Abstract
Background:
Chronic urticaria (CU) is a vexing problem and patients of CU suffer from the morbidity that arise from irritable itch and weals and are also subjected to a huge antihistamine pill burden. The symptoms are more in autoreactive urticaria (AU) where auto-antibodies in blood flares-up the condition. Search for newer effective modalities which can reduce pill burden is a felt need.
Aims:
This study evaluates the effectiveness of autologous serum therapy (AST) in CU and also determines its usefulness in AU.
Materials and Methods:
Double blind, parallel group, randomized, controlled study. Fifty four patients were given AST and 57 patients were given injection normal saline (placebo), along with cetirizine in an on-demand basis in both groups. AST/Placebo was given weekly for nine weeks and followed-up for a total period of 24 weeks. AU was diagnosed by autologous serum skin test. Urticaria total severity score (TSS), Urticaria activity score (UAS), Dermatologic life quality index (DLQI) was used as primary effectiveness variables. Safety parameters assessed were the spontaneously reported adverse events and laboratory parameters.
Results:
TSS showed significant improvement from baseline, 7th week and 8th week onwards in AST group and placebo group respectively. Group comparison showed significant improvement 4th week onwards. UAS showed similar results. DLQI showed significant improvement in AST group compared to placebo at the end of study. Both AU and non-AU patients showed comparable improvement of TSS.
Conclusion:
AST shows promise in treatment of urticaria regardless of the autoreactive nature.
Keywords: Autologous serum skin test, autologous serum therapy, autoreactive urticaria, cetirizine chronic urticaria
Introduction
What was known?
Chronic urticaria has an unpredictable, relentless course and antihistamines are the first line treatment for this distressing condition. The effectiveness of antihistamines is limited during the period of its use; so search for a therapeutic modality that can provide extended relief and reduce pill burden is the need of the hour.
Chronic urticaria has an unpredictable, relentless course. Patients of chronic urticaria suffer from the morbidity that arise from irritable itch and weals and are also subjected to a huge antihistamine pill burden. The patients are at constant apprehension of this temperamental disease which at times waxes and wanes. There have been many studies which compare the efficacy of various second generation antihistamines,[1,2,3] the first line treatment for chronic urticaria.[4] However, the effectiveness of antihistamines is limited during the period of its use; so search for a therapeutic modality that can provide extended relief and reduce pill burden is the need of the hour.
Autologous serum containing tolerance-generating anti-idiotype antibodies to mast cell degranulating antigens has been tried for disease remission in various autoimmune diseases.[5] Non-randomized, non-blinded, short-term studies using autologous serum had been undertaken to note the effectiveness in chronic urticaria also.[6,7] Our study was done to assess the effectiveness and safety of autologous serum therapy (AST) as an adjunctive therapy to standard antihistamine cetirizine compared to patients receiving cetirizine alone. Autoreactive urticaria is a subgroup of chronic urticaria containing circulating functional autoantibodies and AST effectiveness was determined in this subgroup.
Materials and Methods
The study was designed as a single-centre, double-blind, randomized (1:1), placebo-controlled trial. Institutional Ethics Committee permission was obtained prior to the onset of the study and written informed consent was obtained from all study participants. The trial bears the registration number of CTRI/2012/12/003204. The duration of study was from March 2011 to March 2012. Adult patients (>18 years) of either sex suffering from chronic urticaria attending the Dermatology out-patient department of a tertiary care teaching hospital in Eastern India were included. The operational definition of chronic urticaria was taken as itching and weals occurring daily or near daily (≥3 times/week) for ≥6 weeks.[8] Pregnant and lactating women, patients suffering from immunosuppression due to drug or disease, advanced diseases of vital organs, inability to come for weekly follow-ups, addicted to alcohol or other substance abuse, machinery operators, vehicle drivers and in whom sleep/wake cycle alteration could be an issue were excluded from the study.
Visits
Screening visit
At the screening visit, patients were enrolled based on inclusion and exclusion criteria, written informed consent was taken. A run-in period of two days was given prior to the initiation of therapy,[6] when patients were asked to discontinue any antihistamines they had been using. A thorough clinical examination was done to note any associated co-morbidities and relevant investigations were done for their confirmation.
Baseline visit
Autologous serum skin test (ASST) was performed in all study subjects and the result recorded after 30 min (as detailed below). Successive eligible patients were randomized into either treatment arms using a computer generated random number table in 1:1 ratio irrespective of the ASST status. The patients received autologous serum therapy or normal saline as placebo in either treatment group. Cetirizine tablets (5mg) (Brand name: CIT-Z, Manufacturer: Square Pharmaceuticals, Central Medical Store supply, supplied free of cost to the patient from institutional pharmacy) were dispensed to patients in both treatment groups and they were instructed to consume them in an on-demand basis (experiencing wealing or itching) but not more than 1 tablet per day. Any subject experiencing intolerable symptoms of urticaria were instructed to contact the investigator.
Follow-up visits
At each successive weekly follow-up, AST or sterile water was given as per randomization for eight consecutive weeks.[6] At all these eight follow-ups, the effectiveness and safety parameters were noted. The AST therapy was then discontinued and the patients were followed-up for improvement or recurrence of symptoms weekly for the next four weeks. The patients were again asked to come at the end of 24 weeks post randomization. Cetirizine was administered throughout the study period in an on-demand basis even after completion of AST injections. At baseline and at every follow-up effectiveness and safety parameters were noted. Prednisolone tablet (1mg/kg body wt) was kept as rescue medication.
