Significance
Two of the most debilitating and scientifically challenging diseases of the 21st century are cancer and neurodegeneration. Although cancer results from excessive cell growth, neurodegeneration is a consequence of excessive cell loss. Dysfunction of the same key regulators, including oncogenes and tumor suppressors, may cause both diseases. We report that LPS and IFN induce apoptosis-stimulating protein of p53 with signature sequences of ankyrin repeat-, SH3 domain-, and proline-rich region-containing protein 2 (ASPP2) transcription through a signal transducer and activator of transcription 1 (STAT1) -dependent but NF-κB RELA/p65-independent pathway and that ASPP2 mediates LPS-induced apoptosis. Thus, the identified STAT1/ASPP2 pathway reveals an important function of ASPP2 in the cellular response to inflammation and infection and connects neuroinflammation to cell polarity and tumor suppression.
Keywords: TP53BP2, TLR4, multiple sclerosis
Abstract
Inflammation and loss of cell polarity play pivotal roles in neurodegeneration and cancer. A central question in both diseases is how the loss of cell polarity is sensed by cell death machinery. Here, we identify apoptosis-stimulating protein of p53 with signature sequences of ankyrin repeat-, SH3 domain-, and proline-rich region-containing protein 2 (ASPP2), a haploinsufficient tumor suppressor, activator of p53, and regulator of cell polarity, as a transcriptional target of signal transducer and activator of transcription 1 (STAT1). LPS induces ASPP2 expression in murine macrophage and microglial cell lines, a human monocyte cell line, and primary human astrocytes in vitro. LPS and IFNs induce ASPP2 transcription through an NF-κB RELA/p65-independent but STAT1-dependent pathway. In an LPS-induced maternal inflammation mouse model, LPS induces nuclear ASPP2 in vivo at the blood–cerebral spinal fluid barrier (the brain’s barrier to inflammation), and ASPP2 mediates LPS-induced apoptosis. Consistent with the role of ASPP2 as a gatekeeper to inflammation, ASPP2-deficient brains possess enhanced neuroinflammation. Elevated ASPP2 expression is also observed in mouse models and human neuroinflammatory disease tissue, where ASPP2 was detected in GFAP-expressing reactive astrocytes that coexpress STAT1. Because the ability of ASPP2 to maintain cellular polarity is vital to CNS development, our findings suggest that the identified STAT1/ASPP2 pathway may connect tumor suppression and cell polarity to neuroinflammation.
Neurodegenerative disease and cancer are two of the most common aging diseases and major medical challenges of the 21st century. Whereas neurodegeneration is characterized by accelerated cell death and a lack of self-renewal, cancers have an opposing phenotype, with excessive cell growth and dedifferentiation. Support for a molecular link between neurodegenerative disease and cancer is emerging and more intimate than previously understood. Recent studies indicate that patients with a history of cancer display a lower probability of developing Alzheimer’s disease (AD), whereas patients with AD have lower rates of cancer development (1). Compounds that inhibit γ-secretase, the enzyme that generates β-amyloid, have been used as potential therapeutics to treat AD. However, such treatment resulted in higher cancer incidence in a phase III clinical trial of Semagacestat (2). These observations suggest the existence of common molecules controlling cell death and self-renewal programs, which may be deregulated in both cancer and neurodegeneration. One such example is the tumor suppressor p53, the most commonly mutated gene in human cancer.
Several studies have attributed p53-mediated apoptosis to a number of acute and chronic neurodegenerative disorders, including excitotoxicity, AD, Parkinson disease, multiple sclerosis, and Huntington disease (3). Accumulating evidence supports the role of reactive oxygen species in prompting DNA damage in neurodegenerative disease (4), leading to p53-dependent apoptosis. Consistent with a proapoptotic role of p53 in CNS cells, inhibition of p53 by the chemical inhibitor pifithrin-α (5) or deletion of the p53 gene in a mouse model of multiple sclerosis (6) enhanced cell survival. These findings indicate that tumor suppressor pathways involving p53 may be deregulated in neurodegenerative disorders.
Inflammation and cell polarity disruption represent another link between neurodegeneration and cancer. In epithelial cancers, loss of cell polarity is a hallmark of cancer malignancy (7) and often associates with tumor-infiltrating lymphocytes and inflammation (8). Likewise, loss of brain barrier function prompted by neuroinflammation is linked to neurodegenerative disease onset and progression. The blood–brain barrier and blood–cerebral spinal fluid barrier (BCSFB) are the brain’s main barriers to infection (9). Previous studies have shown that a loss of cell polarity at these barriers prompts inflammatory changes, including the intrusion of immune cells and activation of microglia and astrocytes, which contribute to neurodegeneration (10). In the CNS, microglia, astrocytes, and macrophages participate in toll-like receptor (TLR) signaling. Mammalian TLRs are type I transmembrane receptors that recognize microbial pathogen-associated molecular patterns. TLR signaling culminates in the activation of transcription factors, such as NF-κB, signal transducer and activator of transcription 1 (STAT1), and AP-1 (11). Increasing evidence implicates the LPS receptor TLR4 in a number of neurodegenerative diseases and CNS injury (12). In mouse models, systemic injection of LPS leads to progressive neurodegeneration (13). Additionally, the role of viral infection and excessive IFN production in neurodegeneration is underscored by animal models of multiple sclerosis (14, 15) as well as IFN transgenic mouse models. For instance, transgenic mice producing IFN-α1 in GFAP-expressing astrocytes develop progressive neurodegeneration (16). Consistent with the link between neuroinflammation and p53-mediated apoptosis, previous studies indicate that p53 activity is regulated by TLR and IFN signaling. In the murine macrophage cell line RAW264.7, LPS and IFN-γ induce NO synthase and p53-mediated cell death (17). Moreover, a genome-wide in silico search identified most human TLR genes as potential p53 targets (18), suggesting an autoregulation loop between infection and p53 activity. Hence, the apoptotic function of p53 in response to infection plays an important role in controlling the inflammatory response. Given the emerging link between p53-induced apoptosis and inflammation, a better understanding of how cells relay changes in barrier function and cell polarity to cell death signals is critical. We, therefore, hypothesized that p53 regulators and gatekeepers of cell polarity may fulfill these requirements by acting as (i) a sensor that surveys the integrity of cell polarity, (ii) a messenger that communicates changes in cell polarity to cell death machinery, and (iii) a regulator of transcription. We refer to factors that fulfill these three roles as SMRT factors. One p53 regulator that may act as an SMRT factor is apoptosis-stimulating protein of p53 with signature sequences of ankyrin repeat-, SH3 domain-, and proline-rich region-containing protein 2 (ASPP2), a haploinsufficient tumor suppressor, activator of p53, and apical polarity regulator.
