Significance
Several therapeutic strategies have been used to enhance monoamine neurotransmitter signaling. However, many of these interventions have deleterious side effects or lose effectiveness due to off-target actions and system feedback. These undesirable consequences likely occur because of temporal dysregulation of neurotransmitter release and uptake. We demonstrate that increasing vesicular packaging enhances dopamine neurotransmission without this signaling disruption. Mice with elevated vesicular monoamine transporter display increased dopamine release, improved outcomes on anxiety and depressive behaviors, enhanced locomotion, and protection from a Parkinson disease-related neurotoxic insult. The malleable nature of the dopamine vesicle suggests that interventions aimed at enhancing vesicle filling may be of therapeutic benefit.
Keywords: SLC18A2, fast-scan cyclic voltammetry, serotonin, norepinephrine
Abstract
Disruption of neurotransmitter vesicle dynamics (transport, capacity, release) has been implicated in a variety of neurodegenerative and neuropsychiatric conditions. Here, we report a novel mouse model of enhanced vesicular function via bacterial artificial chromosome (BAC)-mediated overexpression of the vesicular monoamine transporter 2 (VMAT2; Slc18a2). A twofold increase in vesicular transport enhances the vesicular capacity for dopamine (56%), dopamine vesicle volume (33%), and basal tissue dopamine levels (21%) in the mouse striatum. The elevated vesicular capacity leads to an increase in stimulated dopamine release (84%) and extracellular dopamine levels (44%). VMAT2-overexpressing mice show improved outcomes on anxiety and depressive-like behaviors and increased basal locomotor activity (41%). Finally, these mice exhibit significant protection from neurotoxic insult by the dopaminergic toxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), as measured by reduced dopamine terminal damage and substantia nigra pars compacta cell loss. The increased release of dopamine and neuroprotection from MPTP toxicity in the VMAT2-overexpressing mice suggest that interventions aimed at enhancing vesicular capacity may be of therapeutic benefit in Parkinson disease.
Faulty monoamine neurotransmission is characteristic of many disorders, including Parkinson disease, depression, dystonia, attention deficit hyperactivity disorder, schizophrenia, addiction, and Huntington disease (1–7). Several strategies have been used to enhance monoamine signaling: administration of precursors to increase synthesis, inhibition of enzymes to prevent metabolism/degradation, inhibition of plasma membrane transporters to increase synaptic lifespan, and administration of receptor agonists to directly activate postsynaptic targets. However, these therapies fail to preserve many, if not all, of the critical aspects of chemical neurotransmission: normal transmitter synthesis, activity-dependent transmitter release and receptor activation, and receptor recovery following signal termination both by transmitter uptake and metabolism. Thus, these approaches often produce deleterious side effects or lose efficacy over time.
Increasing the neurotransmitter content in the synaptic vesicle may represent a therapeutic approach capable of increasing the release of monoamines without the aforementioned adverse effects. The vesicular monoamine transporter 2 (VMAT2, SLC18A2) is responsible for the packaging of neurotransmitter into vesicles for subsequent release from monoaminergic neurons. VMAT2 is an H+-ATPase antiporter, which uses the vesicular electrochemical gradient to drive the packaging of cytosolic transmitter into small synaptic and dense core vesicles (8–10). VMAT2 is also essential for survival of dopamine neurons as cytosolic dopamine is neurotoxic (11, 12). By sequestering intracellular dopamine into vesicles, VMAT2 prevents cytosolic dopamine accumulation and its subsequent conversion to neurotoxic species (13–17). Thus, VMAT2 serves two primary functions: to mediate monoamine neurotransmission and to counteract intracellular toxicity.
Previous data clearly show that disruption of VMAT2 function produces adverse effects. Pharmacological VMAT2 inhibition by reserpine or tetrabenazine results in monoamine depletion and negative behavioral consequences, including akinesia and depressive behaviors (18–20). Genetic reduction of VMAT2 in mice also causes depletion of dopamine, norepinephrine, and serotonin and progressive neurodegeneration in multiple monoaminergic regions (21–25). Similarly, a VMAT2 mutation in humans that dramatically reduces vesicular function was recently linked to an infantile parkinsonism-like condition with symptoms caused by deficits in all of the monoamines (26). SNPs in the VMAT2 gene have also been associated with neurocognitive function in relatives of patients with schizophrenia and even in posttraumatic stress disorder (27, 28). Interestingly, haplotypes within the VMAT2 promoter region that increase VMAT2 expression have been associated with a decreased risk of Parkinson disease (29, 30). Although the detrimental effects of reduced VMAT2 function are recognized, our understanding of the potential benefits of increased VMAT2 function in vivo has been limited to a Drosophila model (31–33). Thus, we generated VMAT2-overexpressing mice using a bacterial artificial chromosome (BAC) to determine whether increased vesicular packaging could provide an elevation of monoamine output in a mammalian system. We report here that VMAT2-overexpressing mice (VMAT2-HI) have increased vesicle capacity, increased synaptic dopamine release, improved outcomes on anxiety-like and depressive-like behaviors, and reduced vulnerability to toxic insult by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). These data demonstrate that the manipulation of vesicular capacity is capable of providing a sustained enhancement of the dopamine system and suggest that the vesicle is a viable therapeutic target for numerous monoamine-deficient disorders.
Results
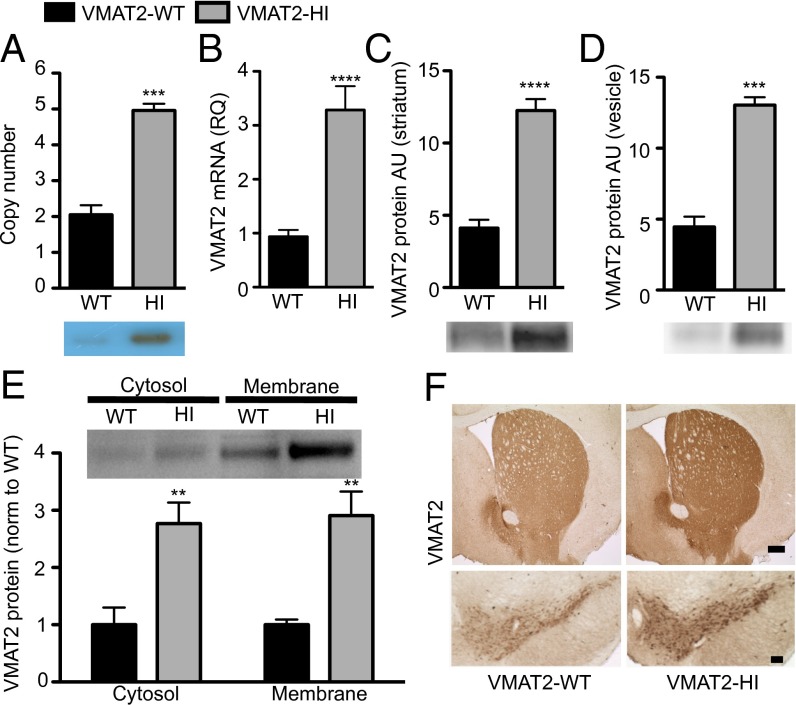
VMAT2 Overexpression in VMAT2-HI Mice.