Autologous serum skin test
Five ml venous blood of the patient was drawn with a sterile, disposable syringe from the antecubital vein in sterile BD Vacutainer® (BD, NJ USA) for serum collection. The blood was subjected to centrifugation using centrifuge machine (R-8C laboratory centrifuge, REMI laboratory instruments, Mumbai, India) at the rate of 2000 rpm for 10 min at room temperature. 0.05 ml of the serum thus separated was injected immediately intra-dermally into the patients’ left flexor forearm 2 inches below the antecubital crease and 0.05 ml sterile normal saline as negative control into right forearm using 31G sterile disposable 1ml BD insulin syringe (BD, NJ USA). The bevelled end of the needle was kept in the upwards direction producing a palpable bleb on the skin. Areas which were involved in spontaneous wealing within last 48 h were avoided. A reading of the weal was taken after 30 min. Patients having weal of more than 1.5mm perpendicular diameter than that of control were considered to be suffering from autoreactive urticaria (ASST positive).[6,9]
Autologous serum therapy
Two ml of the fresh serum separated from the patients’ blood (as stated above) was given deep IM into the upper arm for nine successive weeks (baseline and initial eight follow-up visits).
Blinding
Five ml blood was drawn from all patients irrespective of randomization; serum so separated was injected into the patient in the AST group whereas the patient in the placebo group received normal saline in syringes made opaque by adhesive tape (Leucoplast, Beiersdorf India Pvt. Ltd., Ponda, Goa, India). Thus, all patients were blinded regarding the treatment they received as all of them had their blood drawn and injections given in the same amount and in a similar fashion. The evaluator who assessed the effectiveness and safety parameters at baseline and at follow-ups was another dermatologist who was seated in a separate room and not involved in randomization, drawing, centrifuging, or injection of serum/placebo making the trial double-blind.
Effectiveness parameters
The primary outcome measures for effectiveness were Urticaria activity score (UAS)[10] and Urticaria total severity score (TSS).[6] UAS quantifies disease activity depending on the weal number and size [0: <10 small weals (diameter <3 cm), 1: 10-50 small weals or <10 large weals (diameter >3 cm), 2: >50 small weals or 10-50 large weals, 3: almost whole body covered] and the itch severity score (0: none, 1: mild, 2: moderate, 3: severe). TSS is calculated from the number and size of weals, intensity of pruritus, duration of persistence of lesions, frequency of appearance of lesions and frequency of antihistamine use, with each parameter having a score of 0-3, maximum score being 18. TSS is a more holistic parameter than UAS as it reflects the pill burden along with disease activity; however UAS being simpler to use is more commonly used by dermatologists.
The pill burden was calculated separately using a score (0-none/week, 1 = less than once or once/week, 2 = 2-3 times/week, 3 = daily or almost daily/week) recorded at every visit. The secondary effectiveness parameters were Patients’ global assessment of disease activity improvement and Physicians’ global assessment of disease activity improvement, both recorded on a 5-point Likert scale (0: No improvement, 1: Mild improvement, 2: Moderate improvement, 3: marked improvement, 4: Excellent improvement).[11] These parameters were assessed at baseline, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 24 weeks following randomization.
Safety parameters
Vital signs and spontaneously reported adverse events were assessed at every week of follow-up. Changes in laboratory values of routine hemogram (total count, differential count, hemoglobin percentage, ESR), liver function tests (bilirubin, SGPT, SGOT, alkaline phosphatase, albumin: globulin), serum urea, creatinine, fasting blood glucose were recorded at baseline and at the end of active treatment with AST (8th follow-up).
Quality of life parameter
Quality of life in urticaria patients was assessed by a validated vernacular (Bengali) version of Dermatology Life Quality Index (DLQI)[12] [http://www.dermatology.org.uk/downloads/DLQI_Bengali.pdf] which consisted of ten questions, each scored between 0-3. Prior to use of the questionnaire, permission was obtained from the developer (Andrew Y Finlay).
Statistical analysis
One hundred and twenty was the target sample size, with 60 evaluable subjects in each group. This was calculated considering a conventional trial, to detect a difference of two in TSS between groups, with 80% power and 5% probability of type 1 error, assuming a standard deviation of 3.5 for this parameter and considering possible 20% dropouts. Continuous variables were compared within group by paired t test and between groups by independent samples t test. For comparison of unpaired and paired non-parametric data, Mann Whitney U test and Wilcoxon's matched pairs signed rank test were employed respectively. Friedman's analysis of variance (ANOVA) was carried out with non-parametric data for within group repeated measures comparisons, followed by post-hoc Dunn's test. Categorical data were compared between groups by Chi-squared test or Fisher's exact test, as applicable. MedCalc version 11.6 [Mariakerke, Belgium: MedCalc Software, 2011] and GraphPad Prism version 5 [San Diego, California: GraphPad Software Inc., 20057] software were used for statistical analysis.