ASPP2 belongs to the ASPP family that comprises three members: ASPP1, ASPP2, and iASPP. Although ASPP1 and ASPP2 stimulate the apoptotic function of p53 by promoting the transcription of its proapoptotic target genes, iASPP prevents p53-mediated apoptosis (19). ASPP2 cooperates with p53 to suppress tumor growth in vivo (20). ASPP2-deficient mice lacking exon 3 (ASPP2 Δ3/Δ3) display a loss of neuroepithelial cell polarity and an expansion of CNS neural progenitors (21). ASPP2 Δ3/Δ3 mice die of hydrocephalus and display a loss of tight junctions (TJs) between choroid plexus (CP) epithelial cells, which form the BCSFB. This function of ASPP2 is mediated by its ability to bind Par-3 and maintain the integrity of apical cell polarity and TJs. The importance of ASPP2 in maintaining epithelial polarity is supported by the fact that ASPP2 is a target of CagA, a toxin and oncoprotein of a gastric cancer-associating bacterium Helicobacter pylori (22). Prompted by the emerging roles of cell polarity, inflammation, and p53 in cancer and neurodegeneration, in this study, we tested whether inflammatory stimuli regulate ASPP2 expression.
Results
LPS Induces ASPP2 in Macrophages, Microglia, and Astrocytes.
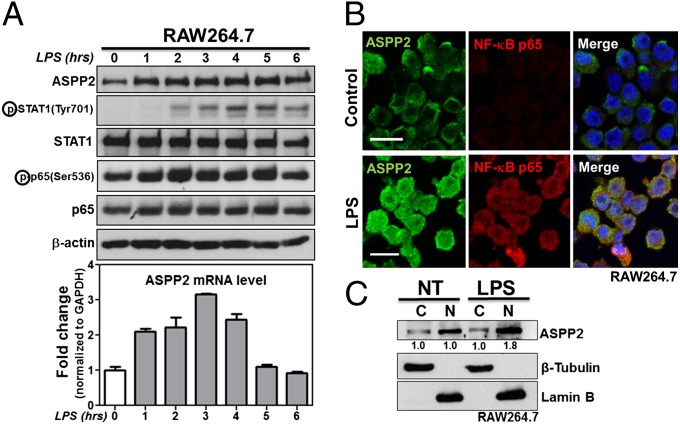
Recent reports support the role of TLR4 in a number of cerebral inflammatory disorders (12, 23). Because Helicobacter pylori infection induces ASPP2 in gastric cancer cells (22), we used the TLR4 ligand LPS to examine whether ASPP2 is responsive to inflammatory signaling. RAW264.7 (mouse macrophage), BV-2 (mouse microglial), and THP-1 (human monocyte) cell lines and primary human astrocytes were treated with 1 µg/mL LPS over the indicated time points. As a positive control for LPS treatment, we first examined the expression level and/or phosphorylation status of major inflammatory signaling transcription factors p65 and STAT1. All primary and secondary antibodies used are listed in Tables S1 and S2. A small increase in phospho-STAT1 and phospho-p65 was detectable by 1 h, and a clear increase was detectable by 2 h (Fig. 1A). Phospho-STAT1 and phospho-p65 decreased 6 h after LPS treatment. Expression of ASPP2 increased in all cell lines examined on LPS treatment (Fig. 1 A and B and Fig. S1 A–C), with maximum induction of ASPP2 apparent at 3 h in RAW264.7 cells. Similar to phospho-STAT1 and phospho-p65, elevated ASPP2 levels began to decrease 6 h after LPS treatment (Fig. 1A).
Fig. 1.
ASPP2 is induced by LPS. (A) LPS time course showing increased ASPP2 expression at protein and mRNA levels in RAW264.7. Expression levels of signaling pathways downstream of LPS were examined, including STAT1 and p65. (B) IF staining of ASPP2 and p65 after 2 h of LPS treatment in RAW264.7. (Scale bars: 10 µm.) (C) Nuclear (N) and cytoplasmic (C) fractionations of ASPP2 on LPS treatment in RAW264.7. Quantification was performed using densitometry analysis. NT, no treatment.
To determine whether LPS regulates ASPP2 at the protein or mRNA level, we examined the effect of LPS treatment on ASPP2 mRNA levels using quantitative RT-PCR (qRT-PCR). Primers used for qRT-PCR are listed in Table S3. Similar to ASPP2 protein level, up-regulation of ASPP2 mRNA was detected in a time-dependent manner after LPS treatment in RAW264.7 cells, with 1 h being the earliest time point of induction (Fig. 1A). Importantly, LPS did not induce iASPP mRNA expression, suggesting that LPS specifically induces ASPP2 expression (Fig. S1D). To determine whether LPS treatment affects ASPP2 protein stability, cycloheximide was used to block protein synthesis. ASPP2 expression levels were measured in RAW264.7 cells over a cycloheximide incubation time course alone or combined with LPS. The presence of LPS had a minimal impact on the kinetics of ASPP2 expression (Fig. S1E). LPS treatment is known to induce p65 nuclear localization (24). In RAW264.7 cells, ASPP2 expression patterns were similar to those of p65 (Fig. 1B). Nuclear and cytoplasmic fractionation indicated that ASPP2 was mainly induced in the nuclear fraction in RAW264.7 cells after 2 h (Fig. 1C). In primary human astrocytes, LPS-induced ASPP2 was mainly cytoplasmic, with a pattern overlapping that of the intermediate filament GFAP (Fig. S1C). The underlying molecular mechanism for this difference in expression pattern in astrocytes is unknown.
