Significance
Timing of vegetative bud dormancy is an environmentally and economically important trait whose importance will grow due to rapid climate changes. However, the underpinning regulatory mechanisms are still poorly understood. We report the identification and characterization of the Early Bud-Break 1 (EBB1) gene in poplar that regulates the timing of bud-break. EBB1 plays a major and integrative role in the reactivation of the shoot apical meristem after winter dormancy. The knowledge about EBB1 function can enable novel approaches for population management, molecular breeding, and genetic engineering of dormancy-associated traits.
Keywords: adaptation, phenology, regeneration, climate change
Abstract
Trees from temperate latitudes transition between growth and dormancy to survive dehydration and freezing stress during winter months. We used activation tagging to isolate a dominant mutation affecting release from dormancy and identified the corresponding gene EARLY BUD-BREAK 1 (EBB1). We demonstrate through positioning of the tag, expression analysis, and retransformation experiments that EBB1 encodes a putative APETALA2/Ethylene responsive factor transcription factor. Transgenic up-regulation of the gene caused early bud-flush, whereas down-regulation delayed bud-break. Native EBB1 expression was highest in actively growing apices, undetectable during the dormancy period, but rapidly increased before bud-break. The EBB1 transcript was localized in the L1/L2 layers of the shoot meristem and leaf primordia. EBB1-overexpressing transgenic plants displayed enlarged shoot meristems, open and poorly differentiated buds, and a higher rate of cell division in the apex. Transcriptome analyses of the EBB1 transgenics identified 971 differentially expressed genes whose expression correlated with the EBB1 expression changes in the transgenic plants. Promoter analysis among the differentially expressed genes for the presence of a canonical EBB1-binding site identified 65 putative target genes, indicative of a broad regulatory context of EBB1 function. Our results suggest that EBB1 has a major and integrative role in reactivation of meristem activity after winter dormancy.
Temporal modifications in plant growth and reproduction in conjunction with cyclical changes in climate are essential for adaptation to variable environments (1). The annual alterations of growth and dormancy in forest trees from boreal and temperate regions in response to changing temperature and/or moisture regimes are well-known examples of such cyclical changes. The molecular mechanisms governing these cycles remain poorly understood (2).
By definition, dormancy is the absence of visible growth in any plant structure containing a meristem (3). The transition from active growth to dormancy in poplar is initiated in the fall by the short-day photoperiod, causing initial cessation of shoot elongation (4). This event is followed by transformation of the apex into a bud (5) and establishment of poorly known physiological changes collectively known as endodormancy, an inability of the meristem and the youngest leaf primordia to respond to growth-promoting signals. Resumption of bud growth, known as bud-break, occurs after meeting a chilling requirement (exposure for several months to low temperatures, with variable species-specific duration) and is controlled almost exclusively by high temperatures (6). The timing of entry and release from dormancy is synchronized with local climates and is highly heritable (7). Most research has focused on the early induction and establishment stages, mainly by seeking homologies to processes and types of dormancies characterized in annual plant species or through transcription profiling (2). For example, because light plays a major role in triggering growth cessation, photoreception via phytochromes has been viewed as an important control point in triggering the process (8, 9). The integration of the light signal transduction into growth response is mediated via the FLOWERING TIME/CONSTANS (FT/CO) module (10, 11) and via regulatory proteins controlling circadian rhythms (12, 13). In Arabidopsis, the ability of FT and other floral integrators to respond to inductive signals is controlled by a suite of MADS (MCM1, AGAMOUS, DEFICIENS, SRF) box genes like SHORT VEGETATIVE PHASE (SVP) and FLOWERING LOCUS C (FLC) (14). Similar MADS box genes, known as DORMANCY-ASSOCIATED MADS (DAM) genes (15), appear to be involved in regulation of bud dormancy in several woody perennial species (6). Involvement of ethylene and abscisic acid signaling in bud formation has also been suggested (16, 17). Modulation of auxin response was also found to be important for the transition to dormancy in poplar (18).
Even less is known about control of endodormancy and reinitiation of bud growth. Studies in Picea, Vitis, and Populus have used transcription profiling to study gene expression during endodormancy and/or resumption of growth (19–21). Homologies to vernalization have been invoked, but critical differences exist because the vernalization-associated epigenetic mechanism requires sustained division, whereas bud dormancy can be imposed and reset in the same meristem cells (2). Recently, it has been shown that the plasmodesmata connections to the meristem are plugged during dormancy and need to be reopened before growth-promoting signals like FT can reach their target’s tissues in the apex (22). Expression of cell-cycle marker genes indicates that after endodormancy establishment, cambium meristem cells are arrested in the G1/S transition and unable to respond to growth-permissive conditions (23). Studies in Arabidopsis have identified many of the regulators of cell proliferation in the shoot apical meristem (SAM) (24), and expression of poplar homologs of these genes correlates with arrest of cell proliferation during dormancy (25). However, functional characterization of these genes in relation to transitions between dormancy and active growth is absent; thus, their role in regulation dormancy characteristics remains unclear.
Here, we report the discovery and characterization of a gene that modifies the timing of bud-break phenology in a woody perennial plant. The gene encodes an APETALA2/Ethylene responsive factor (AP2/ERF) transcription factor that is involved in reactivation of cell division in the meristem and leaf primordia after winter dormancy.
Results
Isolation of Poplar Early Bud-Break Mutant.
We isolated a poplar activation-tagging mutant that showed accelerated bud-break under field conditions (26). All four ramets (vegetative propagules) of the mutant, two pairs of which had been planted in two randomized locations in the ∼1-ha field trial, flushed earlier than WT-717 (Populus tremula × Populus alba Institut National de la Recherche Agronomique 717-IB4) and the large majority of other events that had been transformed with the same vector (Fig. 1A). To validate the field observations, we performed an experiment where we mimicked the induction and release from dormancy under growth chamber conditions (Materials and Methods). Similar to field observations, the mutant showed precocious bud-break approximately twice as fast as WT-717 plants (Fig. 1 B and C). Because of the effect on bud-break, we named the mutant early bud-break 1 dominant (ebb1D) and the corresponding gene EARLY BUD-BREAK 1 (EBB1). Under field conditions, leaves were similar in size to WT-717 and showed slight epinastic curvature (Fig. S1 C–E). Height growth was not affected (P < 0.05) (Fig. S1A), but diameter was slightly but significantly decreased (P < 0.05) compared with WT-717 plants (Fig. S1B).
