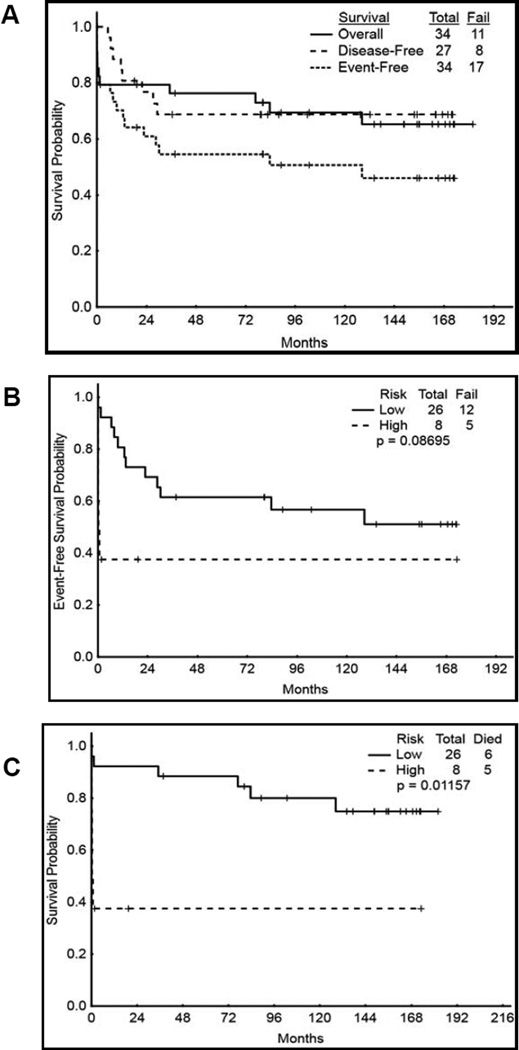
Clinical significance of all-trans retinoic acid (ATRA) in the initial therapy of acute promyelocytic leukemia (APL) is well established.[1],[2] Non-chemotherapeutic strategies have been sought to treat APL in order to avoid the adverse effects of chemotherapy.[3–6] Here, we report the 13 year follow up of a study of single agent liposomal-encapsulated ATRA in the frontline therapy of patients with APL[7]; the data were last updated in 2006.[8] Better pharmacokinetic data with liposomal ATRA [9] as compared with oral ATRA led to this study. The baseline characteristics of the 34 patients enrolled as well as the treatment protocol have been described previously. The complete remission (CR) rates were 79% (27/34) overall, and 92% (24/26) and 38% (3/8) among the low risk (with initial WBC counts <10,000) and in high risk (WBC ≥ 10,000) patients respectively (Table 1). Similar results were observed with respect to molecular response. The median time to hematological and molecular CR was 35 days (range, 24 – 64 days), and 123 days (range, 62 – 133 days), respectively. 27 patients achieved CR and one was lost to follow up. Among the 26 remaining patients, 24 had a molecular CR by qualitative PCR at 3 months. Eighteen patients maintained molecular remission after a median of 142.7 months (range, 18 – 172 months) after CR despite never having received any other antileukemic therapy. After a median follow up of 154.8 months (1.6–181.9 months), eleven patients have died. 5 of 11 deaths (45%) were early and 3 (30%) were due to cerebral hemorrhage. There were 8 relapses (6 alive, 2 dead), and a total of 17 events. The 13-year event-free survival (EFS), disease free survival (DFS) and overall survival (OS) were 46%, 69% and 65% respectively (Figure 1). The EFS and DFS were similar between low and high risk groups, while OS (not shown) was significantly longer in the low risk group (p=0.01). Since the study is limited by the small number of patients with disproportionate distribution among the low and high risk categories, the survival analyses among the risk categories may not be conclusive. Although, 8 patients who relapsed had low risk and none with high risk category relapsed, no definitive conclusions can be made due to the small number of patients. The eight relapses occurred after a median DFS of 11.9 months (range, 5–29 months) and six of the patients who relapsed were in molecular CR prior to relapse. Two relapsed patients underwent stem cell transplantation following salvage therapy with ATO, ATRA, ATO+ATRA, or ATO+ATRA+gemtuzumab ozogamicin, and both remain in remission at 11 and 12 years. Sixteen of the 18 patients who remain in molecular remission had low risk disease. None of the patients had any long term toxicities, in particular secondary myelodysplastic syndrome or AML, second malignancies or cardiotoxicity. These long term data suggest that liposomal ATRA is safe and has significant efficacy in patients with APL, even as monotherapy. We propose that liposomal ATRA is a potential lifesaving alternative to oral ATRA in situations where oral administration is difficult. Prospective studies (with larger cohorts of patients) combining liposomal ATRA with ATO in the frontline setting are warranted.