Genome-wide analysis of LPS-regulated genes has been studied extensively. Because LPS induces ASPP2 mRNA, bioinformatics analysis of ASPP2 mRNA expression in response to inflammatory stimuli was performed using publicly available gene array data in NextBio database (25). In agreement with our findings, LPS-induced ASPP2 mRNA expression was an early response in mouse macrophages (Fig. S1F) and human myeloid lineage cells (Fig. S1G). Additionally, increased ASPP2 mRNA was detected in rheumatoid arthritis or tuberculosis-infected latent, meningeal, or pulmonary human macrophages compared with control macrophages (Fig. S1H). Increased ASPP2 mRNA expression was also found in brain tissue from patients with neurodegenerative disorders, including multiple sclerosis, Parkinson disease, and Huntington disease, compared with control tissue (Fig. S1I). These data support the conclusion that inflammatory stimuli or disease states induce ASPP2 expression in both mouse and human.
ASPP2 Is a Bona Fide Transcriptional Target of STAT1.
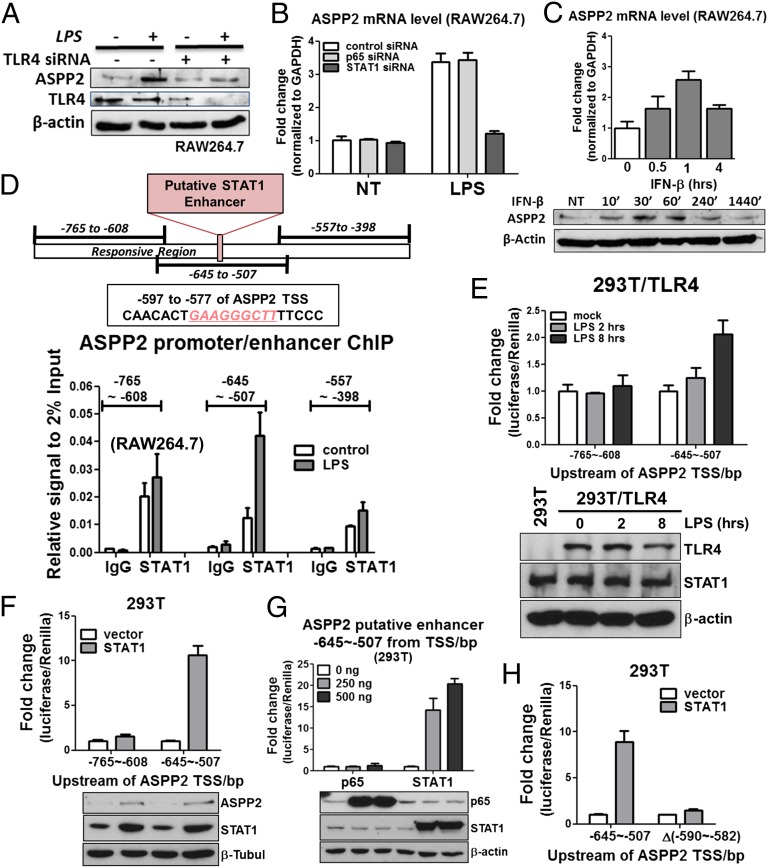
LPS binds TLR4 and its coreceptor MD-2 to initiate the TLR4 signaling cascade (26, 27). We, thus, introduced TLR4 or MD-2 siRNA to RAW264.7 cells in the presence or absence of LPS. As expected, TLR4 siRNA almost prevented LPS from inducing ASPP2 in RAW264.7 cells (Fig. 2A). MD-2 siRNA also dampened LPS-induced ASPP2 in RAW264.7 cells (Fig. S2A). These data suggest that intact TLR4 signaling is required for LPS to induce ASPP2.
Fig. 2.
ASPP2 is a target of STAT1. (A) TLR4 siRNA reduces ASPP2 induction after LPS treatment in RAW264.7 cells. (B) STAT1 siRNA but not p65 siRNA reduces ASPP2 induction after LPS treatment in RAW264.7 cells. (C) IFN-β time course showing increased ASPP2 expression at protein and mRNA levels in RAW264.7. (D) Illustration of the LPS-responsive region of the ASPP2 enhancer region. Putative STAT1 binding site is within the −645 to −507 region. Results of STAT1 ChIP in RAW264.7. The regions corresponding to each of the primer sets are shown. Results are the average of duplicate treatments, and error bars show the range of the duplicates. (E) ASPP2 (−645 to −507) -Luc shows increased activity after LPS treatment for 8 h, whereas ASPP2 (−765 to −608) -Luc shows no response. (F) Only ASPP2 (−645 to −507) -Luc is activated after STAT1 exogenous expression. ASPP2 (−765 to −608) -Luc remains unresponsive. (G) STAT1 but not p65 is able to induce ASPP2 (−645 to −507) -Luc activity. (H) After deletion of the STAT1 binding sequence located at −590 to −582, ASPP2 (−645 to −507) -Luc activity after STAT1 exogenous expression is abolished. NT, no treatment.
The downstream effectors of LPS/TLR4 are canonical MYD88-p65–dependent and noncanonical MYD88-p65–independent pathways. As a result, LPS/TLR4 activates a number of downstream transcription factors, including p65, STAT1, IRF-3, and AP-1. Analysis of the ASPP2 promoter region in conjunction with ENCODE transcription factor binding data (28) suggested that, in addition to the previously identified E2F site (29), both human and mouse ASPP2 promoters contain potential p65 and STAT1 binding sites but do not contain IRF-3 and AP-1 sites (Fig. S2B). In RAW264.7 and THP-1 cells, p65 and STAT1 siRNA reduced STAT1 and p65 expression with similar efficiency. Interestingly, only STAT1 siRNA but not p65 siRNA diminished ASPP2 induction after 3 h of LPS treatment in RAW264.7 cells (Fig. 2B and Fig. S2C) or after 10 h of LPS treatment in THP-1 cells (Fig. S2D). In THP-1 cells, ASPP2 induction after 2 h of LPS treatment was unaffected by p65 or STAT1 depletion, indicating the involvement of other unknown factors at this time point in this cell line. Because IFN is upstream of the JAK/STAT1 pathway, ASPP2 induction was examined with IFN treatment in RAW264.7 and THP-1 cells. IFN-β was able to induce ASPP2 expression in RAW264.7 cells (Fig. 2C), whereas only IFN-γ, not IFN-β, induced ASPP2 in THP-1 cells (Fig. S2 E and F).
