0.27) | 0.24 (0.14, 0.34) | 0.41 (0.25, 0.56) | < .001 |
| Arthritis | 0.03 (-0.01, 0.08) | 0.13 (0.07, 0.18) | 0.04 (−0.02, 0.11) | 0.04 (−0.04, 0.12) | .064 |
| Neck/arm conditions | 0.02 (−0.02, 0.07) | 0.07 (0.02, 0.13) | 0.09 (0.01, 0.17) | 0.23 (0.11, 0.35) | .006 |
| Other | 0.05 (0.01, 0.08) | 0.06 (0.03, 0.10) | 0.06 (0.01, 0.11) | 0.18 (0.09, 0.28) | .085 |
| Women | |||||
| DM | 0.00 (0.00, 0.00) | 0.08 (−0.03, 0.19) | 0.17 (0.07, 0.28) | 0.14 (0.04, 0.25) | .198 |
| Stroke | 0.17 (−0.07, 0.40) | 0.30 (0.09, 0.51) | 0.14 (0.00, 0.28) | 0.29 (0.10, 0.48) | .688 |
| Heart disease | 0.30 (0.06, 0.54) | 0.22 (0.07, 0.37) | 0.18 (0.06, 0.30) | 0.22 (0.07, 0.36) | .859 |
| PVD | 0.19 (−0.06, 0.31) | 0.13 (0.19, 0.57) | 0.38 (0.19, 0.57) | 0.27 (0.06, 0.48) | .501 |
| Cancer | 0.02 (−0.04, 0.08) | 0.07 (0.00, 0.13) | 0.07 (0.00, 0.15) | 0.23 (0.10, 0.36) | .053 |
| CNSLD | 0.09 (0.02, 0.16) | 0.09 (0.02, 0.16) | 0.12 (0.04, 0.20) | 0.22 (0.09, 0.34) | .102 |
| Back pain | 0.15 (0.07, 0.24) | 0.25 (0.16, 0.33) | 0.36 (0.26, 0.47) | 0.49 (0.34, 0.63) | .002 |
| Arthritis | 0.07 (0.01, 0.12) | 0.16 (0.11, 0.21) | 0.16 (0.11, 0.21) | 0.25 (0.18, 0.33) | .003 |
| Neck/arm conditions | 0.10 (0.04, 0.16) | 0.11 (0.06, 0.16) | 0.18 (0.11, 0.24) | 0.09 (0.00, 0.18) | .444 |
| Other | 0.01 (−0.02, 0.03) | 0.03 (0.00, 0.05) | 0.06 (0.02, 0.09) | 0.14 (0.08, 0.21) | .001 |
Note. CI = confidence interval; CNSLD = chronic nonspecific lung disease; DM = diabetes mellitus; PVD = peripheral vascular disease. Regression coefficients representing the disabling impact (in “hazard”) were estimated using multivariable additive regression. Hazards can be transformed into probability using: probability = 1 − exp(−disease hazard). The sample size was n = 24 883.
Table 3 presents the contributions of diseases to the total prevalence of disability for 2 educational groups. The prevalence of disability among men with a tertiary education was estimated at 8.7%. A relatively large part of this total prevalence of disability was attributed to chronic nonspecific lung disease (0.54 percentage points) and heart disease (0.4 percentage points). In men with an elementary education, the prevalence of disability was 27.8%, of which relatively significant parts were attributed to back pain (3.3 percentage points) and neck or arm conditions (2.3 percentage points).
