Significance
Forkhead box P3+ (Foxp3+) regulatory T cells (Tregs) are important for maintaining immune homeostasis and tolerance. The pivotal role of Tregs in immune tolerance demands that they possess a dedicated molecular machinery to maintain stable Foxp3 expression when challenged by an inflammatory environment. Whereas epigenetic mechanisms acting on the foxp3 locus have been extensively implicated in Tregs’ stable expression of Foxp3, the detailed molecular machinery remains elusive. In this study, we show that methyl-CpG binding protein 2 (MeCP2), an X-linked multifunctional epigenetic regulator, is a crucial player in the epigenetic machinery that confers Tregs with resilience against inflammation. Our study provides, to our knowledge, the first mechanistic description by which MeCP2, a molecule best known for its function in the central nervous system, regulates Treg function and immune tolerance.
Keywords: regulatory T-cell stability, immune homeostasis, epigenetic regulation, CNS2 acetylation
Abstract
Forkhead box P3+ (Foxp3+) regulatory T cells (Tregs) are crucial for peripheral tolerance. During inflammation, steady Foxp3 expression in Tregs is essential for maintaining their lineage identity and suppressive function. However, the molecular machinery governing Tregs’ resilience to inflammation-induced Foxp3 destabilization remains elusive. Here, we demonstrate that methyl-CpG binding protein 2 (MeCP2), an eminent epigenetic regulator known primarily as the etiological factor of Rett syndrome, is critical to sustain Foxp3 expression in Tregs during inflammation. In response to inflammatory stimuli, MeCP2 is specifically recruited to the Conserved Non-Coding sequence 2 region of the foxp3 locus, where it collaborates with cAMP responsive element binding protein 1 to promote local histone H3 acetylation, thereby counteracting inflammation-induced epigenetic silencing of foxp3. Consequently, Treg-specific deletion of MeCP2 causes spontaneous immune activation in mice and failure in protection against autoimmunity. Furthermore, we demonstrate that Foxp3 expression in MeCP2-deficient Tregs diminishes with time, resulting in their failure to suppress effector T-cell–mediated colitis. Thus, MeCP2 serves as a critical safeguard that confers Tregs with resilience against inflammation.
Methyl-CpG binding protein 2 (MeCP2) is an X-chromosome linked nuclear protein (1) that binds methylated DNA (2, 3) and has been reported to play bifunctional roles in regulating gene expression (4). As the docking of MeCP2 can recruit histone deacetylases (5) and DNA (cytosine-5)-methyltransferase 1 (6) to methylated CpG elements, this protein has traditionally been considered to be a transcription repressor. However, recent genome-wide studies have revealed that MeCP2 can also bind avidly to unmethylated CpG DNA and facilitate the transcription of a large proportion of genes (4, 7). Specifically, MeCP2 was shown to associate directly with transcription activators such as cAMP responsive element binding protein 1 (CREB1) in promoters, where they synergistically promote gene expression (4). Thus, the current consensus is that, depending on the genomic context, MeCP2 acts as either a transcriptional repressor or activator (8).
Previous studies have centered almost exclusively on MeCP2’s role in the central nervous system. This is attributed to the fact that loss-of-function mutations in the mecp2 locus is the etiological cause of 95% of typical Rett syndrome (RTT) (9, 10). RTT is a devastating disorder that afflicts 1 in 10,000 females and whose symptoms are largely neurodevelopmental (11). Based on the limited immunological studies on RTT patients, however, hints of immunological abnormalities have gradually emerged: Polymorphisms within the human mecp2 locus have recently been associated with an increased susceptibility to systemic lupus erythematosus (12, 13) and primary Sjögren’s syndrome (14), suggesting that mecp2 gene mutations may also contribute to the pathogenesis of inflammatory diseases, and a cohort study also demonstrated significantly elevated levels of IgG against food proteins in the sera of RTT patients (15), which may reflect the possibility of gut inflammation or breakdown of the intestinal barrier; most intriguingly, the transplantation of wild-type (WT) bone marrow successfully arrested RTT disease in MeCP2-null mice (16). Nevertheless, apart from these correlative studies, MeCP2’s causative role in immune regulation remains largely unexplored.
As potent suppressors of inflammation, CD4+ CD25+ regulatory T cells (Tregs) are an indispensable T-cell subset responsible for peripheral tolerance and immune homeostasis (17). Foxp3 is the master regulator of the Treg gene expression program, and consequently Foxp3 mutation in both humans and mice is sufficient to trigger the development of severe lymphoproliferative autoimmune disorders (18–24). Foxp3 is essential both for driving natural Treg (nTreg) development within the thymus and for maintaining lineage identity and suppressive function of peripheral Tregs: Deletion of Foxp3 specifically in postthymic mature Tregs completely abolishes their ability to suppress the onset of effector T-cell–mediated autoimmunity (25). Extensive studies on the molecular mechanisms regulating Foxp3 expression (26) have revealed that many transcription factors, including NFAT (27, 28), AP-1 (27), Smad3 (28), STAT5 (29, 30), NF-κB (31), Ets-1 (32), GATA3 (33), Foxo1/3 (34), and CREB1 (35), bind directly to the foxp3 locus to promote its expression during Treg differentiation, and the Foxp3/Runx1/CBFβ protein complex has recently been suggested to confer the heritable maintenance of Foxp3 expression through an autoregulatory loop (36, 37).
In addition to these trans-regulatory factors, the expression of Foxp3 is also tightly controlled at the epigenetic level (38). Besides the promoter, three cis-regulatory regions including three Conserved Non-Coding sequences (CNSs) within the foxp3 locus are essential in regulating Foxp3’s expression. Interestingly, genetic ablation of these individual elements revealed a division of labor in gene regulation (37): For instance, CNS1, which contains the NFAT–Smad3 binding sites (28), is critical for the peripheral induction of Foxp3 expression in conventional T cells that drives inducible Treg differentiation; on the other hand, CNS2 is specifically required for the maintenance of Foxp3 expression in daughter cells during nTreg cell division. Of note, the CNS2 genomic region is composed of multiple highly conserved CpG islands (35), which suggests that the maintenance of Foxp3 expression is potentially enforced by CpG-associated epigenetic regulators. As MeCP2 is a CpG island-binding protein and known epigenetic regulator, we considered that MeCP2 might potentially play some role in orchestrating foxp3 gene transcription.
In this study, using genetic approaches that delete MeCP2 specifically in Treg cells, we examined the role of MeCP2 in regulating Treg homeostasis. We find that, although MeCP2 is dispensable for the initial induction of Foxp3 expression during thymus-derived nTreg development and in vitro-induced Treg (iTreg) differentiation, it is essential for maintaining the stable expression of Foxp3 and the lineage identity of mature nTregs during inflammation.
Results
Spontaneous T-Cell Activation in Young Adult Mice with Treg-Specific MeCP2 Deletion.
While dissecting the role of the microRNA cluster miR-17–92 in regulating T cells’ effector response, we biochemically identified Mecp2 as a novel target of miR-19b (SI Appendix, Fig. S1A). miR-19b promotes CD4+ T-cell effector responses, in part through suppressing the differentiation of iTregs (39). This led to our hypothesis that miR-19b blocks iTreg conversion by dampening the expression of MeCP2, a foxp3 locus-associated protein (40). To determine this functional linkage, we initially crossed mice carrying conditional mecp2 alleles with mice expressing the Cre recombinase transgene under the control of the proximal Lck promoter. In this way, we attained T-cell–specific deletion of the mecp2 gene as early as the late double negative 2 stage. Contradictory to our initial hypothesis, when CD4+CD25− conventional T cells from Mecp2f/y Lck–Cre mice or their WT littermates were purified by FACS sorting and cultured under various iTreg skewing conditions, we found no significant differences in the generation of Foxp3+ iTreg cells (SI Appendix, Fig. S1 B and C). This indicated that, at least in vitro, it is unlikely that a functional linkage exists between miR-19b and MeCP2 during iTreg differentiation and that MeCP2 is dispensable for the de novo expression of Foxp3 in conventional CD4+ T cells. Furthermore, the thymic nTreg development was entirely intact in the Mecp2f/y Lck–Cre animals (SI Appendix, Fig. S1 D and E).
