The molecular mechanisms by which organisms defend themselves against predators and the molecular weapons the predators use to attack their victims have coevolved over eons of time. Microbial pathogens share the common feature of being covered in a surface coat comprised of carbohydrates (glycans) linked to lipids and proteins or as free polysaccharides. In vertebrates, such defense mechanisms against microbes and parasites include anti-glycan antibodies (1–3), major histocompatibility complex MHCII (4), and MHCI-like molecules to glycolipids (5), as well as a vast army of innate immune receptors, including the pattern recognition receptors that include Toll-like receptors, NOD-like receptors, RIG-I-like receptors, AIM2-like receptors, DNA sensors, C-type lectin receptors, and galectins (6–12). Many of these receptor systems are required to eliminate infectious organisms, and they do so by either promoting their direct killing or through uptake and signaling to bring additional cell-mediated effector mechanisms to eliminate the threat. The search for additional mechanisms of anti-glycan responses has been heightened by new methods in glycosciences, whereby the glycans of both the hosts and their enemies are becoming defined, especially through the use of molecular genetics, MS, and NMR to identify the glycan-related genes and glycan structures they encode as secondary gene products.
In PNAS, Wohlschlager et al. (13) present a very interesting and novel advance in this area through their discovery of the role of Tectonins in both fungi and animals in recognizing specific methylated glycans of both bacteria and nematodes (worms). Tectonins are ancient proteins originally discovered in the slime mold Physarum polycephalum and have been found to have homology to limulus lectins (Tachylectin-1) (14). Such proteins fall within the Tectonin domain-containing superfamily, which contains the 33–37 amino acid consensus Tectonin domain sequence that is predicted to form a four- to six-bladed β-propeller structure with each blade formed by a four-stranded antiparallel β-sheet (15). These proteins have been proposed to play key roles in innate immunity in invertebrates and a human Tectonin is proposed to also be important in innate immunity to pathogens (16). However, the exact specificity of Tectonins for glycan ligands has been unclear. Wohlschlager et al. find that Tectonin 2 from the ectomycorrhizal mushroom Laccaria bicolor (Lb-Tec2) agglutinated some Gram-negative bacteria and was cytotoxic for the free-living nematode Caenorhabditis elegans. Cytotoxity to C. elegans involved binding to intestinal cells in a similar fashion to other nematotoxic fungal lectins (17, 18). A variety of experimental approaches indicated that binding occurred to glycan receptors on the bacteria and nematode. The genome of L. bicolor contains several predicted Tectonin genes, but the authors focus on predicted proteins Tectonin 1 (LbTec1) and Tectonin 2 (LbTec2), both of which lack a signal sequence and are predicted cytoplasmic proteins and both of which contain six tandemly arranged Tectonin domains. The transcription of the gene encoding LbTec2 is highly up-regulated in fruiting bodies compared with vegetative mycelium, whereas there is less up-regulation for LbTec1. Because LbTec2 shares several properties with known fungal defense lectins, the authors tested the recombinant protein for activity toward antifungal insect predators and nematode predators. Importantly, the recombinant LbTec2 was cytotoxic toward Drosophila melanogaster, Aedes aegypti, and C. elegans. Genetic mutants of C. elegans lacking specific types of fucose-containing glycans (either α1,3- or α1,6-linked) and those containing altered glycosphingolipids retained sensitivity to LbTec2, suggesting that glycans containing such sequences were not specific targets. However, mutants exhibiting double and triple mutations of genes involved in Asn-linked oligosaccharides (N-glycan) expression in C. elegans had diminished sensitivity for LbTec2, the first suggestion that N-glycans might be important in recognition by LbTec2. A variety of C. elegans mutant sensitivity studies led to the hypothesis that LbTec2 recognizes a fucose-containing determinant on the outer branches rather than the inner core of complex-type N-glycans.
To identify the key genes in C. elegans encoding the enzymes creating glycans recognized by LbTec2, a forward genetic screen using transposon mutagenesis was performed. This led to the identification of the gene Y54G2A.4 (samt-1), which encodes a protein within the major facilitator superfamily (MFS1). The utility of this mutation was exceptional, because the authors used a biochemical affinity chromatography approach of WT C. elegans and the resistant samt-1(op532)pmk-1(km25) strain to identify N-glycans binding to LbTec2. From the WT C. elegans (actually the mutant lacking the α1,3-core-fucosylation that blocks PNGase F), they identified PNGase F-dependent binding of glycoproteins to immobilized LbTec2. Interestingly, screening for binding of LbTec2 to glycans on the glycan microarrays of the Consortium for Functional Glycomics, which contains >600 immobilized glycans largely of mammalian-type structures, showed that LbTec2 did not recognize any glycans on that microarray. Thus, to identify the types of glycan determinants recognized by LbTec2, the authors analyzed the structures of the N- and O-(Ser/Thr-linked)-glycans of sensitive and resistant C. elegans. Because the authors were aware of the potential for O-methylation of C. elegans N-glycans (19), they perdeuteromethylated the glycans before analysis. This allowed them to identify N-glycans containing methylated residues, and a monosaccharide compositional analysis against defined standards showed the presence of both 3-O-methyl-mannose and 2-O-methyl-fucose. In direct binding studies, the authors show that LbTec2 binds to both allyl 3-O-methyl mannoside and allyl 2-O-methyl fucoside, simple monosaccharide derivatives, with an average Kd value of 21 and 4 mM, respectively.