TABLE 3—
Contribution of Diseases to Educational Disparity in the Prevalence of Disability in Base Situation and Under Counterfactual Assumptions of Equal Disease Prevalence and Equal Disabling Impact: Pooled From the Dutch Permanent Survey of Living Conditions, 2001–2007
| Contributions of Diseases to Total Prevalence of Disability Among Groups with a Tertiary and Elementary Education, Percentage Points |
Difference Between Elementary and Tertiary Education Representing Contribution to Disability Disparity, Percentage Points |
||||||
| Disease/Condition | Tertiary (1) | Elementary (2) | Elementary, Counterfactual With Disease Prevalence of Tertiary (3) | Elementary, Counterfactual With Disabling Impact of Tertiary (4) | Base Situation (No Counterfactual) (2 − 1) | Counterfactual With Disease Prevalence of Elementary Equal to Tertiary (3 − 1) | Counterfactual With Disabling Impact of Elementary Equal to Tertiary (4 − 1) |
| Men | |||||||
| DM | 0.00 | 0.71 | 0.45 | 0.00 | 0.71 | 0.45 | 0.00 |
| Stroke | 0.36 | 1.11 | 0.62 | 0.62 | 0.75 | 0.26 | 0.26 |
| Heart disease | 0.43 | 1.22 | 0.83 | 0.64 | 0.79 | 0.40 | 0.21 |
| PVD | 0.14 | 1.44 | 0.52 | 0.37 | 1.30 | 0.38 | 0.24 |
| Cancer | 0.14 | 0.18 | 0.20 | 0.13 | 0.04 | 0.06 | −0.02 |
| CNSLD | 0.54 | 1.18 | 0.66 | 0.98 | 0.63 | 0.12 | 0.43 |
| Back pain | 0.25 | 3.26 | 1.58 | 0.51 | 3.01 | 1.33 | 0.26 |
| Arthritis | 0.32 | 0.48 | 0.31 | 0.48 | 0.17 | 0.00 | 0.17 |
| Neck/arm conditions | 0.19 | 2.26 | 1.05 | 0.41 | 2.07 | 0.86 | 0.22 |
| Other | 0.53 | 3.56 | 3.12 | 0.61 | 3.03 | 2.59 | 0.08 |
| Background | 5.77 | 12.36 | 12.86 | 13.27 | 6.59 | 7.09 | 7.50 |
| Total (prevalence of disability) | 8.67 | 27.76 | 22.20 | 18.02 | 19.09 | 13.52 | 9.35 |
| Women | |||||||
| DM | 0.00 | 0.89 | 0.21 | 0.00 | 0.89 | 0.21 | 0.00 |
| Stroke | 0.29 | 0.78 | 0.42 | 0.52 | 0.49 | 0.13 | 0.23 |
| Heart disease | 0.80 | 0.96 | 0.55 | 1.31 | 0.16 | −0.25 | 0.51 |
| PVD | 0.25 | 0.70 | 0.33 | 0.54 | 0.44 | 0.08 | 0.28 |
| Cancer | 0.26 | 1.10 | 1.30 | 0.22 | 0.84 | 1.04 | −0.04 |
| CNSLD | 0.23 | 1.44 | 0.98 | 0.33 | 1.21 | 0.76 | 0.10 |
| Back pain | 1.30 | 3.81 | 2.82 | 1.71 | 2.50 | 1.52 | 0.41 |
| Arthritis | 1.16 | 4.85 | 4.17 | 1.32 | 3.68 | 3.00 | 0.15 |
| Neck/arm conditions | 1.19 | 1.28 | 0.91 | 1.62 | 0.09 | −0.28 | 0.43 |
| Other | 0.16 | 3.25 | 2.77 | 0.18 | 3.09 | 2.61 | 0.02 |
| Background | 9.83 | 16.60 | 17.12 | 18.10 | 6.77 | 7.29 | 8.27 |
| Total (prevalence of disability) | 15.48 | 35.66 | 31.59 | 25.85 | 20.18 | 16.12 | 10.37 |
Note. CNSLD = chronic nonspecific lung disease; DM = diabetes mellitus; PVD = peripheral vascular disease. Estimates were weighed and age-standardized to the study population of men and women. The sample size was n = 24 883.
In the counterfactual scenario using the prevalence of diseases among persons with a tertiary education, the prevalence of disability in persons with an elementary education was reduced to 22.2%. In the scenario using the disabling impact of diseases of persons with a tertiary education, the prevalence of disability reduced to 18.0%.