To determine the STAT1 binding site in the ASPP2 promoter/enhancer, we first performed a ChIP assay. Primers used for the ChIP assay are listed in Table S4. An anti-STAT1 antibody was used to precipitate formaldehyde cross-linked STAT1–DNA complexes in RAW264.7 and THP-1 cells treated with or without LPS. The presence of ASPP2 promoter/enhancer DNA sequences was verified by PCR using primers surrounding distinct but overlapping regions of the mouse and human ASPP2 promoter/enhancer. In RAW264.7 cells, three LPS-responsive sites were identified by the ChIP assay: −1,058 to −799, −844 to −577, and −597 to −331 (Fig. S2G). To further locate the responsive sequence, additional primers were designed within the responsive region, and sequence −645 to −507 was identified as the maximally LPS-responsive ASPP2 promoter/enhancer region containing the putative STAT1 binding site (Fig. 2D). Three mouse ASPP2 promoter/enhancer fragments were cloned into a pGL4.23 (luc2/minP) luciferase reporter plasmid. The ASPP2 (−645 to −507) -Luc construct contains the putative STAT1 binding site, whereas ASPP2 (−765 to −608) -Luc does not. ASPP2 Δ(−590 to −582) -Luc contains a deletion of the STAT1 binding sequence that is present in ASPP2 (−645 to −507) -Luc. The responsiveness of these three promoter/enhancer fragments was tested in 293T cells, which enable high transfection efficiency and also lack TLR4 (26, 27). LPS treatment together with transfected TLR4 resulted in a time-dependent increase in ASPP2 (−645 to −507) -Luc activity. Plasmids used are listed in Table S5. Under the same conditions, ASPP2 (−765 to −608) -Luc activity was unchanged (Fig. 2E).
Consistent with the notion that STAT1 is a downstream effector of TLR4, STAT1 overexpression was sufficient to induce endogenous ASPP2 expression in TLR4-deficient 293T cells. Although ASPP2 luciferase activity was induced 9- to 10-fold in the ASPP2 (−645 to −507) -Luc, the ASPP2 (−765 to −608) -Luc showed no change in activity (Fig. 2F). To further confirm that ASPP2 promoter/enhancer activity is responsive to STAT1 and not p65, ASPP2 (−645 to −507) -Luc was transfected with a gradient of p65 or STAT1-expressing plasmids. STAT1, but not p65, induced ASPP2 (−645 to −507) -Luc reporter activity (Fig. 2G). Importantly, exogenous STAT1 expression induced ASPP2 (−645 to −507) -Luc activity but failed to stimulate the transcriptional activity of ASPP2 Δ(−590 to ∼−582) -Luc, in which the binding site was excised (Fig. 2H). A similar approach was used to identify the STAT1 binding site in the human ASPP2 promoter. ChIP assays were performed in human THP-1 cells, which showed two LPS-responsive sites (Fig. S2H). These sites were confirmed using luciferase assays. STAT1 overexpression resulted in increased ASPP2 promoter/enhancer activity in the responsive human fragments (Fig. S2I). These data showed that ASPP2 is a bona fide transcriptional target of STAT1.
LPS Induces Nuclear ASPP2 Expression in a Mouse Model of Maternal Inflammation and Mediates Apoptosis.
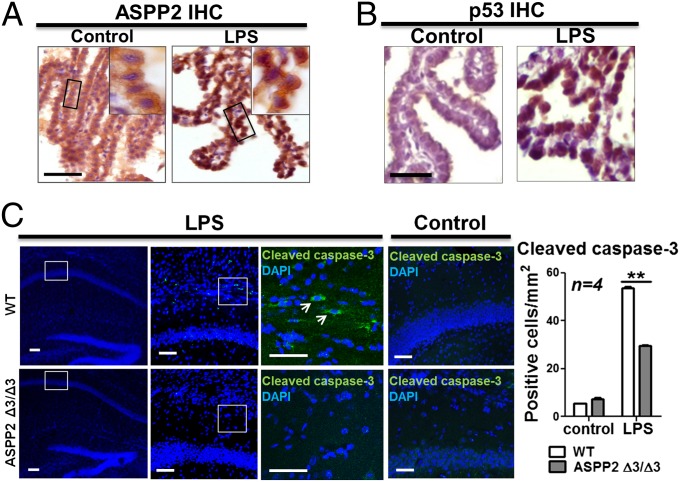
To see whether LPS-induced ASPP2 induction could be observed in vivo and understand the role of LPS-induced ASPP2 transcription in the context of cerebral inflammatory disease, ASPP2 expression was examined in an LPS-induced model of maternal inflammation. In this model, LPS was injected i.p. into pregnant mice carrying embryos at embryonic day (E) 13.5 (30). Pup brains were then examined at postnatal day (P) 8. Under basal conditions, ASPP2 is expressed in the TJs of CP epithelial cells, where it binds Par-3 to maintain cell polarity (21). ASPP2 was expressed at the junctions of CP epithelial cells in animals receiving saline injections as a control. In the LPS injection group, however, ASPP2 expression was up-regulated and accumulated in the nucleus rather than at the TJs (Fig. 3A and Fig. S3A). Nuclear p53 was also observed in the nucleus of CP epithelial cells on LPS treatment (Fig. 3B and Fig. S3B).
Fig. 3.