Fig. 1.
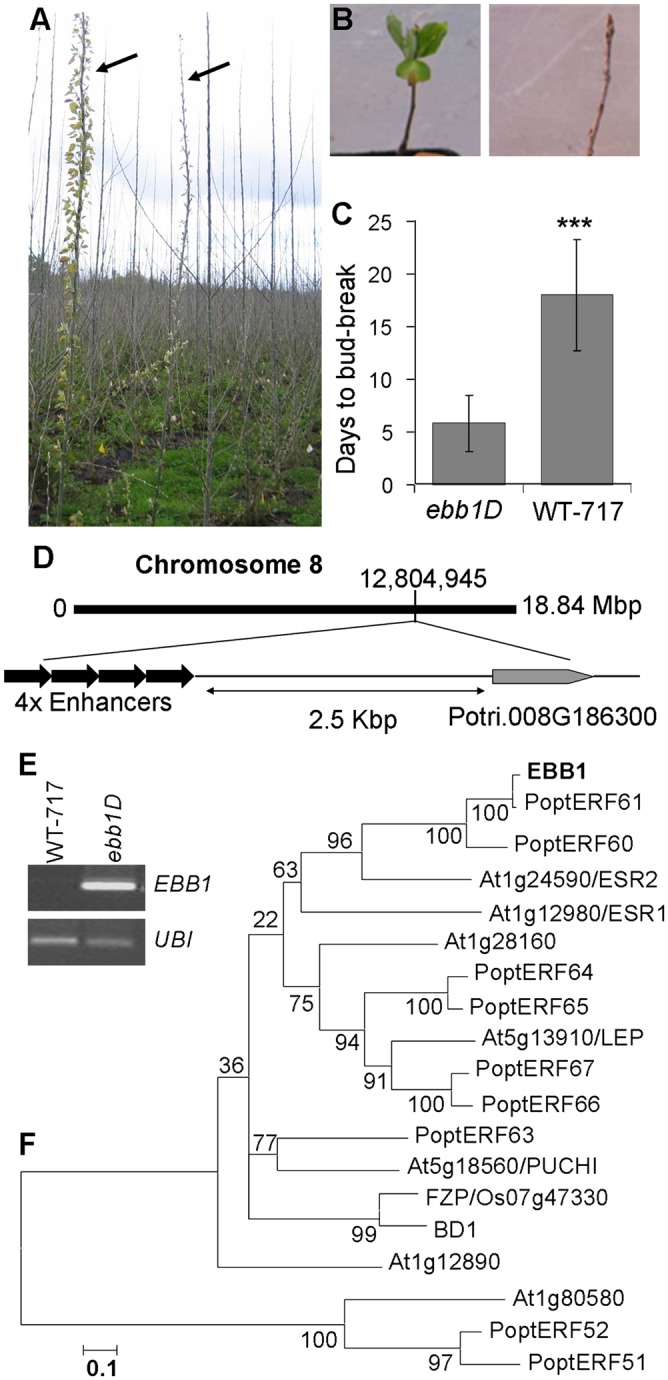
Isolation and molecular characterization of ebb1D poplar mutant. (A) Precocious bud-break of the ebb1D in the field during the start of the second growing season. Mutant plants showed advanced bud-break compared with neighboring transgenic and WT-717 (WT) trees. Arrows point to two ebb1D ramets that show accelerated bud-break compared with the majority of neighboring other activation tagging events and WT-717 plants. (B) Precocious bud-break of an ebb1D mutant plant (Left) compared with a WT-717 plant (Right) after growth chamber photoperiodic induction of dormancy followed by 11 wk of chilling. (C) Average number of days to bud-break in WT-717 and ebb1D plants (detailed description of inductive treatments is provided in Materials and Methods). Bars show 1 SE over a mean of at least 10 ramets per genotype. Significance of differences was tested by the Student t test (***P < 0.001). (D) Genome position of activation tag insertion in ebb1D. 4× Enhancers, enhancers derived from the CaMV35S promoter. (E) Expression of AP2/ERF-tagged gene in WT-717 and ebb1D mutant plants. UBI, ubiquitin gene. (F) Unrooted neighbor-joining tree of proteins from Arabidopsis, poplar, rice, and maize that belong to the same AP2/ERF gene subfamily. Numbers in the branch nodes indicate percentage of bootstrap support of 1,000 iterations. Poptr, Populus trichocarpa; FZP, FRIZZLE PANICLE (rice); BD1, BRANCHED SILKLESS 1 (maize).
Molecular Characterization of ebb1D.
We positioned the tag in the genome sequence on Chr08:12,804,945, and we located a predicted gene model, Potri.008G186300, at ∼2.5 kb upstream of the insertion site (Fig. 1D). No other annotated gene was found within 10 kb in either direction from the enhancers of the inserted transfer DNA (T-DNA) sequence. To verify that the proximal gene was activated, we compared the expression of the candidate gene in WT-717 and mutant plants. The gene corresponding to Potri.008G186300 was highly activated in ebb1D plants and undetectable in WT-717 plants (Fig. 1E).
EBB1 Corresponds to an AP2/ERF-Domain Protein.
We amplified, cloned, and sequenced full-length cDNA of the putative EBB1 gene. Sequence analysis indicated that the gene encodes an AP2/ERF domain putative transcription factor corresponding to a gene in the Populus trichocarpa genome annotated as PoptERF61. AP2/ERF is a superfamily (e.g., at least 147 members in Arabidopsis) of plant-specific transcription factors (27). EBB1 is most similar to a small subfamily of seven members in Arabidopsis, including four that are functionally characterized in Arabidopsis [e.g., ENHANCED SHOOT REGENERATION (ESR1)/DORNROSCHËN (DRN), ESR2/DRN-like (DRNL), LEAFY PETIOLE (LEP), and PUCHI] (28–31). The subfamily is composed of nine members in poplar, none of which has been characterized to date (Fig. 2C). We performed phylogenetic analysis using all nine P. trichocarpa proteins (Table S1); EBB1; all seven Arabidopsis proteins; and two monocot proteins, FIZZLE PANNICLE (FZP; rice) and BRACHLESS SILKLESS (BD1; maize), that have been functionally studied. EBB1, its P. trichocarpa close homolog, PoptERF61, and its putative paralog, PoptERF60, were clustered with very high bootstrap confidence in a common lineage, along with ESR2/DRNL (Fig. 1F).