Effectiveness analysis was done on modified intention-to-treat basis with subjects reporting for at least one post-baseline follow-up visits. The last observation carried forward strategy dealt with the missing values. Pre and post-treatment laboratory values were compared in patients for whom both sets of data were available. For other safety analysis, all subjects who had received at least one dose of a study drug (essentially all 120 subjects) were considered.
Results
Among 156 study participants screened, 120 were randomized equally into two groups receiving AST or placebo in addition to cetirizine SOS. Nine subjects were lost to follow-up (termination of trial prior to one week of follow-up) leaving 111 (92.5%) intention-to-treat dataset. The flow of study participants is depicted in Figure 1.
Figure 1.
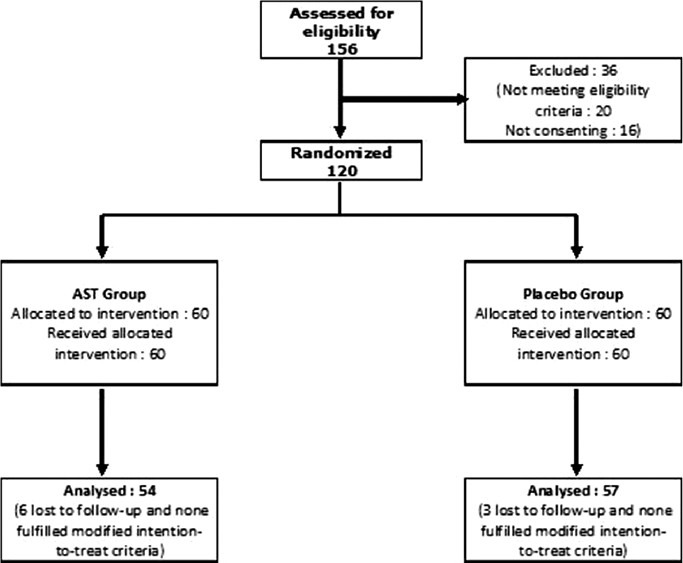
Flow chart of study participants
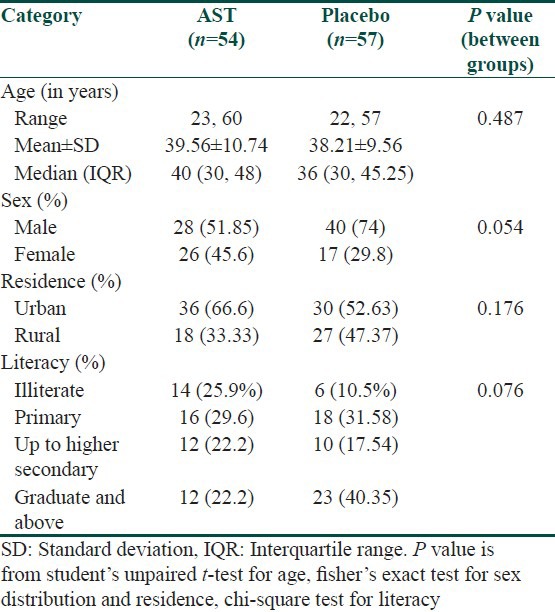
Males outnumbered females in both the treatment arms and were of the late thirties age group. The mean duration of urticaria prior to inclusion in the study was comparable in both the treatment arms. Study groups were comparable at baseline with respect to age, sex, rural-urban status and literacy and occupation categories. Majority of cases in both the treatment arms belonged to idiopathic urticaria (19.8%) and dermatographism (27%). Atopy was the most common co-existent illness and two patients had autoimmune disease (SLE) [Table 1].
Table 1.
Demographic profile of study population
There were no significant changes of laboratory parameters at the end of active treatment (8th follow-up) from baseline. Sedation (27%), dryness of mouth (9%), epigastric pain (2.7%), nausea (2.7%) and diarrhea (1.8%) were the spontaneously reported adverse events, however they were comparable in either group.
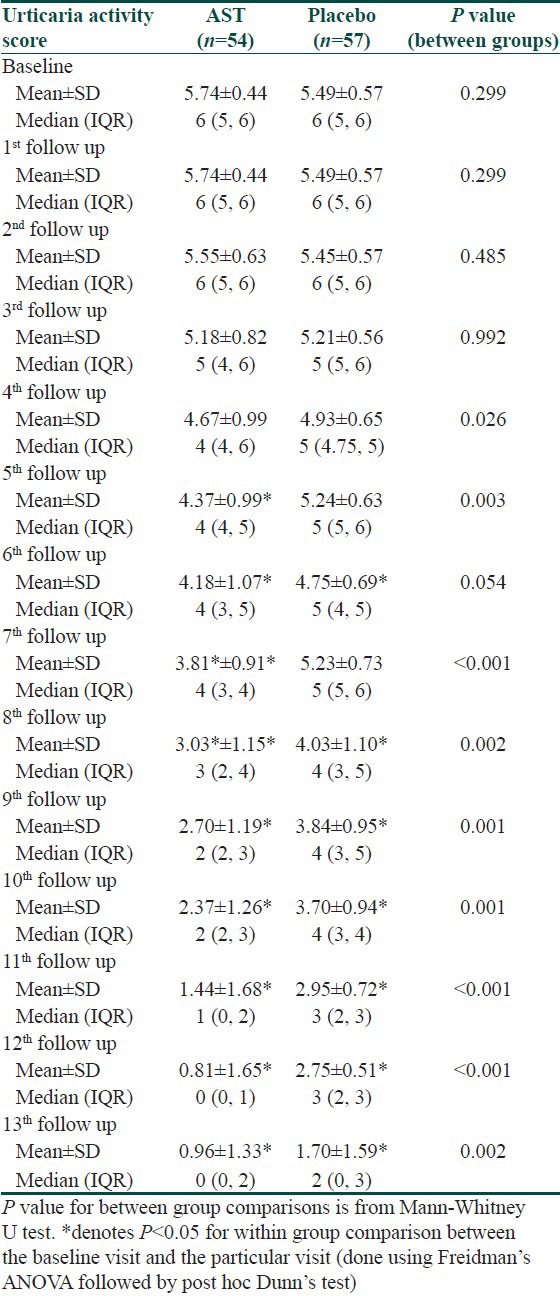
UAS was comparable at baseline, and decreased significantly in the AST group from 5th follow-up and in the placebo group from 6th follow-up onwards. However; intergroup comparison revealed that the decrease was significantly more in the AST group from 4th follow-up and was evident till end of six months [Table 2].
