Abstract
Background
Children with heart disease are frequently exposed to imaging examinations using ionizing radiation. Although radiation exposure is potentially carcinogenic, there are limited data on cumulative exposure and the associated cancer risk. We evaluated the cumulative effective dose (ED) of radiation from all radiation examinations to estimate the lifetime attributable risk (LAR) of cancer in children with heart disease.
Methods and Results
Children ≤6 years of age who had previously undergone 1 of 7 primary surgical procedures for heart disease at a single institution between 2005 and 2010 were eligible. Exposure to radiation-producing examinations was tabulated, and cumulative ED was calculated in millisievert (mSv). These data were used to estimate LAR of cancer above baseline using the approach of the Committee on Biological Effects of Ionizing Radiation VII. The cohort included 337 children exposed to 13,932 radiation examinations. Conventional radiographs represented 92% of examinations, while cardiac catheterization and computed tomography accounted for 81% of cumulative exposure. Overall median cumulative ED was 2.7 mSv (range 0.1–76.9 mSv), and the associated LAR of cancer was 0.07% (range 0.001–6.5%). Median LAR of cancer ranged widely depending on surgical complexity (0.006–1.6% for the 7 surgical cohorts) and was twice as high in females per unit exposure (0.04% versus 0.02% per 1 mSv ED for females versus males, respectively; p<0.001).
Conclusions
Overall radiation exposures in children with heart disease are relatively low, however select cohorts receive significant exposure. Cancer risk estimation highlights the need for limiting radiation dose, particularly for high-exposure modalities.
Keywords: catheterization, imaging, pediatrics, radiography
Introduction
Children with congenital and acquired heart disease typically undergo imaging procedures that may expose them to large amounts of ionizing radiation.1-5 Radiation exposure in childhood is of particular concern because children have immature developing organ and tissue structures. These factors, as well as their potentially longer lifespan, may significantly increase lifetime cancer risk.6-8
Previous studies of radiation exposure in children have largely focused on single exposure from various imaging modalities including computed tomography (CT), fluoroscopy, nuclear medicine, and radiographs.9-15 Investigators have emphasized that the radiation risk from a single imaging modality can be high.3,7,8,10,13,15 However, children with complex heart diseases are often exposed to repetitive imaging.16 According to current guidelines from the International Committee on Radiation Protection (ICRP), stochastic exposure risks (i.e. cancer) increase in a linear, dose-response fashion and therefore repetitive exposures are believed to incrementally increase risk.17 What is currently unknown in young children with heart disease, is the amount of cumulative exposure, the relative contribution of various imaging modalities to cumulative exposure, and the associated lifetime attributable risk (LAR) of cancer.
In a cohort of young children undergoing one of seven operations for congenital and acquired heart disease, we sought to estimate: 1) the cumulative effective dose of radiation exposure across the spectrum of radiation-producing imaging modalities; 2) the relative contribution of various imaging modalities to cumulative effective dose; and 3) the estimated LAR of cancer from cumulative radiation exposure.
Methods
Study Population
Children were eligible for inclusion if they were ≤6 years of age and had previously undergone one of seven different primary surgical procedures for heart disease, including isolated atrial septal defect closure, isolated ventricular septal defect (VSD) closure, atrioventricular canal defect (AVCD) repair (including complete, transitional, and partial AVCD), tetralogy of Fallot repair (excluding patients with tetralogy/AVCD, pulmonary atresia or tetralogy with absent pulmonary valve), isolated arterial switch operation (excluding arterial switch ± VSD and / or coarctation repair), cardiac transplant, and Norwood operation, at a single institution between July 1, 2005, and December 31, 2010. Surgical procedures used for study entry were chosen to represent more commonly performed surgical procedures and also a spectrum of surgical complexity. Patients were grouped according to their initial surgical procedure unless their course ended in a cardiac transplantation, in which case they were analyzed in the transplant group. This study was approved by the Duke University Medical Center institutional review board with waiver of informed consent.
Data Collection
Patient demographics were collected from the electronic medical record and included sex, race, age at operation, and the presence of other congenital anomalies. Radiation exposure data were collected from birth, including all specific examinations with radiation-producing imaging modalities (radiographs, fluoroscopy, nuclear medicine, and CT) for each patient. Exposure data were collated by searching institutional databases and also by searching specific current procedural terminology codes through the electronic medical record. A chart review was performed for 10% of the study population to confirm the accuracy of the search and demonstrated <5% missing data.