LPS induces nuclear ASPP2 expression in a model of maternal inflammation, and ASPP2 mediates apoptosis. (A) On LPS injection, ASPP2 is disrupted from the TJs and relocalized to the nucleus of CP epithelial cells. (Scale bar: 25 µm.) (B) After LPS injection, p53 appears in the nucleus of CP epithelial cells. (Scale bar: 25 µm.) (C) IF staining of cleaved caspse-3 in LPS-injected and control saline-injected WT and ASPP2 Δ3/Δ3 mice. Arrows indicate cleaved caspase-3–positive cells. (Scale bars: 25 µm.) Quantification of the number of cleaved caspase-3–positive cells in the hippocampus after LPS or saline injection (n = 4). **P < 0.01.
Previous studies have shown that STAT1 cooperates with p53 to induce apoptosis by selectively enhancing the transcriptional activity of p53 on p53 target gene promoters bearing an ASPP2 signature, such as Bax (31) and Noxa (32). Because LPS-induced STAT1 activity induces ASPP2 expression, we tested whether LPS-induced nuclear ASPP2 and p53 may play a proapoptotic role in response to inflammatory stimuli. To test if ASPP2 plays a proapoptotic role, ASPP2 siRNA was used in RAW264.7 cells, which express WT p53. On treatment with LPS, the expression level of cleaved polyADP ribose polymerase (PARP) diminished in the ASPP2-depleted cells but not the control cells (Fig. S3C). To further examine the role of ASPP2 in apoptosis, ASPP2-deficient ASPP2 Δ3/Δ3 and WT mice were injected with LPS or saline i.p. Previous studies have shown that systemic administration of LPS can induce apoptosis in the brain, particularly in the hippocampus (33). Levels of cleaved caspase-3 were compared in hippocampal brain sections from ASPP2 Δ3/Δ3 and WT mice compared with saline-injected controls. Cleaved caspase-3–positive cells were quantified and found to be significantly less prevalent in ASPP2 Δ3/Δ3 vs. WT mice injected with LPS, supporting a proapoptotic role of ASPP2. Few cleaved caspase-3–positive cells were found in mice receiving saline injections (Fig. 3C). These data support the role of ASPP2 in mediating LPS-induced apoptosis in vitro and in vivo.
ASPP2 Deficiency Enhances Neuroinflammation in Vivo.
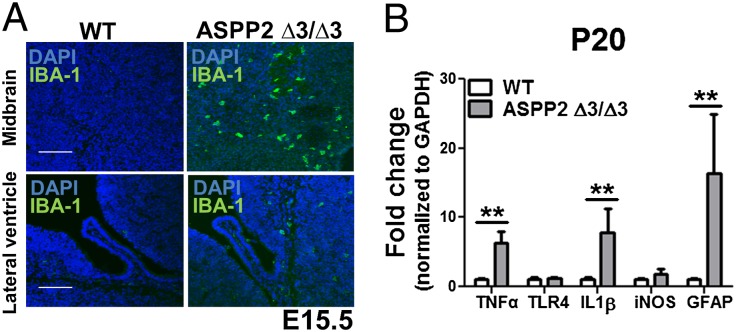
Because ASPP2 was previously shown to maintain the TJs between CP epithelial cells, the brain’s main barrier to inflammation, we examined whether ASPP2 Δ3/Δ3 mice display neuroinflammation. Immunohistochemistry (IHC) staining showed that ASPP2 Δ3/Δ3 mice possess IBA1-positive microglia and GFAP-positive astrocytes throughout the parenchyma at E15.5 (Fig. 4A) and P20 (Fig. S4A). Using qRT-PCR with cortical tissue from ASPP2 Δ3/Δ3 and WT mice, we observed that ASPP2 Δ3/Δ3 mice displayed a significant increase in several proinflammatory cytokines, including TNF-α at E15.5 (Fig. S4B). The extent of neuroinflammation increased by P20 as the production of TNF-α and IL-1β in cortical brain tissues increased dramatically compared with age-matched WT mice (Fig. 4B). Thus, the localization of ASPP2 at the TJs of the BCSFB under basal conditions and the neuroinflammatory phenotype of ASPP2 Δ3/Δ3 mice suggest that ASPP2 may act as a barrier to inflammation.
Fig. 4.
ASPP2-deficient mice possess neuroinflammation. (A) Increased IBA1-positive microglia in ASPP2 Δ3/Δ3 mice at E15.5. (Scale bars: 100 µm.) (B) Increased proinflammatory cytokines in cortical brain tissue of ASPP2 Δ3/Δ3 mice at P20. **P < 0.01.
ASPP2 Is Highly Expressed in Reactive Astrocytes in Mouse Neuroinflammation Models and Human Neuroinflammatory Disease.