Fig. 2.
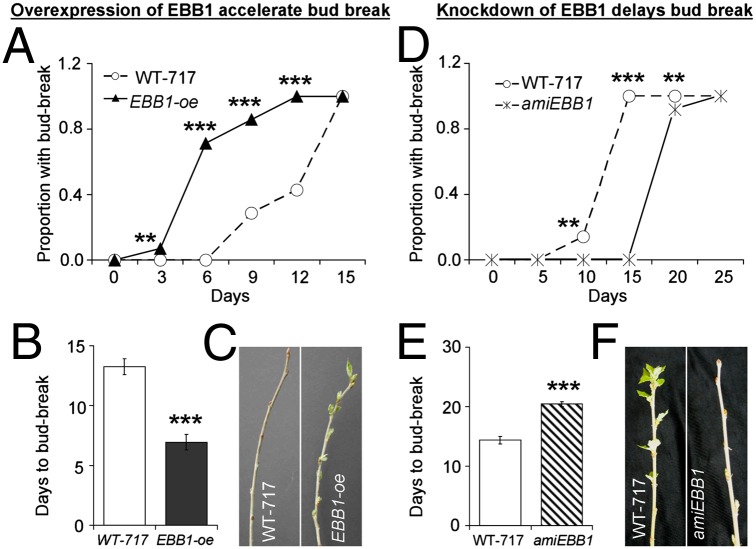
EBB1 is a positive regulator of bud-break. (A–C) Precocious bud-break in EBB1-oe transgenics. (D–F) Delayed bud-break in amiEBB1 transgenic plants. Dynamics of bud-break in EBB1-oe (A) and amiEBB1 (D) transgenics compared with WT-717 plants. Average number of days to bud-break in EBB1-oe (B) and amiEBB1 (E) transgenics compared with WT-717 plants. Bud-break in typical EBB1-oe (C) and amiEBB1 (F) transgenics after 1 wk (C) or 2 wk (F) in long days and at high temperatures following chilling treatment (details are provided in Materials and Methods). Bars in B and E show 1 SE over genotypes’ means (n = 10–15 in A and B, n = 7–12 in D and E). Significance of differences tested by the Fisher’s exact test in A and B or the Student t test in B and E (**P < 0.01; ***P < 0.001).
Recapitulation of ebb1D Phenotype.
To recapitulate the involvement of PoptrERF61/EBB1 in the early bud-break phenotype, we fused its cDNA to the strong CaMV35S promoter and transformed the construct into the same WT-717 poplar genetic background. We recovered 21 independent events, PCR-verified them for the presence of the transgene, and performed RT-PCR on a subset to verify overexpression (hereafter referred to as EBB1-oe transgenics). During in vitro regeneration, we observed an increase in the rate of shoot regeneration (Fig. S2). In addition, the EBB1-oe transgenics produced prolific shoot regeneration from callus tissues produced on the surface of cut stems (Fig. S3). To recapitulate EBB1’s effect on bud-break, we performed a controlled growth chamber experiment with 15 independent events. No effect of EBB1 overexpression was observed on growth cessation and timing of bud-set; however, all EBB1-oe transgenics showed precocious early bud-break (Fig. 2 A–C). On average, EBB1-oe transgenics flushed approximately twice as fast as the WT-717 controls (Fig. 2B). More than one-half (75%) of all EBB1-oe transgenics flushed as early as the first week, and some even as early as 1 d, after transfer to long day (LD) photoperiod and high temperature. Bud-break under field conditions was similarly precocious in all EBB1-oe transgenic events. EBB1-oe transgenics generally showed phenotypic abnormalities more severe than in the original mutant, likely as a result of the stronger and ubiquitous expression expected from the 35S promoter. These included smaller epinastic leaves and an increased proliferation of sylleptic branches (Fig. S4).
Suppression of EBB1 Delays Bud-Break.
Using artificial micro-RNA, we down-regulated expression of EBB1 in four independent lines (called amiEBB1) (Fig. S5). None of the lines with suppressed expression of EBB1 showed unusual phenotypes during in vitro development and the first year of greenhouse growth. We next studied the effect of EBB1 suppression on bud phenology (Materials and Methods). No effect of EBB1 down-regulation was observed on growth cessation, timing of bud-set, or bud formation; however, all EBB1-suppressed plants showed delayed bud-break (Fig. 2 D–F). In contrast to the overexpression events, all amiEBB1 plants flushed significantly later than the WT-717 controls (Fig. 2 E and F).
Anatomical Changes in EBB1 Transgenic Buds and Apices.
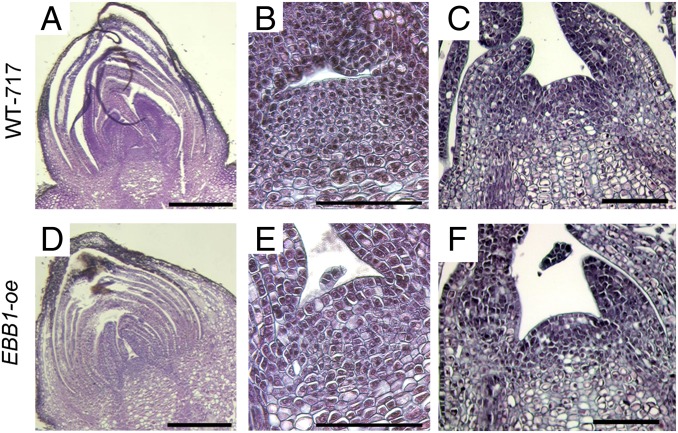
We inspected the cellular and tissue organization of the bud and actively growing apex in WT-717 plants and EBB1-oe transgenics (Fig. 3). The poplar buds consisted of tightly folded and packed bud scales, stipules, and embryonic leaves (16). In all examined EBB1-oe transgenics (10 ramets from five different lines), bud scales and embryonic leaves appeared to be thicker at the base and less folded compared with WT-717 plants (Fig. 3 A, B, D, and E). Thus, the overall bud shape in EBB1-oe transgenics appeared more oval (Fig. 3D), and the meristem was more open and exposed (Fig. 3E). No changes in bud morphology were observed in the EBB1-suppressed plants. We also studied the organization of nondormant actively growing apices. The meristem dome was visibly enlarged (Fig. 3 C and F). We studied if cell division activity was increased in the apex of transgenic plants. Indeed, EBB1-oe transgenics displayed an ∼80% higher cell division rate than WT-717 plants (Fig. S6).
