Abstract
Background:
Recently, the abnormal presence of thyroglobulin antibody (TG-Ab) and thyroid peroxidase antibody (TPO-Ab) has been reported in vitiligo patients, but presence of TG-Ab and TPO-Ab in patients of different ages and gender, and its association with vitiligo and thyroid autoimmunity has rarely been reported. The aim of our research was to determine whether vitiligo was associated with thyroid autoimmunity and figure out its relationship with age and gender.
Materials and Methods:
We analyzed TG-Ab, TPO-Ab in age and gender matched 87 vitiligo patients and 90 healthy controls, the patients of vitiligo who were positive for the presence of TG-Ab and TPO-Ab were followed up to confirm autoimmune thyroid disease subsequently.
Results:
Results showed that the frequencies of TG-Ab (23.0%, 20/87) positivity and TPO-AB (24.1%, 21/87) in vitiligo patients were significantly higher than that in healthy controls (P < 0.05). Moreover, The positivity for of TG-Ab and TPO-Ab was higher in 11-20-year age group and 21-40-year age group than that in age matched healthy controls. We found female patients with vitiligo had higher positive frequencies of TG-Ab and TPO-Ab than healthy female controls. (34.1% vs. 8.8% and 34.1% vs. 11.1%, P = 0.000 and P = 0.011). When 20 patients with TG-Ab and TPO-Ab positivity were followed up for three monthes, 14 of them (70%) were diagnosed as having autoimmune thyroid disease compared with age-matched healthy controls (16.7%, χ2 = 5.4, P = 0.02).
Conclusion:
TG-Ab and TPO-Ab are likely to be found in female teenagers with vitiligo, and are relevant with respect to subsequent development autoimmune thyroid disease.
Keywords: Autoimmune, thyroglobulin antibody, thyroid peroxidase antibody, thyroiditis, vitiligo
Introduction
What was known?
Vitiligo is a systemic autoimmune disease due to the loss of melanocytes from the epidermis.
Thyroid functional disorders and autoimmune thyroid diseases are related to vitiligo and may present in the form of of hyperthyroidism or hypothyroidism.
Vitiligo is a depigmenting disorder characterized by the loss of melanocytes from the cutaneous epidermis with a complex presentation, therapy, and etiology,[1,2] The incidence worldwide is approximately around 0.5-2%.[3] Although, the etiopathogenesis of the disease remains unpredictable, thyroid dysfunction and autoimmunity might be associated with it.[4] Till now, many studies have reported that thyroid functional disorders and autoimmune thyroid diseases are related to vitiligo in the pattern of hyperthyroidism or hypothyroidism.[5,6,7] Iacovelli et al.,[8] have found an elevated incidence of thyroid dysfunction in pediatric patients with non-segmental vitiligo. Zetting et al.,[9] suggested that autoimmune thyroid disease (AITD) was the most frequent autoimmune disease associated with vitiligo. Kasumagic-Halilovic et al.,[10] also demonstrated that the titers of antithyroid autoantibodies significantly increased in vitiligo patients in comparison to healthy subjects.
Many previous studies have been undertaken to study the possibility of vitiligo associated with other autoimmune or endocrine diseases.[7,8,9,10,11,12] As vitiligo usually occurs before the development of thyroid disease, it may be advantageous to screen thyroid function and autoimmune antibodies in all vitiligo patients.[13] Anti-thyroid antibodies are the secondary immune response markers which can reflect the damage to thyroid function. What is more, positivity for thyroid globulin antibody (TG-Ab) and thyroid peroxidase antibody (TPO-Ab) are important diagnostic criteria for AITD, most of which are polyclonal IgG antibodies.[14] Therefore, the presence of TG-Ab and TPO-Ab are major markers for thyroid autoimmunity and by monitoring TG-Ab and TPO-Ab, we can understand the characteristics and extent of AITD.[15] Thus, it seems plausible to screen for thyroid antibodies in vitiligo patients.[16,17]
Thus, by measuring the levels of the anti-thyroid antibodies, the aim of our study was to determine whether vitiligo is associated with autoimmune thyroid diseases, and further illustrate the pathogenesis of vitiligo.
