Abstract
The purpose of this study was to assess the association of age with muscle mass and strength in a group of young adults before and after 12 weeks of progressive resistance training. Eight hundred twenty-six young males and females (age 24.34 ± 5.69 yr, range 18–39 yr) completed a strictly supervised 12-week unilateral resistance training program of the nondominant arm. Isometric (maximal voluntary contraction [MVC]) and dynamic strength (1 repetition maximum [1RM]) of the elbow flexors and cross-sectional area (CSA) of the biceps-brachii using magnetic resonance imaging (MRI) scans were measured before and after training. Pearson correlation coefficients were calculated for size and strength variables and age. In addition, the cohort was divided into groups according to decade of life and differences assessed by analysis of variance. Age correlated significantly and positively with all pretraining measures of muscle size and strength (CSA: r = 0.191, p < 0.001; MVC: r = 0.109, p = 0.002; 1RM: r = 0.109, p = 0.002). Age was not related to the training-induced changes in CSA or MVC but was negatively associated with the change in 1RM (r = −0.217, p < 0.001). The study indicates that age does have a significant positive relationship with muscle size and strength in untrained young adults. Although age was negatively associated with improvements in 1RM, the effect of age was small relative to the improvements induced through resistance training, thus suggesting age does not limit response to training in any practical way during early adulthood.
Keywords: magnetic resonance imaging, muscle cross-sectional area, isometric strength, isotonic strength, supervised resistance training
Introduction
The importance of skeletal muscle in maintaining metabolic and functional health is well established (7,26). Without intervention, decreases in muscle size and strength of 10–15% per decade are observed after the age of 50 years, with increasing rates of loss after the age of 65 (22). This is problematic because muscle strength may be the primary determinant in the risk of falling in the elderly (27). It has also been shown that low muscle strength reduces the force required to cause a fracture after a fall (5,6). As such, maintenance of adequate levels of muscle mass and strength into an advanced age are key components of healthy aging.
Skeletal muscle is a malleable tissue and responds well to resistance training (2). Numerous studies have shown that resistance training can attenuate the negative effects of aging on muscle size and strength. As such, current physical activity recommendations for older adults include the performance of resistance training at a moderate intensity (1). Although master athletes who have accrued years of training remain stronger than their age-matched, nontrained peers (24), those who start resistance training late may experience attenuated gains (13,19,25).
Although the effect of age on muscle size has been studied in younger adults (14), the effect of age on the adaptability of skeletal muscle in individuals under the age of 40 is less well studied. Without this knowledge, the information required to develop a comprehensive long-term strategy to combat age-related changes in muscle morphology and performance is incomplete. The purpose of this study was to use data from the Functional Single Nucleotiode Polymorphisms Associated with Human Muscle Size and Strength Study (FAMuSS), a large multicenter study with sensitive measures of muscle size and strength before and after 12 weeks of supervised resistance training in untrained young adults (18–40 yr), to investigate these questions. It was our hypothesis that an age-related association would not be evident in a group too young to have experienced the endocrine and other changes causing the reduced muscle size and strength seen in older adults.
Methods
Experimental Approach to the Problem
The protocol for the FAMuSS study has been previously described in detail (28). Untrained young adults were tested on both arms for muscle cross-sectional area (CSA) of the upper arm and both isotonic and isometric strength of the biceps brachii. Subsequently, they were put through a 12-week unilateral (nondominant arm) periodized training program of the upper arm, with each session strictly supervised by research staff. Training-induced improvements were assessed by repeating the pretraining battery of tests shortly after completion of the training period.
Subjects
Participants were recruited from each institution’s student population and the local community. Potential participants were considered for enrollment in the study if they had not performed any resistance training for at least 12 months and did not perform a job that required repetitive use of their arms (e.g., server, delivery personnel, shelf stacker). Subjects older than 40 years were excluded to avoid the decrease in testosterone levels and other hormonal changes that affect skeletal muscle in older age groups (29,17). All participants provided written consent as approved by the institutional review board for human subjects experimentation of each institution involved in the project. Participants’ physical characteristics are presented in Table 1.
Table 1.