Effective Dose Calculation
Cardiac catheterization
Organ-specific radiation doses were measured using 2 ATOM family (CIRS, Norfolk, VA) anthropomorphic phantoms (representing 1 and 5 years of age). The phantoms include sectional slabs, each with a thickness of 25 mm, and are manufactured using tissue/organ-equivalent epoxy resins (including bone density formulated to represent a 1- and 5-year-old skeleton). The phantoms each incorporate dosimeters within cancer-susceptible tissue structures including thyroid, lung, breast, thymus, bone marrow, kidney, adrenals, liver, esophagus, pancreas, spleen, stomach, intestine, ovaries, testes, prostate, and bladder. Doses were measured for all conventional angiographic projections. For fluoroscopy assessment the pulsed frame rate was 15 frames per second and for cineangiography the frame rate was 30 frames per second consistent with institutional protocols during the time of the study. Organ-specific data were used to develop a proprietary radiation dose calculator which was then used to determine total catheterization effective dose by entering fluoroscopy and cineangiography times and camera angulation for catheterizations performed upon the patient cohort. The 1 year old calculator was used for exposures ages two years and younger the five year old calculator for exposures between ages three and six years. The relative contribution of anteroposterior versus lateral fluoroscopy exposure was not known retrospectively for the time frame of study; therefore it was assumed that 2/3rds and 1/3rd of the total fluoroscopy exposure came from the anteroposterior angle and lateral angle, respectively. These estimates were validated by reviewing 100 consecutive more recent institutional cardiac catheterizations where mean contribution of fluoroscopy was 35% ± 18% from the lateral camera angle. All phantom data acquisition was performed on a Philips Integris Allura 9 (Philips Healthcare, Netherlands) fluoroscopy system.
Other imaging modalities
Age-specific effective dose estimates for all other radiographic examinations were derived from a combination of previously published institutional data estimated using phantoms (upper gastrointestinal series with small bowel follow through, chest CT, cardiac gated CT angiography, abdomen/pelvis multi-detector array CT, chest CT) and data from the peer-reviewed radiology literature (Appendix 1). A central tendency value was used to define the effective dose of an examination in cases of several source estimates.
Cumulative effective dose estimates were calculated by summing effective doses over each patient's imaging history. The average annual effective dose was defined as the average effective dose per year from birth to the time of last data collection. The post-operative effective dose was defined as the effective dose within the first 3 months after the initial surgical procedure.
LAR Cancer Estimation
Radiation dose was estimated by organ system and summed to estimate effective dose. Cumulative risk of cancer and age- and sex-specific LAR of cancer above baseline was estimated based on the effective dose using the approach of the National Academy of Sciences Committee on Biological Effects of Ionizing Radiation (BEIR) VII.18 The lower and upper limit cancer risk estimations were calculated using the BEIR VII 5% and 95% risk estimates for exposure. These limits were calculated individually for each examination in each patient. The BEIR VII models assume a normal life expectancy, take into account the age at exposure and the sex of the population, and assume that cancer risk is proportional to the radiation dose with no threshold. Therefore, every ionizing radiation-producing procedure performed on an individual produces a corresponding increase in cancer risk. To calculate cancer risk in our high risk population with anticipated shorter life expectancy, excess relative cancer risk was calculated at 0.035 / 1 mSv exposure at mean follow-up of 10 years based on previous epidemiologic data.19 For calculations, exposure was assumed to have occurred at age 5 years. Background cancer rates were based on reported U.S. 5-year cancer incidence for adolescents (ages 15-19 years).20
Statistical Analysis
The unit of observation for this analysis was a subject enrolled in the study. Summary statistics were used to describe the study variables, including means and standard deviations and frequency counts and percentages. Distribution of effective dose and LAR across procedure types were compared using a non-parametric Kruskal Wallis test. All analyses were conducted using Stata 12.0 (College Station, TX), and a 2-tailed p-value<0.05 was considered statistically significant.