In women with a tertiary education, the prevalence of disability was estimated at 15.5%. A relatively significant part was attributed to back pain (1.3 percentage points), neck or arm conditions (1.2 percentage points), and arthritis (1.2 percentage points). In women with an elementary education, the estimated prevalence of disability was 35.7%, of which relatively significant parts were attributed to arthritis (4.9 percentage points) and back pain (3.8 percentage points). In the counterfactual scenarios using the prevalence, and respectively, the disabling impact of diseases of persons with a tertiary education, the estimated prevalence of disability among women with an elementary education decreased to 32.6% and 25.6%.
Table 3 also presents the differences between persons with elementary and tertiary education in the contributions of diseases to the total prevalence of disability as observed and for the 2 counterfactual scenarios. Of the total disparity of 19.1% points in men, 12.5% points (65%) were attributed to the diseases included and 6.6% points (35%) to background. Diseases that contributed most to the disparity were back pain (3.0 percentage points), neck or arm conditions (2.1 percentage points), and peripheral vascular disease (1.3 percentage points).
In the counterfactual scenario using the prevalence of diseases of men with a tertiary education for those with an elementary education, the disability disparity was reduced to 13.5 percentage points, which was a reduction of 29% compared with the noncounterfactual situation. In the counterfactual scenario using equal disabling impacts, the disparity was reduced to 9.4 percentage points, which was a reduction of 51%.
In women, the estimated disparity was 20.2 percentage points. Diseases that contributed most to the disparity were arthritis (3.7 percentage points), back pain (2.5 percentage points), and chronic nonspecific lung disease (1.2 percentage points). In the counterfactual scenario using the prevalence of diseases of women with a tertiary education, the disparity was reduced by 16.1 percentage points, which was a reduction of 20%. In the scenario using the disabling impact of women with a tertiary education, the disparity was reduced to 10.4 percentage points, which was a reduction of 49 percentage points.
DISCUSSION
The aim of this study was to assess the contributions of the prevalence and disabling impact of specific diseases to educational disparities in the burden of disability. An educational gradient existed in the prevalence of disability according to level of education, with a stepwise increase of disability for each step down the educational ladder. Approximately two thirds of the disparity could be attributed to specific chronic diseases. Differences in the disabling impact of chronic diseases contributed most to the educational disparity in disability, whereas differences in their prevalence contributed to a smaller extent. Diseases that contributed most to the disparity in men were back pain, neck or arm conditions, and peripheral vascular disease, whereas arthritis, back pain, and chronic nonspecific lung disease contributed most to the disparity in women.
Selection bias might limit the external validity of the results. We minimized possible selection bias caused by nonresponse (38%) by using individual weights to adjust for selection effects by age, gender, marital status, urbanization grade, province, employment, and health- and smoking status.24
Item nonresponse was 23%, and we evaluated possible associated bias by comparing self-assessed health and activities of daily living disability (ages 55 years and older) in the source and study population. The prevalence of less than good self-assessed health and activities of daily living disability was slightly higher in the source population, suggesting that the prevalence estimates of disability were conservative in our study. However, the level of underestimation did not differ substantially by level of education, suggesting that selection bias did not affect our substantive conclusions to a great extent.
Our study population did not cover the institutionalized population, which might limit the generalizability of our results to this specific population. However, in the Netherlands only a minority of elderly people live in an institution, and the bias caused by excluding the institutionalized population was probably small and negligible at younger ages.25 Studies that investigated contributions of diseases to educational disparities in mortality showed substantial variation across countries.8,26 In Europe, a particular north–south gradient existed in the contribution of ischemic heart disease, whereas the contribution in the United States was in between.26 Similar variation in contributions of diseases to disparities in disability might exist, which might put restrictions on the generalizability of our results to other countries.
In our study, we measured disability as functional limitations as part of the long-term indicator of disabilities developed by the Organisation for Economic Co-operation and Development.18 Because functional limitations represent a relatively mild form of disability, this provided the statistical power required for our analysis. An additional analysis revealed that diseases contribute particularly to the “mobility” domain of the indicator, and to a smaller extent, the “hearing” and “seeing” domains. This suggested that the results of our study were domain-specific and might not be fully generalizable to other domains of disability.