The studies of Wohlschlager et al. highlight the incredible importance of glycan recognition for innate immunity.
The ability of LbTec2 to bind to such methylated sugars was extended to show that it could bind to the polysaccharide of Escherichia coli O8 that has a nonreducing terminal 3-O-methyl group but not to strains lacking the O-antigen or the terminal 3-O-methyl-phosphate-mannose. Finally, the authors show that a related Tectonin (Tectonin L6 from the Japanese horseshoe crab, Tachypleus tridentatus), like LbTec2, also binds to methylated sugars.
The results of this study expand our general knowledge of the incredible diversity of glycan-binding proteins in fungi and animals for recognition of their microbial and helminth enemies. The Tectonin superfamily may comprise a large array of glycan-binding proteins whose binding is selective toward modified glycans, such as methylated species. It is noteworthy that methylated glycans have likely been missed in prior studies because permethylation rather than perdeuteromethylation will hide the modification. Thus, as the authors note, glycan methylation may be much more widespread than commonly appreciated. It is also interesting that Wohlschlager et al. find O-methylated fucose in O-glycans but these do not seem to be important for LbTec2 binding.
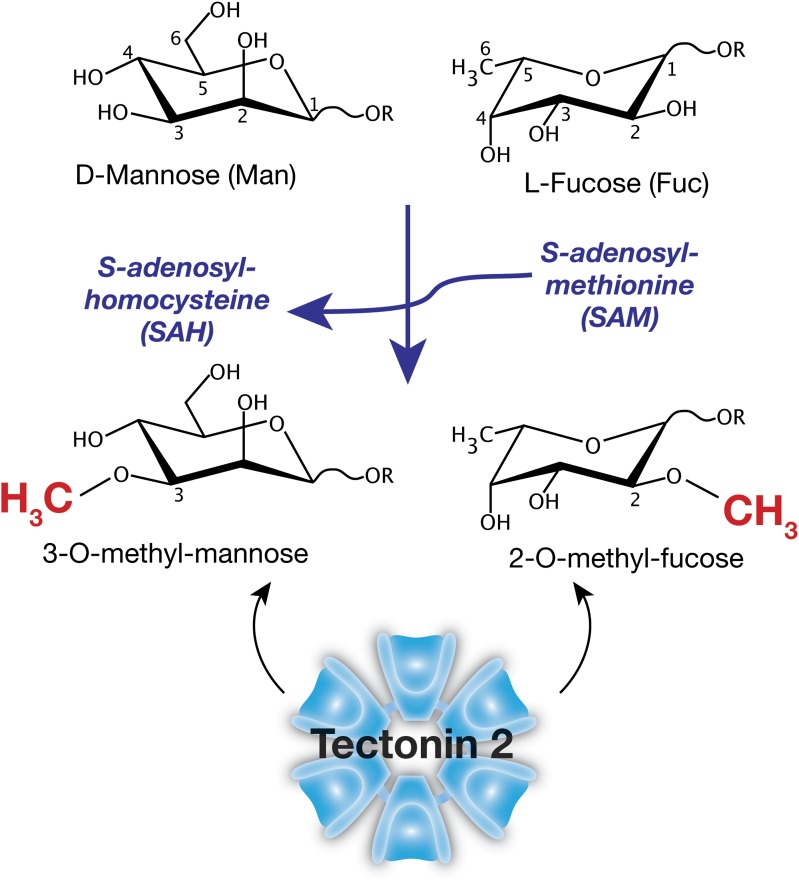
A major question is the synthesis of such methylated glycans, which is presumed to occur in the Golgi apparatus following maturation of glycoproteins and thus is a post-glycosylational event (Fig. 1). However, the details of the entry of S-adenosylmethionine (SAM) into the Golgi and the fate of S-adenosylhomocysteine (SAH) following methyl donation are unknown. The authors make the insightful guess, based on their forward genetic screens, that samt-1 may encode the transporter of SAM required for methylation of glycans, which, if their ongoing studies bear out, would be the first SAM transporter to be described.