BAC transgenic mice were generated by pronuclear injection of linearized BAC clone RP23-292H20, which contains the full-length Slc18a2 sequence and presumably all of the promoter and regulatory sequences for proper spatial and temporal VMAT2 expression (SI Materials and Methods). Founders were identified by PCR and confirmed by Southern blot with probes against the BAC sequence. We observed increased VMAT2 expression and vesicular uptake in multiple C57BL/6 and FVB BAC-positive mouse lines, suggesting that the results presented here are not due to a founder or insertion effect. We focused on one line that exhibited significant VMAT2 overexpression, designated VMAT2-HI. Copy-number estimation by genomic quantitative PCR (qPCR) for Slc18a2 confirmed that BAC-positive VMAT2-HI mice carry three copies of the BAC, bringing their total number of VMAT2 gene copies to five (P < 0.0001) (Fig. 1A, Southern blot Inset). These three BAC copies have likely integrated in tandem at a single locus because we observe Mendelian inheritance patterns of the BAC in the VMAT2-HI colony. VMAT2-HI mice show 3.5-fold higher VMAT2 mRNA compared with wild-type littermates by qPCR (P < 0.0001) (Fig. 1B). They also express threefold higher VMAT2 protein in both striatal homogenate (P < 0.0001) (Fig. 1C) and an isolated vesicle fraction (P < 0.001) than wild-type littermates (Fig. 1D). The relative ratio of VMAT2 between the membrane and cytosolic protein fractions is maintained in the VMAT2-HI mice, suggesting that the increased protein is properly trafficked (Fig. 1E). VMAT2 overexpression was confirmed in midbrain dopamine pathways (Fig. 1F) and in serotonergic and noradrenergic cell bodies (Fig. S1) by immunohistochemistry. We observed no changes in expression of other presynaptic dopamine markers, including tyrosine hydroxylase (TH) and the dopamine transporter (DAT) as measured by immunoblotting and immunohistochemistry (Fig. S2). We also saw no overt health concerns, reproductive or fecundity issues, or premature deaths in the VMAT2-HI mice and have shown that they have normal body weight (Fig. S1B).
Fig. 1.
Increased VMAT2 expression in the VMAT2-HI mice. (A) The VMAT2-HI genome contain three insertions of the BAC by genomic qPCR (n = 4). Southern blot against Slc18a2 (Inset). (B) VMAT2-HI mice have 3.5-fold more VMAT2 mRNA than wild-type by qPCR (n = 12–13). (C and D) VMAT2-HI mice show a threefold increase in VMAT2 expression by immunoblot of striatal tissue (n = 6) and whole brain vesicular fraction (n = 6). (E) VMAT2-HI mice show a threefold increase in VMAT2 expression in both cytosolic and membrane fractions. (F) VMAT2 immunohistochemistry of striatal and midbrain sections. (Scale bar: 200 µm.)
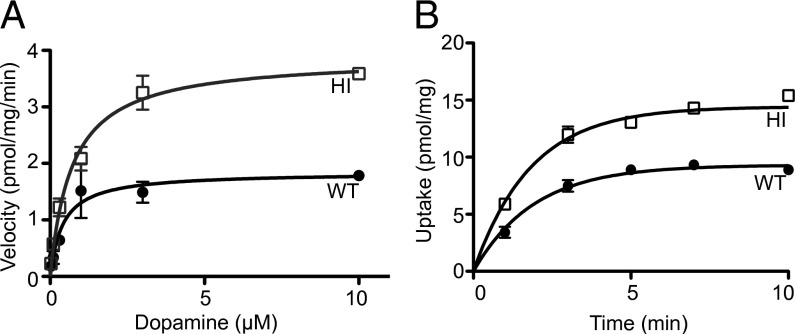
Increased Vesicular Uptake and Capacity in VMAT2-HI Mice.
VMAT2-HI mice showed a twofold increase in vesicular dopamine uptake in isolated vesicles (Vmax, P < 0.0001), without a change in Km (P = 0.202) (Fig. 2A). The higher VMAT2 levels in the VMAT2-HI mice increased transport speed while also augmenting the vesicular capacity for dopamine as measured in an uptake time course (56% increase in maximum capacity at 1 μM dopamine, P < 0.001) (Fig. 2B).
Fig. 2.
Increased vesicular uptake and capacity in the VMAT2-HI mice. (A) VMAT2-HI mice show a twofold increase in [3H]-DA uptake in purified vesicles (n = 3). (B) VMAT2-HI mice have an increased vesicle capacity by time course of [3H]-DA uptake (1 µM final dopamine concentration) (n = 3).