Fig. 3.
Bud and apex morphology of EBB1-oe transgenics. Dormant bud (A, B, D, and E) and actively growing vegetative SAM (C and F) in WT-717 plants (A–C) and EBB1-oe transgenics (D–F). Note the difference in the shape of scales in the transgenic line, which form a more open area around the meristem. In WT buds, the meristem is more compactly surrounded by buds’ scales. (B and E) Close-up magnifications of the same sections shown in A and D. (Scale bars: A and D, 500 μm; B, C, E, and F, 100 μm.)
EBB1 Native Expression and Localization.
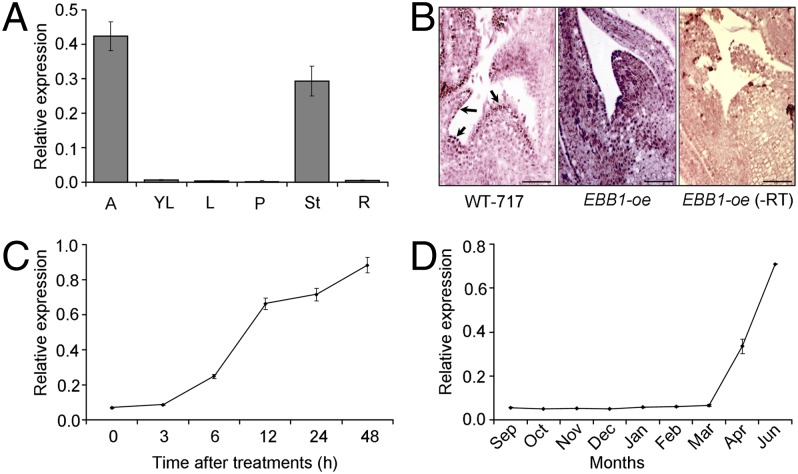
We studied EBB1 expression in WT-717 plants. EBB1 transcript was detected in the apex and stems, with highest expression in the apices (Fig. 4A). Because EBB1 expression was highest in growing apices, we performed in situ RT-PCR to understand its tissue localization in WT-717 plants (Fig. 4B). We used an EBB1-oe transgenic as a positive control to verify that the transgene was expressed in all apical tissues. As expected, we detected ubiquitous and high expression throughout the EBB1-oe transgenic shoot apex. No signal was detected in an RT-negative control. In WT-717 apices, EBB1 transcript was detected in the L1/L2 layers of the meristem dome, extending into the emerging leaf primordia. We also found that EBB1 was cytokinin-induced and required treatment with an auxin, such as 2,4-dichlorophenoxyacetic acid (2,4-D); cytokinin or 2,4-D alone was unable to induce EBB1 expression (Fig. 4C). During natural bud-set to bud-break in wild aspen (Populus tremuloides) trees, EBB1 expression was undetectable for most of the dormancy period but increased before bud-break (Fig. 4D).
Fig. 4.
EBB1 expression and localization. (A) EBB1 expression in various organs. Tissues were collected from WT-717 plants at the same time of the day and correspond to the following: 1-cm root tips (R); 2- to 3-mm apical shoot, including meristem and subtending leaf primordia (A); unexpanded young LPI 1–2 leaves (YL); fully expanded leaf plastochron index (LPI) 5–10 leaves (L); petioles of fully expanded leaves (P); and whole stem collected from LPI 5–10 (St). (B) In situ RT-PCR localization of EBB1 transcript in actively growing apices of WT-717 plants (Left) and EBB1-oe transgenics (Center). A negative (−RT) control was performed on EBB1-oe apices (Right). Arrows indicate the localization of the EBB1 transcript in the L1/L2 layers of the meristem and leaf primordia. (Scale bars: 50 μm.) (C) EBB1 is induced by a combination of cytokinin and auxin treatment (more details are provided in Materials and Methods). (D) Expression of EBB1 in vegetative buds of wild aspen (Populus tremuloides) trees. Expression of EBB1 in actively growing apices (June–August) is at the same level as shown for June. Relative expression for all experiments was normalized for loading differences using UBI. Bars and data points show mean ± 1 SE of at least three independent biological replicates for all experiments except D, where two individual trees were used as biological replications.
EBB1 Transgenic Modifications Lead to Major Genome-Wide Transcriptome Changes.
We used microarrays to study the transcriptomic changes in the EBB1-modified transgenics. Our analysis focused on apices because of the native predominant expression of EBB1 in this tissue. We first identified differentially expressed genes in the transgenic plants. The significantly regulated genes were then subjected to coexpression analysis with the expression of the EBB1 gene in the three genotypes (e.g., WT-717, EBB1-oe, amiEBB1). A total of 971 differentially expressed genes that correlated with EBB1 expression in the three genotypes were identified (Dataset S1). Of the 971 genes, 416 were positively correlated and 555 were negatively correlated with EBB1 expression (Dataset S1). The expression changes identified through the microarray studies were successfully validated by RT-PCR for a subset of 15 genes (Fig. S7 and Table S2). We performed functional classification of the differentially expressed genes using gene ontology (GO) analysis (Dataset S2). Several groups of categories of biological processes were significantly affected in the EBB1 transgenics. For example, genes involved in regulation of hormone level and response, receptor-linked signaling pathways, and growth and development were significantly enriched (Dataset S2).
Identification of EBB1 Putative Direct Targets.
In Arabidopsis, the EBB1 close orthologs (DRN/ESR1 and DRNL/ESR2) have been found to be positive regulators that bind to a GCC box sequence (32). Therefore, to identify putative direct targets of EBB1, we searched the promoter regions (−3,000 bp) of the 416 positively regulated genes for presence of a GCC box. A total of 65 genes were identified (Dataset S3). Interestingly, GO analysis of the EBB1 putative target genes identified enrichment of a large number (n = 43) of biological processes (Dataset S4). Among the most represented/enriched were nitrogen metabolic processes (13), developmental process (11), response to stimulus (12), and regulation of transcription (6).
Dormancy Induction and EBB1 Share Common Regulons.