Table 2.
Changes in urticaria activity score during the study period
At baseline, TSS was comparable in both treatment arms. Significant decline in TSS was observed in the AST group and placebo group from the 5th and 6th follow-up respectively. TSS too showed significant decrease in the AST group as compared to placebo from 4th follow-up till study end [Table 3]. Similar observations were noted with Physicians’ and Patients’ global assessment of disease improvement scales [Figures 2 and 3].
Table 3.
Changes in urticaria total severity score during the study period
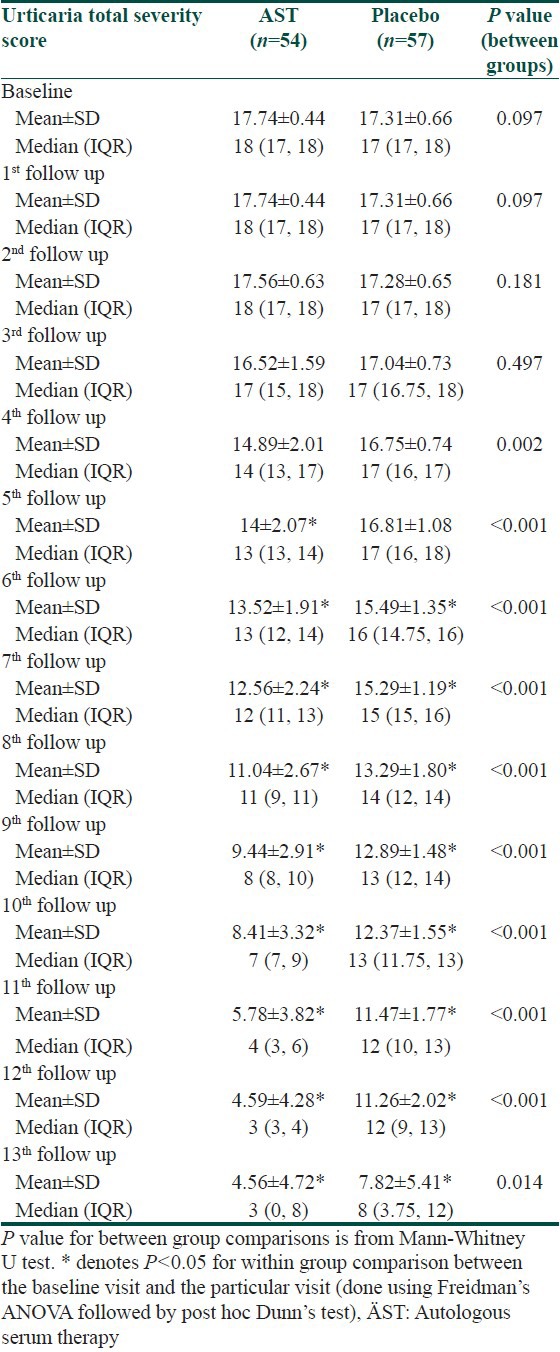
Figure 2.
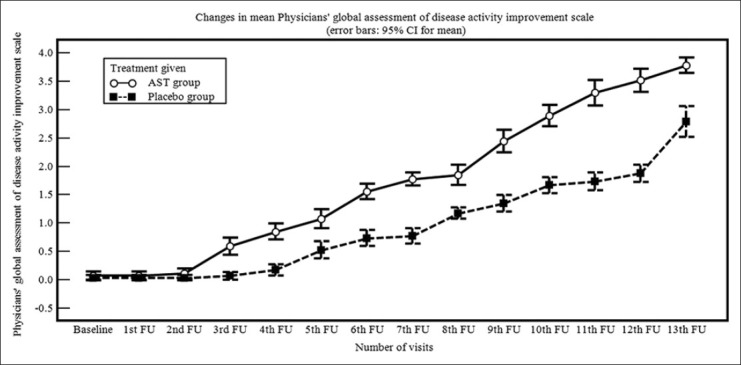
Line diagram showing changes in mean Physicians’ global assessment of disease activity improvement scale during treatment period
Figure 3.