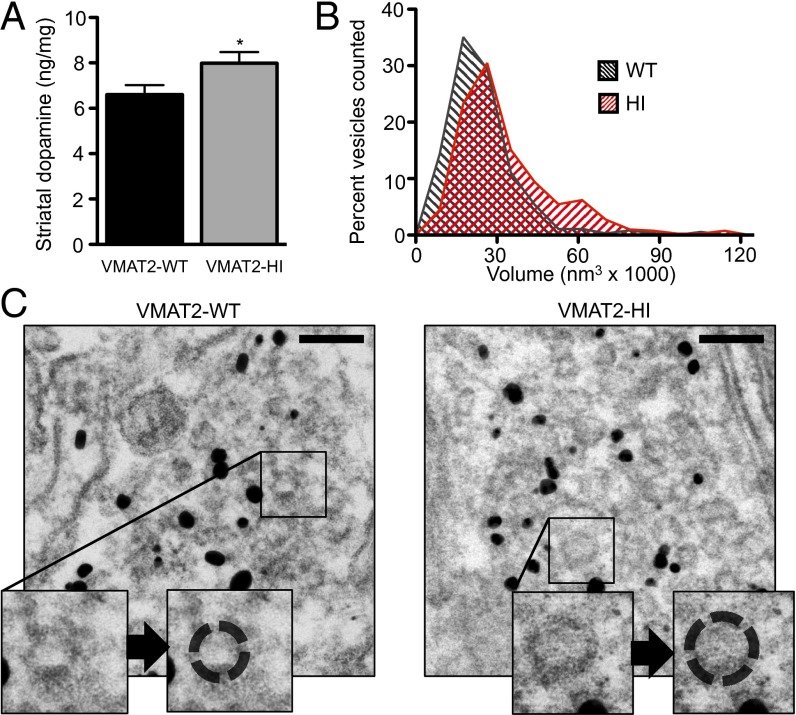
Increased Vesicular Capacity for Dopamine Increases Striatal Dopamine Content in VMAT2-HI Mice.
VMAT2-HI mice showed a 21% increase in striatal dopamine content as measured by HPLC (P < 0.05, one-tailed t test) (Fig. 3A). Stereological counts of both nigral tyrosine hydroxylase-positive (TH+) neurons (WT v. HI, 4,884 ± 399 v. 5,121 ± 396, P = 0.68, n = 6) and Nissl-positive neurons (WT v. HI, 3,206 ± 503 v. 3,293 ± 591, P = 0.91) showed no difference between genotypes, confirming that this enhanced dopamine content is not due to an increase in the number of midbrain neurons.
Fig. 3.
Increased striatal dopamine content and vesicle size in the VMAT2-HI mice. (A) VMAT2-HI mice show increased striatal dopamine content as measured by HPLC (n = 20). (B) VMAT2-HI mice show a 33% increase in average vesicle volume and a significantly different vesicle volume distribution, with a second peak of vesicles with an approximately doubled volume than wild-type animals (n = 3). (C) Representative striatal TH+ immunogold-electron micrographs with sample vesicle size tracings (Insets). (Scale bar: 100 nm.)
Increased Striatal Vesicle Volume in VMAT2-HI Mice.
Using measurements from immunogold electron micrographs from TH+ terminals in the striatum, we showed that the average vesicle volume in VMAT2-HI mice is ∼32,000 nm3, a 33% increase over the wild-type vesicular volume of ∼24,000 nm3 (P < 0.0001, Mann–Whitney test) (Fig. 3 B and C). Furthermore, the distribution of vesicle volumes in VMAT2-HI mice was significantly different from that of wild-type animals (P < 0.0001, Kolmogorov–Smirnov test), with a second peak of vesicles with an approximately doubled volume of 55,000 nm3. We observed these large-volume vesicles within a close proximity to the active zone in the VMAT2-HI mice, suggesting that they may contribute to the readily releasable vesicle pool.
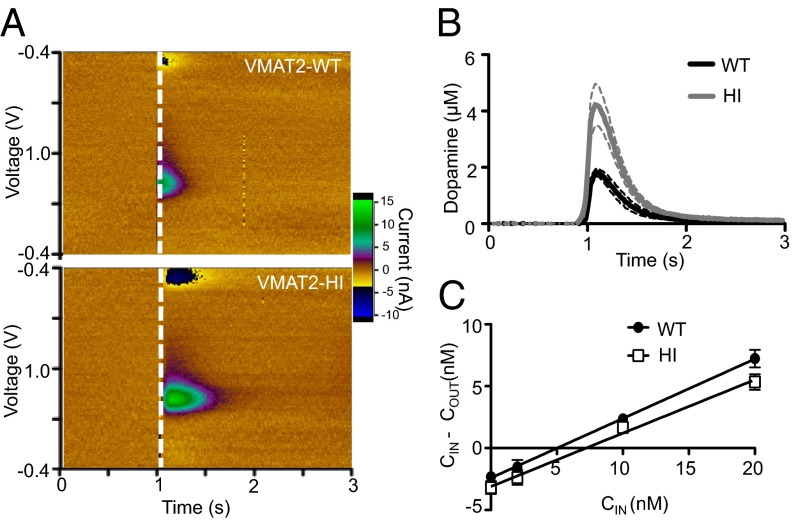
Increased Synaptic Dopamine Release in VMAT2-HI Mice.
To determine whether the increased vesicular dopamine capacity in the VMAT2-HI mice leads to enhanced neurotransmitter release, we assessed dopamine terminal function using fast-scan cyclic voltammetry (FSCV) in striatal slices. VMAT2-HI mice had an 84% increase in stimulated dopamine release in the dorsal striatum (P < 0.05) (Fig. 4 A and B), demonstrating that enhanced vesicular capacity translates to increased synaptic release of dopamine.
Fig. 4.
Increased striatal dopamine release and extracellular dopamine in the VMAT2-HI mice. VMAT2-HI mice show an 84% increase in stimulated dopamine release as measured by fast-scan cyclic voltammetry in striatal slice (n = 5–6). (A) Representative colorplots (uncalibrated to electrode sensitivity). (B) Average stimulated dopamine-release traces. (C) Basal striatal extracellular dopamine levels are also increased as measured by no-net flux microdialysis (n = 6).
Increased Extracellular Dopamine in VMAT2-HI Mice.
To determine whether the increase in synaptic dopamine release translates to altered extracellular levels of dopamine, we also performed in vivo no-net flux microdialysis in awake behaving mice. VMAT2-HI mice showed a 44% increase in extracellular dopamine compared with wild-type mice (P < 0.05, one-tailed t test) (Fig. 4C). VMAT2-HI mice showed no compensatory changes in extracellular dopamine clearance as indicated by the extraction fraction as measured by microdialysis (P = 0.2917) or the uptake rate constant (tau) as measured by voltammetry (34) (WT v. HI, 0.265 ± 0.038 v. 0.256 ± 0.016, P = 0.862). These findings are consistent with other unchanged dopamine markers in VMAT2-HI mice: striatal DAT and TH levels (Fig. S2), midbrain TH+ neuron number, and striatal dopamine metabolites as measured by HPLC (Fig. S3).
Reduced Anxiety-Like and Depressive-Like Behaviors in VMAT2-HI Mice.
