To the Editor
Soccer is the most popular sport in the world, with more than 250 million active players.1 It is the only sport in which the unprotected head is a primary point of contact when heading the ball. In other contact sports, the deleterious long-term effects of repetitive traumatic brain injury (TBI), such as impaired white matter integrity,2 are well recognized.3 However, whether frequent subconcussive blows to the head lead to TBI remains controversial,4,5 although evidence suggests impaired neuropsychological function in soccer players.5 We evaluated concussion-naive soccer players using high-resolution diffusion tensor imaging (DTI), which is highly sensitive for detecting alterations in white matter architecture.
Methods
All right-handed male soccer players from 2 training groups of an elite-level soccer club in Germany were approached to participate. All were trained since childhood for a career in professional soccer. A comparison cohort of swimmers, which is a sport with low exposure to repetitive brain trauma, was recruited from competitive clubs to match on age (group-matched), handedness, and sex. Exclusion criteria were history of concussion or any other neuropsychiatric disorder. The local ethics committee approved the study and written informed consent was obtained.
A DTI sequence with 64 diffusion directions was acquired on a 3T magnetic resonance scanner (Verio, Siemens Healthcare) in July and August 2011. Group analyses were performed using automated whole-brain, tract-based spatial statistics6 for the following measures of diffusivity: fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity. Fractional anisotropy and mean diffusivity have been shown to be sensitive markers for mild TBI.
Axial diffusivity and radial diffusivity measure axonal and myelin pathology. Voxel-wise statistics were used to investigate group differences at a 2-sided significance level of P < .05, corrected for multiple comparisons. Voxels with a significant group difference were merged into a single cluster. Diffusivity measures were obtained for each individual and a linear regression model was applied to test for group differences adjusted for age and years of training. SPSS version 20 (SPSS Inc) was used.
Results
Twelve of 40 soccer players (mean [SD] age, 19.7 [1.6] years; mean duration of playing soccer, 13.3 [2.9] years) and 11 of 20 swimmers (mean [SD] age, 21.4 [2.8] years; mean duration of training, 9.3 [2.9] years) met the inclusion criteria. Three swimmers were excluded from the statistical analyses for anatomic or technical problems.
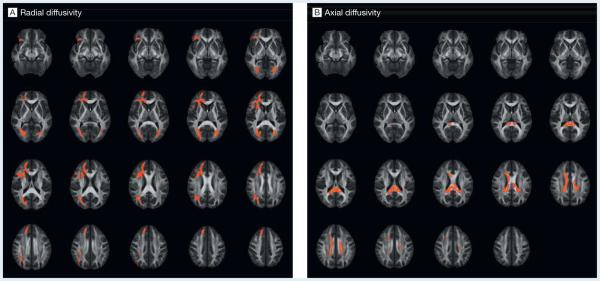
Widespread differences between groups were found, with increased radial diffusivity in soccer players (mean, 0.000444 [95% CI, 0.000427-0.000461] mm2/s vs 0.000368 [95% CI, 0.000356-0.000381] mm2/s in swimmers) in the right orbitofrontal white matter, the genu and anterior portions of the corpus callosum, association fibers involving bilateral inferior fronto-occipital fasciculus, bilateral optic radiation, and bilateral anterior cingulum, right anterior, right superior, and bilateral posterior corona radiata, right anterior limb of the internal capsule, right external capsule, and right superior frontal gyrus (Figure 1A).
Figure 1. Results of the Tract-Based Spatial Statistics (TBSS) Analysis.
The diffusion tensor for each voxel was estimated by the multivariate linear fitting algorithm, and the tensor matrix was diagonalized to obtain 3 pairs of eigenvalues (λ1, λ2, λ3) and eigenvectors. Voxelwise summary parameters included fractional anisotropy, radial diffusivity ([λ2 + λ3]/2), axial diffusivity (A1), and mean diffusivity (λ1 + λ2 + λ3). Group analyses were performed using whole-brain TBSS6 for the following measures of diffusivity: fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity. For all analyses, threshold-free cluster enhancement was used to obtain significant differences between groups at P < .05. After accounting for multiple comparisons using the family-wise error rate, the voxels highlighted in red demonstrate significantly increased radial diffusivity and axial diffusivity values for the soccer group compared with swimmers.