Results
Clinical characteristics are presented by surgical subgroup in Table 1. The study cohort consisted of 337 children undergoing one of seven surgical procedures of interest. For the overall cohort, median age at surgery was 88 days (5th–95th percentile 3–819), and median duration of follow-up from birth was 23.9 months (5th–95th percentile 1.6–60.9).
Table 1.
Clinical Characteristics of Total Cohort and Surgical Groups
| Surgical cohort | n (%) | Male, n (%) | Median age at operation (days)* | Median duration of follow-up (days)* |
|---|---|---|---|---|
| ASD | 21 (6.2%) | 9 (42.9%) | 551 (85, 1490) | 1156 (289, 1872) |
| VSD | 83 (24.6%) | 41 (49.4%) | 125 (35, 439) | 819 (83, 1899) |
| AVCD | 52 (15.4%) | 19 (36.5%) | 133 (48, 913) | 1014 (100, 1786) |
| TOF | 63 (18.7%) | 42 (66.7%) | 116 (4, 242) | 653 (71, 1769) |
| ASO | 24 (7.1%) | 22 (91.7%) | 7 (3, 12) | 654 (22, 1630) |
| Cardiac transplant | 10 (3.0%) | 5 (50.0%) | 129 (12, 570) | 1061 (117, 1822) |
| Norwood | 84 (24.9%) | 51 (60.7%) | 6 (2, 19) | 353 (27, 1774) |
| Total | 337 (100.0%) | 189 (56.1%) | 88 (3, 819) | 716 (47, 1826) |
Data represent median (5%, 95%).
ASD = atrial septal defect; VSD = ventricular septal defect; AVCD = atrioventricular canal defect; TOF = tetralogy of Fallot; ASO = atrial switch operation.
The numbers of radiation-producing examinations, average annual, and cumulative effective dose per operative group are listed in Table 2. In total, 13,932 examinations were performed with a median of 17 examinations (5th–95th percentile 4–158) per child and a median cumulative effective dose of 2.7 mSv (5th–95th percentile 0.1–76.9) per child. Radiation exposure varied widely across surgical cohorts: those with more complex heart disease (i.e., cardiac transplant and Norwood cohorts) received substantially greater cumulative exposure. In terms of timing of examinations, the majority were performed in the first 3 months after the entry surgical procedure (6,992/13,932, 50%), but these immediate post-operative examinations accounted for only 26% of cumulative exposure (range 23–36% for the 7 surgical sub-groups). The transplant patients represent a unique cohort in that they frequently have complex pre-transplant medical needs, particularly in those with a prior history of congenital heart disease (70% of our cohort). In these patients, post-transplant radiation accounted for the majority of exposure (72%) with a median post-transplant cumulative effective dose of 45.8 mSv (5th–95th percentile 7.4-154.2).
Table 2.
Number of Ionizing Radiation-producing Examinations, Annual Effective Dose, and Cumulative Effective Dose per Patient According to Operative Group
| Exams per patient (n)* | Average annual effective dose (mSv/year)* | Cumulative effective dose (mSv)* | |
|---|---|---|---|
| ASD (n=21) | 6 (4, 30) | 0.09 (0.02, 6.93) | 0.19 (0.07, 29.28) |
| VSD (n=83) | 9 (4, 79) | 0.20 (0.01, 12.01) | 0.36 (0.06, 23.60) |
| AVCD (n=52) | 11 (4, 111) | 0.26 (0.02, 29.45) | 0.49 (0.07, 30.38) |
| TOF (n=63) | 10 (5, 63) | 0.27 (0.02, 22.59) | 0.55 (0.08, 33.30) |
| ASO (n=24) | 18 (10, 47) | 0.29 (0.05, 3.56) | 0.60 (0.18, 8.19) |
| Cardiac transplant (n=10) | 117 (50, 247) | 42.54 (8.34, 396.31) | 63.79 (9.93, 190.01) |
| Norwood (n=84) | 63 (19, 271) | 20.08 (0.66, 181.98) | 28.93 (0.70, 113.61) |
| Total (n=337) | 17 (4, 158) | 1.34 (0.03, 83.24) | 2.67 (0.08, 76.93) |
Data represent median (5%, 95%).
ASD = atrial septal defect; VSD = ventricular septal defect; AVCD = atrioventricular canal defect; TOF = tetralogy of Fallot; ASO = atrial switch operation.