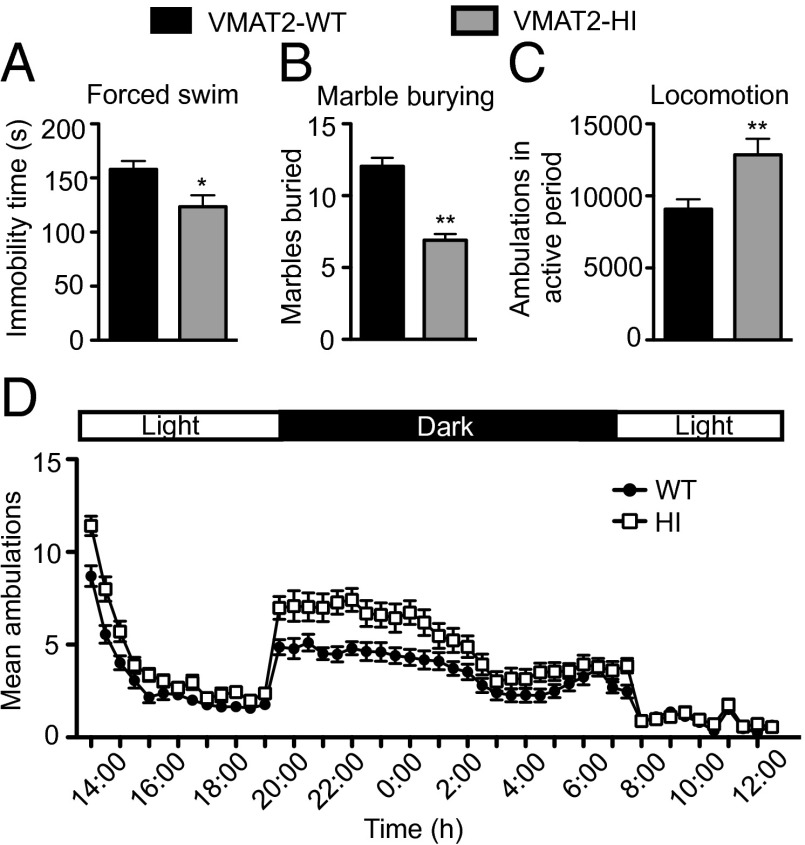
We performed a behavioral battery to assess overall behavioral health (Fig. 5 and Table S1). No significant difference was found between genotypes on the elevated plus maze (Table S1), suggesting that VMAT2-HI mice do not have increased anxiety-like behavior by this measure. Instead, VMAT2-HI mice showed reduced immobility time on the forced-swim test (P < 0.05) (Fig. 5A) and reduced marble burying in the marble-burying assay (P < 0.01) (Fig. 5B), suggesting that VMAT2-HI mice actually show improved outcomes on other anxiety and depressive-like measures.
Fig. 5.
Improved depressive-like and anxiety-like behaviors and increased locomotor activity in the VMAT2-HI mice. (A) VMAT2-HI mice show decreased immobility time on the forced-swim test (n = 21). (B) VMAT2-HI mice show decreased marble burying on the marble-burying assay (n = 11). (C) VMAT2-HI mice show elevated total ambulations in the active (dark) period. (D) Average 24-h circadian locomotor activity trace (1-h bins; n = 27).
Increased Locomotor Activity in VMAT2-HI Mice.
Next, we evaluated whether the VMAT2-HI mice display increased locomotor activity, a dopamine-mediated behavior (35). The VMAT2-HI mice showed a 41% increase in total ambulations in the active period (dark cycle) compared with wild-type littermates (P < 0.01) (Fig. 5 C and D). No difference in locomotor activity was observed in the inactive period.
Reduced MPTP Neurotoxicity in VMAT2-HI Mice.
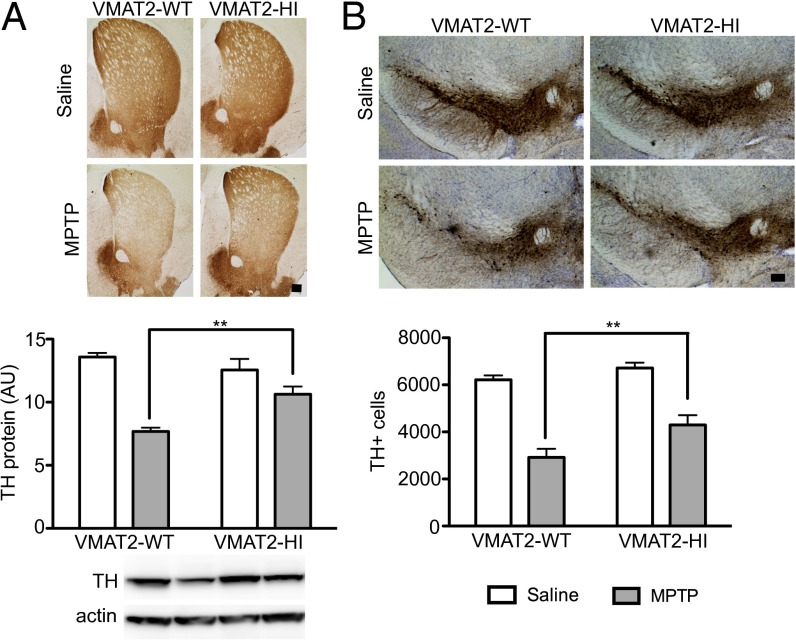
Finally, we examined whether VMAT2-HI mice are protected from the dopaminergic toxicant MPTP. Following a low dose of MPTP (2 × 15 mg/kg s.c., 12 h apart), VMAT2-HI mice showed protection from both striatal TH and DAT loss, indicative of sparing of striatal dopamine terminals (P < 0.05) (Fig. 6A and Fig. S4). Following a multiday MPTP regimen known to cause ∼50% cell body loss in the SNpc (5 × 20 mg/kg s.c., 24 h apart) (36), VMAT2-HI mice showed significant protection from TH+ cell loss as measured by unbiased stereology (P < 0.05) (Fig. 6B). VMAT2-HI mice were also protected from striatal TH loss following this high MPTP dosing regimen (P < 0.05) (Fig. S4).
Fig. 6.