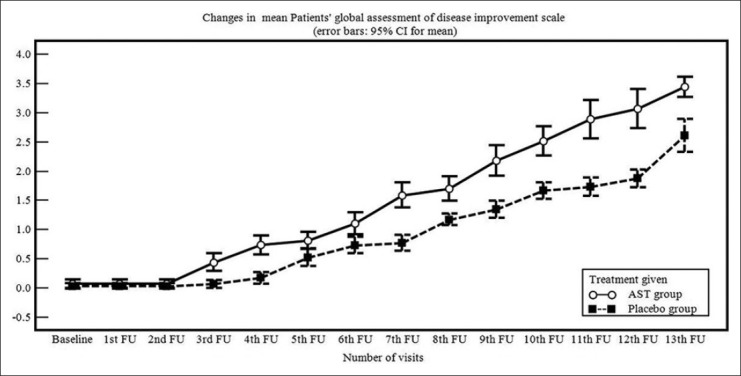
Line diagram showing changes in Patients’ global assessment of disease activity improvement scale during treatment period
Autoreactive urticaria subgroup (ASST positive) comprised of 45 (40.5%) patients of our study population, of whom 20 (18%) patients got randomized to AST group and the rest (22.5%) received placebo. A notably significant decrease in TSS was found those receiving AST and was evident from 4th follow-up onwards [Table 4].
Table 4.
Difference in TSS in autoreactive urticaria (ASST positive) subgroup (n=45) during the study period
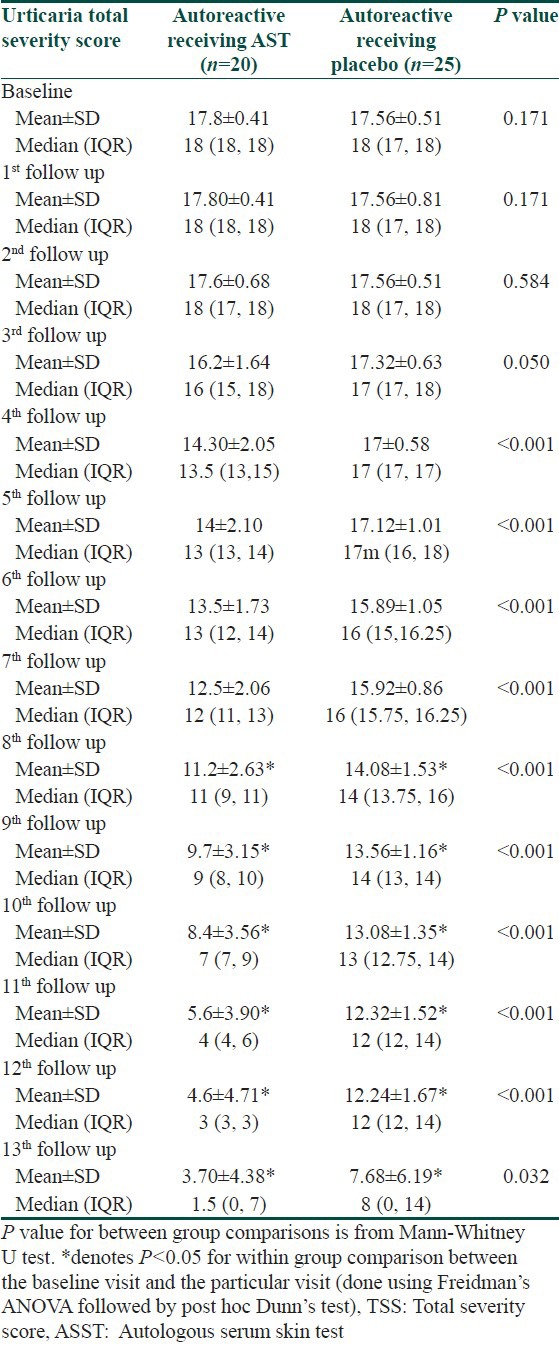
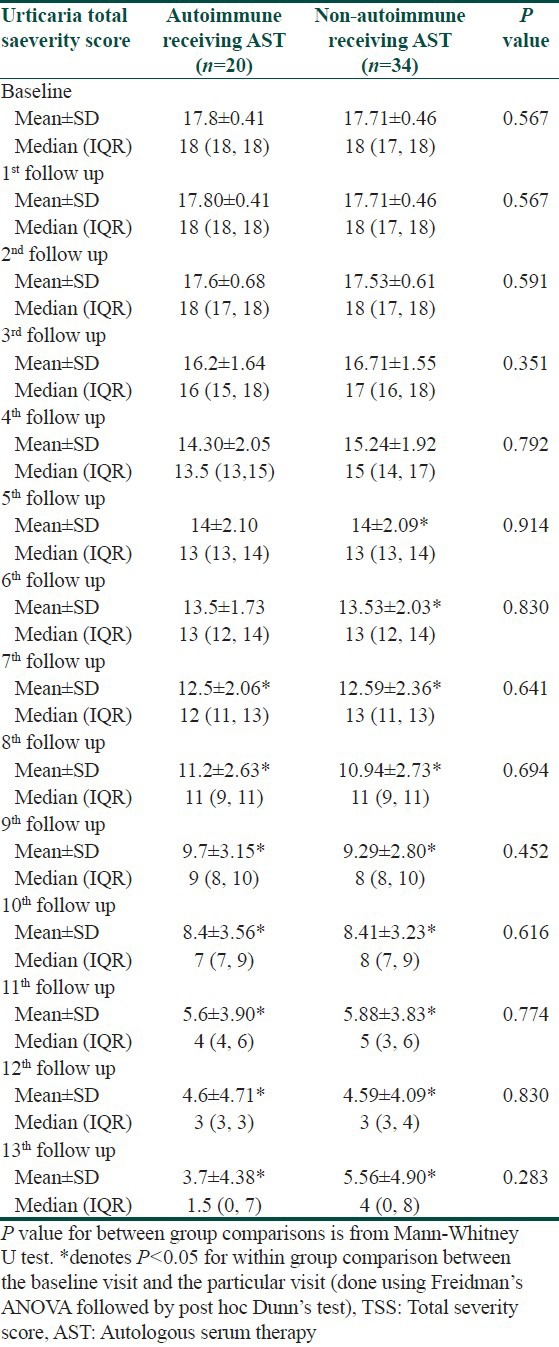
Subgroup analysis of those who received AST revealed significant decline irrespective of their autoreactive urticaria status. Autoreactive urticaria took two weeks more time for significant decline from baseline TSS than non- autoreactive variety, though the intergroup comparison showed no significant difference at every follow-up [Table 5].