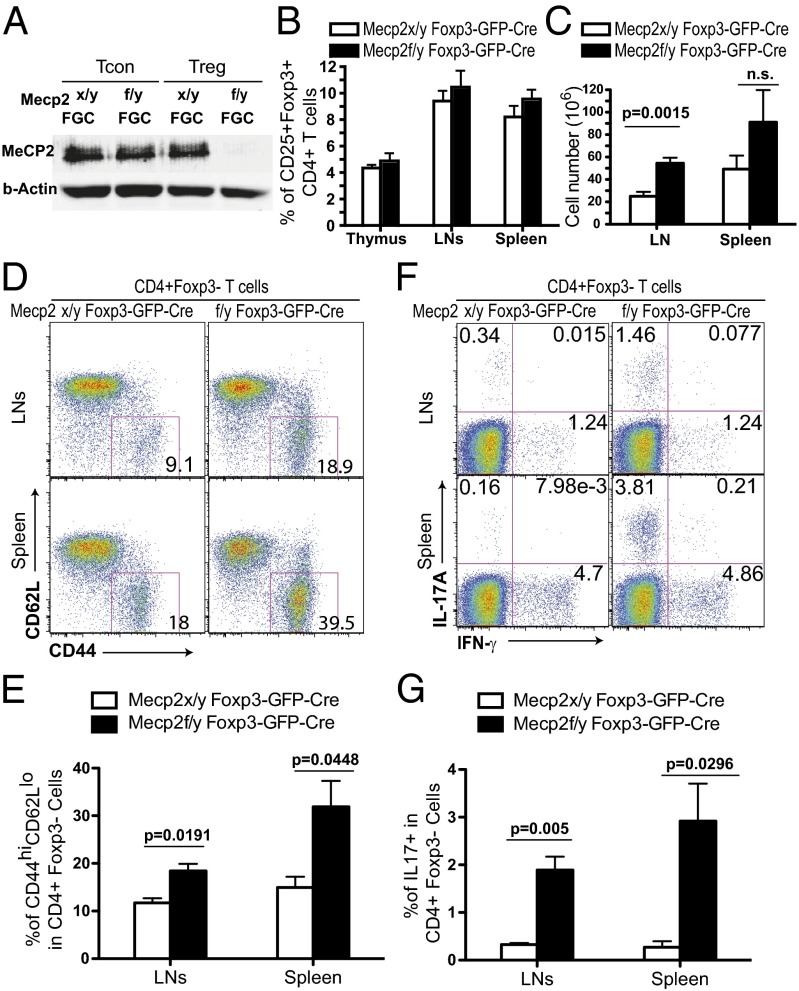
To examine the role of MeCP2 specifically in mature nTregs, we crossed mice carrying conditional mecp2 alleles with BAC transgenic mice expressing Cre and GFP proteins under the control of the foxp3 promoter (41). This allows us to specifically ablate the mecp2 gene after T cells establish their commitment into the Treg lineage (Fig. 1A). The ablation of the MeCP2 protein did not change the relative size of the CD25+Foxp3+ population within the pool of CD4+ T cells (Fig. 1B). However, in mice as young as 8–10 wk of age, we observed a significant increase in the total number of cells in the lymph nodes (LNs) from Mecp2f/y Foxp3–GFP–Cre mice (Fig. 1C). Although the percentages of CD4 and CD8 single positive cells in thymi and peripheral lymphoid organs were comparable to their WT littermates (SI Appendix and Fig. 2 A and B), the proportion of CD4+ T cells adopting an activated phenotype (CD44hi CD62Llo) in the spleens and LNs of Mecp2f/y Foxp3–GFP–Cre mice was significantly increased (Fig. 1 D and E). We then examined the cytokine production of the CD4+Foxp3− T cells in these mice: Treg-specific deletion of MeCP2 resulted in significantly elevated levels of IL-17–producing conventional CD4 T cells (Fig. 1 F and G). When these mice were aged for more than 6 mo, although there were no immunopathologies of the kidney, liver, or lung, Mecp2f/y Foxp3–GFP–Cre mice frequently developed skin lesions near the neck area, and further histological analysis unveiled extensive inflammatory infiltration (SI Appendix, Fig. S2C). Collectively, these data suggest that MeCP2 expression in Tregs is required to enforce immune homeostasis in vivo.
Fig. 1.
Mild immune activation in young adult Mecp2f/y Foxp3–GFP–Cre mice. (A) MeCP2 expression was examined by Western blot in sorted CD4+CD25−GFP− Tcon and CD4+CD25+GFP+ Treg cells from the spleens and LNs of Mecp2x/y Foxp3–GFP–Cre or Mecp2f/y Foxp3–GFP–Cre mice. (B and C) Lymphocytes from the thymus, LNs, and spleens of 8–10-wk-old Mecp2f/y Foxp3–GFP–Cre or littermate control mice were isolated for enumeration of cell numbers and flow cytometry analysis. Data are shown as means ± SEM (n = 5). (B) Percentage of CD25+Foxp3+ cells in CD4+ T cells. (C) Absolute cell numbers in the LNs and spleens. (D and E) Expression of CD44 and CD62L on the surface of CD4+Foxp3− T cells from LNs and spleens. (F and G) Lymphocytes isolated freshly ex vivo were stained for intracellular cytokines after 4 h of stimulation with 0.9 nM PdBU and 0.5 μg/mL ionomycin in the presence of 5 μg/mL brefeldin A and 2 μM monensin.
Fig. 2.
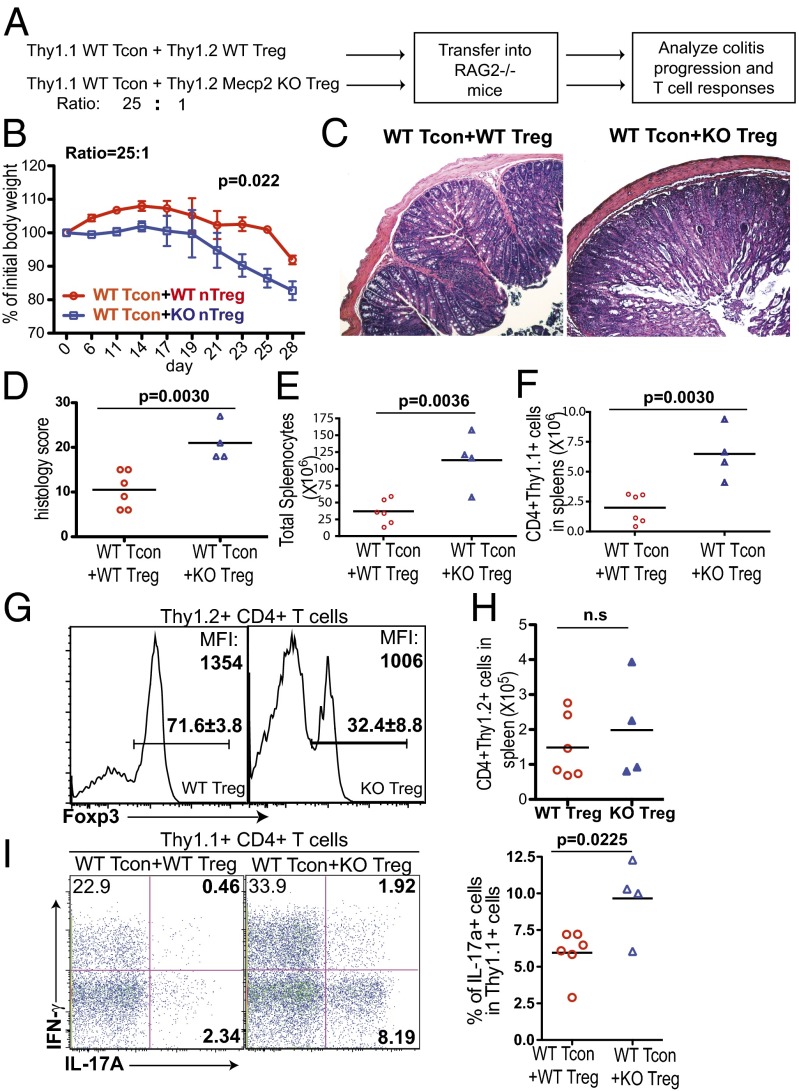
MeCP2-deficient Tregs are impaired in suppressing effector T-cell–mediated colitis. (A–I) The 5.0 × 105 CD25− CD45RBhi CD4 T cells from WT Thy1.1+ B6 mice (WT Tcon) were sorted and mixed with 2.0 × 104 Thy1.2+ CD4+CD25+ GFP+ Tregs from Mecp2f/y Foxp3–GFP–Cre (n = 4) or Mecp2x/y Foxp3–GFP–Cre (n = 6) littermate control mice, before cotransfer into RAG2−/− recipient mice. (A) Schematic representation of the workflow. (B) Weight changes of recipient mice after adoptive transfer were normalized to their initial body weights before transfer. Data show means ± SEM. (C and D) Recipient mice were euthanized 4 wk later. Colon tissues were isolated for histopathologic analysis. (C) Representative images of colon tissues with H&E staining. (D) Summary of the histopathologic scores. (E) Absolute number of splenocytes. (F) Absolute number of CD4+Thy1.1+ cells in spleens. (G) Percentage of Foxp3+ cells among Thy1.2+ cells and mean fluorescence intensity (MFI) of Foxp3 staining in Foxp3+ Tregs. (H) Absolute number of CD4+Thy1.2+ cells in spleens. (I) Thy1.1+ T cells from spleens were stimulated by PdBU and ionomycin for 4 h and stained for intracellular cytokines. Left, representative FACS plot; Right, summary from experimental groups.
MeCP2-Deficient nTregs Fail to Suppress Effector T-Cell–Mediated Colitis in Vivo.
Although immune activation in Mecp2f/y Foxp3–GFP–Cre mice is apparent, this inflammation is relatively mild compared with the severe lymphoproliferation in Treg-depleted mice (42). In agreement with this, Mecp2f/y Foxp3–GFP–Cre mice and their WT littermate controls had comparable percentages of CD25+Foxp3+ CD4 T cells in the peripheral lymphoid organs as well as in the thymus (Fig. 1B). It is worth noting that the expression level of Foxp3 in Tregs from Mecp2f/y Foxp3–GFP–Cre mice was moderately reduced (SI Appendix, Fig. S2D). Loss of MeCP2-deficient Tregs over time might be obscured by the inflammation-induced compensatory expansion of Tregs (33, 43) or the continuous Treg output from the thymus. To rule out these possibilities, we examined the functionality of MeCP2-deficient Tregs using a classical T-cell adoptive transfer model for the induction of systemic colitis in lymphopenic hosts.