Pretraining characteristics.
| All subjects (n = 826)
|
Male (n = 346)
|
Female (n = 480)
|
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (yr) | 24.34 | 5.69 | 24.66 | 5.48 | 24.12 | 5.82 |
| Weight (kg) | 70.85 | 15.75 | 78.08 | 16.16 | 65.65 | 13.20 |
| Height (cm) | 169.79 | 9.07 | 176.58 | 7.52 | 164.92 | 6.63 |
| Body mass index (kg/m2) | 24.43 | 4.64 | 24.95 | 4.74 | 24.06 | 4.53 |
Procedures
Isometric Biceps Strength Testing
Isometric strength, as measured by a maximal voluntary contraction (MVC) of the elbow flexor muscles of each arm, was determined separately, before and after 12 weeks of strength training, using a specially constructed, modified preacher bench and strain gauge (model 32628CTL, Lafayette Instrument Company, Lafayette, IN, USA). Pretraining measures of MVC were assessed on 3 separate days spaced no more than 2 days apart. The first of these sessions was used for familiarization to the testing protocol, so the pretraining value was taken as the average of the results obtained on the second and third testing days. Post-training measures of MVC were assessed immediately before the last training session or 24 to 48 hours after the last training session.
One Repetition Maximum Biceps Strength Testing
The dynamic strength of the elbow flexor muscles of each arm was assessed separately by determining the maximum amount of weight with which a subject could perform 1 repetition maximum (1RM) of the 1-arm preacher curl exercise using a standardized protocol (4). The 1RM testing was performed only once after the final isometric tests both before and after the 12-week training program.
Measurement of Muscle Cross-Sectional Area
Magnetic resonance imaging (MRI) scan was performed before and after exercise training to assess changes in the biceps brachii CSA. The procedure for standardization of the MRI measurement (pretraining vs. post-training) has been explained in detail previously (28). Pre- and post-training MRI scans were performed either before the strength tests or 48 hours after the MVC and 1RM tests. This was done to avoid postexertional swelling that might spuriously increase muscle size and affect the validity of the measurements. Post-training images were taken 48 to 96 hours after the final training session.
Pre- and post-training MRI scans were obtained separately from both the dominant and nondominant arms. Images were analyzed using a custom-designed interactive processing and visualization program that operates in Matlab (The Math Works, Inc., Natick, MA, USA). On the basis of the MRI scan acquisition data (i.e., field of view and matrix resolution), the CSA (cm2) of region of interest was then calculated.
Exercise Training Program
Subjects underwent gradually progressive, supervised strength training 2 times per week of their nondominant arm only, which was defined as the nonwriting arm. The exclusion of the dominant arm from the 12-week training program allowed the dominant arm to act as a control. The 1RM measured during pretraining testing was used to estimate the amount of weight that could be lifted for 12, 8, and 6 repetitions using standard formulas (4). Each training session followed the same sequence of exercises: biceps preacher curl, overhead triceps extension, biceps concentration curl, triceps kickback, and standing biceps curl. All exercises were performed with dumbbells (Powerblocks, Intellbell, Inc., Owatonna, MN, USA), and some exercises used a preacher curl bench (Yukon International, Inc., Cleveland, OH, USA).
All training sessions were supervised and lasted approximately 45 to 60 minutes. The program periodization used the following weekly training protocol: weeks 1 to 4: 12 repetitions of the 12RM weight; weeks 5 to 8: 8 repetitions of the 8RM weight; weeks 9 to 12: 6 repetitions of the 6RM weight. Subjects performed 3 sets of each exercise throughout all 12 weeks with a mandatory 2-minute rest interval between sets. The weight lifted was increased when subjects could perform 2 extra repetitions on the third set of a given exercise beyond the goal repetitions for that week; the subject would then increase the weight lifted during the subsequent training session for that given exercise.
Dietary Control Procedures
Subjects were instructed to maintain their habitual dietary intake over the course of the study. Individuals who supplemented their diet with additional protein or any dietary supplement reported to build muscle or to cause weight gain within the 3 previous months were excluded.