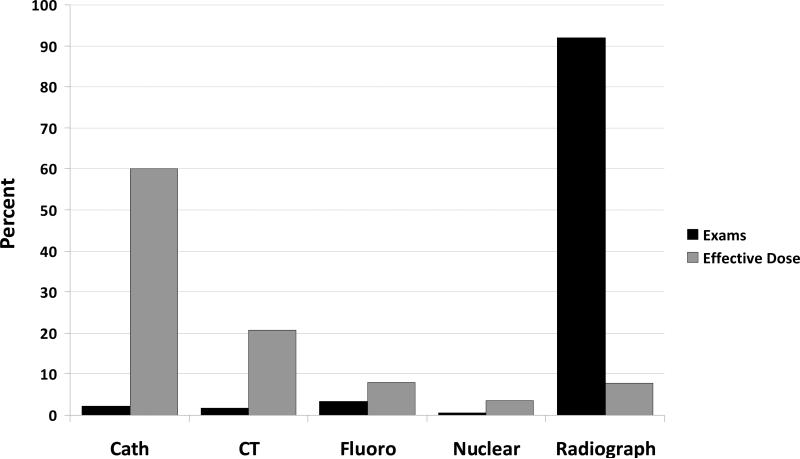
Table 3 shows the relative contribution of radiation-producing examinations to the total cumulative effective dose. Conventional radiographic examinations represented 92% of total examinations but accounted for only 8% of the cumulative effective dose. Conversely, cardiac catheterization procedures represented 1.5% (n=303/13,932) of all examinations but contributed 60% of total radiation exposure (Figure 1). CT angiography of the chest, followed by interventional catheterization examinations, accounted for the highest effective dose per study (Table 3).
Table 3.
Medical Imaging Examinations and Relative Contribution to Cumulative Effective Dose
| Examination | Total number (n) | Effective dose per exam (mSv) | Proportion of cumulative effective dose (%)* |
|---|---|---|---|
| Intervention catheterization | 138 | 13.77 | 35.2 |
| Diagnostic catheterization | 117 | 9.10 | 20.5 |
| Gated CT angiography chest with/without contrast | 21 | 18.28 | 6.8 |
| CT head without contrast | 119 | 2.54 | 5.6 |
| Biopsy catheterization | 48 | 4.65 | 4.3 |
| Portable abdomen flat and upright | 1126 | 0.17 | 3.1 |
| Fluoroscopy tube placement | 152 | 1.00 | 2.7 |
| CT abdomen with contrast | 24 | 5.62 | 2.4 |
| Upper GI | 91 | 1.49 | 2.4 |
| Gastric emptying liquid | 61 | 2.11 | 2.3 |
| Portable KUB anteroposterior | 1517 | 0.08 | 2.2 |
| CT pelvis with contrast | 22 | 4.60 | 1.8 |
| Portable anteroposterior chest | 8847 | 0.01 | 1.6 |
| CT chest without contrast | 16 | 4.77 | 1.4 |
| CT chest with contrast | 8 | 4.77 | 0.7 |
| Barium swallow esophagus | 27 | 1.49 | 0.7 |
| Barium enema colon | 17 | 1.98 | 0.6 |
| Chest posteroanterior and lateral | 1161 | 0.03 | 0.6 |
| CT angiography head with/without contrast | 4 | 5.07 | 0.4 |
| Upper GI with small bowel follow through | 16 | 1.49 | 0.4 |
| CT temporal bone with contrast | 8 | 2.54 | 0.4 |
| Hepatobiliary imaging study | 2 | 9.14 | 0.3 |
| Lasix renal scan | 7 | 2.45 | 0.3 |
| CT angiography abdomen with/without contrast | 3 | 5.61 | 0.3 |
| Perfusion lung scan | 11 | 1.30 | 0.3 |
Proportion of cumulative effective dose by examination calculated for the total cohort.
CT = computed tomography; GI = gastrointestinal; KUB = kidneys ureters bladder.
Figure 1.
Percentage contribution of each imaging modality to number of examinations performed and cumulative effective dose. Cath = catheterization; CT = computed tomography; fluoro = fluoroscopy.