We sorted Thy1.2+ CD4+CD25+GFP+ Treg cells from Mecp2f/y Foxp3–GFP–Cre and Mecp2x/y Foxp3–GFP–Cre mice, mixed them with WT Thy1.1+ naïve conventional CD4 T cells, and transferred them into RAG2−/− recipients (Fig. 2A). As expected, WT Tregs were capable of mitigating the development of colitis in recipient mice, monitored by both weight changes and histological analysis. In contrast, mice receiving cotransfers of conventional T cells and MeCP2-deficient Tregs manifested more severe colitis symptoms characterized by dramatic weight loss (Fig. 2B), massive leukocyte infiltration, and severe mucosal tissue damage in the colon (Fig. 2 C and D). Accompanying this inflammation, the numbers of total splenocytes (Fig. 2E) and, more specifically, Thy1.1+ effector T cells (Fig. 2F) were significantly elevated in recipients cotransferred with MeCP2-deficient Tregs. Furthermore, upon MeCP2 deletion, although the number of Thy1.2+ cells was comparable (Fig. 2H), we observed a significant reduction of the percentage of Foxp3+ Tregs in the Thy1.2+ population and dampened Foxp3 expression at the individual cell level (Fig. 2G). Consequently, the frequency of inflammatory cytokine-producing cells of conventional T-cell origin (Thy1.1+) was significantly increased (Fig. 2I). As a control, when MeCP2-deficient Tregs were transferred alone into RAG2−/− recipients, they were unable to generate any obvious weight loss or immunopathology for up to 8 wk (SI Appendix, Fig. S3 C–E). Although it remains possible that colitis requires a longer time for development in this experiment, we reasoned that the colitis symptoms associated with MeCP2-deficient Tregs observed previously were mainly caused by the loss of protection from conventional T-cell–elicited immune activation and cytokine storms, rather than the intrinsic inflammatory characteristics of MeCP2-deficient Tregs themselves. Overall, these data suggest that MeCP2 expression in Tregs critically supports their ability to suppress T effector cell-mediated inflammation in vivo.
MeCP2-Deficient Tregs Are Competent in Suppressing Effector T-Cell Activation During Short-Term Culture.
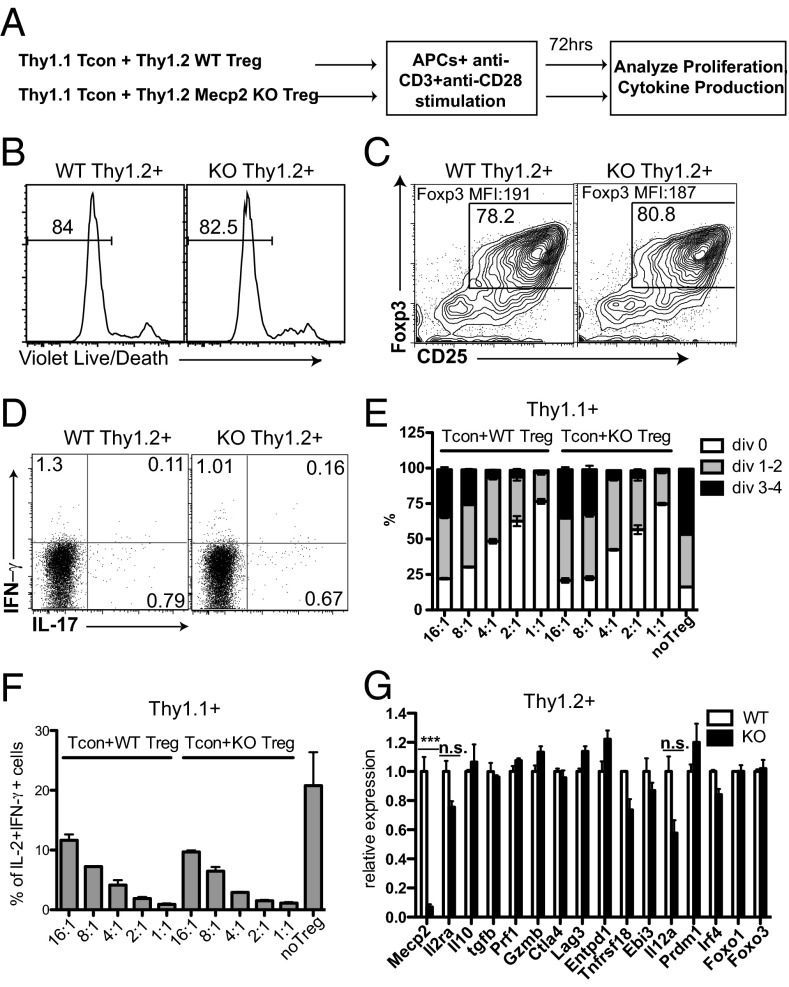
At least two potential mechanisms may account for the failure of MeCP2-deficient nTregs in suppressing inflammation: First, either independent of or in conjunction with Foxp3, MeCP2 acts as a master regulator controlling the expression of effector molecules that directly execute immune suppression; alternatively, MeCP2 may be essential in maintaining Foxp3 expression to enforce the nTreg lineage identity, specifically under inflammatory conditions. We first examined whether MeCP2 modulates effector molecule expression with a standard in vitro suppression assay. Thy1.2+ MeCP2-deficient nTregs were cocultured with Thy1.1+ WT conventional T cells at various ratios and stimulated with soluble anti–CD3/CD28 in the presence of antigen-presenting cells for a short time (3 d) in vitro (Fig. 3A). Under these conditions, the absence of MeCP2 did not affect the viability of Tregs (Fig. 3B), expression of Foxp3 (Fig. 3C), or ratio of conversion from regulatory to effector T cell (Fig. 3D). Using effector T-cell proliferation as well as the IL-2 and IFN-γ cytokine production as functional readouts of Treg-mediated immune inhibition, we found no significant differences in the suppressive capacities of WT and MeCP2-deficient nTregs (Fig. 3 E and F and SI Appendix, Fig. S4 A and D). However, under this lineage-unbiased condition, we consistently observe a moderate, but reproducible, increase in IL-17 production from conventional T cells when cocultured with MeCP2-deficient Tregs (SI Appendix, Fig. S4 B and C), which mirrored the enhanced IL-17 production by conventional T cells in intact Mecp2f/y Foxp3–Cre–GFP mice (Fig. 1 F and G).
Fig. 3.
MeCP2-deficient Tregs are competent in suppressing effector T-cell activation during short-term culture in vitro. CD4+CD25− conventional T cells from LNs and spleens of Thy1.1+ B6 mice were FACS sorted, labeled with CFSE, mixed with Thy1.2+ CD4+CD25+ Tregs from LNs and spleens of Mecp2f/y Lck–Cre or littermate control mice at the indicated ratios, and stimulated with 0.5 μg/mL anti-CD3 and 0.5 μg/mL anti-CD28 in the presence of T-cell–depleted splenocytes (as APCs) from B6 mice for 72 h. (A) Schematic representation of the workflow. (B–D) The survival (B), Foxp3 maintenance (C), and cytokine production (D) from WT and MeCP2-deficient Tregs (Thy1.2+) as shown by LIVE/DEAD Fixable Violet Dead Cell Staining and intracellular staining, respectively. Data shown represent three independent experiments. (E and F) The suppression of effector T-cell (Thy1.1+) proliferation (E) and cytokine production (F) by MeCP2-sufficient or -deficient Tregs (Thy1.2+) as shown by CFSE dilution and intracellular staining. Bar graphs show means ± SEM of three independent experiments. (G) Viable WT and MeCP2-deficient Thy1.2+ Treg cells from the 4:1 Tcon:Treg culture were sorted, and expression of genes that are critical for Treg suppressive function was determined by qPCR. Data show means ± SEM of two independent experiments.
At the end of these mixed cultures, we sorted the remaining viable Thy1.2+ Tregs to further analyze their expression of a panel of molecules known to be crucial for Tregs’ suppressive function. This panel included the effector molecules (44) CD25, IL-10, TGFβ, perforin, granzyme B, CTLA4, LAG3, CD39, GITR, and IL-35, as well as their upstream regulators Blimp1 (45), IRF4 (45) (for Il10), and Foxo family transcription factors (for ctla4) (46). Consistent with comparable functional outcomes, we found that at the transcriptional level, the suppressive machinery of MeCP2-deficient Tregs was largely intact (Fig. 3G).
Because DNA methylation is crucial in orchestrating the transcriptome and function of Tregs (47) and MeCP2 was a well-characterized epigenetic factor involved in CpG methylation, we further examined whether the global gene expression pattern is perturbed in MeCP2-deficient Tregs. Surprisingly, when over 39,000 transcripts are profiled from in vitro-activated WT or MeCP2-deficient Tregs, there are only 154 genes whose expressions are significantly altered, and we failed to link any of these genes to Treg-associated immunosuppression (SI Appendix, Table S1). These data collectively suggested that, at least over the course of a few days, the immunosuppressive nature and the associated transcription architecture of MeCP2-deficient Tregs are well preserved.
MeCP2 Is Critical for Maintaining Foxp3 Expression in nTregs During Inflammation in Vitro.