Axial diffusivity was higher in the corpus callosum in soccer players (mean, 0.00156 [95% CI, 0.00154-0.00158] mm2/s vs 0.00143 [95% CI, 0.00140-0.00146] mm2/s in swimmers; Figure 1B). No significant differences were found for fractional anisotropy or mean diffusivity. Cluster analysis revealed significantly higher radial and axial diffusivity in soccer players; age and years of training had no significant association with diffusivity values (Figure 2). Structural images as read by a neuroradiologist showed no abnormalities.
Figure 2. Diffusivity Measures for Each Individual.
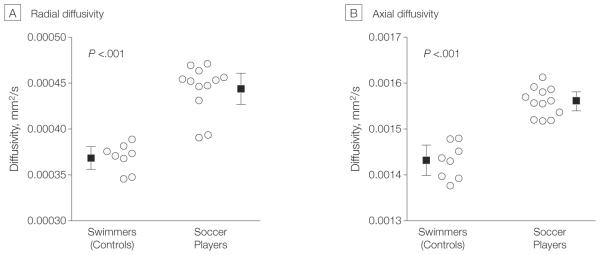
Voxels with a significant group difference as revealed by Tract-Based Spatial Statistics (Figure 1) were merged to a single cluster. Circles indicate individual values, squares indicate mean values, and error bars indicate 95% confidence intervals. Diffusivity measures were obtained for each individual and plotted for the 2 study groups. Linear regression showed no significant association of age or years of training with radial diffusivity (P = .13 and P = .12, respectively) or for axial diffusivity values (P = .22 and P = .54, respectively).
Comment
This study found differences in white matter integrity in a small sample of soccer players compared with swimmers. Although only participants without previous symptomatic concussion were included, advanced DTI revealed widespread increase in radial diffusivity in soccer players, consistent with findings observed in patients with mild TBI, and suggesting possible demyelination.
The etiology of the findings, however, is not clear. One explanation may be the effect of frequent subconcussive brain trauma, although differences in head injury rates, sudden accelerations, or even lifestyle could contribute. Additionally, soccer players showed increased axial diffusivity in the absence of increased radial diffusivity limited to the corpus callosum, possibly resulting from specialized training or neuroinflammation.
Limitations of the study include small sample size, single cross-sectional evaluation, and lack of information regarding functional outcomes. Future studies are needed to confirm the results and elucidate the etiology and effects of white matter alterations in soccer players.
Acknowledgments
Funding/Support:
This study was supported by the Else Kröner-Fresenius Stiftung and the Deutsche Akademischer Austauschdienst (Dr Koerte). This work was also supported in part by the Intrust Posttraumatic Stress Disorder and Traumatic Brain Injury Clinical Consortium, which is funded by Department of Defense Psychological Health/Traumatic Brain Injury Research Program grant X81XWH-07-CC-CS-DoD (Drs Zafonte and Shenton), and by grant R01 NS 078337 from the National Institute of Neurological Disorders and Stroke (Dr Shenton).
Role of the Sponsor:
The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Additional Contributions:
We thank the following colleagues for their contributions to this research: Robert A. Stern, PhD (Center for The Study of Traumatic Encephalopathy, Boston University School of Medicine, Boston, Massachusetts), for his support in interpreting our results; David Kaufmann, MD, Stefanie Immler, MD, and Denise Steffinger, certified magnetic resonance imaging technician (Institute for Clinical Radiology, Ludwig-Maximilians-University, Munich, Germany), for assistance with participant recruitment and data acquisition; Marc Muehlmann (Institute for Clinical Radiology, Ludwig-Maximilians-University), and Maulik Purohit, MD, MPH (Department of Physical Medicine and Rehabilitation, Harvard Medical School, Boston, Massachusetts), for assistance in data analyses and preparation of the figures; Marek Kubicki, MD, PhD, Alexander Lin, PhD, Sylvain Bouix, PhD, Yogesh Rathi, PhD, and Ofer Pasternak, PhD (Psychiatry Neuroimaging Laboratory, Brigham and Women’s Hospital, Boston, Massachusetts), for guidance with the methods; Ryan Eckbo, MSc (Psychiatry Neuroimaging Laboratory, Brigham and Women’s Hospital), and Ruediger Laubender, MA, MPH (Institute of Medical Informatics, Biometry, and Epidemiology, Ludwig-Maximilians-University), for assistance in the data analyses; Florian Heinen, MD (Department of Pediatric Neurology and Developmental Medicine, Ludwig-Maximilians-University), and Erin D. Bigler, PhD (Department of Psychology, Brigham Young University, Provo, Utah), for assistance in interpreting our results. None of the individuals acknowledged were compensated for their contributions.