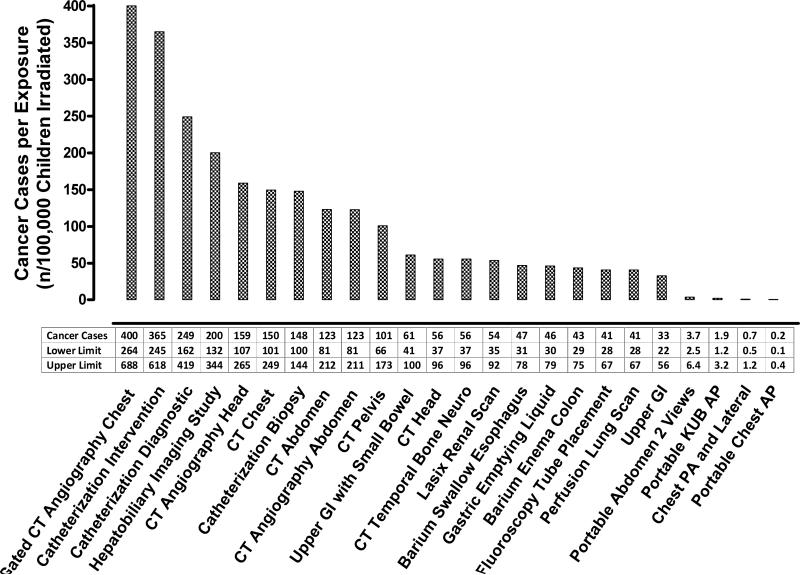
The estimated LAR of cancer above baseline per operative group and examination modality is listed in Table 4. Median LAR across surgical cohorts was 65 cases/100,000 children exposed. Lower (43 cases per 100,000 exposed) and upper limits (112 cases / 100,000 exposed) of LAR represent the median cohort 5% and 95% LAR respectively based on the BEIR VII confidence intervals. LAR of cancer per unit exposure was substantially greater in females (41/100,000 versus 22/100,000 per 1 mSv effective dose for females versus males respectively, p<0.001), primarily reflecting increased breast and thyroid cancer risk. The LAR per individual radiation-producing examination varied widely depending on examination, exceeding 350 cases/100,000 children exposed to a CT angiography of the chest and interventional catheterization but only 0.2 cases/100,000 children exposed to a portable chest x-ray (Figure 2).
Table 4.
Lifetime Attributable Risk of Cancer above Baseline According to Operative Group
| Cancer Cases / 100,000 Exposed | ||
|---|---|---|
| Median (LL, UL) | 5,95% | |
| ASD | 6 (4,11) | 2,836 |
| VSD | 12 (8,20) | 2, 846 |
| ASO | 13 (9,22) | 4,180 |
| TOF | 14 (9,23) | 2, 1311 |
| AVCD | 20 (13,32) | 2, 698 |
| Norwood | 799 (536,1354) | 15, 3043 |
| Cardiac transplant | 1677 (1125,2815) | 405, 6467 |
| Total | 65 (43, 112) | 2, 2319 |
LL = lower limit; UL = upper limit; these values were calculated using the BEIR VII confidence intervals. A LL and UL was calculated for each patient, and the data presented represent the median cohort values for these limits. The 5% and 95% estimates represent the overall cohort 5% and 95% limits calculated using the standard BEIR VII age and sex specific cancer estimates. ASD = atrial septal defect; VSD = ventricular septal defect; AVCD = atrioventricular canal defect; TOF = tetralogy of Fallot; ASO = atrial switch operation.
Figure 2.
The lifetime attributable risk of cancer above baseline for specific radiation-producing examinations. The lifetime attributable risk of cancer was calculated using median measured exposure for all cardiac catheterization procedures and estimated exposure based on previously published data for all other radiation-producing examinations.Appendix 1 The lower and upper limits are based on the BEIR VII confidence intervals. A lower and upper limit estimate was calculated for each examination on each patient, and the data presented represent the median cohort values for these limits. CT = computed tomography; GI = gastrointestinal; KUB = kidneys ureters bladder; AP = anteroposterior; PA = posteroanterior.