Table 5.
Changes in TSS in autoreactive and non-autoreactive urticaria receiving AST
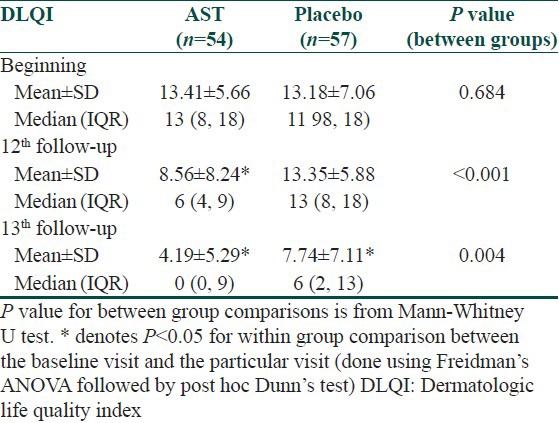
DLQI score was comparable in both the treatment arms at baseline. Significant improvement in quality of life was evident at 12th follow-up in the AST group; whereas placebo group showed no such change. At 24 weeks, observation was similar [Table 6].
Table 6.
Changes in DLQI over the treatment period
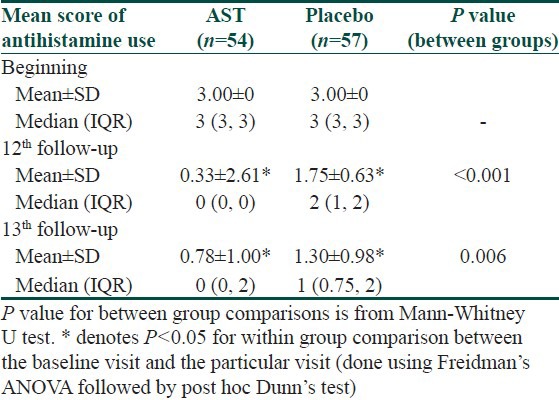
The antihistamine pill burden was significantly less in the AST group compared to the patients receiving placebo [Table 7].
Table 7.
Changes in mean score of antihistamine use over the treatment period
Discussion
Since, the time of Heberden who described urticaria and said “…the greatest number (of patients) experience no other evil from it besides the intolerable anguish arising from the itching.”,[13] till this 21st century, urticaria has shown to have significant impact of patient quality-of-life on the aspect of emotion, functioning as well as symptoms.[14,15] Mast cell degranulation plays the pivotal role in the pathogenesis of urticaria, however the reason for degranulation is shrouded in mystery in a large fraction of cases and the term “idiopathic” is used to describe this entity. In recent times antibody to high-affinity IgE receptor (FcεRI) is identified and is found to degranulate the mast cell by crosslinking the IgE receptors. This entity is regarded as autoreactive urticaria and is found to prevail in 27-61% of chronic urticaria.[16,17,18,19] In our study population, 40.5% was found to be suffering from autoreactive urticaria. The gold standard for diagnosing the antibodies to FcεRI is basophil histamine release and the closest in-vivo analogue of it is autologous skin test (ASST).[20]
Degranulation leads to release of both pre-formed and newly formed mediators like histamine, leukotrienes, prostaglandins, platelet activating factor, cytokines, proteases, etc., The pharmacotherapy for urticaria rests on two important pillars; one being neutralizing the effect of products of degranulation and the other is to prevent degranulation. Antihistamines and leukotriene inhibitors, are till date the mainstay for urticaria management which primarily target two of the many mediators and thus at times insufficient in controlling urticaria. There are times when immunosuppressive agents (e.g., corticosteroids, cyclosporine, methotrexate, adalimumab etc.) are needed for controlling urticaria symptoms and they aim to avert degranulation by preventing antibody formation. The use of these agents is limited by virtue of their side-effect profile(s) and/or by their prohibitory cost.[4]
Chronic urticaria is a disease with unpredictable course and the treatment is continued till the disease goes into remission. The need for newer therapeutic modality to supplement the antihistamines and leukotriene inhibitors is long felt and any adjuvant therapy that can reduce the pill-burden while achieving symptom free period is welcome by the patients and physicians alike. Our study explored the long forgotten art of autologous whole blood therapy with some modification. The use of serum in place of whole blood is the modification that has been used by Bajaj et al.[6] Whole blood therapy had been tried any many autoimmune diseases including pemphigus,[21] severe dry eye[22] (due to Sjogren's syndrome, rheumatoid arthritis, etc) and diseases like viral illness like herpes, cancer, allergic diseases like atopic dermatitis.[23,24,25,26] Potential of whole blood in the treatment of urticaria was documented by Fleck M[27] and Stuabach et al.,[23] in separate studies and later use of serum in the treatment of urticaria was highlighted by Bajaj et al.[6] The plausible mechanism of action of AST was thought be induction of anti-idiotypes, which have recently been shown to inhibit the function of disease-inducing antibodies in pemphigus and also to shift the Th2 cytokine profile to Th1 in ASST+ patients.[5,23] The present study revealed that AST has enormous potential in the treatment of urticaria. The symptoms of urticaria showed significant reduction by 5th week of initiation of therapy and the control was far better than what could be achieved by on-demand antihistamine use. The reduction in the symptom is accompanied by reduction in pill-burden and decrease in TSS score.