Materials and Methods
Participants
This study was carried out according to the Helsinki guidelines. An informed consent was taken from all patients participating in the study, and the protocol was approved by the research ethics committee of the Second Xiangya Hospital of Central South University. A total of 87 patients with vitiligo (44 males and 43 females), including 77 vulgaris and 10 segmental with an average age of 32.9 (range 4-49) years and the median duration of 2 years (range 0-27 years), were randomly selected from patients diagnosed at the Second Xiangya Hospital of Central South University from Jan 2006 to Oct 2007. Another, 90 healthy individuals age and sex-matched with the vitiligo patients (43 males, 47 females, mean age 33.2 years, range 5-71 years) were also enrolled in this study as healthy control subjects, who had no family history of vitiligo and other autoimmune diseases. When the participants were classified according to thyroid volume, there were 80.5% of normal ones, 17.2% of first degree of swelling, and 2.3% of second degree of swelling in the patients with vitiligo, while 78.9%, 17.8% and 3.3% in healthy control subjects, respectively.
FT3, FT4, TSH, TG-Ab and TPO-Ab assay
Serum anti-thyroid autoantibodies (TG-Ab and TPO-Ab) and thyroid hormones Free T3 (FT3), free T4(FT4) and thyroid stimulating hormone (TSH) were assayed by chemiluminescence (Bayer AG, Leverkusen, Germany). The cutoff values of positivity for TG-Ab and TPO-Ab were 60 IU/ml. The normal range for FT3, FT4, and TSH were 3.5-6.5 pg/ml, 11.5-23.2 pmol/L, 0.35-5.5 pmol/L, respectively.
Thyroid ultrasonography together with physical examination was performed to check thyroid volume which can be divided into I, II and III degrees swelling. The diagnosis of autoimmune thyroid disease was according to endocrinology by Eryuan et al.[18]
Statistical analysis
Results are shown as mean ± SD. The unpaired t-test was used for normally distributed data. The difference between classified variables was tested using Chi-square test or Fishers exact test, if the expected number of subjects in any cell was less than 5. P values less than 0.05 were considered significant.
Results
Thyroid parameters alteration in vitiligo patients and healthy controls
The occurence of TG-Ab and TPO-Ab was higher in vitiligo patients than in control subjects (χ2 = 9.4 and 8.9, P = 0.002 and 0.003). The TSH levels were significantly higher in vitiligo patients than in control subjects (P = 0.045). The FT3 and FT4 levels were also slightly higher in the vitiligo patients. What is more, patients with positive TG-Ab and TPO-Ab had vitiligo vulgaris. Among 13 vitiligo patients with elevated TSH, 12 (92.3%) were the patients with vitiligo vulgaris and 1 (7.6%) had segmental vitiligo [Table 1].
Table 1.
Comparison of altered thyroid parameters between vitiligo and control groups
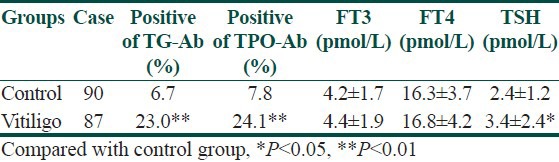
TG-Ab and TPO-Ab distribution in vitiligo patients and healthy controls
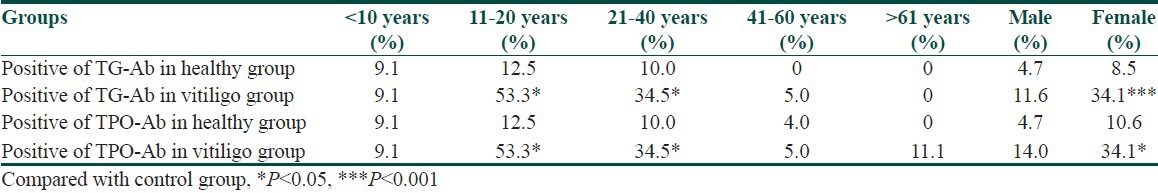
According to the age, the vitiligo patients and healthy controls were divided into five subgroups (<11 years, 11-20 years, 21-40 years, 41-60 years and > 60 years, respectively). The proportions in vitiligo patients were 12.7%, 17.2%, 33.3%, 25.3% and 11.5% and the corresponding were 12.2%, 17.8%, 33.3%, 26.7% and 10.0% in healthy controls.