We compared the differentially expressed genes in the EBB1 transgenics with recently published genes regulated during induction of bud dormancy in the same poplar genotype that we used in this study (25). This analysis discovered 265 (132 positively regulated and 133 negatively regulated) common genes (Fig. 5A), representing a significant enrichment of bud dormancy-related genes in EBB1 transgenics (27.3% of all differentially expressed genes; P < 0.001, Fisher exact test; Dataset S5). Classification of the common gene set by GO (Dataset S6) identified many genes that have been associated with entry into dormancy, including response to water deprivation, response to temperature, light exposure, red/far red light quality, and abscisic acid signaling. We calculated the mean of expression for all 265 commonly regulated genes separately for negatively regulated and positively regulated genes in EBB1 transgenics, and studied their expression dynamics during the 6 wk of dormancy induction using data from a previously published study (25) (Fig. 5B). Surprisingly, large numbers of genes (over 130 genes in each category) showed distinct and opposing patterns in their expression during poplar bud dormancy induction. Genes that were up-regulated in EBB1-oe transgenics and down-regulated in the EBB1-suppressed plants showed reduced expression during dormancy induction. Conversely, genes that were down-regulated in EBB1-oe transgenics but up-regulated in the amiEBB1 plants showed elevated expression during dormancy induction. Therefore, EBB1 appears to have a negative effect on the expression of genes that are typically up-regulated during dormancy induction and to have a positive impact on the transcript abundance of genes that are normally repressed during dormancy induction.
Fig. 5.
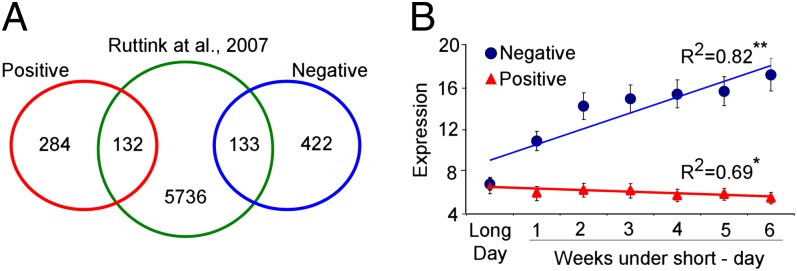
Dormancy induction and EBB1 share common and opposing regulons. (A) Venn diagram of the common gene set between differentially expressed genes in EBB1 transgenic apices and genes that are differentially expressed in apices of the same genotype during sort day-induced dormancy (25). (B) Trends in the expression of the common gene set during the 5 wk of the dormancy induction period. Data points and error bars represent the mean and SE over the averaged expression of the common genes. R2 represents the coefficient of determination for goodness of fit for the calculated linear trend line. *P < 0.05 and **P < 0.01, linear regression significances. In A and B, genes with positive and negative correlation to EBB1 expression are denoted as “Positive” and “Negative,” respectively.
Discussion
We report the isolation and characterization of the EBB1 gene that regulates the reinitiation of shoot growth after winter dormancy in Populus. Two main lines of experimental evidence strongly suggest that EBB1 plays an important role in the resumption of growth after winter dormancy. First, overexpression of the gene is sufficient to accelerate bud-burst, whereas down-regulation delays bud-break. Second, EBB1 transcript levels in buds are undetectable during the majority of the dormancy period but sharply increase prior to and during bud-break. There is very little information on the regulation of bud-break. To date, the only gene other than EBB1 that has been implicated in control of bud-break is the poplar ortholog of CENTRORADIALIS (CEN) (PopCEN1). CEN is known as TERMINAL FLOWER1 (TFL1) in Arabidopsis and is a repressor of flowering (33). Similar to Arabidopsis, PopCEN1 overexpression delayed flowering, whereas RNAi suppression of both paralogs (PopCEN1 and PopCEN2) accelerated flowering in poplar (34). In support of the growing body of evidence for a link between regulation of flowering and bud phenology in perennials (35), PopCEN1 overexpressors displayed delayed bud-break, whereas RNAi transgenics showed precocious bud flush (34). It is still unclear if EBB1 plays a part in this regulatory module. EBB1 shows highest sequence homology to DRN/ESR1 and DRNL/ESR2 from Arabidopsis (30). To our knowledge, there has been no information to date of a mechanistic connection between the flowering time integrators and DRN/DRNL.
In Arabidopsis, DRN and DRNL are considered paralogs with largely overlapping but some distinct functions (36). Comparative sequence analysis indicates that despite the recent whole-genome duplication (37), the poplar genome has the same number of orthologous genes: EBB1/PoptERF61 and PoptERF60 (Fig. 2C). The phylogenetic sequence analysis indicates that both EBB1/PoptERF61 and PoptERF60 are more similar to DRNL. However, EBB1 tissue localization and expression in response to hormones is reminiscent of those of the DRN gene (discussed more below) (28). Thus, it is difficult to draw functional parallels between the paralogous pairs in poplar and Arabidopsis based solely on their sequence orthology. DRN and DRNL genes are considered to be part of one of the three independent pathways that maintain growth and organization of the SAM (24). In vitro, DRN ectopic expression causes enhanced shoot regeneration from root cultures, suggesting that DRN promotes de novo meristem formation and activity. EBB1 shows expression and localization that resemble those of DRN. EBB1, like DRN, was induced by a combination of cytokinin and auxin treatment (28), and it was expressed predominantly in the L1/L2 layers of SAM and leaf primordia (30). Similar to the positive effect of DRN on shoot regeneration from root cultures (28), EBB1 up-regulation caused spontaneous regeneration from tissues that typically do not produce shoots and enhanced shoot regeneration from leaf disks (Figs. S2 and S3). Increased cell division activity in the SAM of EBB1-oe transgenics (Fig. S6) further supports the role of EBB1 in activation of SAM via stimulation of cell proliferation. Therefore, we believe that EBB1 is involved in bud-break after winter dormancy through restarting of cell proliferation in SAM and leaf primordia.
The localization of EBB1 in the L1/L2 layers of SAM and leaf primordia implies that growth after winter dormancy reinitiates in L1/L2 layers. L1/L2, particularly L1, from which epidermis is derived, may promote and restrict growth of the entire leaf/shoot by spreading growth-promoting signals to the inner layers (38). The enrichment of genes that encode epidermal cell fate specification and leaf shape in EBB1 transgenics supports this putative function. Furthermore, because L1 interfaces most closely with the ambient environment, it may have a unique role in perception of environmental cues (38), particularly changes in temperature that typically are the sole drivers of bud-break. Consistent with this hypothesis, our microarray analysis indicates significant enrichment in EBB1 transgenics of GO categories associated with perception of various environmental cues, including temperature (discussed below).