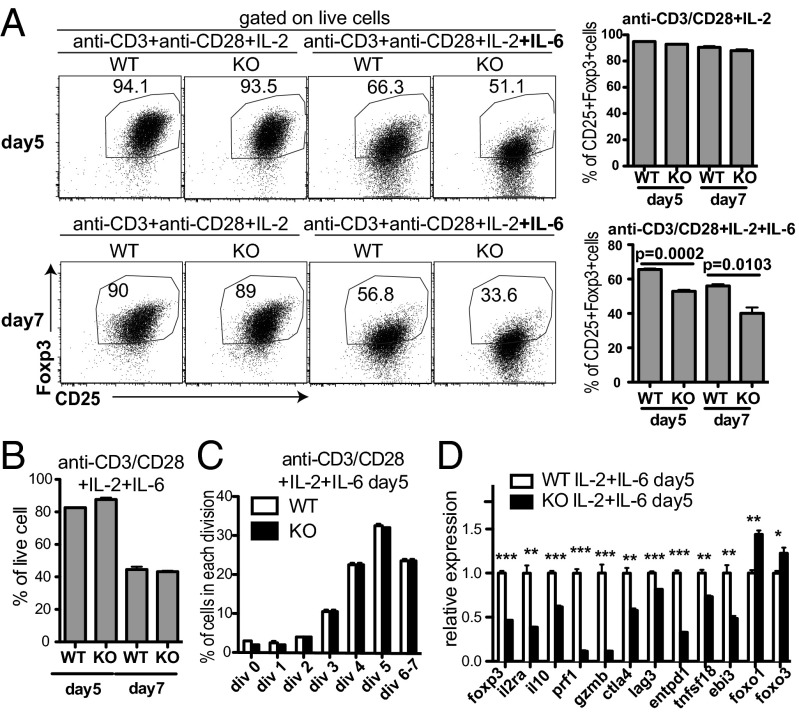
We next examined the alternative hypothesis that MeCP2 is essential for the long-term maintenance of Foxp3 expression during inflammation. Yang et al. previously showed that in vitro treatment of nTregs with inflammatory cytokines, such as IL-6, causes a gradual loss of Foxp3 expression (48). To determine whether MeCP2 ablation facilitated this process, we stimulated MeCP2-sufficient or -deficient nTregs with anti-CD3/28, IL-2, and IL-6. As a noninflammatory control, these nTregs were also cultured with only anti-CD3/28 and IL-2. We found that without IL-6, both WT and MeCP2-deficient Tregs could maintain Foxp3 expression. However, in the presence of IL-6, MeCP2-deficient nTregs demonstrated an accelerated loss of Foxp3 expression, both at the protein level (Fig. 4A and SI Appendix, Fig. S5A) and mRNA level (Fig. 4D). We also monitored the survival and proliferation of Tregs to exclude the possibility that this decrease in percentage was caused by altered cell death or proliferation of MeCP2-deficient Tregs (Fig. 4 B and C and SI Appendix, Fig. S5 B and C). In addition, accompanying the accelerated loss of Foxp3 expression, IL-17 production from MeCP2-deficient Tregs was significantly elevated (SI Appendix, Fig. S5D). This elevated Treg-to-effector conversion supports our hypothesis that MeCP2 preserves the characteristics of Tregs in response to IL-6–mediated foxp3 silencing in vitro.
Fig. 4.
MeCP2 is required for the maintenance of Foxp3 expression in Tregs during inflammatory cytokine stimulation in vitro. (A–C) CD4+CD25+GFP+ Tregs from LNs and spleens of Mecp2f/y Foxp3–GFP–Cre or their WT littermate control mice were sorted, labeled with the CellTrace proliferation dye, and stimulated with 1 μg/mL plate-bound anti-CD3 and 1 μg/mL plate-bound anti-CD28 in the presence of 50 U/mL IL-2 with or without 50 ng/mL IL-6 for 5–7 d (n = 3). (A) The percentages of viable cells retaining CD25 and Foxp3 expression at the indicated time points were measured by flow cytometry. Data show means ± SEM. (B) The percentages of viable cells at the indicated time points were determined by LIVE/DEAD Fixable Violet Dead Cell Staining. Data show means ± SEM. (C) Proliferation of viable cells at the indicated time points were determined by dilution of the CellTrace proliferation dye. Data show means ± SEM. (D) Viable WT and MeCP2-deficient Treg cells after 5 d of culture with anti-CD3/CD28, IL-2, and IL-6 were sorted, and expression of Treg signature genes was determined by qPCR. Data show means ± SEM (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
Because Foxp3 is required to enforce the transcriptional program of Tregs (49), we also assessed whether the expression of Treg signature genes was disrupted by MeCP2 deficiency in this inflammatory setting. As expected, accompanying the significant reduction of foxp3 transcripts in MeCP2-deficient Tregs, many Treg signature genes that regulate Treg function were down-regulated (Fig. 4D). In contrast, genes that have been suggested to act in parallel with Foxp3 to control Treg function, such as foxo1 (50) and foxo3, were either unchanged or even slightly elevated (Fig. 4D).
MeCP2 Is Critical for Maintaining Foxp3 Expression in nTregs During Inflammation in Vivo.
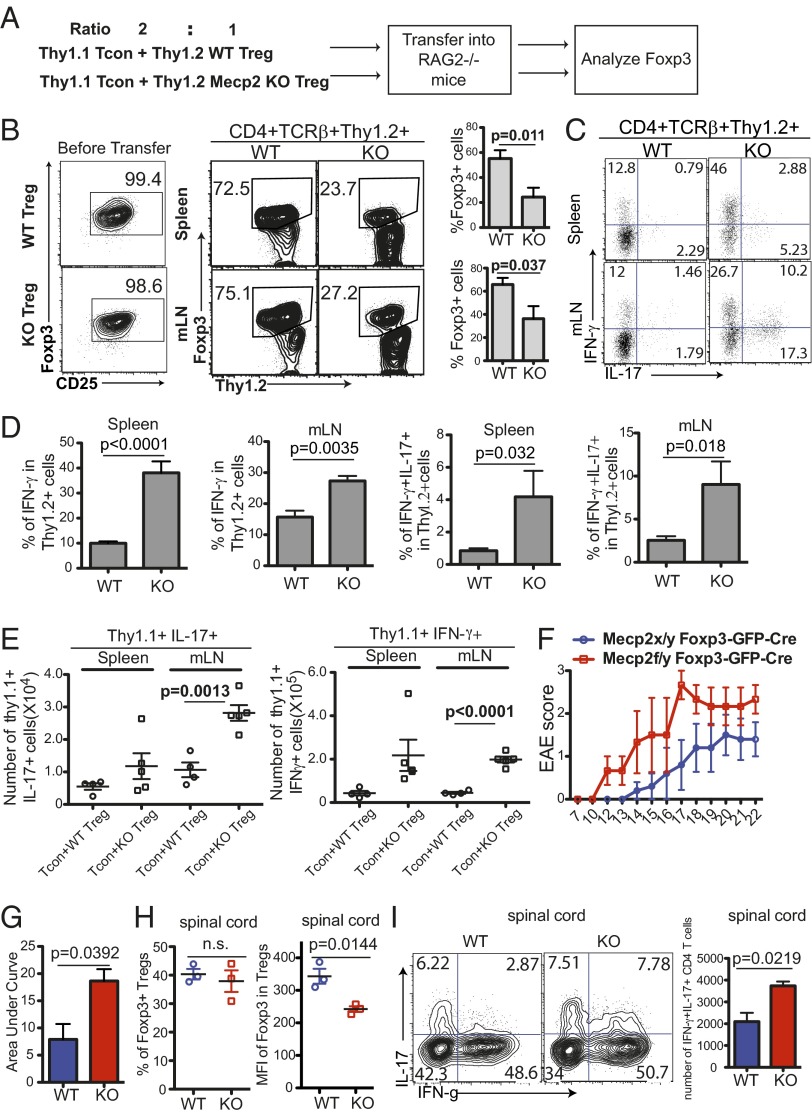
We took a similar adoptive transfer approach to examine the role of MeCP2 in sustaining Foxp3 expression in vivo. We sorted Thy1.2+ CD25+GFP+ nTreg cells from Mecp2f/y Foxp3–GFP–Cre or Mecp2x/y Foxp3–GFP–Cre mice and mixed them with Thy1.1+ CD4+CD25− conventional T (Tcon) cells at a 1:2 ratio before cotransfer into RAG2−/− mice (Fig. 5A). A 1:2 ratio of nTreg:Tcon allowed us to recover enough Thy1.2+ T cells for reliable endpoint analysis after a long-term transplantation. Three weeks after transfer, the mice were killed and T cells of the Thy1.2+ nTreg origin were analyzed. In mice carrying WT nTreg, a majority of the Thy1.2+ population remained Foxp3+ (Fig. 5B). In contrast, the MeCP2-deficient Tregs exhibited a dramatic loss in Foxp3 expression (Fig. 5B). A significant portion of these ex-Tregs were converted into IL-17– and/or IFN-γ−producing effector cells (Fig. 5 C and D). Coordinately, conventional T cells cotransferred with MeCP2-deficient Tregs also showed augmented proinflammatory cytokine production (Fig. 5E).
Fig. 5.
MeCP2 is required for the maintenance of Foxp3 expression in Tregs in vivo. (A–E) The 2.0 × 105 CD4+CD25− T cells from Thy1.1+ WT B6 mice were sorted and mixed with 1.0 × 105 Thy1.2+ CD4+CD25+ GFP+ Tregs from Mecp2f/y Foxp3–GFP–Cre (KO) or Mecp2x/y Foxp3–GFP–Cre (WT) littermate control mice, and the mixture was cotransferred into RAG2−/− mice. Recipient mice were euthanized 3 wk after transfer for flow cytometry analysis. Bar graphs in B and D show means ± SEM of six mice. Data represent three independent experiments. (A) Schematic view of the workflow. (B) Percentage of Thy1.2+CD4 T cells before transfer and those that maintain Foxp3 expression in the mesenteric LNs (mLNs) and spleens of recipient mice (n = 6). (C and D) Cytokine production from Thy1.2+ cells in the mLNs and spleens of recipient mice as determined by intracellular staining following 4 h of PdBU and ionomycin stimulation (n = 6). (E) Absolute number of Thy1.1+ effector T cells that produce IL-17 and IFN-γ in the mLNs and spleens of recipient mice (WT, n = 4; KO, n = 5). (F–I) EAE was induced in Mecp2f/y Foxp3–GFP–Cre (KO) or Mecp2x/y Foxp3–GFP–Cre (WT) littermate control mice by immunization with MOG peptide emulsified in CFA, and mice were killed at day 22 for cell analysis. (F) Clinical scores over time. (G) Area under curve (Right). (H) Percentage of Foxp3+ Tregs and MFI of Foxp3 expression in Foxp3+ Tregs from the spinal cord of WT or KO mice. (I) Percentage and number of CD4+Foxp3− cells that produce proinflammatory cytokines in spinal cords of WT and KO mice. All pooled data from F–I are represented as means ± SEM from three mice per group.