In 11-20 years and 21-40 years subgroups of vitiligo patients, the positive frequencies of TG-Ab and TPO-Ab were significantly higher than the corresponding subgroups of healthy controls (χ2 = 5.7 and 7.4, both P < 0.05). Further analysis showed that among vitiligo patients, the positive frequencies of TG-Ab and TPO-Ab in 11-20 years age subgroup was significantly higher compared to < 11 years, 41-60 years and > 60 years subgroups (÷2 = 5.3 and 6.7,11.2 and 13.5,4.7 and 6.0, all P < 0.05). The positive frequency of TG-Ab in 21-40 years age subgroup was significantly higher than 41-60 years and > 60 years (χ2 = 7.2 and 4.5, both P < 0.05), while positive frequency of TPO-Ab was significantly higher than 41-60 years subgroup (χ2 = 7.2, P < 0.05) in vitiligo patients. As to the presence of TG-Ab and TPO-Ab, there was no difference neither between 11-20 years and 21-40 years subgroups in vitiligo patients nor among different age subgroups in healthy controls. Besides, the positive frequencies of TG-Ab and TPO-Ab in female vitiligo patients were significantly higher than female healthy controls (χ2 = 7.1 and 9.4, both P < 0.05) [Table 2].
Table 2.
TG-Ab and TPO-Ab distribution of the vitiligo and control groups
Clinical diagnosis
Twenty vitiligo patients and six healthy subjects with positive TPO-Ab and TG-Ab were followed-up every 3 month for 3 years. We found that 14 cases (70%) of vitiligo patients had been diagnosed with autoimmune thyroid disease after an average 2.5 years (2.5 ± 0.5 years). Among them, except for 1 Graves’ disease, the other 13 patients were Hashimoto's thyroiditis. Only 1 male (16.7%) was diagnosed as Hashimoto's thyroiditis (HT) in 6 healthy subjects. There was significant difference between the two groups (χ2 = 5.4, P = 0.02). Among 66 cases of vitiligo patients and 83 cases of healthy subjects with negative TG-Ab and TPO-Ab, only 2 subjects were diagnosed with subclinical hypothyroidism (one was male vitiligo patient, another was female healthy subject).
Discussion
Vitiligo is a systemic autoimmune disease due to the loss of melanocytes from the epidermis. Nowadays, autoimmunity is considered to be a major etiological factor. And the presence of the TG-Ab and TPO-Ab not only sustained autoimmune inflammation, but it also might be the key factor for several autoimmune diseases with chronic features.[15] Thus, it is of no doubt that there is a close relationship between the serum TG-Ab and TPO-Ab concentrations and chronic thyroid inflammation.
Our study confirmed previous findings that the differences in terms of serum thyroid parameters between vitiligo patients and healthy controls did exist. We found that the occurence of TG-Ab and TPO-Ab were signficantly higher in vitiligo patients compared to the healthy subjects, and all of the patients positive for TG-Ab and TPO-Ab had vitiligo vulgaris. In this study, the presence of TG-Ab and TPO-Ab in different gender and age subgroups of vitiligo patients and in sex and age matched healthy controls was also were analyzed. The TG-Ab and TPO-Ab were significantly higher in female vitiligo patients compared to female controls. We found that compared to healthy controls, the presence of TG-Ab and TPO-Ab were higher in 11-20 years and 21-40 years age group. In addition, the highest positivity of these two antibodies was in 11-20 years age group of vitiligo patients, which was consistent with other studies. However, we did not find the increased ocurrence of TG-Ab and TPO-Ab in < 10 years age groups, which was potentially attributable to the vitiligo patients enrolled in the different studies, and can be explained by the diversity of ethnic groups.