Fig. 1.
The predicted methylation reaction to generate 2-O-methyl-fucose and 3-O-methyl-mannose using SAM as the methyl donor. The methylated sugars produced are within intact glycans (R represents other sugars) and are key determinants recognized by Lb-Tec2, a lectin (Tectonin) expressed by the ectomycorrhizal mushroom L. bicolor.
Overall, the studies of Wohlschlager et al. highlight the incredible importance of glycan recognition for innate immunity and also provide further insights into the key roles of glycan modification, e.g., methylation, phosphorylation, sulfation, and acetylation, in creating the modified glycan determinants uniquely recognized by the growing world of glycan-binding proteins.
Supplementary Material
Footnotes
The author declares no conflict of interest.
See companion article on page E2787.
References
- 1.Prasanphanich NS, Mickum ML, Heimburg-Molinaro J, Cummings RD. Glycoconjugates in host-helminth interactions. Front Immunol. 2013;4:240. doi: 10.3389/fimmu.2013.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Diepen A, Van der Velden NS, Smit CH, Meevissen MH, Hokke CH. Parasite glycans and antibody-mediated immune responses in Schistosoma infection. Parasitology. 2012;139(9):1219–1230. doi: 10.1017/S0031182012000273. [DOI] [PubMed] [Google Scholar]
- 3.Kaul A, et al. Serum anti-glycan antibody biomarkers for inflammatory bowel disease diagnosis and progression: A systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18(10):1872–1884. doi: 10.1002/ibd.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avci FY, Li X, Tsuji M, Kasper DL. Carbohydrates and T cells: A sweet twosome. Semin Immunol. 2013;25(2):146–151. doi: 10.1016/j.smim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 2014;32:323–366. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- 6.Sukhithasri V, Nisha N, Biswas L, Anil Kumar V, Biswas R. Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol Res. 2013;168(7):396–406. doi: 10.1016/j.micres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 7.van Vliet SJ, García-Vallejo JJ, van Kooyk Y. Dendritic cells and C-type lectin receptors: Coupling innate to adaptive immune responses. Immunol Cell Biol. 2008;86(7):580–587. doi: 10.1038/icb.2008.55. [DOI] [PubMed] [Google Scholar]
- 8.Wevers BA, Geijtenbeek TB, Gringhuis SI. C-type lectin receptors orchestrate antifungal immunity. Future Microbiol. 2013;8(7):839–854. doi: 10.2217/fmb.13.56. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9(7):465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21(4):317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stowell SR, et al. Microbial glycan microarrays define key features of host-microbial interactions. Nat Chem Biol. 2014;10(6):470–476. doi: 10.1038/nchembio.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasta GR, Ahmed H, Tasumi S, Odom EW, Saito K. Biological roles of lectins in innate immunity: Molecular and structural basis for diversity in self/non-self recognition. Adv Exp Med Biol. 2007;598:389–406. doi: 10.1007/978-0-387-71767-8_27. [DOI] [PubMed] [Google Scholar]
- 13.Wohlschlager T, et al. Methylated glycans as conserved targets of animal and fungal innate defense. Proc Natl Acad Sci USA. 2014;111:E2787–E2796. doi: 10.1073/pnas.1401176111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huh CG, et al. Cloning and characterization of Physarum polycephalum tectonins. Homologues of Limulus lectin L-6. J Biol Chem. 1998;273(11):6565–6574. doi: 10.1074/jbc.273.11.6565. [DOI] [PubMed] [Google Scholar]
- 15.Low DH, et al. A novel human tectonin protein with multivalent beta-propeller folds interacts with ficolin and binds bacterial LPS. PLoS ONE. 2009;4(7):e6260. doi: 10.1371/journal.pone.0006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Low DH, et al. Molecular interfaces of the galactose-binding protein Tectonin domains in host-pathogen interaction. J Biol Chem. 2010;285(13):9898–9907. doi: 10.1074/jbc.M109.059774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butschi A, et al. Caenorhabditis elegans N-glycan core beta-galactoside confers sensitivity towards nematotoxic fungal galectin CGL2. PLoS Pathog. 2010;6(1):e1000717. doi: 10.1371/journal.ppat.1000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schubert M, et al. Plasticity of the β-trefoil protein fold in the recognition and control of invertebrate predators and parasites by a fungal defence system. PLoS Pathog. 2012;8(5):e1002706. doi: 10.1371/journal.ppat.1002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altmann F, Fabini G, Ahorn H, Wilson IB. Genetic model organisms in the study of N-glycans. Biochimie. 2001;83(8):703–712. doi: 10.1016/s0300-9084(01)01297-4. [DOI] [PubMed] [Google Scholar]