Studies that investigated the validity of self-report of chronic conditions showed that this was generally fairly accurate, and that level of education did not have a significant effect on reporting behavior.27–29 Self-report of disability was shown to strongly correlate with performance-based measures independent of the level of education.30–32 Therefore, it was expected that self-report would not affect our substantive conclusions regarding which disease contributed most to the disparities.
Using a less stringent definition of disability, which was defined as 1 or more items answered with “at least minor difficulty,” resulted in a larger part of the disparities explained by background. However, relative contributions of diseases to educational disparities in the burden of disability remained unchanged. Because of a lack of power, using a more stringent cut-level could not be evaluated.
The cross-sectional nature of our methods and data did not allow identifying cases in which disability was present before the onset of the disease. In these cases, the disability might be falsely attributed to the disease. However, because the risk for disability before the onset of a particular disease was likely equal to the risk among persons without onset of this disease, such false attribution was expected not to have biased our results.
It was decided not to exclude conditions such as “dizziness with falling” and “involuntary loss of urine,” which have an intermediate position between diseases and disability, but to add them in a separate category named “other.” The main contributors to the educational disparities in men were “dizziness with falling,” “migraine or frequent severe headache,” and “involuntary loss of urine.” In women, these were “involuntary loss of urine” and “migraine or frequent severe headache” (data available on request).
We lacked a valid indicator of mental conditions, but to obtain an impression of the importance of mental illness for disparities in disability, we performed a sensitivity analysis that included a score of 60 or lower on the RAND Mental Health Inventory as a disease.33 In this analysis, 19% of the disparities in men and 18% in women were attributed to mental health problems, suggesting that mental illness might contribute substantially to educational disparities in disability. However, these results should be interpreted with caution because the RAND Mental Health Inventory was designed to assess mental health status at population level and might lack validity to diagnose individual mental disorders.
Comparison with Previous Studies
Our study was the first to our knowledge to show that differences in the disabling impact were more important than differences in disease prevalence in the explanation of educational disparities in the prevalence of disability. Similar to earlier studies, we found that back pain and arthritis substantially contributed to educational disparity in the prevalence of disability. In addition, we showed important contributions of neck or arm conditions.15–17
In contrast to the findings of Sainio et al.,15 our study suggested that peripheral vascular disease also contributed significantly to disability in men, and in agreement with Nusselder et al.,17 we showed that chronic nonspecific lung disease was important in women.
Interpretation of Findings
Factors that might explain the educational disparity in the burden of disability include health behaviors,15,16,34–36 psychosocial factors,36 work-related factors,15,35 and wealth.35 These factors might have an effect on the prevalence or the disabling impact of diseases, but probably most factors affect both. To illustrate, the physical demands for jobs that require a relatively low level of education re commonly higher than that for jobs that require a high level of education.37 These physical demands are associated with a more frequent occurrence of musculoskeletal conditions in low socioeconomic groups.37 In addition, in persons who have a musculoskeletal condition, unfavorable conditions at work could lead to an increased severity of the disease, causing disability.38–41 Consequently, unfavorable conditions at work might contribute to the burden of disability by an effect on both the prevalence and disabling impact of musculoskeletal conditions. In our analysis, the disabling impact of arthritis was highest in individuals who completed upper secondary education. A possible explanation might be that some diseases, such as arthritis, particularly interfere with jobs requiring a relatively high level of education. Another example is obesity, which is clustered among groups with a low level of education and is a major cause of diabetes.42 In persons who have diabetes, obesity has been shown to be a risk factor for functional decline.42 Thus, obesity might contribute to the educational inequality in disability via an effect on the prevalence and disabling impact of diabetes.