Reduced MPTP toxicity in the nigrostriatal pathway of male VMAT2-HI mice. (A) Following a 2 × 15 mg/kg MPTP dose, VMAT2-HI mice show protection from striatal TH loss by immunoblotting and immunohistochemistry (n = 4). (B) Following a 5 × 20 mg/kg MPTP dose, VMAT2-HI mice show a significantly higher number of remaining TH+ neurons in the substantia nigra pars compacta (n = 6). No difference was seen in Nissl+ cells between genotypes (P > 0.05). (Scale bar: 200 µm.)
Discussion
Until now, it was unknown whether overexpression of a vesicular monoamine transporter would induce a sustained and beneficial enhancement of the mammalian monoamine system. The results presented here show that the overexpression of VMAT2 leads to increased vesicular capacity and size, elevated transmitter release and neurotransmission, and improved behavioral outcomes. Furthermore, this enhanced vesicular filling also results in reduced vulnerability to the parkinsonism-inducing toxicant MPTP. These results highlight a previously untapped reserve of monoamine vesicle capacity.
VMAT2-HI Mice Reveal an Unexpected Enhancement of Vesicular Storage Capacity.
Here, we show that the overexpression of VMAT2 enhances vesicular uptake and capacity in vivo (Fig. 2). We observed that vesicles from wild-type mice never reach the same level of vesicular filling as those from VMAT2-HI mice, no matter the length of time allowed for this uptake in a time-course experiment (Fig. 2B). These results are in contrast to some common perceptions about in vivo vesicle capacity, which had assumed that baseline vesicular storage might be at its maximum once equilibrium is reached across the membrane. In this scenario, the amount of VMAT2 would have no effect on vesicle content. However, in vitro work has shown in both chromaffin cells and dopamine neurons that VMAT2 overexpression alters quantal size independent of activity (i.e., regardless of the speed of vesicle recycling) (37). Recent evidence, including the in vivo results presented here with the VMAT2-HI mice, suggests that membrane equilibrium is not a limiting factor to monoamine vesicle filling (37–39).
Increased Vesicular Transport Increases Vesicle Size but Does Not Alter Presynaptic Dopaminergic Terminal Integrity.
We also demonstrate that VMAT2 overexpression increases the volume of vesicles in striatal dopamine synapses (Fig. 3 B and C). Because the majority of dopamine in the brain is stored in vesicles, the increase in vesicular volume in the VMAT2-HI mice presumably allows for the sustained increase in dopamine capacity (Fig. 2B) and striatal dopamine content (Fig. 3A). VMAT2-HI mice also show increased vesicle size, supporting previous in vitro studies (39, 40). Despite these vesicular changes, VMAT2-HI mice have no alterations in the structural integrity of the synapse and nonvesicular measures nor in the number of synaptic vesicles per striatal dopamine terminal as measured by electron micrographs (Table S2). These findings demonstrate that the presynaptic dopaminergic terminals in VMAT2-HI mice are intact despite morphological alterations to vesicle size.
VMAT2 Overexpression Increases Dopamine Release and Neurotransmission Without Compensatory Changes to Other Presynaptic Functions.
Overexpression of VMAT2 has been shown to increase dopamine release by increasing quantal size in both chromaffin cells and primary midbrain culture (37, 38). Similarly, VMAT2-HI mice show an 84% increase in stimulated striatal dopamine release (Fig. 4 A and B), a corresponding 44% increase in basal extracellular dopamine levels (Fig. 4C), and elevated locomotor output in the active period (Fig. 5). This enhanced transmitter release and locomotion suggest elevated dopamine neurotransmission in these mice, a finding reflected in previous work on the overexpression of the Drosophila vesicular monoamine transporter (DVMAT-A) (31–33). Together with previous findings demonstrating reduced dopamine release and locomotion in VMAT2-deficient animals, the results from the VMAT2-HI mice suggest that there is a gene dosage-dependent regulation of vesicular capacity and dopamine output (21, 41, 42).
Evidence suggests that enhanced dopamine release via molecules like amphetamine or cocaine can change DAT expression and localization (43). Because VMAT2-HI mice show both increased dopamine release and increased extracellular dopamine levels, we anticipated that there would be compensatory changes to DAT or other dopamine mediators. However, VMAT2-HI mice show no differences in levels of DAT or TH (Fig. S2), nor in indirect estimates of transmitter clearance by voltammetry (rate constant, tau) and microdialysis (extraction fraction). These data suggest that the enhancement of the dopamine system is sustained without major compensatory changes to other mediators of presynaptic dopamine dynamics in the VMAT2-HI mice. In addition, VMAT2-HI mice display and maintain monoamine-mediated behavioral phenotypes, suggesting that receptor levels or function have not overcome these presynaptic alterations.
VMAT2-HI Phenotypes Contrast with Vesicular Transporter Overexpression in Nonmonoamine Transmitter Systems.
We present many positive effects of VMAT2 overexpression; however, overexpression of other vesicular transporters has produced mixed results. Vesicular acetylcholine transporter (VAChT) overexpression increases EPSCs in Xenopus (44) and increases acetylcholine release in mouse hippocampal slice (45). ChAT–ChR2–EYFP mice, which also carry several copies of the VAChT gene, show severe cognitive deficits in attention and memory (46). Evidence from the glutamate system had suggested that compensatory changes would override any increased monoamine output in the VMAT2-HI mice. Overexpression of the vesicular glutamate transporter (VGLUT1) increases excitatory synaptic transmission in hippocampal culture (47). Additionally, DVGLUT overexpression in Drosophila increases both quantal size and synaptic vesicle volume (48). However, these changes are accompanied by compensatory decreases in the number of synaptic vesicles released, resulting in no net change in neurotransmission. Thus, the enhanced dopamine release and beneficial behavioral outcomes in the VMAT2-HI mice, an intact mammalian model also subject to compensation, indeed provide an unexpected contrast to the acetylcholine and glutamate systems.
VMAT2 Overexpression Improves Outcomes in Measures of Depressive-Like and Anxiety-Like Behaviors.