We further examined the role of MeCP2 in sustaining Foxp3 expression in intact Mecp2x/y or f/y Foxp3–GFP–Cre mice during experimental autoimmune encephalomyelitis (EAE), which is characterized by strong Th17- and Th1-mediated inflammation in the central nervous system. We found that upon disease induction, although the percentages of Tregs in the spinal cord were comparable between Mecp2x/y Foxp3–GFP–Cre (WT) and Mecp2f/y Foxp3–GFP–Cre mice (KO) mice (Fig. 5H), Foxp3 expression in individual MeCP2-deficient Tregs was consistently reduced (Fig. 5H). Coordinately, Mecp2f/y Foxp3–GFP–Cre mice exhibited accelerated disease onset, more severe symptoms (Fig. 5 F and G), as well as enhanced proinflammatory cytokine production (in particular, an increased IL-17+IFN-γ+ population) from effector T cells in the spinal cords (Fig. 5I). Taken together, these data suggest that MeCP2 is critical to maintain Foxp3 expression in Tregs during inflammation in vivo.
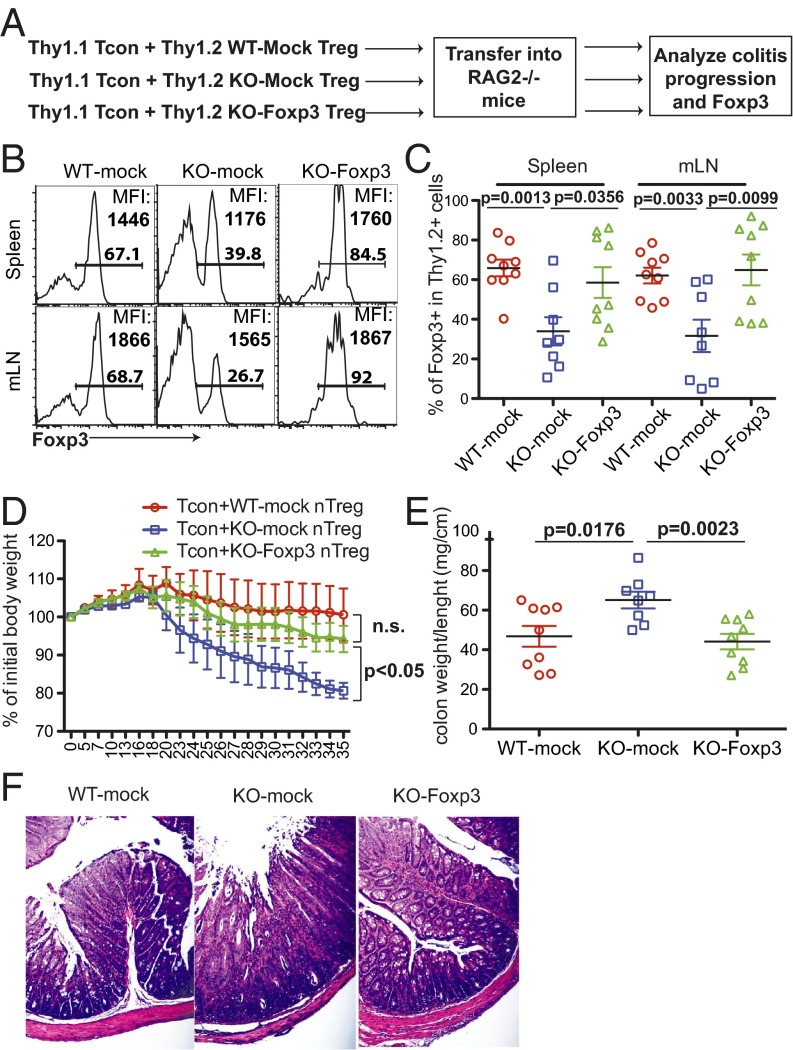
Restoring Foxp3 Expression Sufficiently Rescues the Competence of MeCP2-Deficient Tregs to Suppress Inflammation in Vivo.
Recent studies demonstrated that, besides the expression of Foxp3, epigenetically, nTreg development also requires a Treg-specific CpG hypomethylation (47). In our case, the incompetence of MeCP2-deficient Tregs in suppressing inflammation could also be influenced by certain undetected epigenetic modifications. To further determine causality between MeCP2, Foxp3, and immune protection, we transduced MeCP2-deficient Tregs with a retrovirus that ectopically expresses Foxp3 transcripts and a GFP marker. Continued use of GFP+ Tregs from Foxp3–GFP–Cre mice would preclude the identification of cells that have been transduced with the exogenous Foxp3–GFP coexpression vector; to circumvent this technical limitation, we instead purified Tregs from WT or Mecp2f/yLck–Cre mice based on CD25 expression. Even though this purification strategy could contaminate Treg pools with a low percentage of conventional T cells, we validated that, as a group, these MeCP2-deficient CD4+CD25+ cells recapitulated the phenotypic defects of sorted GFP+CD25+ cells from Mecp2f/yFoxp3–GFP–Cre mice. They failed to protect against T-cell transfer-induced colitis (SI Appendix, Fig. S3 A and B) and failed to maintain Foxp3 expression during inflammatory cytokine stimulation (SI Appendix, Fig. S5A). We examined whether restoring Foxp3 expression in MeCP2-deficient Tregs could rescue their immunosuppressive capacity with the aforementioned adoptive transfer model (Fig. 6A). Five weeks after the initial transfer, we confirmed that the retroviral expression restored Foxp3 protein expression in MeCP2-deficient Tregs to that of WT levels (Fig. 6B). Coordinately, we observed a complete rescue of Treg identity at the population level (Fig. 6C) and, more importantly, a complete rescue of their competence to suppress colitis development, as reflected by the maintained body weight (Fig. 6D) and diminished colon pathology (Fig. 6 E and F). Taken together, these data indicated that the critical role of MeCP2 in immune protection is to maintain a stable Foxp3 expression.
Fig. 6.
Ectopic expression of Foxp3 restores the capacity of MeCP2-deficient Tregs to suppress effector T-cell–mediated colitis in vivo. Thy1.2+ CD4+CD25+ Tregs from LNs and spleens of Mecp2f/y Lck–Cre or their WT littermate control mice were sorted and stimulated with 1 μg/mL anti-CD3 and anti-CD28 in the presence of 50 U/mL IL-2. Eighteen hours later, cells were transduced with a retrovirus that encodes the foxp3 coding sequence together with GFP (Foxp3) or GFP alone (mock). Two days later, 5.0 × 104 Thy1.2+GFP+ Tregs were sorted and mixed with 5.0 × 105 naïve conventional T cells (CD25− CD45RBhi CD4+) from WT Thy1.1+ B6 donors, and then transferred into RAG2−/− mice. Colon pathology and phenotype of transferred cells were analyzed 5 wk after adoptive transfer (n ≥ 8). (A) Schematic view of the workflow. (B and C) Percentage of recovered Foxp3+ cells from the Thy1.2+ origin and MFI of Foxp3 expression in Foxp3+ Tregs. (B) Representative FACS plots. (C) Summary of results from various experimental groups. Each symbol represents one single recipient mouse. (D) Weight changes during the colitis progression. The weight of recipients at different time points was normalized to the initial body weight of the individual mouse before transfer. Data show means ± SEM. (E and F) Colon immunopathology of recipient mice was indicated by the ratio of colon weight to length (E) and illustrated by H&E staining (F).
MeCP2 Opposes foxp3 Gene Silencing by Recruiting CREB1 and Enforcing Local Histone Acetylation in the foxp3 CNS2 Region.
Our data indicated that MeCP2 was important for bolstering foxp3 expression specifically in response to inflammation-induced transcriptional silencing, but not for de novo transcription of foxp3 per se. It was previously reported that, during the development of acute murine graft-versus-host disease, STAT3 activation downstream of IL-6 stimulation destabilizes Foxp3 expression in Tregs (51). We examined whether the deletion of the mecp2 gene enhances IL-6 signaling. In MeCP2-deficient Tregs, as reflected by Tyr705 phosphorylation, there were no obvious alterations in STAT3 activation in response to IL-6 stimulation (SI Appendix, Fig. S6A).