The precise function of DRN/DRNL in SAM organization is unclear; however, in Arabidopsis, DRN/DRNL is known to act via the auxin signal transduction pathway and physically interacts with BES1-INTERACTING MYC-LIKE 1 (BIM1), a brassinosteroid-regulated basic helix-loop-helix (bHLH) transcription factor (39, 40). Both auxin and brassinosteroid action in the SAM is localized in the L1 layer (38), where we find the highest expression of EBB1. The putative positive interaction of EBB1 with auxin and brassinosteroid signaling is also supported by our microarray analysis. We found among the differentially expressed genes a significant enrichment of GO processes involved in hormone-mediated signaling pathways. These were dominated by genes involved in auxin signaling and brassinosteroid biosynthesis. In addition, two of the putative EBB1 gene targets encode SHORT INTERNODE RELATED SEQUENCES (SRS), which are transcription factors that have been implicated in regulation of auxin biosynthesis in Arabidopsis through activation of the YUCCA gene (41, 42). In support of our findings, recent evidence suggests that auxin is important for reinitiating growth after dormancy (43). Therefore, our work and information about the orthologous gene in Arabidopsis suggest a possible connection of EBB1 with auxin and brassinosteroid signaling.
Consistent with EBB1 function in regulation of bud-break, the EBB1− transgenic transcriptome shows significant enrichment of biological categories associated with responses to various environmental cues (Dataset S2). The EBB1 differentially regulated genes were also significantly enriched in processes typical for actively growing apices, such as those associated with various metabolic processes, meristem growth, and regulation of hormone levels. Consistent with a putative function of EBB1 in SAM regulation, we found genes associated with meristem growth in the L1 layer. For example, poplar homologs of ERECTA-LIKE 1, GLABROUS 3, GLABROUS 2, LITTLE ZIPPER 3, and HOMEOBOX 51 were all up-regulated by EBB1 overexpression. EBB1-oe transgenics also showed enhanced expression of genes that maintain meristem identity, such as APETALA2-like. APETALA2 in conjunction with other transcription factors regulates the stem cell niche in the SAM, and involves interaction with the WUSCHEL-CLAVATA3 (WUS-CLV3) pathway (44). The seemingly multifunctional role of EBB1 in the SAM corresponds well with its self-sufficiency to organize and stimulate meristem activity de novo from differentiated tissues in the transgenic poplar plants (Figs. S1 and S2).
We also found a striking and significant overlap (more than one-quarter of all regulated genes) of the differentially expressed genes in EBB1 transgenics with ones that were previously found to be significantly changed in abundance during dormancy induction in the same genetic background (e.g., WT-717) (25). Even more striking are the opposing trends in the expression patterns among the common set of regulated genes (Fig. 5). This suggests that EBB1 and/or the pathways it affects suppress mechanisms that are associated with induction and preparation for dormancy while promoting responses that are associated with actively growing apices at the same time. For example, the closest poplar ortholog of SVP, one of the DAM genes (Potri.007G010800, PtpAffx.4750.1.S1_at, Dataset S1; PU01890, Dataset S5) was specifically and strongly down-regulated in EBB1-oe transgenics but up-regulated during dormancy induction (25). Down-regulation of DAM genes is necessary for release from endodormancy (45) and could be linked to the early bud-break phenotype of EBB1-oe transgenics.
Using the microarray data, coupled with promoter analysis, we identified 65 putative EBB1 target genes. Interestingly and in support of the validity of our analysis, we found a significant enrichment among the 65 putative target genes of SHI RELATED SEQUENCE (SRS) genes (P < 0.001, Fisher’s exact test). In Arabidopsis, SRS genes like SHORT INTERNODES (SHI) and STYLISH (STY) were demonstrated to be direct targets of DRN/ESR1 activation (32). Enrichment of various GO categories among the putative target genes suggests a broad regulatory context of EBB1 function. For example, the most enriched GO category among the target genes was nitrogen metabolism. Nitrogen mobilization/remobilization during growth/dormancy cycles is essential for the long-term survival of woody perennials (46). Enrichment of processes linked to development and response to stimulus in the target genes is consistent with EBB1 meristem function and response to environmental/hormonal clues. Finally, enrichment of processes linked to transcription regulation, which are mainly represented by transcription factors of various families, suggests that EBB1 mediates its response through regulation of other transcription factors. In addition to transcription factors, the EBB1-mediated regulatory mechanisms likely involve other signaling pathways. For example, the most significant correlation among the target genes with EBB1 expression was found for a gene encoding a Candidate G Protein Coupled Receptor 1 (CAND1). In Arabidopsis, CAND1 was shown to interact physically with GTP-binding protein alpha subunit 1 (GPA1), the only G alpha-encoding gene in the Arabidopsis genome (47). GPA1 is part of the abscisic acid, brassinosteroid, gibberellin, and sugar signaling and response pathways (reviewed in ref. 48). Furthermore, in maize, a GPA1 ortholog regulates meristem activity in a CLAVATA-dependent manner (49).
The experimental evidence presented here, as well as the known functions of the close homologs DRN/DRNL in Arabidopsis, suggests that EBB1 has a dual function in the SAM; it appears that it regulates both meristem cell proliferation and stem cell maintenance (24). This type of regulation is unusual, because many other meristem genes have highly specialized roles [e.g., WUS, CLV, SHOOT MERISTEMLESS (STM)]). Accordingly, the absence of EBB1 during establishment of dormancy allows progression through the physiological, developmental, and adaptive changes leading to dormancy, whereas the expression of EBB1 in specific cell layers before bud-break enables reactivation of growth in the SAM and leaf primordial and reentry into the active growth phase.
Vegetative bud dormancy is an important adaptive and economic trait, whose significance is likely to grow as a result of rapid climate change. Most cold injuries in trees occur due to frost damage as a result of either late spring frosts around the time of bud-break or early fall frosts around the time of growth cessation (50). Late frost damage after bud-break is more likely to cause damage than is injury due to late bud-set (51, 52). Through analysis of EBB1 and the physiological processes of which it is a part, it should be possible to gain new insights into control of dormancy release in perennial plants. This will enable novel approaches for population management, molecular breeding, and genetic engineering of dormancy-associated traits.