The reduction in pill-burden gives a sense of well-being that is reflected in the improvement of quality of life (measured by DLQI) which was found to be significant in those receiving AST. The improvement that was evident from 5th week of therapy continued even at six months which speaks for itself the usefulness of this therapeutic modality.
Chronic urticaria patients with a positive ASST (autoreactive subgroup) are more likely to be associated with HLA DR4, to have autoimmune thyroid disease, a more prolonged disease course and may be recalcitrant to H1-antihistamine treatment than those with a negative ASST.[9] The autoreactive category of patients (40.5%) in our study was higher than that observed by Godse from Mumbai (26.67%)[16] and lower than Bajaj from Allahabad (49.5%).[6]
We found that improvement in urticaria symptoms were evident in both autoreactive and non-autoreactive urticaria patients but those with autoreactive urticaria required more time to experience the benefit of AST. It should be focused that in autoreactive urticaria, a subgroup of urticaria otherwise refractory to conventional therapy, AST has proved itself as an excellent adjuvant therapy. The goal of therapy in chronic urticaria is to maintain a symptom free period and to ensure that the treatment is not associated with least hazards and monetary burden.
Conclusion
In patients with chronic urticaria, AST is a useful adjunct which reduces the pill burden and improves the quality of life. The effect of nine weekly injections of autologous serum was found to persist even four months after cessation of therapy. Autoreactive urticaria patients also benefited from this method and thus AST finds its place in the therapeutic armamentarium of clinicians treating chronic urticaria.
What is new?
Autologous serum therapy (AST) is a useful adjunct to antihistamines in chronic unticaria. The effect of nine weekly injections of autologous serum was found to persist even four months after cessation of therapy and thus AST reduces the pill burden and improves the quality of life.
Acknowledgement
The support provided by the residents and staffs of the Department of Dermatology of the institution during conduction of the study is duly acknowledged. The authors would also like to acknowledge Prof. Andrew. Y. Finlay for giving permission to use Bengali version of Dermatologic life quality index (DLQI).
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Sil A, Tripathi SK, Chaudhuri A, Das NK, Hazra A, Bagchi C, et al. Olopatadine versus levocetirizine in chronic urticaria: An observer-blind, randomized, controlled trial of effectiveness and safety. J Dermatolog Treat. 2013 doi: 10.3109/09546634.2012.750414. In press. [DOI] [PubMed] [Google Scholar]
- 2.Okubo Y, Shigoka Y, Yamazaki M, Tsuboi R. Double dose of cetirizine hydrochloride is effective for patients with urticaria resistant: A prospective, randomized, non-blinded, comparative clinical study and assessment of quality of life. J Dermatolog Treat. 2013;24:153–60. doi: 10.3109/09546634.2011.608783. [DOI] [PubMed] [Google Scholar]
- 3.Anuradha P, Maiti R, Jyothirmai J, Mujeebuddin O, Anuradha M. Loratidine versus levocetirizine in chronic idiopathic urticaria: A comparative study of efficacy and safety. Indian J Pharmacol. 2010;42:12–6. doi: 10.4103/0253-7613.62399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuberbier T, Asero R, Bindslev-Jensen C, Canonica G, Church MK, Gimenez-Arnau AM, et al. EAACI/GA2LEN/EDF/WAO guideline: Management of urticaria. Allergy. 2009;64:1427–43. doi: 10.1111/j.1398-9995.2009.02178.x. [DOI] [PubMed] [Google Scholar]
- 5.Alvarado-Flores E, Avalos-Diaz E, Diaz L, Herrera-Esparza R. Anti-idiotype antibodies neutralize in vivo the blistering effect of pemphigus foliaceus IgG. Scand J Immunol. 2001;53:254–8. doi: 10.1046/j.1365-3083.2001.00863.x. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj AK, Saraswat A, Upadhyay A, Damisetty R, Dhar S. Autologous serum therapy in chronic urticaria: Old wine in a new bottle. Indian J Dermatol Venereol Leprol. 2008;74:109–13. doi: 10.4103/0378-6323.39691. [DOI] [PubMed] [Google Scholar]