Standardization Between Sites
Adaptations to resistance training are highly specific to the training protocol. Therefore, to control for any difference among sites, each site used an identical training protocol, and identical exercise equipment was purchased from the same manufacturers. The techniques for MRI, strength and anthropometric measurements, and exercise training were videotaped, and each site’s research personnel were required to review the videotaped procedures before the start of each cohort. All sites also met semi--annually to review standardized measurement and training techniques.
Statistical Analyses
Data were analyzed using SPSS (version 12.0, Chicago, IL, USA). A one-way analysis of variance was used to test for differences between age groups, classified by their decade of life. For the analysis of the response to training, delta scores were calculated (post–pre) and used as the dependant variable with baseline measures as a covariate. Significant F ratios were probed with a Tukey post hoc test. Pearson correlation coefficients were used to determine the relation between age and a) pretraining strength and size measurements, and b) the change in size and strength variables. The alpha level was set at 0.05 for all analyses. All data are presented as means ± SD.
Results
Pretraining Associations with Age
In the entire cohort, age was significantly and positively correlated with all pretraining measures of muscle size and strength in the elbow flexors of the nondominant arm (CSA: r = 0.191, p < 0.001, n = 605; MVC: r = 0.109, p = 0.002, n = 788; 1RM: r = 0.109, p = 0.002, n = 819). Comparable results were seen in the dominant arm. There was also a significant difference in muscle CSA between both the third and fourth decade of life compared with the second. There were no age-related differences in muscle strength in the dominant arm, whether assessed by MVC or 1RM (Figure 1).
Figure 1.
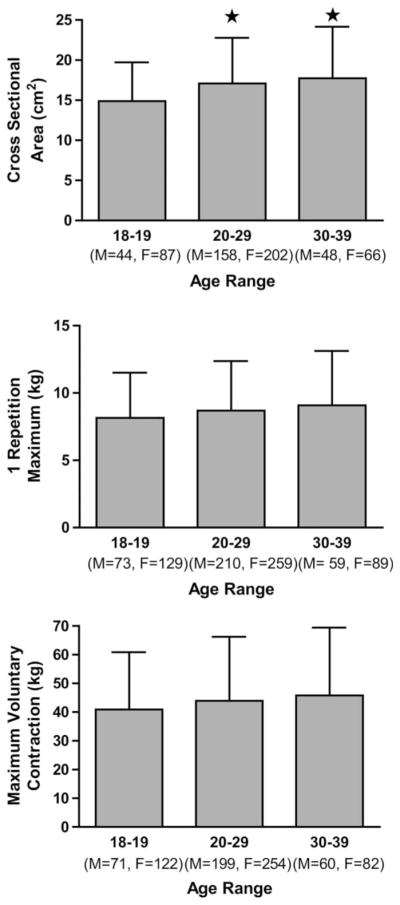
Comparison of A) biceps brachii cross-sectional area B) 1 repetition maximum C) maximum voluntary contraction across the second to fourth decade in nondominant arm of untrained individuals. *Greater than 18 to 19, p < 0.05.
Effect of 12 Weeks of Resistance Training
As previously described, the 12-week resistance training program resulted in positive adaptations in all measures of muscle size and strength in the trained arm (12). In short, increases of 18.9% ± 9.42 in CSA, 54.34% ± 33.46 in 1RM, and 20.66 ± 20.20 in MVC were seen in the trained arm.
In the entire cohort, age was related to change in 1RM but not in muscle size or MVC (Table 2). A sex-specific pattern of adaptation to this protocol has previously been reported (12). We therefore also analyzed these data according to sex, but the relationship with age was not different to that of the entire cohort.
Table 2.