In order to compare the thyroid function of vitiligo patients and healthy controls, we test FT3, FT4 and TSH. Compared to total T3 and total T4, FT3 and FT4 could be unaffected on serum thyroid-binding globulin (TBG) which can directly reflect the thyroid function and have better sensitivity and specificity.[15] We found that TSH level was slightly higher in vitiligo patients, which indicated a mild damage. Furthermore, in subsequent clinical follow-up, we found that around 70% TG-Ab and TPO-Ab positive vitiligo patients were diagnosed as having autoimmune thyroid diseases at average 2.5 years. Besides one case of Grave's disease, the rest had Hashimoto's thyroiditis, which was consistent with previous elevated TSH level in vitiligo patients.
Vitiligo is frequently associated with other autoimmune diseases, particularly autoimmune thyroid diseases including Hashimoto's thyroiditis and Graves’ disease, rheumatoid arthritis, type 1 diabetes, psoriasis, pernicious anemia, systemic lupus erythematosus, Addison's disease, and alopecia areata. This indicates the presence of genetically determined susceptibility to not only vitiligo but also to other autoimmune disorders.[19] The association between vitiligo and thyroid autoimmunity may be explained by the presence of a genetic background of shared susceptibility. As T-cell mediate autoimmune diseases, Graves’ disease and Hashimoto's thyroiditis have lymphocytic infiltration in the thyroid parenchyma.[20] Similarly, skin biopsies from vitiligo patients show dermal and epidermal lymphocytic infiltrate, consisting of activated T-cells, which are thought to cause melanocyte destruction. Studies have also identified immunogenetic associations with vitiligo and autoimmune thyroid diseases. Vitiligo is associated with HLA-DR4, and thyroid disease is associated with Class 1 and Class II HLA including HLA-DR.[21] Graves’ disease has been associated with HLA-DR3.[22] In comparison, Hashimoto's thyroiditis has not had a consistent HLA-association, but HLA-DR has been ocassionally associated. Therefore, vitiligo and AITD are closely associated epidemiologically, and it is likely that, as additional susceptibility genes are identified for both of these autoimmune diseases, many will be shared in common, leading to a convergence of shared autoimmune susceptibility genes, as well as sets of specific genes and ultimately environmental triggers that determine disease specificity and onset.
Our study indicates a potential risk of autoimmune thyroid disease in vitiligo patients. For young patients with vitiligo vulgaris, especially females screening for TG-Ab and TPO-Ab is of importance for early diagnosis and treatment of autoimmune thyroid diseases.
Young female patients with vitiligo have higher positivity of TG-Ab and TPO-Ab, and have a trend towards developing autoimmune thyroid disease.
What is new?
The female teenagers with vitiligo tend to have autoimmune thyroid disease, and screening TG-Ab and TPO-Ab in young vitiligo patients is of importance for early diagnosis and treatment of autoimmune thyroid diseases.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Kovacs SO. Vitiligo. J Am Acad Dermatol. 1998;38(5 Pt 1):647–66. doi: 10.1016/s0190-9622(98)70194-x. quiz 667-8. [DOI] [PubMed] [Google Scholar]
- 2.Boissy RE, Nordlund JJ. Vitiligo: Current medical and scientific understanding. G Ital Dermatol Venereol. 2011;146:69–75. [PubMed] [Google Scholar]
- 3.Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. PigmentCellRes. 2003;16:208–14. doi: 10.1034/j.1600-0749.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 4.Kemp EH, Waterman EA, Weetman AP. Autoimmune aspects of vitiligo. Autoimmunity. 2001;34:65–77. doi: 10.3109/08916930108994127. [DOI] [PubMed] [Google Scholar]