The monoamines have long been associated with affective disorders. The “monoamine hypothesis” of depression was largely based on the dramatic mood changes observed in patients taking the VMAT2 inhibitor reserpine (49). Increased monoamines also have been implicated in a variety of psychiatric conditions, including bipolar disorder, mania, schizophrenia, and psychosis, all of which include symptoms of mood disturbances (50). Due to these examples, we hypothesized that the increased dopamine signaling in the VMAT2-HI mice would result in anxiety or mania-like behaviors (51). We have shown that the VMAT2-HI mice display no increase in anxiety-like behaviors (Fig. 5 and Table S1). Instead, the VMAT2-HI mice display decreased anxiety-like behavior in the marble-burying assay and decreased depressive-like behavior on the forced-swim test. Both of these results suggest that the VMAT2-HI mice actually have improved outcomes in these measures. Although elevated locomotor activity may confound the results from the VMAT2-HI mice on the forced-swim test, the output from the marble-burying assay is not dependent on activity. Although the behavioral paradigms described here undoubtedly involve additional monoamine neurotransmitters beyond dopamine (e.g., serotonin and norepinephrine), these findings suggest that elevated VMAT2 function in vivo may have benefits in altering mood and affective behaviors.
VMAT2 Overexpression Protects Against MPTP-Induced Toxicity in Dopamine Neurons.
VMAT2 protects against the parkinsonism-inducing neurotoxicant MPTP by sequestering its active metabolite, MPP+, into the vesicular lumen away from its site of action at complex I of the mitochondria (52–54). VMAT itself was first identified due to its protective effects against MPP+ toxicity in culture (10, 52). Our results show that increased VMAT2 confers significant protection from damage to the nigrostriatal dopamine system following MPTP dosing even in the presence of elevated dopamine levels. Thus, VMAT2’s neuroprotective properties via sequestration of both exogenous (e.g., MPTP) and endogenous (e.g., cytosolic dopamine) toxicants could be particularly relevant to vulnerable dopamine neurons.
Enhancement of Vesicular Function Is a Putative Therapeutic Target.
Here, we show that increasing vesicular transporter levels enhances dopamine output while preserving appropriate activity-dependent release and signal termination. Furthermore, the dopaminergic system maintains this heightened state without significant compensatory changes in dopamine pathways that are often the result of supraphysiological amounts of transmitter in the synaptic cleft and uncontrolled activation of postsynaptic targets. Enhancing monoamine signaling by increasing vesicle capacity has the potential to positively affect a range of behaviors, from locomotion to mood; these results are particularly relevant for hypomonoaminergic conditions such as Parkinson disease or unipolar depression. Together, this work suggests that enhanced vesicular filling can be sustained over time and may be a viable therapeutic approach for a variety of monoamine-mediated CNS disorders.
Materials and Methods
Animals.
VMAT2-HI founders were bred to Charles River C57BL/6 breeders for characterization. Four- to 6-mo-old male and female mice were used for all studies except where noted; 6- to 8-mo-old mice were used for voltammetry and MPTP studies. All procedures were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Emory University. See SI Materials and Methods for further detail on the generation of the VMAT2-HI mice.
Southern Blotting.
Genomic DNA was digested overnight with EcoRI. Nitrocellulose membranes were hybridized in buffer (Clontech) with a radiolabeled probe generated by PCR amplification with primers corresponding to sequences within Slc18a2 (5′-ggaacagagatgctgcatagc; 5′-agcgtgaggcagaaggcaat).
Genomic qPCR.
DNA was isolated from tail clips. Relative quantification of gene target VMAT2 and a reference gene, Tfrc, was obtained using the GoTaq qPCR Master Mix (Promega) on an Applied Biosystems 7500 Real-Time PCR System (55). Allelic copy number of VMAT2 was determined by the comparative CT method. VMAT2 primers were as follows: forward, 5′ccgtggtgtccgtgattatta; reverse, gcaacgcctgtggatttatg. Tfrc primers were as follows: forward, 5′-cagtcatcagggttgcctaata; reverse, 5′-atcacaacctcaccatgtaact.
qPCR.
Reagents were purchased from Life Technologies. mRNA was isolated from ventral mesencephalon using the Dynabeads mRNA Direct Kit. Residual DNA was digested with TURBO DNA-free. Reverse transcription was carried out with a SuperScript Vilo Master Mix. Quantitative qPCR was performed on an Applied Biosystems 7500 Fast Real Time PCR system with TaqMan Universal Master Mix II with UNG. The following TaqMan Gene Expression Assays were used: Mm_00553058_m1 (Slc18a2; VMAT2) and Mn_99999915_g1 (GAPDH). Data were analyzed by the comparative CT method using SDS software.
Western Blotting.
Western blots were performed as previously described (23). For antibody information, see SI Materials and Methods.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (23). For antibody information, see SI Materials and Methods. Nissl stain was performed by a 2-min Cresyl Violet dip of mounted sections before dehydration, xylene clearing, and coverslipping. Images were acquired with NeuroLucida (MicroBrightField).
Vesicular [3H]-DA Uptake.
Two whole brains from each genotype were homogenized in homogenization buffer for each statistical n. Homogenates from each genotype were pooled, and vesicular uptake was performed as previously described (23).
HPLC Determination of Tissue Dopamine Content.
Striatal tissue was flash frozen, and dopamine levels were measured by liquid chromatography with electrochemical detection after batch alumina extraction at the Clinical Neurochemistry Laboratory of the Clinical Neurocardiology Section in Intramural National Institute of Neurological Disorders and Stroke (56).
Electron Microscopy.
For preembedding description, see SI Materials and Methods. After embedding, dorsal striatal tissue was cut into 70-nm sections and stained with uranyl acetate and lead citrate. Sections were imaged at ×15,000 using a JEOL JEM-1400 Transmission Electron Microscope. Vesicle area was calculated by tracing vesicle circumference in TH-positive terminals in ImageJ software. Volume was calculated using the spherical volume equation (V = 4/3πr3). Additional measurements (mitochondrial area, synaptic cleft distance, terminal area, vesicles per terminal) were also made.
Stereological Analysis.
Stereological sampling was performed using the Stereo Investigator software as previously described, and the number of neurons in the SNpc was estimated using the optical fractionator method (MicroBrightField) (24). Parameters, cell-type definition, and counting intervals were also the same as previously described (24).
Fast-Scan Cyclic Voltammetry in Striatal Slice.
Slice FSCV was performed as previously described (57) (SI Materials and Methods). A five-recording survey of four different dorsal striatal release sites was taken for each animal with a 5-min rest interval between each synaptic stimulation (2.31 V). Maximal release at striatal sites in a slice was averaged. Carbon-fiber microelectrodes were calibrated with dopamine standards using a flow-cell injection system. Kinetic constants were extracted using nonlinear regression analysis of release and uptake of dopamine.