Correlation between age and change from pretraining values (post-training – pretraining) in nondominant (trained) arm after 12 weeks of resistance training.
| Muscle cross-sectional area
|
1 repetition maximum
|
Maximum voluntary contraction
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | All | Male | Female | All | Male | Female | |
| r | 0.036 | −0.008 | 0.005 | −0.217 | −0.216 | −0.255 | 0.079 | 0.079 | 0.039 |
| p | NS | NS | NS | <0.001 | <0.001 | <0.001 | NS | NS | NS |
| n | 597 | 249 | 348 | 728 | 302 | 426 | 683 | 285 | 398 |
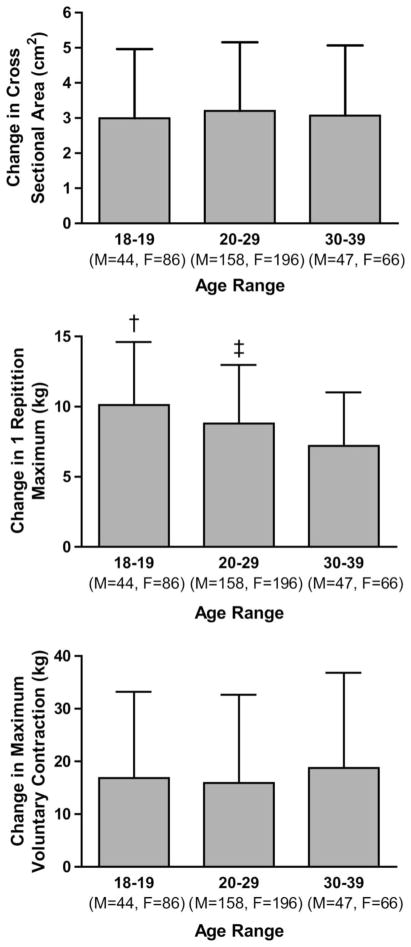
After controlling for baseline values, improvements in 1RM in the second decade were greater than the improvements in both the third and forth decades. In turn, greater improvements were seen in the third decade than the forth (Figure 2). Although this analysis showed statistical significance, the effect was small, with the parameter estimates of a difference of just 3.07 kg across the entire cohort.
Figure 2.
Comparison of absolute improvements in A) biceps brachii cross-sectional area B) 1 repetition maximum C) maximum voluntary contraction after 12 weeks of progressive periodized resistance training across second to fourth decade in nondominant arm of previously untrained individuals. †Greater than 20 to 29 and 30 to 39, p < 0.05. ‡Greater than 30 to 39, p < 0.05.
Discussion
It is well established that age affects muscle size and strength in middle- and older-aged adults. However, we believe the present study is the first to combine such a large sample size, a sensitive measure of muscle CSA (MRI), the use of measures of both isometric and dynamic strength, and a strictly monitored, standardized program of resistance training in an uncommonly studied age group (age 18–40) to examine the association of primary age on muscle size and strength in both the trained and untrained state.
Our data show that age does have a significant positive association with biceps muscle CSA in untrained individuals between the ages of 18 and 40 years. Although our results indicate a positive relationship that was statistically significant, the association was not strong, being responsible for 3.6% of the variance between individuals in biceps muscle size. It has previously been shown that the musculature of the upper body is maintained better with increased age than the lower body, suggesting that age may have a variable relationship with muscle size depending on the location of the muscle (14). This may be the explanation for the contrast of the present study with that of Lexell at al. (20), which showed the onset of age-related atrophy of the vastus lateralis at 25 years of age.
The relationship between age and pretraining strength (isometric and isotonic) was weaker than the relationship with CSA but still statistically significant. These data showed age was responsible for only 1% of the observed variance. As such, when the cohort was grouped by decade, the association was not strong enough to reveal a statistically significant difference across the 3 age groups. Several studies have shown that muscular strength is improved or maintained until approximately 40 years (3,15,18,23), whereas other studies have shown significant decrements before the age of 40 (11,16,21). Larsson et al. (18) showed increases in isometric and dynamic strength up to the end of the third decade, with strength maintained thereafter until the sixth decade in the quadriceps muscle. Kallman et al. (15) found that grip strength peaked by the end of the fourth decade, with a decline starting during the fifth decade and accelerating thereafter. Furthermore, the present data corroborate the findings of Metter et al. (23), who found that grip strength did not change significantly in a large cohort of adults between the ages of 18 to 40 years. The inconsistency in these observations suggests that the relationship between age and muscle size and function may differ according to anatomic location.