Table -1.
Distribution of patients among low and high risk categories
| Variables | Low Risk | High Risk | Total |
|---|---|---|---|
| Proportions n (%) | 26 (76) | 8 (24) | 34 (100) |
| Age (years) (Median; Range) | 48 (10–77) | 54 (22–72) | 50 (10–77) |
| Age >60 years, n (%) | 4 (12) | 2 (6) | 6 (18) |
| WBC K/uL (Median; Range) | 1.4 (0.4–8.2) | 17 (10–42) | 2.2 (0.4–42) |
| Platelet K/uL (Median; Range) | 31.5 (11–157) | 15 (7–13) | 29 (7–157) |
| Zubrod PS >1, n (%) | 5 (15) | 7 (20) | 12 (35) |
| Complete Remission (CR) n (%) | 24 (89) * | 3 (11) * | 27 (100) |
| No response n (%) | 0 | 2 (6) | 2 (6) |
| Median CR duration (months) | 85 (5.3–172) | 18 (0.3–172) | 102 (0.3–172) |
| Induction Deaths n (%) | 0 | 2 (6) | 2 (6) |
| Late Relapses | 8 (24) | 0 | 8 (24) |
| Deaths n (%) | 6 (17) | 5 (15) | 11 (32) |
| Median Overall Survival (OS) (months) | 142 (0.1–182) | 0.6 (0–173) | 115 (0–182) |
When CR is calculated within the risk categories the values were (92% vs 38% in low and high risk respectively)
Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Statement
P.J., H.K., E.E., S.P., J.C., G.L.B. and F.R. contributed to the overall design of the research and wrote the paper.
H.K., E.E., J.C., and F.R were involved in the care of the patients and contributed clinical samples and data.
Disclosures
All co-authors do not have any conflict to report.
References
- 1.Estey E, et al. Treatment of newly diagnosed acute promyelocytic leukemia without cytarabine. J Clin Oncol. 1997;15(2):483–490. doi: 10.1200/JCO.1997.15.2.483. [DOI] [PubMed] [Google Scholar]
- 2.Shen ZX, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2004;101(15):5328–5335. doi: 10.1073/pnas.0400053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mi JQ, et al. How to manage acute promyelocytic leukemia. Leukemia. 2012;26(8):1743–1751. doi: 10.1038/leu.2012.57. [DOI] [PubMed] [Google Scholar]
- 4.Mathews V, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J Clin Oncol. 2010;28(24):3866–3871. doi: 10.1200/JCO.2010.28.5031. [DOI] [PubMed] [Google Scholar]
- 5.Ravandi F. Acute promyelocytic leukemia can be treated successfully without cytotoxic chemotherapy. Oncology (Williston Park) 2011;25(8):741–743. [PubMed] [Google Scholar]
- 6.Ravandi F, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27(4):504–510. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estey EH, et al. Molecular remissions induced by liposomal-encapsulated all-trans retinoic acid in newly diagnosed acute promyelocytic leukemia. Blood. 1999;94(7):2230–2235. [PubMed] [Google Scholar]
- 8.Tsimberidou AM, et al. Single-agent liposomal all-trans retinoic acid can cure some patients with untreated acute promyelocytic leukemia: an update of The University of Texas M. D. Anderson Cancer Center Series. Leuk Lymphoma. 2006;47(6):1062–1068. doi: 10.1080/10428190500463932. [DOI] [PubMed] [Google Scholar]
- 9.Ozpolat B, et al. Pharmacokinetics of intravenously administered liposomal all-trans-retinoic acid (ATRA) and orally administered ATRA in healthy volunteers. J Pharm Pharm Sci. 2003;6(2):292–301. [PubMed] [Google Scholar]