Materials and Methods
A detailed description of the materials and methods used in the paper are provided in SI Materials and Methods. The following items are included: generation of the activation tagging population; the implementation of the field trial and screening for bud phenology; plant material and treatments; plasmid rescue and positioning of the tag; sequence analyses; expression analyses; generation of binary vector constructs and transformation; microscopy and in situ RT-PCR analysis; and microarray hybridization and data analyses. These items are shown in the above-mentioned order.
Supplementary Material
Acknowledgments
We thank Chamini Illangasinghe for production of binary constructs and RT-PCR analyses, and Petio Kotov and Sharon Junttila for help with histological analysis. This research was supported, in part, by grants from the Office of Science Biological and Environmental Research (BER) program at the US Department of Energy (Grants DE-FG02-06ER64185, DE-FG02-05ER64113, and DE-SC0008462); US Department of Agriculture (USDA)-National Resources Inventory Plant Genome Program (Grant 2003-04345); Consortium for Plant Biotechnology Research, Inc. (Grant GO12026-203A); USDA Biotechnology Risk Assessment Research Grants Program (Grant 2004-35300-14687); USDA McIntire Stennis Fund (Grant 1001498); and industrial members of the Tree Biosafety and Genomics Research Cooperative at Oregon State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession nos. GSE16495 and GSE55813).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1405621111/-/DCSupplemental.
References
- 1.Peñuelas J, Filella I. Phenology. Responses to a warming world. Science. 2001;294(5543):793–795. doi: 10.1126/science.1066860. [DOI] [PubMed] [Google Scholar]
- 2.Rohde A, Bhalerao RP. Plant dormancy in the perennial context. Trends Plant Sci. 2007;12(5):217–223. doi: 10.1016/j.tplants.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Lang GA. Dormancy: A new universal terminology. HortScience. 1987;22(5):817–820. [Google Scholar]
- 4.Howe G, Hackett W, Furnier G, Klevorn R. Photoperiodic responses of a northern and southern ecotype of black cottonwood. Physiol Plant. 1995;93(4):695–708. [Google Scholar]
- 5.Rohde A, et al. Molecular Biology of Woody Plants. Vol 1. Dordrecht, The Netherlands: Kluwer; 2000. Molecular aspects of bud dormancy in trees; pp. 89–134. [Google Scholar]
- 6.Cooke JEK, Eriksson ME, Junttila O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012;35(10):1707–1728. doi: 10.1111/j.1365-3040.2012.02552.x. [DOI] [PubMed] [Google Scholar]
- 7.Frewen BE, et al. Quantitative trait loci and candidate gene mapping of bud set and bud flush in populus. Genetics. 2000;154(2):837–845. doi: 10.1093/genetics/154.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu B, Coleman GD. Phytochrome-mediated photoperiod perception, shoot growth, glutamine, calcium, and protein phosphorylation influence the activity of the poplar bark storage protein gene promoter (bspA) Plant Physiol. 2001;126(1):342–351. doi: 10.1104/pp.126.1.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingvarsson PK, García MV, Hall D, Luquez V, Jansson S. Clinal variation in phyB2, a candidate gene for day-length-induced growth cessation and bud set, across a latitudinal gradient in European aspen (Populus tremula) Genetics. 2006;172(3):1845–1853. doi: 10.1534/genetics.105.047522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Böhlenius H, et al. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science. 2006;312(5776):1040–1043. doi: 10.1126/science.1126038. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CY, et al. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc Natl Acad Sci USA. 2011;108(26):10756–10761. doi: 10.1073/pnas.1104713108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos A, et al. Winter disruption of the circadian clock in chestnut. Proc Natl Acad Sci USA. 2005;102(19):7037–7042. doi: 10.1073/pnas.0408549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson ME, Webb AAR. Plant cell responses to cold are all about timing. Curr Opin Plant Biol. 2011;14(6):731–737. doi: 10.1016/j.pbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Li D, et al. A repressor complex governs the integration of flowering signals in Arabidopsis. Dev Cell. 2008;15(1):110–120. doi: 10.1016/j.devcel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Reighard GL, Abbott AG, Bielenberg DG. Dormancy-associated MADS genes from the EVG locus of peach [Prunus persica (L.) Batsch] have distinct seasonal and photoperiodic expression patterns. J Exp Bot. 2009;60(12):3521–3530. doi: 10.1093/jxb/erp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohde A, et al. PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar. Plant Cell. 2002;14(8):1885–1901. doi: 10.1105/tpc.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruonala R, et al. Transitions in the functioning of the shoot apical meristem in birch (Betula pendula) involve ethylene. Plant J. 2006;46(4):628–640. doi: 10.1111/j.1365-313X.2006.02722.x. [DOI] [PubMed] [Google Scholar]
- 18.Baba K, et al. Activity-dormancy transition in the cambial meristem involves stage-specific modulation of auxin response in hybrid aspen. Proc Natl Acad Sci USA. 2011;108(8):3418–3423. doi: 10.1073/pnas.1011506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathiason K, et al. Transcript profiling in Vitis riparia during chilling requirement fulfillment reveals coordination of gene expression patterns with optimized bud break. Funct Integr Genomics. 2009;9(1):81–96. doi: 10.1007/s10142-008-0090-y. [DOI] [PubMed] [Google Scholar]
- 20.Rohde A, et al. Gene expression during the induction, maintenance, and release of dormancy in apical buds of poplar. J Exp Bot. 2007;58(15-16):4047–4060. doi: 10.1093/jxb/erm261. [DOI] [PubMed] [Google Scholar]
- 21.Yakovlev IA, Fossdal CG, Johnsen O, Junttila O, Skroppa T. Analysis of gene expression during bud burst initiation in Norway spruce via ESTs from subtracted cDNA libraries. Tree Genet Genomes. 2006;2(1):39–52. [Google Scholar]
- 22.Rinne PLH, et al. Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-beta-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell. 2011;23(1):130–146. doi: 10.1105/tpc.110.081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espinosa-Ruiz A, et al. Differential stage-specific regulation of cyclin-dependent kinases during cambial dormancy in hybrid aspen. Plant J. 2004;38(4):603–615. doi: 10.1111/j.1365-313X.2004.02070.x. [DOI] [PubMed] [Google Scholar]