- 5.Abanmi A, Al Harthi F, Al Baqami R, Al Assaf S, Zouman A, Arfin M, et al. Association of HLA loci alleles and antigens in Saudi patients with vitiligo. Arch Dermatol Res. 2006;298:347–52. doi: 10.1007/s00403-006-0699-4. [DOI] [PubMed] [Google Scholar]
- 6.Hegedüs L, Heidenheim M, Gervil M, Hjalgrim H, Høier-Madsen M. High frequency of thyroid dysfunction in patients with vitiligo. Acta Derm Venereol. 1994;74:120–3. doi: 10.2340/0001555574120123. [DOI] [PubMed] [Google Scholar]
- 7.Shriya D, Mariette D'S, Devinder MT, Reddy KS, Zachariah BB. High frequencyof thyroid dysfunction in Indian patients with vitiligo. Indian J Dermatol. 2003;48:68–72. [Google Scholar]
- 8.Iacovelli P, Sinagra JL, Vidolin AP, Marenda S, Capitanio B, Leone G, et al. Relevance of thyroiditis and of other autoimmune diseases in children with vitiligo. Dermatology. 2005;210:26–30. doi: 10.1159/000081479. [DOI] [PubMed] [Google Scholar]
- 9.Zettinig G, Tanew A, Fischer G, Mayr W, Dudczak R, Weissel M. Autoimmune diseases in vitiligo: Do anti-nuclear antibodies decrease thyroid volume? Clin Exp Immunol. 2003;131:347–54. doi: 10.1046/j.1365-2249.2003.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasumagic-Halilovic E, Prohic A, Begovic B, Ovcina-Kurtovic N. Association between Vitiligo and Thyroid Autoimmunity. J Thyroid Res. 2011;2011:938257. doi: 10.4061/2011/938257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopal KV, RamaRao GR, Kumar YH, Appa Rao MV, Vasudev P, Srikant Vitiligo: A part of a systemic autoimmune process. Indian J Dermatol Venereol Leprol. 2007;73:162–5. doi: 10.4103/0378-6323.32710. [DOI] [PubMed] [Google Scholar]
- 12.Sedighe M, Gholamhossein G. Thyroid dysfunction and thyroid antibodies in Iranian patients with vitiligo. Indian J Dermatol. 2008;53:9–11. doi: 10.4103/0019-5154.39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sehgal VN, Srivastava G. Vitiligo: Auto-immunity and immune responses. Int J Dermatol. 2006;45:583–90. doi: 10.1111/j.1365-4632.2005.02651.x. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Lin X, Fu W, Luo X, Kang K. An approach to the correlation between vitiligo and autoimmune thyroiditis in Chinese children. Clin Exp Dermatol. 2010;35:706–10. doi: 10.1111/j.1365-2230.2009.03671.x. [DOI] [PubMed] [Google Scholar]
- 15.Radetti G, Gottardi E, Bona G, Corrias A, Salardi S, Loche S Study Group for Thyroid Diseases of the Italian Society for Pediatric Endocrinology and Diabetes (SIEDP/ISPED) The natural history of euthyroid Hashimoto's thyroiditis in children. J Pediatr. 2006;149:827–32. doi: 10.1016/j.jpeds.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 16.Carle A, Laurberg P, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, et al. Thyroid peroxidase and thyroglobulin auto-antibodies in patients with newly diagnosed overt hypothyroidism. Autoimmunity. 2006;39:497–503. doi: 10.1080/08916930600907913. [DOI] [PubMed] [Google Scholar]
- 17.Shong YK, Kim JA. Vitiligo in autoimmune thyroid disease. Thyroidology. 1991;3:89–91. [PubMed] [Google Scholar]
- 18.Liao EY. Beijing, China: People's Health Press; 2001. Endocrinology; pp. 614–47. [Google Scholar]
- 19.Oiso N, Suzuki T, Fukai K, Katayama I, Kawada A. Nonsegmental vitiligo and autoimmune mechanism. Dermatol Res Pract 2011. 2011:518090. doi: 10.1155/2011/518090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neerja P. Correspondence: A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247–8. doi: 10.4103/0019-5154.96227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–25. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 22.Kraiem Z, Newfield RS. Graves’ disease in childhood. J Pediatr Endocrinol Metab. 2001;14:229–43. doi: 10.1515/jpem.2001.14.3.229. [DOI] [PubMed] [Google Scholar]