As the cardiac transplant and Norwood cohorts may not have a normal anticipated life expectancy, we also estimated relative cancer risk in the short term for these two cohorts. Based on cumulative exposure, the median 10-year sex-averaged relative risk of any cancer compared to an unexposed population was 3.2 (5%, 95%: 1.4-7.7) for the transplant cohort and 2.0 (5%, 95%:1.0-5.0) for the Norwood cohort. Based on background cancer incidence among U.S adolescents, this translates to a median 5-year sex-averaged all-cancer incidence [between the ages of 15 and 19] of 69.4 and 43.4 per 100,000 for the two cohorts respectively.
Discussion
This is the largest study evaluating cumulative radiation exposure across the spectrum of imaging modalities to estimate the associated LAR of cancer in children with heart disease. While commonly performed, radiographs contribute a relatively small proportion to total radiation exposure. Conversely, less commonly performed but higher exposure imaging modalities such as catheterization and chest CT are the most important contributors to cumulative radiation exposure and, therefore, lifetime cancer risk.
In the United States and internationally, use of radiation-producing imaging examinations in children continues to rise.7 Although children benefit from advanced imaging procedures for more accurate diagnosis and less-invasive treatment, radiation has potential health risks. Several studies have shown that for a given dose of radiation, children are 3–4 times more likely than adults to develop malignancies.2,6,18
Risk associated with radiation exposure is particularly relevant for children with more complex heart diseases who often receive repetitive imaging with high-exposure modalities. Even in the limited time frame studied, the estimated LAR of cancer above baseline was as high as 6.5%. Shortened anticipated lifespan in these high risk cohorts does not mitigate cancer mortality and morbidity risks as they have a significantly increased relative risk of cancer even within the first 10 years following exposure. These data are consistent with epidemiologic data demonstrating that relative risk of cancer is highest in the early years after exposure.18 Conversely, for children with lower complexity heart disease, and a presumably less complicated course, exposure was reassuringly low. For five of the seven procedure cohorts, the median annual ED (0.09 - 0.29 mSv) from imaging procedures was substantially below the annual background exposure within the United States (3.0-3.5 mSv).21 Nonetheless, LAR of cancer exceeded 0.5% at the upper limits of exposure for 6/7 cohorts with the notable exception of children following arterial switch operation.
These data provide actionable information that could be employed to reduce exposure and suggest that the greatest risk reduction can be achieved with a targeted approach focused on minimizing radiation use during high-exposure examinations such as catheterization and CT. Although less frequently performed, these modalities are the main contributors to cumulative effective dose and can contribute up to 1,800 times as much effective dose per examination as a standard radiograph. In our cohort, higher-risk patients were frequently exposed to these high-exposure imaging modalities repeatedly beyond the immediate post-operative period and consequently had much higher average annual effective dose. Conversely, conventional radiographic examinations were primarily performed during the immediate post-operative period and, although high in volume, contributed a relatively small amount of cumulative effective dose. This finding is consistent with prior publications.16 It is also important to recognize that risk of cancer was substantially higher in the female population due to the increased risk of breast and thyroid cancer.18
Strengths of this current analysis include the large sample size, and our comprehensive approach to estimating effective dose. Our effective dose data are particularly robust as many of the effective dose calculations (including the highest exposure modalities—chest CT and catheterization) were obtained using data from dosimeters placed over vital tissue structures in anthropomorphic phantoms.15 In the case of the catheterization procedures, these data were then combined with actual patient data on fluoroscopic and cineangiographic times and camera angles. This allowed us to directly measure organ-specific exposures. The median effective dose from therapeutic and diagnostic cardiac catheterization procedures (13.77 mSv and 9.10 mSv) was higher than in previous publications.2-4 However, these previous publications all used simulation models to calculate an effective dose in mSv from the reported skin exposure represented in milligray.2-4 Estimates of skin exposure calculated by the equipment from technical parameters may introduce error depending on the equipment. External exposure data also fails to account for beam attenuation and other factors that alter absorbed radiation dose. Therefore these data are generally less accurate. Although phantom data are more robust, there are also limits as phantoms do not perfectly approximate the clinical setting where factors including body habitus, ergonomics, anatomy and variation in imaging parameters all uniquely affect exposure.