- 7.Patil S, Sharma N, Godse K. Autologous serum therapy in chronic urticaria. Indian J Dermatol. 2013;58:225–6. doi: 10.4103/0019-5154.110833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greaves MW, Sabroe RA. Allergy and the skin I: Urticaria. Br Med J. 1998;316:1147–50. doi: 10.1136/bmj.316.7138.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P, Grattan CE. EAACI/GA2LEN task force consensus report: The autologous serum skin test in urticaria. Allergy. 2009;64:1256–68. doi: 10.1111/j.1398-9995.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 10.Erbagci Z. The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria: A single-blind, placebo-controlled, crossover clinical study. J Allergy Clin Immunol. 2002;110:484–8. doi: 10.1067/mai.2002.126676. [DOI] [PubMed] [Google Scholar]
- 11.Bonifati C, Berardesca E. Clinical outcome measures of psoriasis. Reumatismo. 2007;59(Suppl 1):64–7. doi: 10.4081/reumatismo.2007.1s.64. [DOI] [PubMed] [Google Scholar]
- 12.Finlay AY. Quality of life indices. Indian J Dermatol Venereol Leprol. 2004;70:143–8. [PubMed] [Google Scholar]
- 13.Heberden W. Of the nettle rash. Med Trans. 1772;2:173. [Google Scholar]
- 14.Maurer M, Ortonne JP, Zuberbier T. Chronic urticaria: A patient survey on quality-of-life, treatment usage and doctor-patient relation. Allergy. 2009;64:581–8. doi: 10.1111/j.1398-9995.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- 15.Grob JJ, Gaudy-Marqueste C. Urticaria and quality of life. Clin Rev Allergy Immunol. 2006;30:47–51. doi: 10.1385/CRIAI:30:1:047. [DOI] [PubMed] [Google Scholar]
- 16.Godse KV. Autologous serum skin test in chronic idiopathic urticaria. Indian J Dermatol Venereol Leprol. 2004;70:283–4. [PubMed] [Google Scholar]
- 17.Fiebiger E, Maurer D, Holub H, Reininger B, Hartmann G, Woisetschlδger M, et al. Serum IgG autoantibodies directed against the alpha chain of Fc epsilon RI: A selective marker and pathogenetic factor for a distinct subset of chronic urticaria patients? J Clin Invest. 1995;96:2606–12. doi: 10.1172/JCI118325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer M, Kinet JP, Kaplan AP. Comparative studies of functional and binding assays for IgG anti Fc epsilon Riα (α-subunit) in chronic urticaria. J Allergy Clin Immunol. 1998;101:672–8. doi: 10.1016/s0091-6749(98)70176-9. [DOI] [PubMed] [Google Scholar]
- 19.Piconi S, Trabattoni D, Iemoli E, Fusi ML, Villa ML, Milazzo F, et al. Immune profiles of patients with chronic idiopathic urticaria. Int Arch Allergy Immunol. 2002;128:59–66. doi: 10.1159/000058004. [DOI] [PubMed] [Google Scholar]
- 20.Grattan CE, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol. 2002;46:645–57. doi: 10.1067/mjd.2002.122759. [DOI] [PubMed] [Google Scholar]
- 21.Alvarado-Flores E, Avalos-Diaz E, Diaz L, Herrera-Esparza R. Anti-idiotype antibodies neutralize in vivo the blistering effect of pemphigus foliaceus IgG. Scand J Immunol. 2001;53:254–8. doi: 10.1046/j.1365-3083.2001.00863.x. [DOI] [PubMed] [Google Scholar]
- 22.Noble BA, Loh RS, MacLennan S, Pesudovs K, Reynolds A, Bridges LR, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88:647–52. doi: 10.1136/bjo.2003.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staubach P, Onnen K, Vonend A, Metz M, Siebenhaar F, Tschentscher I, et al. Autologous whole blood injections to patients with chronic urticaria and a positive autologous serum skin test: A placebo-controlled trial. Dermatology. 2006;212:150–9. doi: 10.1159/000090656. [DOI] [PubMed] [Google Scholar]
- 24.Bocci V. Autohaemotherapy after treatment of blood with ozone. A reappraisal. J Int Med Res. 1994;22:131–44. doi: 10.1177/030006059402200301. [DOI] [PubMed] [Google Scholar]
- 25.Pittler MH, Armstrong NC, Cox A, Collier PM, Hart A, Ernst E. Randomized, doubleblind, placebo-controlled trial of autologous blood therapy for atopic dermatitis. Br J Dermatol. 2003;148:307–13. doi: 10.1046/j.1365-2133.2003.04921.x. [DOI] [PubMed] [Google Scholar]
- 26.Olwin JH, Ratajczak HV, House RV. Successful treatment of herpetic infections by autohemotherapy. J Altern Complement Med. 1997;3:155–8. doi: 10.1089/acm.1997.3.155. [DOI] [PubMed] [Google Scholar]
- 27.Fleck M. Urticaria. Dermatologie und Venerologie. In: Gottron H, Schönfeld W, editors. Vol. 3. Stuttgart: Thieme; 1959. pp. 265–98. [Google Scholar]