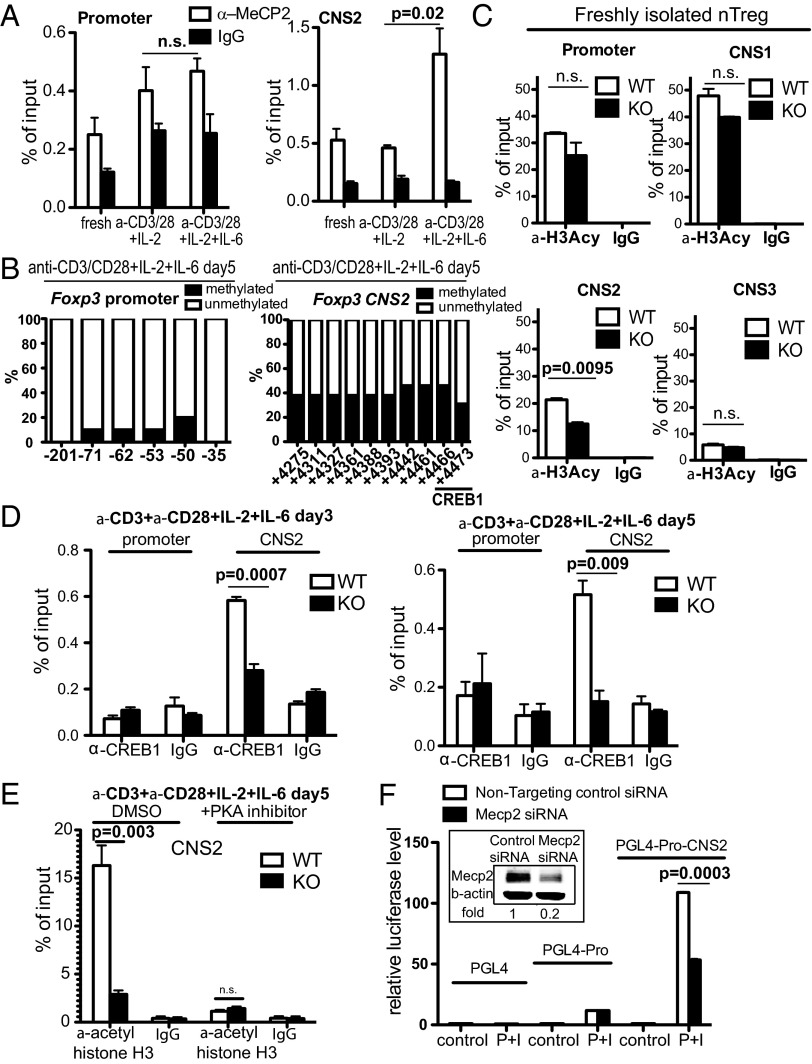
Among the identified cis-elements known to be important for foxp3 gene regulation, the promoter and CNS2 region contain CpG islands (37) that could potentially recruit MeCP2. Using chromatin immunoprecipitation (ChIP), we examined the interaction between MeCP2 and cis-elements under different conditions. In freshly isolated WT nTreg cells, CpG islands within the promoter and CNS2 regions of foxp3 were almost completely unmethylated (SI Appendix, Fig. S7 A and B). Somewhat surprisingly, the CNS2 region was partially occupied by the MeCP2 protein (Fig. 7A), agreeing with the notion that MeCP2 can bind to unmethylated DNA elements as well (4, 7). However, in the presence of T-cell receptor (TCR) stimulation and inflammatory cytokines, although the DNA sequence within the foxp3 promoter remained largely unmethylated, methylation was rapidly initiated across the entire CNS2 region (Fig. 7B and SI Appendix, Fig. S8). Accordingly, MeCP2 protein was preferentially recruited to and accumulated within the CpG-methylated CNS2 region (Fig. 7A). This recruitment of MeCP2 by CNS2 depended on inflammatory stimuli. In Tregs stimulated with anti-CD3, anti-CD28, and IL-2 only, no enhanced MeCP2 binding was detected (Fig. 7A).
Fig. 7.
MeCP2 occupies the foxp3 CNS2 region and regulates its chromatin accessibility through recruitment of CREB1. (A) Freshly isolated Tregs from LNs and spleens of WT B6 mice or Tregs that were stimulated with anti-CD3/CD28 and 50 U/mL IL-2, in the presence or absence of 50 ng/mL IL-6 for 5 d, were subject to ChIP analysis for MeCP2 enrichment at the foxp3 promoter and CNS2 region. The bar graph shows means ± SEM (n = 3). (B) CD4+CD25+GFP+ nTregs were sorted from LNs and spleens of Mecp2x/y Foxp3–GFP–Cre (WT) and stimulated with anti-CD3/CD28, 50 U/mL IL-2, and 50 ng/mL IL-6 for 5 d. The methylation status in the promoter and CNS2 of foxp3 locus was analyzed by bisulfite sequencing. Numbers on the x axes indicate the position of CpGs relative to the TSS of the foxp3 gene. Bar graphs show the data quantified from sequencing at least 10 clones for each sample. Data shown represent three independent experiments. (C) Freshly isolated CD4+CD25+GFP+ Tregs sorted from LNs and spleens of Mecp2f/y Foxp3–GFP–Cre mice (KO) or Mecp2x/y Foxp3–GFP–Cre mice (WT) were analyzed for histone H3 acetylation at various cis-elements within the foxp3 locus by ChIP (n = 3). Means ± SEM are shown. (D) ChIP analysis for the enrichment of CREB1 at the foxp3 promoter and CNS2 region in WT or KO Tregs that were stimulated with anti-CD3/CD28, IL-2, and IL-6 for 3 or 5 d. The bar graph shows means ± SEM (n = 3). (E) Histone H3 acetylation of CNS2 in WT or KO Tregs that were stimulated with anti-CD3/CD28, IL-2, and IL-6 for 5 d in the presence or absence of 5 μM PKA pathway inhibitor H89 (Calbiochem) (n = 3). Means ± SEM are shown. (F) Luciferase reporter assays. Reporter constructs of PGL4, PGL4 linked with the Foxp3 promoter (PGL4–Pro), or PGL4 linked with the Foxp3 promoter and CNS2 (PGL4–Pro–CNS2) were transfected into Jurkat T cells together with nontargeting control siRNA or siRNA against MeCP2. Forty-eight hours later, cells were either untreated or treated with PdBU and ionomycin for 16–24 h, and lysed for analysis of luciferase activity (n = 3). (Inset) The efficiency of siRNA treatment was examined by Western blot 48 h after transfection. Data shows means ± SEM (n = 3).
To determine the functional importance of inflammation-induced MeCP2 recruitment to the foxp3 locus, we first examined the status of DNA CpG methylation in freshly isolated MeCP2-deficient nTregs. The loss of MeCP2 resulted in a comparable, or even a slight reduction in, DNA methylation at both the promoter and CNS2 region (SI Appendix, Fig. S7 A and B). Therefore, methylation cannot explain the protective effect of MeCP2 on foxp3 gene expression. To identify a possible positive gene regulatory function for MeCP2, we next scanned the status of histone H3 acetylation—a known mediator of chromatin accessibility—in all regulatory regions of the foxp3 gene. In freshly isolated Tregs, the ablation of MeCP2 did not affect H3 acetylation in the promoter, CNS1, and CNS3 region, but reduced H3 acetylation in the CNS2 region by 43% (see Fig. 7C and SI Appendix, Fig. S9A for quality controls). When the MeCP2-deficient Tregs were stimulated with anti-CD3/28 and inflammatory cytokines for 5 d, the H3 acetylation defect in the CNS2 region was even more drastic, reaching a mere 18% of WT levels (Fig. 7E, DMSO panel). However, this observed diminishment in H3 acetylation at the CNS2 region may not necessarily be the cause of Foxp3 destabilization, but could instead simply be a consequence of an enrichment of ex-Treg cells due to MeCP2 deficiency (as shown in Fig. 4A). To distinguish between these two possibilities, we examined H3 acetylation of CNS2 in Tregs 3 d post-TCR and inflammatory cytokine stimulation. At this time point, similar percentages of WT and MeCP2-deficient Tregs remained Foxp3-positive (SI Appendix, Fig. S9B); however, ChIP analysis revealed significantly reduced CNS2 H3 acetylation in MeCP2-deficient Tregs (SI Appendix, Fig. S9C). Because epigenetic programming precedes Foxp3 loss in MeCP2-deficient Tregs, we reasoned that the loss of H3 acetylation is the cause, rather than the consequence, of Foxp3 destabilization in MeCP2-deficient Tregs. Overall, these data suggested that MeCP2 recruitment to the CNS2 region might be important for sustaining chromatin accessibility via H3 histone acetylation and that inflammation-induced CNS2 methylation might specifically recruit MeCP2 to counteract methylation-mediated chromatin silencing.
Consistent with our data from the MeCP2 ChIP, mice with a knock-in deletion of foxp3’s CNS2 demonstrated that CNS2 is primarily responsible for the maintenance of Foxp3 transcription, in contrast to its initial induction (37). Various transcription factors, including STAT5, NF-κB, Ets-1, GATA3, Foxo1/3, the Foxp3/Runx/CBFβ complex, and CREB1, have been identified to operate within this region (26). Among these transcription factors, CREB1 forms a histone acetyltransferase complex with CBP/p300 and is also known to partner with MeCP2 to synergistically activate the transcription of somatostatin in neurons (4). We therefore hypothesized that in nTregs exposed to inflammatory stimuli, MeCP2-mediated protection of foxp3 expression might also depend on CREB1. To this end, we examined the binding of CREB1 to foxp3 regulatory elements under inflammatory stimuli in the presence or absence of MeCP2. CREB1 expression levels were similar between WT and MeCP2-deficient Tregs (SI Appendix, Fig. S10), and CREB1 bound avidly to the foxp3 CNS2 in the presence of MeCP2; however, in the absence of MeCP2, this binding was greatly diminished after 3 d of inflammatory stimulation and completely abolished when inflammation was extended by 2 additional days (Fig. 7D). Consistent with a possible role for CREB1 in MeCP2-dependent histone accessibility, we observed that H3 acetylation within the CNS2 region was reduced by 5.6-fold in the MeCP2-ablated Tregs. Furthermore, when CREB1 activation was blocked with the PKA pathway-specific inhibitor H89 (52), the MeCP2-dependent H3 acetylation of CNS2 was largely abrogated (Fig. 7E). Taken together, these results suggest that at the foxp3 locus, recruitment of the CREB1 transcription factor/acetyltransferase complex is MeCP2-dependent and that epigenetic regulation enacted by MeCP2 in response to inflammation is mediated through CREB1.