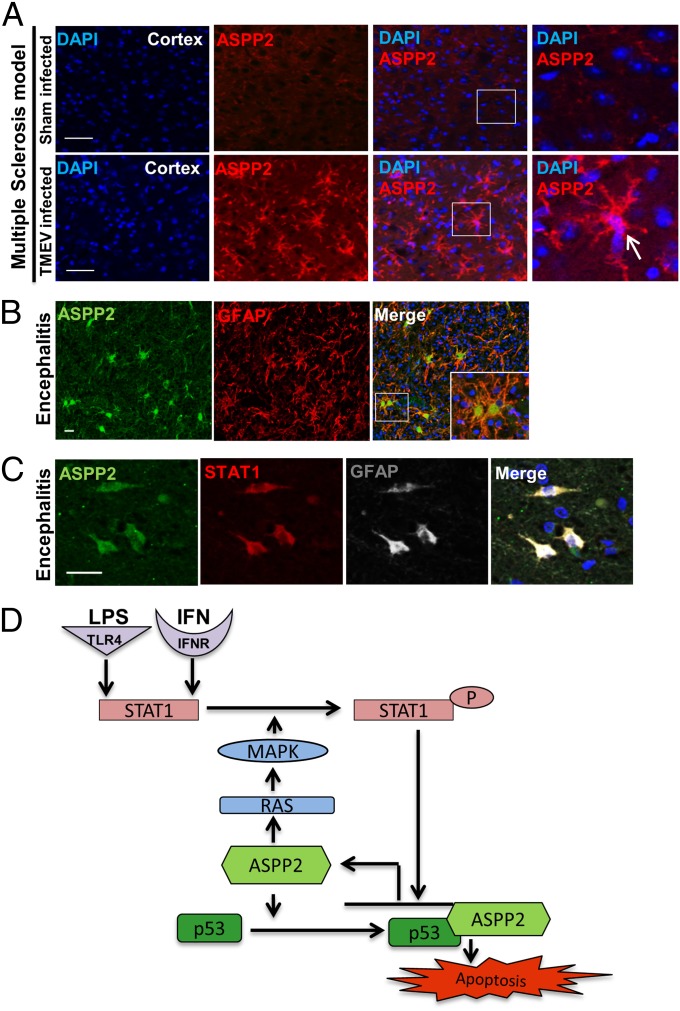
To test whether ASPP2 is involved in neuroinflammatory and neurodegenerative disorders involving STAT1 signaling, we used mouse models in which IFN activation is implicated in the disease pathology, including multiple sclerosis and stroke (34, 35). Tissue sections were obtained from an animal model of experimental stroke, middle cerebral artery occlusion, and the Theiler's murine encephalomyelitis virus infection model of multiple sclerosis. ASPP2 IHC revealed marked up-regulation in cells with astrocytic morphology found in the dentate gyrus of the hippocampus ipsilateral to the cerebral infarct in the middle cerebral artery occlusion model (Fig. S5A) and the cerebral cortex of the Theiler's murine encephalomyelitis virus model (Fig. 5A). ASPP2 was not as highly expressed on the hemisphere contralateral to the cerebral infarct, indicating that ASPP2 up-regulation was specific to areas of the brain in which major damage was present.
Fig. 5.
ASPP2 is up-regulated in mouse models and human neuroinflammatory disorders. ASPP2 induction in (A) an animal model of multiple sclerosis and (B) human encephalitis. (C) ASPP2 is up-regulated in GFAP-positive reactive astrocytes. STAT1 (red) and ASPP2 (green) coexpression in GFAP-positive reactive astrocytes (white). (Scale bars: 25 µm.) (D) Proposed mechanism of ASPP2/STAT1-induced apoptosis in response to inflammatory stimuli.
We next examined human tissue samples obtained from a variety of disorders associated with neuroinflammation and neurodegeneration. IHC staining showed low to no expression of ASPP2 in noninflamed tissue. However, high expression of ASPP2 was observed in cerebral infarct (Fig. S5B) and subacute/chronic encephalitis (Fig. S5C). Morphological analysis suggested that reactive astrocytes were the dominant ASPP2-expressing cell type. To confirm that ASPP2 is expressed in astrocytes, we performed double immunofluorescence (IF) staining with anti-ASPP2 and anti-GFAP antibodies on biopsy tissue, which enabled ASPP2 antigen preservation. ASPP2 was found to be highly expressed in the cytoplasm of reactive GFAP-positive astrocytes, particularly those with gemistocytic morphology (Fig. 5B and Fig. S5D). The detected increase in ASPP2 expression is specific, because iASPP was not highly expressed in either encephalitis or control tissue (Fig. S5E). The number of ASPP2-positive cells was quantified per cell subtype and revealed that ∼83% of GFAP-positive cells had high ASPP2 expression (Fig. S5F). Because encephalitis is known to arise from viral infection and because IFN secretion in encephalitis is linked to neuronal dysfunction (36), we carried out triple IF staining to examine whether STAT1-mediated ASPP2 induction was present in astrocytes in human tissue samples. In agreement with this hypothesis, ASPP2 was found to be up-regulated in STAT1- and GFAP-expressing reactive astrocytes (Fig. 5C). The role of ASPP2 induction in astrocytes was tested by treating primary human astrocytes with IFN-β for 24 h, after which time they began to undergo apoptosis. Increased ASPP2 expression was found in cells that also express Annexin V and cleaved caspase-3 (Fig. S5 G and H). These findings are consistent with the conclusions that ASPP2 is a transcriptional target of STAT1 and that it plays a proapoptotic role in response to inflammatory stimuli such as LPS and IFN.
Discussion
We identify ASPP2, a known tumor suppressor, activator of p53, and regulator of cell polarity, as a bona fide transcriptional target of STAT1 and regulator of neuroinflammation. Its dynamic cellular localization and diverse functions place ASPP2 in an ideal position to act as an SMRT factor that can sense inflammatory stimuli at the apical cell membrane, act as a messenger to transcriptional machinery in the nucleus, and determine cell fate as a regulator of transcription. When ASPP2 binds Par-3 through its N terminus, it maintains the integrity of the apical polarity complex and the TJs of the BCSFB. In this way, ASPP2 may act as a defender against infection and inflammation. The identification of ASPP2 as a novel transcriptional target of STAT1 and the finding that LPS and IFNs can induce ASPP2 expression suggest that it may also act as a sensor of infection. ASPP2 may also promote phosphorylation of STAT1 through the potentiating RAS-MAPK pathway (37), which forms an autoregulation loop (Fig. 5D). The kinetics of ASPP2 induction by LPS suggest that ASPP2 is likely to be involved in the early phase of infection through its ability to induce apoptosis, a type of cell death that halts additional inflammation, in contrast to necroptosis, where a failure to eliminate damage may further propagate inflammation (38). Consistent with this hypothesis, ASPP2-deficient mice possess enhanced neuroinflammation and a reduced apoptotic response after LPS injection.