Presently, age has been seen to have no association with the magnitude of change in muscle CSA or isometric strength after 12 weeks of resistance training. Although obtained in a different muscle group, these results agree with the findings of Ivey et al. (13), who showed that the change in muscle volume, measured by MRI, with 9 weeks of dynamic strength training was not different in younger (20–29 yr of age) compared with older (65–75 yr of age) men.
Lemmer et al. (19) have previously shown that younger (20–30 yr of age) subjects had a significantly greater increase in 1RM strength than older (65–75 yr of age) adults. It is perhaps surprising that age was negatively associated with the improvement in strength in the much younger cohort in the present study. In addition, there was a statistically significant trend in the magnitude of improvement in 1RM across the age groups. It is known that the potential for improvement from training is in part determined by the initial level of performance (10). Because age was positively associated with strength in the untrained state, this could explain the reduced adaptability. However, even after controlling for pretraining strength, the observed trend remained significant.
It is important to note that even though the present study supported the often-observed decrease in adaptability of skeletal muscle with advanced age, the parameter estimates for improvement in 1RM showed a difference of only 3.07 kg between the youngest and oldest age groups. The capacity for improvement in skeletal muscle performance is great, often as much as 200–300% as a result of short-term resistance in those as old as their eighth decade (9,8). In the present study, the average increase in the entire cohort was 8.84 kg. In the oldest group, the average increase in 1RM was 7.21 kg. Therefore, although age is statistically associated with a decreased improvement in strength, this association is small compared with the observed improvements even in the oldest group
In the present study, care was taken to ensure the correct principles were used in the resistance training protocol. However, the unilateral design and focus on a relatively small muscle mass mean the protocol was atypical and may limit generalizability. These decisions were made a priori to maximize control of the study, specifically the unilateral aspect of the training protocol. In addition, the muscles of the arms are nonweight bearing, and therefore we could better control for extracurricular levels of physical activity, both before enrollment and during active participation. However, this extra level of control may limit the ability to extrapolate these findings to the effects of age under a more typical training protocol. It is possible that the greater level of recruited muscle mass and the resulting systemic endocrine and neural responses elicited from a more typical (i.e., whole body) training protocol could alter the adaptations observed presently as well as the relationship between age and the response to training. A further limitation of the study is the short (12-wk) training protocol. It is known that master athletes have considerable advantages over their nontrained age-matched peers (24). Therefore, it is not clear whether a training protocol of longer duration would have altered the small effect of age seen in the present study.
In conclusion, the present data suggest that age has a small, but positive, impact on muscle size and strength in untrained young adults. In addition, age was associated with a slight attenuation of the improvement in dynamic strength in response to 12 weeks of resistance training. Importantly, the size of this effect is very small in comparison with the enduring ability of skeletal muscle to adapt to resistance training, and so age does not affect the response to training in a meaningful way through early adulthood.
Practical Applications
Skeletal muscle has a tremendous capacity for adaptation. The association between age and muscle size and performance demonstrated in the present study can be quickly overcome by the rapid adaptations made during a resistance training program. Furthermore, the very slight negative association age has with skeletal muscle’s adaptability is considerably less than the enduring capacity for adaptation, even in the fourth decade of life. The present data suggest that the muscle size and strength response to resistance training is not influenced by age in any practical manner through the fourth decade of life.
Acknowledgments
The Functional Polymorphisms Associated with Muscle Size and Strength Study (FAMuSS) is funded by a grant from the National Institute Health (5RO1NS040606-03).