Conclusions
About half of the educational disparity in disability was caused by a difference in the disabling impact of chronic diseases, and 20% to 30% was caused by a difference in the prevalence of diseases. For health policymakers who are aiming to reduce disability disparities, this implies that interventions to reduce the disabling impact among groups with a low socioeconomic position are crucial. Evidence showed that interventions, such as high-intensive exercise therapy and orthopedic rehabilitation, can reduce the disabling impact by improving personal capacity, are increasingly available.43,44 However, because disability is not merely a personal characteristic, but more of a gap between personal capability and environmental demand, interventions involving activity accommodation, environmental modifications, psychological coping, and external support might aid in further reduction of the disabling impact.45 The evidence for such interventions is still limited. Given the substantial contribution of musculoskeletal diseases to the disparity in disability, reducing the disabling impact of these conditions seems a priority. The potential gains of effective interventions are substantial. Elimination of the disparity in the disabling impact of musculoskeletal conditions would reduce the total disparity by 25%.
Acknowledgments
This study was part of the project “Living longer in good health,” which was financially supported by Netspar, an independent network for research, education and knowledge exchange in the field of pensions, aging, and retirement. This work was supported by the European Public Health Programme (2006109) and the Erasmus MC, University Medical Center Rotterdam.
Human Participant Protection
No protocol approval was needed for this study because we used secondary data collected by Statistics Netherlands.
References
- 1.Mackenbach JP, Stirbu I, Roskam AJ et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358(23):2468–2481. doi: 10.1056/NEJMsa0707519. [DOI] [PubMed] [Google Scholar]
- 2.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(suppl 1):S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosseinpoor AR, Stewart Williams JA, Gautam J et al. Socioeconomic inequality in disability among adults: a multicountry study using the World Health Survey. Am J Public Health. 2013;103(7):1278–1286. doi: 10.2105/AJPH.2012.301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoeni RF, Martin LG, Andreski PM, Freedman VA. Persistent and growing socioeconomic disparities in disability among the elderly: 1982–2002. Am J Public Health. 2005;95(11):2065–2070. doi: 10.2105/AJPH.2004.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackenbach JP, Bos V, Andersen O et al. Widening socioeconomic inequalities in mortality in six Western European countries. Int J Epidemiol. 2003;32(5):830–837. doi: 10.1093/ije/dyg209. [DOI] [PubMed] [Google Scholar]
- 6.Singh GK, Siahpush M. Widening socioeconomic inequalities in US life expectancy, 1980–2000. Int J Epidemiol. 2006;35(4):969–979. doi: 10.1093/ije/dyl083. [DOI] [PubMed] [Google Scholar]
- 7.Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P Consortium for the European Review of Social Determinants of Health and the Health Divide. WHO European review of social determinants of health and the health divide. Lancet. 2012;380(9846):1011–1029. doi: 10.1016/S0140-6736(12)61228-8. [DOI] [PubMed] [Google Scholar]
- 8.Huisman M, Kunst AE, Bopp M et al. Educational inequalities in cause-specific mortality in middle-aged and older men and women in eight western European populations. Lancet. 2005;365(9458):493–500. doi: 10.1016/S0140-6736(05)17867-2. [DOI] [PubMed] [Google Scholar]