This study has several additional limitations. First, there are inherent limitations to a single-center observational study. In particular, the surgical cohorts were relatively small and heterogeneous with the lifetime radiological history derived from hospital records over a 5-year period with a median per-patient follow-up of approximately 2 years. The follow-up periods varied for the specific cohorts, and a meaningful number of patients died during follow up. These factors lead unavoidably to an approximation that likely underestimates the total radiation exposure and may bias relative estimates in select cohorts. Second there is variability in the dose of each radiation examination and while phantom data provide the most accurate estimate of our institutional exposure, they may not be directly generalizable to institutions using different imaging protocols or equipment.15,22 A third limitation is that cancer estimates using BEIR VII tables are subject to sources of uncertainty due to inherent limitations in epidemiological data and in the general understanding of how radiation exposure increases the risk of cancer. Moreover, we used effective dose to calculate LAR, whereas the BEIR VII data use summed cancer risks for individual organs following a total-body exposure. Although the use of effective dose in this context is not strictly correct, it has been shown that the 2 approaches yield similar values of LAR.23-26
Conclusions
The effective dose from radiation-producing imaging examinations varies greatly across the spectrum of imaging modalities. Overall, for our patient cohort, cumulative effective dose was relatively low, less than the annual background exposure in the U.S. However select children with complex heart disease can be exposed to large cumulative doses that increase the estimated LAR of cancer to up to 6.5% above baseline, even in the limited time frame studied. High-exposure imaging modalities such as catheterization and CT are the most important contributors to the cumulative effective dose. To reduce long term cancer risk, providers should target reducing radiation exposure in the highest risk cohorts including those children that will require repetitive high-exposure imaging and females because of their increased cancer risk. Providers can consider our relative exposure estimates when choosing between various radiation producing imaging modalities. Ultimately novel technologies and / or therapies are needed to mitigate risk of radiation exposure. With a burgeoning population of children with heart disease surviving into adulthood, these advances will have a very meaningful public health impact.
Supplementary Material
Acknowledgments
Sources of Funding
Research reported in this publication was supported by:
1. The National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001117.
2. The Mend A Heart Foundation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Mend A Heart Foundation
Footnotes
Disclosures
TKY receives support from the U.S. Nuclear Regulatory Commission, The U.S. Department of Energy, and from a Coulter Research Grant from Duke University for his work on Radiation Dosimetry.
None of the remaining authors have disclosures relevant to the topic of the manuscript
References
- 1.Andreassi MG. Radiation risk from pediatric cardiac catheterization: friendly fire on children with congenital heart disease. Circulation. 2009;120:1847–1849. doi: 10.1161/CIRCULATIONAHA.109.904458. [DOI] [PubMed] [Google Scholar]
- 2.Beels L, Bacher K, De Wolf D, Werbrouck J, Thierens H. γ-H2AX foci as a biomarker for patient x-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risk? Circulation. 2009;120:1903–1909. doi: 10.1161/CIRCULATIONAHA.109.880385. [DOI] [PubMed] [Google Scholar]
- 3.Bacher K, Bogaert E, Lapere R, De Wolf D, Thierens H. Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation. 2005;111:83–89. doi: 10.1161/01.CIR.0000151098.52656.3A. [DOI] [PubMed] [Google Scholar]
- 4.El Sayed MH, Roushdy AM, El Farghaly H, El Sherbini A. Radiation exposure in children during the current era of pediatric cardiac intervention. Pediatr Cardiol. 2012;33:27–35. doi: 10.1007/s00246-011-0064-z. [DOI] [PubMed] [Google Scholar]
- 5.Yakoumakis E, Kostopoulou H, Makri T, Dimitriadis A, Georgiou E, Tsalafoutas I. Estimation of radiation dose and risk to children undergoing cardiac catheterization for the treatment of a congenital heart disease using Monte Carlo simulations. Pediatr Radiol. 2013;43:339–346. doi: 10.1007/s00247-012-2510-3. [DOI] [PubMed] [Google Scholar]