- 24.Carles CC, Fletcher JC. Shoot apical meristem maintenance: The art of a dynamic balance. Trends Plant Sci. 2003;8(8):394–401. doi: 10.1016/S1360-1385(03)00164-X. [DOI] [PubMed] [Google Scholar]
- 25.Ruttink T, et al. A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell. 2007;19(8):2370–2390. doi: 10.1105/tpc.107.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busov V, et al. Activation tagging is an effective gene tagging system in Populus. Tree Genet Genomes. 2011;7(1):91–101. [Google Scholar]
- 27.Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006;140(2):411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banno H, Ikeda Y, Niu QW, Chua NH. Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell. 2001;13(12):2609–2618. doi: 10.1105/tpc.010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Graaff E, Dulk-Ras AD, Hooykaas PJJ, Keller B. Activation tagging of the LEAFY PETIOLE gene affects leaf petiole development in Arabidopsis thaliana. Development. 2000;127(22):4971–4980. doi: 10.1242/dev.127.22.4971. [DOI] [PubMed] [Google Scholar]
- 30.Kirch T, Simon R, Grünewald M, Werr W. The DORNROSCHEN/ENHANCER OF SHOOT REGENERATION1 gene of Arabidopsis acts in the control of meristem cell fate and lateral organ development. Plant Cell. 2003;15(3):694–705. [Google Scholar]
- 31.Hirota A, Kato T, Fukaki H, Aida M, Tasaka M. The auxin-regulated AP2/EREBP gene PUCHI is required for morphogenesis in the early lateral root primordium of Arabidopsis. Plant Cell. 2007;19(7):2156–2168. doi: 10.1105/tpc.107.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eklund DM, et al. Expression of Arabidopsis SHORT INTERNODES/STYLISH family genes in auxin biosynthesis zones of aerial organs is dependent on a GCC box-like regulatory element. Plant Physiol. 2011;157(4):2069–2080. doi: 10.1104/pp.111.182253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. Inflorescence commitment and architecture in Arabidopsis. Science. 1997;275(5296):80–83. doi: 10.1126/science.275.5296.80. [DOI] [PubMed] [Google Scholar]
- 34.Mohamed R, et al. Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. Plant J. 2010;62(4):674–688. doi: 10.1111/j.1365-313X.2010.04185.x. [DOI] [PubMed] [Google Scholar]
- 35.Horvath D. Common mechanisms regulate flowering and dormancy. Plant Sci. 2009;177(6):523–531. [Google Scholar]
- 36.Cole M, et al. Live imaging of DORNRÖSCHEN and DORNRÖSCHEN-LIKE promoter activity reveals dynamic changes in cell identity at the microcallus surface of Arabidopsis embryonic suspensions. Plant Cell Rep. 2013;32(1):45–59. doi: 10.1007/s00299-012-1339-4. [DOI] [PubMed] [Google Scholar]
- 37.Tuskan GA, et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313(5793):1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- 38.Savaldi-Goldstein S, Chory J. Growth coordination and the shoot epidermis. Curr Opin Plant Biol. 2008;11(1):42–48. doi: 10.1016/j.pbi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandler JW, Cole M, Flier A, Werr W. BIM1, a bHLH protein involved in brassinosteroid signalling, controls Arabidopsis embryonic patterning via interaction with DORNROSCHEN and DORNROSCHEN-LIKE. Plant Mol Biol. 2009;69(1-2):57–68. doi: 10.1007/s11103-008-9405-6. [DOI] [PubMed] [Google Scholar]
- 40.Cole M, et al. DORNROSCHEN is a direct target of the auxin response factor MONOPTEROS in the Arabidopsis embryo. Development. 2009;136(10):1643–1651. doi: 10.1242/dev.032177. [DOI] [PubMed] [Google Scholar]
- 41.Sohlberg JJ, et al. STY1 regulates auxin homeostasis and affects apical-basal patterning of the Arabidopsis gynoecium. Plant J. 2006;47(1):112–123. doi: 10.1111/j.1365-313X.2006.02775.x. [DOI] [PubMed] [Google Scholar]
- 42.Eklund DM, et al. The Arabidopsis thaliana STYLISH1 protein acts as a transcriptional activator regulating auxin biosynthesis. Plant Cell. 2010;22(2):349–363. doi: 10.1105/tpc.108.064816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petterle A, Karlberg A, Bhalerao RP. Daylength mediated control of seasonal growth patterns in perennial trees. Curr Opin Plant Biol. 2013;16(3):301–306. doi: 10.1016/j.pbi.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Würschum T, Gross-Hardt R, Laux T. APETALA2 regulates the stem cell niche in the Arabidopsis shoot meristem. Plant Cell. 2006;18(2):295–307. doi: 10.1105/tpc.105.038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasaki R, et al. Functional and expressional analyses of PmDAM genes associated with endodormancy in Japanese apricot. Plant Physiol. 2011;157(1):485–497. doi: 10.1104/pp.111.181982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooke JEK, Weih M. Nitrogen storage and seasonal nitrogen cycling in Populus: Bridging molecular physiology and ecophysiology. New Phytol. 2005;167(1):19–30. doi: 10.1111/j.1469-8137.2005.01451.x. [DOI] [PubMed] [Google Scholar]
- 47.Gookin TE, Kim J, Assmann SM. Whole proteome identification of plant candidate G-protein coupled receptors in Arabidopsis, rice, and poplar: Computational prediction and in-vivo protein coupling. Genome Biol. 2008;9(7):R120. doi: 10.1186/gb-2008-9-7-r120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urano D, Chen JG, Botella JR, Jones AM. Heterotrimeric G protein signalling in the plant kingdom. Open Biol. 2013;3(3):120186. doi: 10.1098/rsob.120186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bommert P, Je BI, Goldshmidt A, Jackson D. The maize Gα gene COMPACT PLANT2 functions in CLAVATA signalling to control shoot meristem size. Nature. 2013;502(7472):555–558. doi: 10.1038/nature12583. [DOI] [PubMed] [Google Scholar]
- 50.Derory J, et al. Transcriptome analysis of bud burst in sessile oak (Quercus petraea) New Phytol. 2006;170(4):723–738. doi: 10.1111/j.1469-8137.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 51.Timmis R, Flewelling J, Talbert C. Frost injury prediction model for Douglas-fir seedlings in the Pacific Northwest. Tree Physiol. 1994;14(7_9):855–869. doi: 10.1093/treephys/14.7-8-9.855. [DOI] [PubMed] [Google Scholar]
- 52.Ningre F, Colin F. Frost damage on the terminal shoot as a risk factor of fork incidence on common beech (Fagus sylvatica L.) Ann Sci. 2007;64(1):79–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.