No-Net Flux Microdialysis.
No-net flux microdialysis and HPLC were performed as previously described (58) (SI Materials and Methods).
Circadian Locomotor Activity.
Locomotor activity was assessed using an automated system (San Diego Instruments) with infrared photobeams that recorded ambulations (consecutive beam breaks). Mice were placed into individual chambers and recorded for 24 h. Data are presented in 1-h bins.
Forced-Swim Test.
After 7 d of individual housing, mice were placed in glass cylinders (24 × 16 cm) with 15 cm of water maintained at 25 °C for 6 min. After the first 2 min, the total duration of time spent immobile was recorded during a 4-min test. The mouse was deemed immobile when it was floating passively.
Marble-Burying Assay.
Mice were individually housed for 7 d before behavioral battery. Mice were placed into their home cage that contained 6 inches of lightly pressed bedding. Within each tub, 20 marbles were evenly arranged. The mouse was placed into the cage for 30 min, after which the number of marbles at least 2/3 covered with bedding was counted.
MPTP Treatment.
Male mice were injected (s.c.) with either MPTP-HCl (M0896, Sigma,) or saline (0.9%). The “terminal” lesion consisted of two injections of 15 mg/kg MPTP (freebase) with an interinjection interval of 12 h. Mice were killed 7 d after the final dose. The “cell body” lesion consisted of five injections of 20 mg/kg MPTP (freebase) with an interinjection interval of 24 h. Mice were killed 21 d after the final injection.
Statistical Analysis.
All data were analyzed in GraphPad Prism. Differences between genotypes were compared by two-tailed t tests, except where indicated. One-tailed t tests, assuming increases in the VMAT2-HI mice, were used in analyses where lower VMAT2 function has previously been shown to result in reduced outcomes (e.g., HPLC) (17, 23). Stereological counts and densitometric analysis were analyzed using two-way ANOVA (with treatment and genotype as factors) with Bonferroni post hoc tests. Outliers were defined by the Grubbs’ test for outliers (α = 0.05). All errors shown are SEM.
Supplementary Material
Acknowledgments
We thank Minagi Ozawa and Patricia Sullivan for their excellent technical assistance; the Emory University Transgenic Mouse and Gene Targeting Core for BAC DNA isolation, DNA preparation, and pronuclear injections; and the Rodent Behavioral Core Facility for assistance with the locomotor activity assay. This work was supported by National Institutes of Health Grants P01ES016731, P30ES019776, T32ES012870, DA015040, T32GM008605, F31NS084739, P50AG025688, and P50NS071669; Canadian Institutes of Health Research Grant 210296; and the Lewis Dickey Memorial Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402134111/-/DCSupplemental.
References
- 1.Carlsson A. Biochemical and pharmacological aspects of Parkinsonism. Acta Neurol Scand Suppl. 1972;51:11–42. [PubMed] [Google Scholar]
- 2.Freis ED. Mental depression in hypertensive patients treated for long periods with large doses of reserpine. N Engl J Med. 1954;251(25):1006–1008. doi: 10.1056/NEJM195412162512504. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg J, Asnis GM, van Praag HM, Vela RM. Effect of tyrosine on attention deficit disorder with hyperactivity. J Clin Psychiatry. 1988;49(5):193–195. [PubMed] [Google Scholar]
- 4.Song CH, Fan X, Exeter CJ, Hess EJ, Jinnah HA. Functional analysis of dopaminergic systems in a DYT1 knock-in mouse model of dystonia. Neurobiol Dis. 2012;48(1):66–78. doi: 10.1016/j.nbd.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192(4238):481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 6.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine self-administration appears to be mediated by dopamine uptake inhibition. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(2-3):233–239. doi: 10.1016/0278-5846(88)90040-1. [DOI] [PubMed] [Google Scholar]
- 7.Klawans HL, Jr, Goodman RM, Paulson GW, Barbeau A. Levodopa in the presymptomatic diagnosis of Huntington’s chorea. Lancet. 1972;2(7766):49. doi: 10.1016/s0140-6736(72)91314-1. [DOI] [PubMed] [Google Scholar]
- 8.Erickson JD, Eiden LE, Hoffman BJ. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci USA. 1992;89(22):10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson JD, Eiden LE. Functional identification and molecular cloning of a human brain vesicle monoamine transporter. J Neurochem. 1993;61(6):2314–2317. doi: 10.1111/j.1471-4159.1993.tb07476.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Roghani A, Edwards RH. Gene transfer of a reserpine-sensitive mechanism of resistance to N-methyl-4-phenylpyridinium. Proc Natl Acad Sci USA. 1992;89(19):9074–9078. doi: 10.1073/pnas.89.19.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: A review. Neurotox Res. 2000;1(3):181–195. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DS, et al. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem. 2013;126(5):591–603. doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alter SP, Lenzi GM, Bernstein AI, Miller GW. Vesicular integrity in Parkinson’s disease. Curr Neurol Neurosci Rep. 2013;13(7):362. doi: 10.1007/s11910-013-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Shachar D, Zuk R, Glinka Y. Dopamine neurotoxicity: Inhibition of mitochondrial respiration. J Neurochem. 1995;64(2):718–723. doi: 10.1046/j.1471-4159.1995.64020718.x. [DOI] [PubMed] [Google Scholar]
- 15.Mosharov EV, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62(2):218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulusoy A, Björklund T, Buck K, Kirik D. Dysregulated dopamine storage increases the vulnerability to α-synuclein in nigral neurons. Neurobiol Dis. 2012;47(3):367–377. doi: 10.1016/j.nbd.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Caudle WM, Colebrooke RE, Emson PC, Miller GW. Altered vesicular dopamine storage in Parkinson’s disease: A premature demise. Trends Neurosci. 2008;31(6):303–308. doi: 10.1016/j.tins.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Kirshner N. Uptake of catecholamines by a particulate fraction of the adrenal medulla. Science. 1962;135(3498):107–108. doi: 10.1126/science.135.3498.107. [DOI] [PubMed] [Google Scholar]
- 19.Kirshner N, Rorie M, Kamin DL. Inhibition of dopamine uptake in vitro by reserpine administered in vivo. J Pharmacol Exp Ther. 1963;141:285–289. [PubMed] [Google Scholar]
- 20.Pettibone DJ, Totaro JA, Pflueger AB. Tetrabenazine-induced depletion of brain monoamines: Characterization and interaction with selected antidepressants. Eur J Pharmacol. 1984;102(3-4):425–430. doi: 10.1016/0014-2999(84)90562-4. [DOI] [PubMed] [Google Scholar]