Finally, to determine whether MeCP2-mediated epigenetic modulation at the CNS2 region functionally impacts foxp3 gene transcription, luciferase reporter constructs containing either the foxp3 promoter alone or the foxp3 promoter together with the CNS2 region were transfected into Jurkat T cells as described previously (33). MeCP2 siRNA was cotransfected to assess the role of MeCP2 in regulating CNS2 activity. Transfected Jurkat T cells were then treated with Phorbol 12,13-dibutyrate (PdBU) plus ionomycin to mimic TCR activation. In agreement with its previously reported enhancer activity, the presence of the CNS2 region significantly boosted TCR-induced luciferase reporter activity. When MeCP2 was silenced to 20% of its normal expression level (Fig. 7F, Inset), foxp3 promoter activity was not affected, but we observed a significant reduction in CNS2-dependent transcription (Fig. 7F). Therefore, we argue that MeCP2-dependent chromatin remodeling specifically at the CNS2 region is an important contributor to foxp3 expression, especially in the face of inflammatory signals that act to silence foxp3.
Discussion
Tregs’ pivotal role in immune tolerance demands that they possess a dedicated molecular mechanism to maintain stable Foxp3 expression when challenged by an inflammatory environment. In this study, we show that MeCP2 is a crucial player in the epigenetic machinery that determines Tregs’ resilience in the face of inflammation. Interestingly, the function of MeCP2 is dedicated specifically to the maintenance as opposed to the induction of Foxp3. In the absence of immune activation, MeCP2 is dispensable for Foxp3 expression, both during nTreg and iTreg lineage commitment. In contrast, upon inflammation, MeCP2 is crucial for Foxp3 maintenance and Treg-enforced immune homeostasis. Mechanistically, the distinguishing feature is that MeCP2 binds to methylated DNA with enhanced affinity. TCR activation and inflammatory cytokine signaling subject the foxp3 locus to gene silencing by promoting DNA methylation in its CNS2 region, and whereas this modification is known to occlude transcription factor binding (53), we show that it also reciprocally recruits MeCP2 to associate specifically with CNS2 and thereby rescues foxp3 transcription via local histone H3 acetylation. Specifically, the docking of MeCP2 achieves this by recruiting CREB1 and potentially the CREB1 binding partner CBP/p300 (54) histone acetyltransferase to CNS2. Previous studies using mass spectrometry have demonstrated that the direct interaction between CREB1 and MeCP2 synergistically promotes the expression of a wide range of genes in the hypothalamus (4). In nTregs, we detected simultaneous and MeCP2-dependent binding of these two proteins to the CNS2 region. This interaction between CREB1 and MeCP2 may be direct or indirect. It may also be possible that MeCP2 associates with other coactivators to maintain a locally accessible chromatin structure, thereby indirectly facilitating the binding of CREB1 in the absence of physical interaction. In either case, the result is that MeCP2 provides an important epigenetic safeguard that confers Tregs with resistance to inflammation-induced foxp3 silencing.
MeCP2 has been shown to serve multifunctional roles, both as a transcription activator or repressor, presumably dependent upon the genomic context in which it operates. How then do cues in the genomic milieu control this functional switch? Genome-wide analyses have suggested that for MeCP2-targeted genes, the loci activated by MeCP2 are largely unmethylated or only partially methylated, whereas repressed loci are enriched in fully methylated elements. This suggests that the pivotal factor dictating MeCP2 function may lie in the status of local DNA methylation. Our data are consistent with this notion. CNS2, the primary region controlling the stability of Foxp3 expression, is completely unmethylated in naïve nTregs and becomes partially methylated upon inflammatory stimulation. Under these conditions, MeCP2 functions as a coactivator. In contrast, the CNS2 region is fully methylated in naïve Tcon cells, a situation in which MeCP2 plays no role during iTreg induction. We speculate that, in addition to Tregs, MeCP2 may exert a much broader impact on the lineage specificity and plasticity of other CD4+ T-cell subsets, which calls for further investigation in the future.
Due to its association with RTT, prior studies on MeCP2 have been centered almost exclusively on the central nervous system. Patients with RTT show no overt abnormalities in the first 6 mo of life, but they subsequently develop a profound neurodevelopmental disorder characterized by devastating periods of regression, which result in permanent cognitive impairment. Although an immune attack against the central nervous system was suspected to be the etiological factor of RTT in the early 1990s, in-depth pathological analysis failed to provide any evidence for autoimmunity. Using a mouse model of Treg-specific MeCP2 gene ablation, we have identified spontaneous CD4+ T-cell activation in young adult mice, a secondary effect of defective Treg-mediated immune suppression. Interestingly, this inflammatory phenotype vanished when mecp2 was deleted in all T cells using CD4–Cre or Lck–Cre to drive gene depletion. This was a strong indication that the loss of MeCP2 in other T-cell subsets might compensate for Treg dysfunction and prevent the onset of overt autoimmunity. Indeed, we have examined the function of MeCP2 in effector T-cell lineages and found that MeCP2-deficient effector CD4 T cells are severely impaired in their production of inflammatory Th1 and Th17 cytokines (55). Nonetheless, it is clear that T-cell immunity is not normal in these animals. Further analysis of the role of MeCP2 in other immune lineages will help to elucidate the regulatory nature of this protein in the immune system, and possibly in the neural system as well.
Materials and Methods
Mice.
Mice homozygous for the floxed mecp2 allele (B6.129P2-MeCP2tm1Bird/J) and nonobese diabetic mice were purchased from The Jackson Laboratory. Lck–Cre [B6.Cg-Tg(lck–cre)1Cwi N9] mice were from Taconic. Foxp3–GFP–Cre BAC transgenic mice were kindly provided by Xiaoping Zhong (Duke University Medical Center, Durham, NC). T-cell–specific and Treg-specific MeCP2-deficient mice were generated by crossing floxed mecp2 mice with lck–cre mice or Foxp3–GFP–Cre mice for more than eight generations. All mice experiments were approved by the Institutional Animal Care and Use Committee at Duke University.
Plasmids and Retrovirus Transduction.
The retroviral Foxp3 expression plasmid (Addgene plasmid 24067) was kindly provided by Dan Littman (New York University, New York). For retrovirus transduction, CD4+CD25+ Tregs were sorted and stimulated with 1 μg/mL anti-CD3 and anti-CD28 in the presence of 50 U/mL IL-2. Eighteen hours later, cells were transduced with a retrovirus in a 24-well plate by spin inoculation at a speed of 1,258 × g for 90 min at 37 °C.
RNA Extraction and Quantitative PCR.
Total RNA was isolated with miRVana extraction kit (Ambion) according to the manufacturer’s instructions. Reverse transcription was performed with qScript Flex cDNA Kit (Quanta Biosciences). Gene expression was quantified by SYBR Green-based quantitative PCR (qPCR) analysis and normalized to the level of succinate dehydrogenase complex, subunit A, flavoprotein as internal control.
FACS, Intracellular Staining, and Flow Cytometry Analysis.
Cell sorting was performed using a MoFlo Legacy sorter, and purity of sorted cells was determined by postsorting to be at least 98% for each experiment. Foxp3 staining was performed using the Foxp3/Transcription Factor Staining Buffer Set and an anti-mouse/rat Foxp3 antibody (clone FJK-16s) from eBioscience based on the suggested protocol. For intracellular cytokine staining, cells were stimulated with 0.9 nM PdBU and 0.5 μg/mL ionomycin (Sigma–Aldrich) in the presence of 5 μg/mL brefeldin A and 2 μM monensin (eBioscience) for 4 h. Cells were fixed with 2% paraformaldehyde, followed by permeabilization with 0.1% saponin (Sigma-Aldrich) and antibody staining. Fluorescence-conjugated antibodies for CD4, CD8, CD25, CD44, CD62L, IL-17A, IFN-γ, IL-2, CD90.1(Thy1.1), and CD90.2(Thy1.2) were purchased from Biolegend. The survival of cells was assessed by the LIVE/DEAD Fixable Violet Dead Cell Stain Kit from Invitrogen.
Adoptive Transfer and Inflammatory Bowel Disease Model.
To monitor the maintenance of Foxp3 in vivo, 1 × 105 MeCP2-sufficient or -deficient Thy1.2+ Tregs were sorted and mixed with 2 × 105 CD4+CD25− conventional T cells from Thy1.1+ B6 mice, and the mixture was cotransferred into RAG2−/− mice i.v. Mice were euthanized 3 wk later for analysis of Foxp3 expression. For the inflammatory bowel disease (IBD) model, 2 × 104 or 5 × 104 Thy1.2+ MeCP2-sufficient or -deficient Tregs were mixed with 5 × 105 Thy1.1+ CD4+CD25−CD45RBhi naïve T cells. For IBD experiments testing the effects of ectopically expressed Foxp3, Thy1.2+CD4+CD25+ Tregs from LNs and spleens of Mecp2f/y Lck–Cre or their WT littermate control mice were sorted and stimulated with 1 μg/mL anti-CD3 and anti-CD28 in the presence of 50 U/mL IL-2. Eighteen hours later, cells were transduced by spin infection with a retrovirus that encodes the foxp3 coding sequence together with GFP (Foxp3) or GFP alone (mock). Two days later, 5.0 × 104 Thy1.2+GFP+ Tregs were sorted and mixed with 5.0 × 105 CD25− CD45RBhi CD4 T cells from WT Thy1.1+ B6 mice and then transferred into RAG2−/− mice i.p. Recipient mice were monitored closely for changes in body weight for 3–7 wk and then euthanized for immunological and histological analysis.