- 9.Steenland K, Henley J, Thun M. All-cause and cause-specific death rates by educational status for two million people in two American Cancer Society cohorts, 1959-1996. Am J Epidemiol. 2002;156(1):11–21. doi: 10.1093/aje/kwf001. [DOI] [PubMed] [Google Scholar]
- 10.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585–1592. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 11.Kunst AE, Groenhof F, Mackenbach JP, Health EW. Occupational class and cause specific mortality in middle aged men in 11 European countries: comparison of population based studies. EU Working Group on Socioeconomic Inequalities in Health. BMJ. 1998;316(7145):1636–1642. doi: 10.1136/bmj.316.7145.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung-Choi K, Khang YH, Cho HJ. Socioeconomic differentials in cause-specific mortality among 1.4 million South Korean public servants and their dependents. J Epidemiol Community Health. 2011;65(7):632–638. doi: 10.1136/jech.2009.100651. [DOI] [PubMed] [Google Scholar]
- 13.van Rossum CT, Shipley MJ, van de Mheen H, Grobbee DE, Marmot MG. Employment grade differences in cause specific mortality. A 25 year follow up of civil servants from the first Whitehall study. J Epidemiol Community Health. 2000;54(3):178–184. doi: 10.1136/jech.54.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalstra JA, Kunst AE, Borrell C et al. Socioeconomic differences in the prevalence of common chronic diseases: an overview of eight European countries. Int J Epidemiol. 2005;34(2):316–326. doi: 10.1093/ije/dyh386. [DOI] [PubMed] [Google Scholar]
- 15.Sainio P, Martelin T, Koskinen S, Heliovaara M. Educational differences in mobility: the contribution of physical workload, obesity, smoking and chronic conditions. J Epidemiol Community Health. 2007;61(5):401–408. doi: 10.1136/jech.2006.048306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koster A, Penninx BW, Bosma H et al. Is there a biomedical explanation for socioeconomic differences in incident mobility limitation? J Gerontol A Biol Sci Med Sci. 2005;60(8):1022–1027. doi: 10.1093/gerona/60.8.1022. [DOI] [PubMed] [Google Scholar]
- 17.Nusselder WJ, Looman CW, Mackenbach JP et al. The contribution of specific diseases to educational disparities in disability-free life expectancy. Am J Public Health. 2005;95(11):2035–2041. doi: 10.2105/AJPH.2004.054700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McWhinnie JR. Disability assessment in population surveys: results of the OECD common development effort. Rev. Epidemiol. Sante Publique. 1981;29(4):413–419. [PubMed] [Google Scholar]
- 19.Nusselder WJ, Looman CW. Decomposition of differences in health expectancy by cause. Demography. 2004;41(2):315–334. doi: 10.1353/dem.2004.0017. [DOI] [PubMed] [Google Scholar]
- 20.Davies PT, Tso MK-S. Procedures for reduced rank regression. Appl Stat. 1982;31(3):244–255. [Google Scholar]
- 21.Yee TW, Hastie TJ. Reduced-rank vector generalized linear models. Stat Model. 2003;3(1):15–41. [Google Scholar]
- 22.Fletcher R. A new approach to variable metric algorithms. Comput J. 1970;13(3):317–322. [Google Scholar]
- 23.Chiang CL. Competing risks in mortality analysis. Annu Rev Public Health. 1991;12:281–307. doi: 10.1146/annurev.pu.12.050191.001433. [DOI] [PubMed] [Google Scholar]
- 24.Stam S, Knoops K. Lange tijdreeksen gezonde levensverwachting. Beschikbaarheid van enquetedata gezondheidsindicatoren. 2009. Available at: http://www.cbs.nl/NR/rdonlyres/1B82D3E7-523E-4AB2-9E59-705C9390D858/0/2009langetijdreeksengezondelevensverwachtingart.pdf. . Accessed September 1, 2013.
- 25.The Netherlands Institute for Social Research. Raportage ouderen 2006. 2006. Available at: http://www.scp.nl/Publicaties/Alle_publicaties/Publicaties_2006/Rapportage_ouderen_2006. Accessed September 1, 2013.