Footnotes
Author Contributions:
Drs Koerte and Shenton had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Koerte, Reiser, Shenton.
Acquisition of data: Koerte, Ertl-Wagner, Reiser.
Analysis and interpretation of data: Koerte, Ertl-Wagner, Zafonte, Shenton.
Drafting of the manuscript: Koerte.
Critical revision of the manuscript for important intellectual content: Koerte, Ertl-Wagner, Reiser, Zafonte, Shenton.
Statistical analysis: Koerte.
Obtained funding: Koerte, Ertl-Wagner, Reiser.
Administrative, technical, or material support: Koerte, Ertl-Wagner, Reiser, Shenton.
Study supervision: Koerte, Ertl-Wagner, Reiser, Zafonte, Shenton.
Conflict of Interest Disclosures:
The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Koerte reported receiving a fellowship from the German Academic Exchange Service and Else Kröner-Fresenius Stiftung; having a grant pending with the National Institutes of Health; and receiving travel expenses and accommodations from the Society for Neuro-pediatrics, Germany. Dr Ertl-Wagner reported serving on advisory boards for Springer Publishing, Bracco, and Philips Radiology; serving as a consultant to Munich Medical International; receiving grants to her institution from Deutsches Zentrum fuer Neurodegenerative Erkrankungen, Friedrich-Baur Stiftung, Arbeitsgemeinschaft Botulinumtoxin, Deutsche Forschungsgemeinschaft, Omnibus Stiftung, Guerbet, Merck-Serono, and the Radiological Society of North America; receiving payment for lectures from Philips, Siemens, Deutsche Roentgengesellschaft, Alfried-Krupp-Stiftung, and Bayer-Schering; receiving payment for manuscript preparation from Siemens, Springer Publishing, and Thieme Medical Publishers; receiving royalties from Springer Publishing and Thieme Medical Publishers; receiving payment for the development of educational presentations from Springer Publishing, Bracco, and Siemens; owning stock in Siemens; and receiving travel expenses and accommodations from the European Society of Radiology, the Radiological Society of North America, and Asklepios Clinics. Dr Reiser reported receiving grants to his institution from Deutsche Forschungsgemeinschaft, Eurobioimaging, the German National Cohort, Munich Cluster of Excellence M4 Imaging, and BMBG German Centers for Lung Diseases and Cardiovascular Diseases. Dr Zafonte reported receiving grants to his institution from the National Institutes of Health and the Department of Defense; serving on a board for the International Brain Injury Association; providing expert testimony for a US attorney; receiving royalties from Demos Books for a brain injury textbook; and serving on the editorial board for Elsevier. Dr Shenton reported receiving a grant to her institution from Intrust, the National Institutes of Health, the US Department of Veteran Affairs, the US Department of Defense, the National Alliance for Medical Imaging, the National Institute of Mental Health, National Alliance for Research in Schizophrenia and Depression, Fogarty International Center, National Health and Medical Research Council, and Silvio Conte Centers for Basic Translational Mental Health Research; and receiving travel expenses and accommodations from the 9th World Congress on Brain Injury.
References
- 1.Fédération Internationale de Football Fédération Internationale de Football website. http://www.fifa.com. Accessibility verified October 9, 2012.
- 2.Henry LC, Tremblay J, Tremblay S, et al. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28(10):2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- 3.Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- 4.Kirkendall DT, Jordan SE, Garrett WE. Heading and head injuries in soccer. Sports Med. 2001;31(5):369–386. doi: 10.2165/00007256-200131050-00006. [DOI] [PubMed] [Google Scholar]
- 5.Matser EJ, Kessels AG, Lezak MD, Jordan BD, Troost J. Neuropsychological impairment in amateur soccer players. JAMA. 1999;282(10):971–973. doi: 10.1001/jama.282.10.971. [DOI] [PubMed] [Google Scholar]
- 6.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]