The function of ASPP2 as a gatekeeper could be caused by its ability to regulate cell polarity and RAS signaling through its N terminus (37). In polarized epithelial cells, including CP epithelial cells, ASPP2 is located at TJs (21). On RAS activation, ASPP2 is translocated to the cytoplasm and enhances the apoptotic function of p53 (37). The ability of ASPP2 to stimulate the apoptotic function of p53 and p73 is mediated by its C terminus, which is localized in the nucleus (19). The up-regulation of ASPP2 in the LPS-induced maternal inflammation model shows that inflammatory stimuli could induce ASPP2 in vivo. When the TJs of the BCSFB are disrupted, ASPP2 is displaced from the cell junctions and relocalizes to the nucleus. Like many other ankyrin repeat-containing proteins without an identifiable nuclear localization signal, ASPP2 may enter the nucleus through a newly identified RanGDP/Ankyrin Repeats binding nuclear import pathway (39).
Bacterial and viral infections induce inflammatory cellular responses through TLRs. LPS and IFN are often used as inflammatory stimuli to mimic infections induced by Gram-negative bacteria and viral RNA, respectively. LPS and IFN were previously found to have cell- and context-dependent pro- or antiapoptotic functions. LPS is able to induce MYD88-mediated antiapoptotic pathways through p65 (40), and LPS/TLR4 can also induce STAT activation through an MYD88-independent and IFN-dependent pathway. Interestingly, STAT1 has been shown to potentiate p53- and p73-induced apoptosis by selectively enhancing its transcriptional activity on proapoptotic genes, such as Bax (31) and Noxa (32). However, it remains unclear how STAT1 selectively enhances the apoptotic function of p53 family members. The identification of ASPP2 as a transcriptional target of STAT1 explains how STAT1 signaling could be guided to a proapoptotic path. Because ASPP2 is a common activator of the p53 family, the status of ASPP2 may play a key role in dictating the response of the cell to inflammatory stimuli. Consistent with the role of ASPP2 in apoptosis, reduced ASPP2 expression dampened LPS-induced apoptosis in RAW264.7 cells, and ASPP2-deficient mice displayed less LPS-induced apoptosis in the hippocampus.
Interestingly, we also observed a biphasic induction of ASPP2 in human THP-1 cells by LPS. The underlying mechanism for the biphasic induction is currently unknown. Also, in THP-1 cells, only IFN-γ induced ASPP2 expression and not IFN-β. The failure of IFN-β to induce ASPP2 is not because of a lack of activity in THP-1 cells, because both IFN-γ and IFN-β induce phopspho-STAT1 expression in THP-1 cells with similar kinetics. The induction of ASPP2 by IFN-γ alone may indicate that, in THP-1 cells, IFN-γ specifically induces the formation of STAT1–STAT1 homodimers that translocate to the nucleus and bind interferon-gamma activated site (GAS) elements (41) that are present in the promoter/enhancer of ASPP2, thereby initiating the transcription of ASPP2. Also, the identified STAT1 binding sites in the mouse (-590GAAGGGCTT-582) and human (-1090GAAAGAATT-1081) ASPP2 promoter/enhancer are GAS elements (Fig. S2B). In RAW264.7 cells and human astrocytes, IFN-β also induces the formation of STAT1–STAT1 homodimers (41) and binds GAS elements in the ASPP2 promoter/enhancer. However, in THP-1 cells, IFN-β may fail to do so. Furthermore, our data indicate that STAT1 induces transcription of ASPP2 in its activated tyrosine phosphorylated form on activation by IFNs or overexpression.
The identification of ASPP2 as a transcriptional target of STAT1 in response to LPS and IFN signaling reveals an important function of ASPP2 in the response of the cell to infection and inflammation. Increased ASPP2 expression in mouse neuroinflammation models and human neuroinflammatory disorders as well as the finding that ASPP2 Δ3/Δ3 mice have reduced apoptosis in response to systemic LPS injection also support the potential importance of ASPP2 in sensing, integrating, and dictating the cellular response to inflammatory stimuli. Because ASPP2 is a haploinsufficient tumor suppressor, cell polarity regulator, and activator of p53, the identified STAT1/ASPP2 pathway provides an important link between infection, inflammation, cell polarity, and tumor suppression.
Materials and Methods
ASPP2 Δexon3 C57BL/6Jx129SvJ mice were backcrossed in a BALB/c background for nine generations. All animal procedures were approved by the University of Oxford's ethical review committee and licensed by the UK Home Office (license number PPL 30/2862). SI Materials and Methods provides complete experimental methods. It includes reagents and details of IHC, IF, immunoblotting, RNA extraction, cDNA preparation, qRT-PCR, cell culture, ChIP assay, luciferase assay, and statistical analysis.
Supplementary Material
Acknowledgments
We thank Thao Do for her generous help with the primary human astrocyte culture. We also thank Dr. Doug Golenbock for the TLR4 construct and Dr. Nancy Rice for the p65 construct. This work was primarily supported by the Ludwig Institute for Cancer Research Ltd. C.T. is funded by the National Institutes of Health–Oxford Scholars Program. Y.W. is supported by Medical Research Council Grant MR/J000930/1. S.N.C. is supported by Fondation Contre le Cancer, Salus Sanguinis Programs IAP P7/43 BeMGI and ARC 10/15-027. H.B.S. and Z.M. were supported by St. John’s College, the European Union Neurobid Consortium, and the Medical Research Council. F.G.S. is supported by National Institutes of Health Grant R01 NS-42253. O.A. and X.L. are partly funded by the Cancer Research–United Kingdom Oxford Cancer Centre Development Fund. The Thomas Willis Oxford Brain Bank is supported by the Oxford National Institute for Health Research Biomedical Research Centre and the Medical Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407898111/-/DCSupplemental.