- 26.Mackenbach JP, Cavelaars AE, Kunst AE, Groenhof F. Socioeconomic inequalities in cardiovascular disease mortality: an international study. Eur Heart J. 2000;21(14):1141–1151. doi: 10.1053/euhj.1999.1990. [DOI] [PubMed] [Google Scholar]
- 27.Kriegsman DM, Penninx BW, van Eijk JT, Boeke AJ, Deeg DJ. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients’ self-reports and on determinants of inaccuracy. J Clin Epidemiol. 1996;49(12):1407–1417. doi: 10.1016/s0895-4356(96)00274-0. [DOI] [PubMed] [Google Scholar]
- 28.Leikauf J, Federman AD. Comparisons of self-reported and chart-identified chronic diseases in inner-city seniors. J Am Geriatr Soc. 2009;57(7):1219–1225. doi: 10.1111/j.1532-5415.2009.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson CF, Boyd CM, Carlson MC, Griswold ME, Guralnik JM, Fried LP. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: factors that modify agreement. J Am Geriatr Soc. 2004;52(1):123–127. doi: 10.1111/j.1532-5415.2004.52021.x. [DOI] [PubMed] [Google Scholar]
- 30.van den Brink CL, Tijhuis M, Kalmijn S et al. Self-reported disability and its association with performance-based limitation in elderly men: a comparison of three European countries. J Am Geriatr Soc. 2003;51(6):782–788. doi: 10.1046/j.1365-2389.2003.51258.x. [DOI] [PubMed] [Google Scholar]
- 31.Kivinen P, Sulkava R, Halonen P, Nissinen A. Self-reported and performance-based functional status and associated factors among elderly men: the Finnish cohorts of the Seven Countries Study. J Clin Epidemiol. 1998;51(12):1243–1252. doi: 10.1016/s0895-4356(98)00115-2. [DOI] [PubMed] [Google Scholar]
- 32.Daltroy LH, Larson MG, Eaton HM, Phillips CB, Liang MH. Discrepancies between self-reported and observed physical function in the elderly: the influence of response shift and other factors. Soc Sci Med. 1999;48(11):1549–1561. doi: 10.1016/s0277-9536(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 33.Berwick DM, Murphy JM, Goldman PA, Ware JE, Jr, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care. 1991;29(2):169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Ramsay SE, Whincup PH, Morris RW, Lennon LT, Wannamethee SG. Extent of social inequalities in disability in the elderly: results from a population-based study of British men. Ann Epidemiol. 2008;18(12):896–903. doi: 10.1016/j.annepidem.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martikainen P, Stansfeld S, Hemingway H, Marmot M. Determinants of socioeconomic differences in change in physical and mental functioning. Soc Sci Med. 1999;49(4):499–507. doi: 10.1016/s0277-9536(99)00135-5. [DOI] [PubMed] [Google Scholar]
- 36.Koster A, Bosma H, Broese van Groenou MI et al. Explanations of socioeconomic differences in changes in physical function in older adults: results from the Longitudinal Aging Study Amsterdam. BMC Public Health. 2006;6:244. doi: 10.1186/1471-2458-6-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aittomäki A, Lahelma E, Rahkonen O, Leino-Arjas P, Martikainen P. The contribution of musculoskeletal disorders and physical workload to socioeconomic inequalities in health. Eur J Public Health. 2007;17(2):145–150. doi: 10.1093/eurpub/ckl121. [DOI] [PubMed] [Google Scholar]
- 38.Harrison MJ, Tricker KJ, Davies L et al. The relationship between social deprivation, disease outcome measures, and response to treatment in patients with stable, long-standing rheumatoid arthritis. J Rheumatol. 2005;32(12):2330–2336. [PubMed] [Google Scholar]
- 39.Eachus J, Chan P, Pearson N, Propper C, Davey Smith G. An additional dimension to health inequalities: disease severity and socioeconomic position. J Epidemiol Community Health. 1999;53(10):603–611. doi: 10.1136/jech.53.10.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brekke M, Hjortdahl P, Kvien TK. Severity of musculoskeletal pain: relations to socioeconomic inequality. Soc Sci Med. 2002;54(2):221–228. doi: 10.1016/s0277-9536(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 41.Brekke M, Hjortdahl P, Thelle DS, Kvien TK. Disease activity and severity in patients with rheumatoid arthritis: relations to socioeconomic inequality. Soc Sci Med. 1999;48(12):1743–1750. doi: 10.1016/s0277-9536(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 42.Anton SD, Karabetian C, Naugle K, Buford TW. Obesity and diabetes as accelerators of functional decline: can lifestyle interventions maintain functional status in high risk older adults? Exp Gerontol. 2013;48(9):888–897. doi: 10.1016/j.exger.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287(8):1022–1028. doi: 10.1001/jama.287.8.1022. [DOI] [PubMed] [Google Scholar]
- 44.Bachmann S, Finger C, Huss A, Egger M, Stuck AE, Clough-Gorr KM. Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;340:c1718. doi: 10.1136/bmj.c1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38(1):1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]