EAE Model.
For the induction of EAE, 6–10-wk-old Mecp2f/y Foxp3–GFP–Cre mice or their littermate controls were injected with 200 ng pertussis toxin in 200 μL of PBS per mouse i.p. on day 0 and day 2. On day 0, each mouse was immunized with 100 μg myelin oligodendrocyte glycoprotein (MOG) peptide emulsified in complete Freund's adjuvant (CFA) s.c. Mice were then monitored closely for disease severity using the standard scale: 0, no clinical signs; 1, limp tail; 2, weak paraparesis (weak and incomplete paralysis of one or two hind limbs); 3, paraplegia (complete paralysis of two hind limbs); 4, paraplegia with forelimb weakness or paralysis; 5, death.
In Vitro Treg Suppression Assay.
The 1.0 × 105 CD4+CD25− conventional T cells from LNs and spleens of Thy1.1+ B6 mice were FACS sorted, labeled with carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen) at a concentration of 10 μM, and then mixed with sorted Thy1.2+ CD4+CD25+ Tregs from LNs and spleens of Mecp2f/y Lck–Cre or littermate control mice at different ratios. The mixture of cells was then stimulated for 72 h with 0.5 μg/mL soluble anti-CD3 (BioXCell) and 0.5 μg/mL soluble anti-CD28 (BioXCell) in the presence of 1 × 105 T-cell–depleted splenocytes from B6 mice, which had been pretreated with 50 μg/mL mitomycin C (Sigma) at 37° for 1 h to serve as antigen presenting cells (APCs). The proliferation of conventional T cells was analyzed by CFSE dilution. Cytokine production from conventional T cells or Tregs was analyzed by intracellular staining following 4 h of PdBU and ionomycin stimulation.
Luciferase Reporter Assay.
The PGL4, PGL4–Foxp3 Promoter (Pro), and PGL4–Pro–CNS2 constructs were kindly provided by Yisong Wan (University of North Carolina at Chapel Hill, Chapel Hill, NC) as previously described. In short, the foxp3 promoter [−500 bp to 100 bp of transcription start site (TSS)] or foxp3 CNS2 (4,001 bp to 4,820 bp of TSS) in conjugation with the promoter was cloned into the PGL4 basic vector upstream of the luciferase gene. Jurkat T cells were transfected by Nucleofector Device (Lonza) with a nontargeting control siRNA or siRNA against human MeCP2 (150 pmol per sample) (Thermo Scientific Dharmacon), together with 1 μg of reporter plasmid or 1 μg of thymidine kinase promoter-Renilla luciferase reporter plasmid as an internal control. Cells were either untreated or treated, 48 h after transfection, with 0.9 nM PdBU and 0.5 μg/mL ionomycin (Sigma) for an additional 16–24 h. Firefly and Renilla Luciferase activities were then determined using a dual luciferase assay kit (Promega).
DNA Methylation Analysis and ChIP.
Genomic DNA was purified with GenElute Mammalian Genomic DNA Miniprep Kit (Sigma, cat. no. G1N79). Methylation analysis was quantified by genomic DNA sequencing after bisulfite conversion using the MethylDetector Kit (Active Motif), PCR amplification, and cloning. ChIP was done based on a standard protocol with anti–acetyl-histone H3 rabbit polyclonal antibody (06-599, Millipore), MeCP2 (D4F3) XP rabbit monoclonal antibody (3456, Cell Signaling), CREB1 (48H2) rabbit monoclonal antibody (9197, Cell Signaling), or a nonspecific rabbit anti-mouse IgG (315-005-003, Jackson ImmunoResearch Laboratories). For H3 acetylation ChIP, micrococcal nuclease was used to digest chromosomes into single or double nucleosomes. For MeCP2 and CREB1 ChIP, sonication was used to break crosslinked chromatin/protein complexes into small fragments. The amount of DNA immunoprecipitated by antibodies was quantified by qPCR using primers specific for the indicated gene regulatory regions and normalized to the input before immunoprecipitation.
Histology.
Tissues were excised, fixed with 4% (vol/vol) paraformaldehyde in PBS overnight at room temperature, placed in 70% (vol/vol) ethanol, and then embedded in paraffin before H&E staining. The histologic scores of colon in the IBD model were assessed as previously described in a double-blinded setting. In short, the percent area of involvement (scale of 0–4) was multiplied by the percent involvement for each of the three following histological features: inflammation severity (scale of 0–3), inflammation extent (scale of 0–3), and Crypt damage (scale of 0–4). Therefore, the final score ranges from 0 to 40.
Statistics.
Two-tailed Student t tests were used to determine whether the difference between a given set of means was statistically significant unless otherwise mentioned. Differences with P values of less than 0.05 were considered statistically significant. n.s. indicates not significant.
Supplementary Material
Acknowledgments
We thank Drs. Bingtao Hao and Mike Krangel (Duke) for technical support on the ChIP experiment. We thank Nancy Martin from the Duke Cancer Institute Flow Core Facility for cell sorting services. We thank Dr. Yisong Wan (University of North Carolina at Chapel Hill) for his foxp3-luciferase reporters and Dr. Dan Littman (New York University) for the Foxp3 expression vector (MIGR–mFoxP3). We thank Dr. Peter. J. R. Ebert and Regina Lin for critical suggestions and reading of the manuscript. Q.-J.L. is a Whitehead Family Foundation Scholar and is supported by grants from the American Cancer Society (RSG-10-157-01-LIB), the American Diabetes Association (1-10-JF-28), and the National Institute of Allergy and Infectious Diseases (R01AI091878).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401505111/-/DCSupplemental.
References
- 1.Quaderi NA, et al. Genetic and physical mapping of a gene encoding a methyl CpG binding protein, Mecp2, to the mouse X chromosome. Genomics. 1994;22(3):648–651. doi: 10.1006/geno.1994.1442. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JD, et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69(6):905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- 3.Meehan RR, Lewis JD, Bird AP. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 1992;20(19):5085–5092. doi: 10.1093/nar/20.19.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chahrour M, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320(5880):1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nan X, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393(6683):386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 6.Kimura H, Shiota K. Methyl-CpG-binding protein, MeCP2, is a target molecule for maintenance DNA methyltransferase, Dnmt1. J Biol Chem. 2003;278(7):4806–4812. doi: 10.1074/jbc.M209923200. [DOI] [PubMed] [Google Scholar]
- 7.Yasui DH, et al. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci USA. 2007;104(49):19416–19421. doi: 10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guy J, Cheval H, Selfridge J, Bird A. The role of MeCP2 in the brain. Annu Rev Cell Dev Biol. 2011;27:631–652. doi: 10.1146/annurev-cellbio-092910-154121. [DOI] [PubMed] [Google Scholar]
- 9.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 10.Neul JL, et al. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008;70(16):1313–1321. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neul JL, et al. RettSearch Consortium Rett syndrome: Revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68(6):944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawalha AH, et al. Common variants within MECP2 confer risk of systemic lupus erythematosus. PloS One. 2008;3(3):e1727. doi: 10.1371/journal.pone.0001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb R, et al. Variants within MECP2, a key transcription regulator, are associated with increased susceptibility to lupus and differential gene expression in patients with systemic lupus erythematosus. Arthritis Rheum. 2009;60(4):1076–1084. doi: 10.1002/art.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobb BL, et al. Genetic association between methyl-CpG binding protein 2 (MECP2) and primary Sjogren’s syndrome. Ann Rheum Dis. 2010;69(9):1731–1732. doi: 10.1136/ard.2009.122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reichelt KL, Skjeldal O. IgA antibodies in Rett syndrome. Autism. 2006;10(2):189–197. doi: 10.1177/1362361306062024. [DOI] [PubMed] [Google Scholar]
- 16.Derecki NC, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484(7392):105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Chatila TA, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106(12):R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 20.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27(1):18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 21.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 22.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 23.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 25.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8(3):277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 26.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: Mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantel PY, et al. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176(6):3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 28.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9(2):194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 29.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178(1):280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 30.Yao Z, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109(10):4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31(6):921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Mouly E, et al. The Ets-1 transcription factor controls the development and function of natural regulatory T cells. J Exp Med. 2010;207(10):2113–2125. doi: 10.1084/jem.20092153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35(3):337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouyang W, et al. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11(7):618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 35.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: A role for DNA methylation. J Exp Med. 2007;204(7):1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudra D, et al. Runx-CBFbeta complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2009;10(11):1170–1177. doi: 10.1038/ni.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463(7282):808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: The key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9(2):83–89. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 39.Jiang S, et al. Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood. 2011;118(20):5487–5497. doi: 10.1182/blood-2011-05-355644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lal G, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182(1):259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205(9):1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9(6):632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 44.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Cretney E, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12(4):304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 46.Kerdiles YM, et al. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33(6):890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohkura N, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37(5):785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29(1):44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Y, et al. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445(7130):936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 50.Ouyang W, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491(7425):554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laurence A, et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37(2):209–222. doi: 10.1016/j.immuni.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chijiwa T, et al. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265(9):5267–5272. [PubMed] [Google Scholar]
- 53.Polansky JK, et al. Methylation matters: Binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J Mol Med (Berl) 2010;88(10):1029–1040. doi: 10.1007/s00109-010-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chrivia JC, et al. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365(6449):855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 55.Jiang S, et al. MeCP2 reinforces STAT3 signaling and the generation of effector CD4+ T cells by promoting miR-124-mediated suppression of SOCS5. Sci Signal. 2014;7(316):ra25. doi: 10.1126/scisignal.2004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.