- 21.Fon EA, et al. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19(6):1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 22.Wang YM, et al. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19(6):1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 23.Caudle WM, et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27(30):8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor TN, Alter SP, Wang M, Goldstein DS, Miller GW. Reduced vesicular storage of catecholamines causes progressive degeneration in the locus ceruleus. Neuropharmacology. 2014;76(Pt A):97–105. doi: 10.1016/j.neuropharm.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi N, et al. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci USA. 1997;94(18):9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rilstone JJ, Alkhater RA, Minassian BA. Brain dopamine-serotonin vesicular transport disease and its treatment. N Engl J Med. 2013;368(6):543–550. doi: 10.1056/NEJMoa1207281. [DOI] [PubMed] [Google Scholar]
- 27.Simons CJP, van Winkel R. GROUP Intermediate phenotype analysis of patients, unaffected siblings, and healthy controls identifies VMAT2 as a candidate gene for psychotic disorder and neurocognition. Schizophr Bull. 2013;39(4):848–856. doi: 10.1093/schbul/sbs067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solovieff N, et al. Genetic association analysis of 300 genes identifies a risk haplotype in SLC18A2 for post-traumatic stress disorder in two independent samples. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glatt CE, Wahner AD, White DJ, Ruiz-Linares A, Ritz B. Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum Mol Genet. 2006;15(2):299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brighina L, et al. Analysis of vesicular monoamine transporter 2 polymorphisms in Parkinson’s disease. Neurobiol Aging. 2013;34(6):1712.e9–1712.e13. doi: 10.1016/j.neurobiolaging.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang H-Y, et al. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol Psychiatry. 2006;11(1):99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- 32.Lawal HO, et al. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol Dis. 2010;40(1):102–112. doi: 10.1016/j.nbd.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sang TK, et al. A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine. J Neurosci. 2007;27(5):981–992. doi: 10.1523/JNEUROSCI.4810-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: Analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202(2):158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res. 1983;287(2):173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 36.Kaur D, et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: A novel therapy for Parkinson’s disease. Neuron. 2003;37(6):899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 37.Pothos EN, et al. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J Neurosci. 2000;20(19):7297–7306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pothos EN. Regulation of dopamine quantal size in midbrain and hippocampal neurons. Behav Brain Res. 2002;130(1-2):203–207. doi: 10.1016/s0166-4328(01)00419-3. [DOI] [PubMed] [Google Scholar]
- 39.Colliver TL, Pyott SJ, Achalabun M, Ewing AG. VMAT-mediated changes in quantal size and vesicular volume. J Neurosci. 2000;20(14):5276–5282. doi: 10.1523/JNEUROSCI.20-14-05276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong LW, Hafez I, Alvarez de Toledo G, Lindau M. Secretory vesicles membrane area is regulated in tandem with quantal size in chromaffin cells. J Neurosci. 2003;23(21):7917–7921. doi: 10.1523/JNEUROSCI.23-21-07917.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel J, Mooslehner KA, Chan PM, Emson PC, Stamford JA. Presynaptic control of striatal dopamine neurotransmission in adult vesicular monoamine transporter 2 (VMAT2) mutant mice. J Neurochem. 2003;85(4):898–910. doi: 10.1046/j.1471-4159.2003.01732.x. [DOI] [PubMed] [Google Scholar]
- 42.Travis ER, Wang YM, Michael DJ, Caron MG, Wightman RM. Differential quantal release of histamine and 5-hydroxytryptamine from mast cells of vesicular monoamine transporter 2 knockout mice. Proc Natl Acad Sci USA. 2000;97(1):162–167. doi: 10.1073/pnas.97.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur J Pharmacol. 2003;479(1-3):153–158. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 44.Song H, et al. Expression of a putative vesicular acetylcholine transporter facilitates quantal transmitter packaging. Neuron. 1997;18(5):815–826. doi: 10.1016/s0896-6273(00)80320-7. [DOI] [PubMed] [Google Scholar]
- 45.Nagy PM, Aubert I. Overexpression of the vesicular acetylcholine transporter increased acetylcholine release in the hippocampus. Neuroscience. 2012;218:1–11. doi: 10.1016/j.neuroscience.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 46.Kolisnyk B, et al. ChAT-ChR2-EYFP mice have enhanced motor endurance but show deficits in attention and several additional cognitive domains. J Neurosci. 2013;33(25):10427–10438. doi: 10.1523/JNEUROSCI.0395-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson NR, et al. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25(26):6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniels RW, et al. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 2004;24(46):10466–10474. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swerdlow NR, Koob GF. Dopamine, schizophrenia, mania, and depression: Toward a unified hypothesis of cortico-striatopallido-thalamic function. Behav Brain Sci. 1987;10:197–208. [Google Scholar]
- 51.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13(10):1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, et al. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70(4):539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- 53.Gainetdinov RR, et al. Increased MPTP neurotoxicity in vesicular monoamine transporter 2 heterozygote knockout mice. J Neurochem. 1998;70(5):1973–1978. doi: 10.1046/j.1471-4159.1998.70051973.x. [DOI] [PubMed] [Google Scholar]
- 54.Mooslehner KA, et al. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: Potential mouse model for parkinsonism. Mol Cell Biol. 2001;21(16):5321–5331. doi: 10.1128/MCB.21.16.5321-5331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ballester M, Castelló A, Ibáñez E, Sánchez A, Folch JM. Real-time quantitative PCR-based system for determining transgene copy number in transgenic animals. Biotechniques. 2004;37(4):610–613. doi: 10.2144/04374ST06. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein DS, et al. Catechols in post-mortem brain of patients with Parkinson disease. Eur J Neurol. 2011;18(5):703–710. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kile BM, et al. Optimizing the temporal resolution of fast-scan cyclic voltammetry. ACS Chem Neurosci. 2012;3(4):285–292. doi: 10.1021/cn200119u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan X, Hess EJ. D2-like dopamine receptors mediate the response to amphetamine in a mouse model of ADHD. Neurobiol Dis. 2007;26(1):201–211. doi: 10.1016/j.nbd.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.