- 6.Andreassi MG, Ait-Ali L, Botto N, Manfredi S, Mottola G, Picano E. Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. Eur Heart J. 2006;27:2703–2708. doi: 10.1093/eurheartj/ehl014. [DOI] [PubMed] [Google Scholar]
- 7.Dorfman AL, Fazel R, Einstein AJ, Applegate KE, Krumholz HM, Wang Y, Christodoulou E, Chen J, Sanchez R, Nallamothu BK. Use of medical imaging procedures with ionizing radiation in children: a population based study. Arch Pediatr Adolesc Med. 2011;165:458–464. doi: 10.1001/archpediatrics.2010.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. 2006;36:121–125. doi: 10.1007/s00247-006-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gogos KA, Yakoumakis EN, Tsalafoutas IA, Makri TK. Radiation dose considerations in common paediatric x-ray examinations. Pediatr Radiol. 2003;33:236–240. doi: 10.1007/s00247-002-0861-x. [DOI] [PubMed] [Google Scholar]
- 10.Frush DP. Radiation, thoracic imaging, and children: radiation safety. Radiol Clin North Am. 2011;49:1053–1069. doi: 10.1016/j.rcl.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Strauss KJ, Kaste SC. The ALARA (as low as reasonably achievable) concept in pediatric interventional and fluoroscopic imaging: striving to keep radiation dose as low as possible during fluoroscopy of pediatric patients – a white paper executive summary. Pediatr Radiol. 2006;36:110–112. doi: 10.1007/s00247-006-0184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahey FH, Treves ST, Adelstein SJ. Minimizing and communicating radiation risk in pediatric nuclear medicine. J Nucl Med Technol. 2012;40:13–24. doi: 10.2967/jnumed.109.069609. [DOI] [PubMed] [Google Scholar]
- 13.Brody AS, Frush DP, Huda W, Brent RL, American Academy of Pediatrics Section on Radiology Radiation risk to children from computed tomography. Pediatrics. 2007;120:677–682. doi: 10.1542/peds.2007-1910. [DOI] [PubMed] [Google Scholar]
- 14.Brenner DJ, Elliston CD, Hall EJ, Berdon WE. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR. 2001;176:289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 15.Hollingsworth CL, Yoshizumi TT, Frush DP, Chan FP, Toncheva G, Nguyen G, Lowry CR, Hurwitz LM. Pediatric cardiac-gated CT angiography: assessment of radiation dose. AJR. 2007;189:12–18. doi: 10.2214/AJR.06.1507. [DOI] [PubMed] [Google Scholar]
- 16.Ait-Ali L, Andreassi MG, Foffa I, Spadoni I, Vano E, Picano E. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart. 2010;96:269–274. doi: 10.1136/hrt.2008.160309. [DOI] [PubMed] [Google Scholar]
- 17.The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Nuclear and Radiation Studies Board, Division on Earth and Life Studies. National Research Council of the National Academies . Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. The National Academies Press; Washington, DC: 2006. [Google Scholar]
- 19.Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes BG, Giles GG, Wallace AB, Anderson PR, Guiver TA, McGale P, Cain TM, Dowty JG, Bickerstaffe AC, Darby SC. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. doi: 10.1136/bmj.f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [October 28th, 2013];Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. Accessed at < http://seer.cancer.gov/publications/aya>.
- 21.National Council on Radiation Protection and Measurements . Ionizing radiation exposure of the population of the United States. National Council on Radiation Protection report no. 160. National Council on Radiation Protection and Measurements; Bethesda, Md: 2009. [Google Scholar]
- 22.Podberesky DJ, Angel E, Yoshizumi TT, Toncheva G, Salisbury SR, Alsip C, Barelli A, Egelhoff JC, Anderson-Evans C, Nguyen GB, Dow D, Frush DP. Radiation dose estimation for prospective and retrospective ECG-gated cardiac CT angiography in infants and small children using a 320-MDCT volume scanner. AJR. 2012;199:1129–1135. doi: 10.2214/AJR.12.8480. [DOI] [PubMed] [Google Scholar]
- 23.Smith-Bindman R, Lipson J, Marcus R, Kim K, Mahesh M, Gould R, Berrington de Gonzalez A, Miglioretti DL. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCollough CH, Christner JA, Kofler JM. How effective is effective dose as a predictor of radiation risk? AJR. 2010;194:890–896. doi: 10.2214/AJR.09.4179. [DOI] [PubMed] [Google Scholar]
- 25.Martin CJ. Effective dose: how should it be applied to medical exposures? Br J Radiol. 2007;80:639–647. doi: 10.1259/bjr/25922439. [DOI] [PubMed] [Google Scholar]
- 26.Hendee WR, O'Connor MK. Radiation risks from medical imaging: separating fact from fancy. Radiology. 2012;264:312–321. doi: 10.1148/radiol.12112678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.