References
- 1.Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC. Alzheimer disease and cancer. Neurology. 2005;64(5):895–898. doi: 10.1212/01.WNL.0000152889.94785.51. [DOI] [PubMed] [Google Scholar]
- 2.Doody RS, et al. A phase 3 trial of Semagacestat for treatment of Alzheimer's disease. N Engl J Med. 2013;369(4):341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 3.Chang JR, et al. Role of p53 in neurodegenerative diseases. Neurodegener Dis. 2012;9(2):68–80. doi: 10.1159/000329999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beal MF. Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol. 1996;6(5):661–666. doi: 10.1016/s0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- 5.Davenport CM, Sevastou IG, Hooper C, Pocock JM. Inhibiting p53 pathways in microglia attenuates microglial-evoked neurotoxicity following exposure to Alzheimer peptides. J Neurochem. 2010;112(2):552–563. doi: 10.1111/j.1471-4159.2009.06485.x. [DOI] [PubMed] [Google Scholar]
- 6.Li J, et al. Inhibition of p53 transcriptional activity: A potential target for future development of therapeutic strategies for primary demyelination. J Neurosci. 2008;28(24):6118–6127. doi: 10.1523/JNEUROSCI.0184-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royer C, Lu X. Epithelial cell polarity: A major gatekeeper against cancer? Cell Death Differ. 2011;18(9):1470–1477. doi: 10.1038/cdd.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaykhiev R, Bals R. Interactions between epithelial cells and leukocytes in immunity and tissue homeostasis. J Leukoc Biol. 2007;82(1):1–15. doi: 10.1189/jlb.0207096. [DOI] [PubMed] [Google Scholar]
- 9.Coisne C, Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid Redox Signal. 2011;15(5):1285–1303. doi: 10.1089/ars.2011.3929. [DOI] [PubMed] [Google Scholar]
- 10.Bednarczyk J, Lukasiuk K. Tight junctions in neurological diseases. Acta Neurobiol Exp (Warsz) 2011;71(4):393–408. doi: 10.55782/ane-2011-1861. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Okun E, et al. Toll-like receptors in neurodegeneration. Brain Res Brain Res Rev. 2009;59(2):278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin L, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Star BJ, et al. In vitro and in vivo models of multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11(5):570–588. doi: 10.2174/187152712801661284. [DOI] [PubMed] [Google Scholar]
- 15.Hemmer B, Cepok S, Nessler S, Sommer N. Pathogenesis of multiple sclerosis: An update on immunology. Curr Opin Neurol. 2002;15(3):227–231. doi: 10.1097/00019052-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Akwa Y, et al. Transgenic expression of IFN-alpha in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol. 1998;161(9):5016–5026. [PubMed] [Google Scholar]
- 17.Messmer UK, Brüne B. Nitric oxide-induced apoptosis: p53-dependent and p53-independent signalling pathways. Biochem J. 1996;319(Pt 1):299–305. doi: 10.1042/bj3190299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menendez D, et al. The Toll-like receptor gene family is integrated into human DNA damage and p53 networks. PLoS Genet. 2011;7(3):e1001360. doi: 10.1371/journal.pgen.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trigiante G, Lu X. ASPP [corrected] and cancer. Nat Rev Cancer. 2006;6(3):217–226. doi: 10.1038/nrc1818. [DOI] [PubMed] [Google Scholar]
- 20.Vives V, et al. ASPP2 is a haploinsufficient tumor suppressor that cooperates with p53 to suppress tumor growth. Genes Dev. 2006;20(10):1262–1267. doi: 10.1101/gad.374006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sottocornola R, et al. ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev Cell. 2010;19(1):126–137. doi: 10.1016/j.devcel.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Buti L, et al. Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc Natl Acad Sci USA. 2011;108(22):9238–9243. doi: 10.1073/pnas.1106200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehnardt S, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100(14):8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89(3):413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 25.Kupershmidt I, et al. Ontology-based meta-analysis of global collections of high-throughput public data. PLoS ONE. 2010;5(9):pii:e13066. doi: 10.1371/journal.pone.0013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274(16):10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Young DW, Gusovsky F, Chow JC. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J Biol Chem. 2000;275(27):20861–20866. doi: 10.1074/jbc.M002896200. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein BE, et al. ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fogal V, et al. ASPP1 and ASPP2 are new transcriptional targets of E2F. Cell Death Differ. 2005;12(4):369–376. doi: 10.1038/sj.cdd.4401562. [DOI] [PubMed] [Google Scholar]
- 30.Stolp HB, et al. Reduced ventricular proliferation in the foetal cortex following maternal inflammation in the mouse. Brain. 2011;134(Pt 11):3236–3248. doi: 10.1093/brain/awr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soond SM, et al. STAT1 regulates p73-mediated Bax gene expression. FEBS Lett. 2007;581(6):1217–1226. doi: 10.1016/j.febslet.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 32.Townsend PA, et al. STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. J Biol Chem. 2004;279(7):5811–5820. doi: 10.1074/jbc.M302637200. [DOI] [PubMed] [Google Scholar]
- 33.Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30(2-3):144–157. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Rubio N, Palomo M, Alcami A. Interferon-alpha/beta genes are up-regulated in murine brain astrocytes after infection with Theiler’s murine encephalomyelitis virus. J Interferon Cytokine Res. 2010;30(4):253–262. doi: 10.1089/jir.2009.0050. [DOI] [PubMed] [Google Scholar]
- 35.Takagi Y, Harada J, Chiarugi A, Moskowitz MA. STAT1 is activated in neurons after ischemia and contributes to ischemic brain injury. J Cereb Blood Flow Metab. 2002;22(11):1311–1318. doi: 10.1097/01.WCB.0000034148.72481.F4. [DOI] [PubMed] [Google Scholar]
- 36.Sas AR, Bimonte-Nelson H, Smothers CT, Woodward J, Tyor WR. Interferon-alpha causes neuronal dysfunction in encephalitis. J Neurosci. 2009;29(12):3948–3955. doi: 10.1523/JNEUROSCI.5595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, et al. ASPP1 and ASPP2 bind active RAS, potentiate RAS signalling and enhance p53 activity in cancer cells. Cell Death Differ. 2013;20(4):525–534. doi: 10.1038/cdd.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 39.Lu M, et al. A code for RanGDP binding in ankyrin repeats defines a nuclear import pathway. Cell. 2014;157(5):1130–1145. doi: 10.1016/j.cell.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Lombardo E, Alvarez-Barrientos A, Maroto B, Boscá L, Knaus UG. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-alpha autocrine signalling. J Immunol. 2007;178(6):3731–3739. doi: 10.4049/jimmunol.178.6.3731. [DOI] [PubMed] [Google Scholar]
- 41.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.