References
- 1.American College of Sports Medicine Position Stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;30:992–1008. [PubMed] [Google Scholar]
- 2.American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 3.Backman E, Johansson V, Hager B, Sjoblom P, Henriksson KG. Isometric muscle strength and muscular endurance in normal persons aged between 17 and 70 years. Scand J Rehabil Med. 1995;27:109–117. [PubMed] [Google Scholar]
- 4.Baechle TR, Earle RW, Wathen D. Resist Train. 2000;2:395–426. [Google Scholar]
- 5.Bean N, Bennett KM, Lehmann AB. Habitus and hip fracture revisited: skeletal size, strength and cognition rather than thinness? Age Ageing. 1995;24:481–484. doi: 10.1093/ageing/24.6.481. [DOI] [PubMed] [Google Scholar]
- 6.Chandler JM, Hadley EC. Exercise to improve physiologic and functional performance in old age. Clin Geriatr Med. 1996;12:761–784. [PubMed] [Google Scholar]
- 7.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 8.Frontera WR, Meredith CN, O’Reilly KP, Evans WJ. Strength training and determinants of VO2max in older men. J Appl Physiol. 1990;68:329–333. doi: 10.1152/jappl.1990.68.1.329. [DOI] [PubMed] [Google Scholar]
- 9.Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- 10.Hakkinen K. Factors influencing trainability of muscular strength during short term and prolonged training. Natl Strength Cond Assoc J. 1985;7:32–34. [Google Scholar]
- 11.Hortobagyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA. The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol A Biol Sci Med Sci. 1995;50:B399–B406. doi: 10.1093/gerona/50a.6.b399. [DOI] [PubMed] [Google Scholar]
- 12.Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005;37:964–972. [PubMed] [Google Scholar]
- 13.Ivey FM, Tracy BL, Lemmer JT, NessAiver M, Metter EJ, Fozard JL, Hurley BF. Effects of strength training and detraining on muscle quality: age and gender comparisons. J Gerontol A Biol Sci Med Sci. 2000;55:B152–B157. doi: 10.1093/gerona/55.3.b152. [DOI] [PubMed] [Google Scholar]
- 14.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45:M82–M88. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- 16.Kubo A. Changes in abdominal muscle strength with respect to aging. Nippon Ronen Igakkai Zasshi. 1994;31:525–531. doi: 10.3143/geriatrics.31.525. [DOI] [PubMed] [Google Scholar]
- 17.Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez JL, Candas B. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–328. doi: 10.1016/s0039-128x(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 18.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 19.Lemmer JT, Hurlbut DE, Martel GF, Tracy BL, Ivey FM, Metter EJ, Fozard JL, Fleg JL, Hurley BF. Age and gender responses to strength training and detraining. Med Sci Sports Exerc. 2000;32:1505–1512. doi: 10.1097/00005768-200008000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 21.Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654áwomen and men aged 20–93 yr. J Appl Physiol. 1997;83:1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 22.Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol. 2004;91:450–472. doi: 10.1007/s00421-003-0991-3. [DOI] [PubMed] [Google Scholar]
- 23.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52:B267–B276. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 24.Ojanen T, Rauhala T, Hakkinen K. Strength and power profiles of the lower and upper extremities in master throwers at different ages. J Strength Cond Res. 2007;21:216–222. doi: 10.1519/00124278-200702000-00039. [DOI] [PubMed] [Google Scholar]
- 25.Rogers MA, Evans WJ. Changes in skeletal muscle with aging: effects of exercise training. Exerc Sport Sci Rev. 1993;21:65–102. [PubMed] [Google Scholar]
- 26.Snow-Harter C, Bouxsein M, Lewis B, Charette S, Weinstein P, Marcus R. Muscle strength as a predictor of bone mineral density in young women. J Bone Miner Res. 1990;5:589–595. doi: 10.1002/jbmr.5650050608. [DOI] [PubMed] [Google Scholar]
- 27.Szulc P, Beck TJ, Marchand F, Delmas PD. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men–the MINOS study. J Bone Miner Res. 2005;20:721–729. doi: 10.1359/JBMR.041230. [DOI] [PubMed] [Google Scholar]
- 28.Thompson PD, Moyna N, Seip R, Price T, Clarkson P, Angelopoulos T, Gordon P, Pescatello L, Visich P, Zoeller R, Devaney JM, Gordish H, Bilbie S, Hoffman EP. Functional polymorphisms associated with human muscle size and strength. Med Sci Sports Exerc. 2004;36:1132–1139. doi: 10.1249/01.mss.0000132274.26612.23. [DOI] [PubMed] [Google Scholar]
- 29.Vermeulen A, Rubens R, Verdonck L. Testosterone secretion and metabolism in male senescence. J Clin Endocrinol Metab. 1972;34:730–735. doi: 10.1210/jcem-34-4-730. [DOI] [PubMed] [Google Scholar]