Abstract
Leydig cells are the testosterone-producing cells of the testis. The adult Leydig cell (ALC) population ultimately develops from undifferentiated mesenchymal-like stem cells present in the interstitial compartment of the neonatal testis. Distinct stages of ALC development have been identified and characterized. These include stem Leydig cells (SLCs), progenitor Leydig cells, immature Leydig cells, and ALCs. This review describes our current understanding of the SLCs in the fetal, prenatal, peripubertal, adult, and aged rat testis, as well as recent studies of the differentiation of steroidogenic cells from the stem cells of other organs.
Keywords: stem cell, Leydig cell, aging
INTRODUCTION
Testosterone, produced by Leydig cells of the mammalian testis, is important throughout the lifetime of the male. High levels of testosterone are produced by the fetal testis, exerting organizational effects on the morphogenesis of specific organs and programming effects on neural functions and enzyme activities that are expressed later in life (Forest, 1983; Huhtaniemi and Pelliniemi, 1992; Scott et al., 2009). The cells that are responsible for testosterone production in the fetal testis, the fetal Leydig cells (FLCs), do not require luteinizing hormone (LH) for their formation, though late in gestation they attain LH receptors (LHRs) and responsiveness to LH (Baker and O’Shaughnessy, 2001; Migrenne et al., 2001). The Leydig cells of the adult also produce high levels of testosterone and are under the regulation of LH. These cells, which are first seen during puberty, form from stem cells in the postnatal testis (Ge et al., 2006).
The Leydig cells of the adult testis represent a stable population of cells that rarely divide or die (Teerds et al., 1989). The adult Leydig cells (ALCs) can be selectively eliminated by a single injection of the alkylating agent, ethane dimethane sulphonate (EDS) (Kerr et al., 1985; Jackson et al., 1986; Morris et al., 1986). Following their elimination, a new generation of Leydig cells appears. The new cells produce testosterone at the same high level of the cells they replace. The origin of the new generation of cells remains uncertain. With aging, Leydig cell testosterone production diminishes (Chen et al., 1994). The aged cells also can be eliminated with EDS, after which there is repopulation of the aged testis with a new generation of Leydig cells (Chen et al., 1996). The repopulated cells, though residing in aged animals, produce testosterone at the high level of young cells. Key questions remain to be answered about the nature of the stem/progenitor cells in the young and aged testes, their location, whether or not they age, why they are normally quiescent, and why they divide and differentiate upon elimination of the ALC population.
This article reviews our current understanding of the stem Leydig cells (SLCs) in the fetal, prenatal, peripubertal, adult and aged rat testis, focusing on the differentiation of ALCs through a four-stage model, from stem to progenitor to immature to ALCs. We then discuss the issue of whether or not the SLCs age. Finally, we review recent studies of the differentiation of steroidogenic cells from the stem cells of other nonsteroidogenic organs.
LEYDIG CELL POPULATIONS: FROM FETUS TO ADULT
Fetal Leydig Cells
In mammalian species, two distinct populations of Leydig cells have been identified, FLCs and ALCs. These cells appear sequentially from development through adulthood. During gestation, prior to the formation of a definitive fetal mouse testis (embryonic day 11.5–12.5), there is a mixture of primordial germ cells and somatic progenitor cells (Brennan and Capel, 2004). The progenitor cells express steroidogenic factor 1 (SF-1), among other transcription factors. The SF-1-positive progenitor cells give rise to the FLCs during gestation, as well as to Sertoli cells. The differentiation of FLCs has been shown to be regulated at least in part by three signaling molecules or pathways: platelet-derived growth factor A (PDGFA), desert hedgehog (DHH), and Notch signaling (Brennan and Capel, 2004; Ross and Capel, 2005; Barsoum and Yao, 2006; Tang et al., 2008). Knockout of PDGFR has been shown to result in reduced FLC differentiation (Brennan et al., 2003). Testes of DHH knockout embryos develop fewer FLCs than wild-type mice, and the adults lack Leydig cells (Bitgood et al, 1996; Clark et al, 2000). Results such as these suggest that there may be a relationship between the FLCs and ALCs. On the other hand, the apparently different origins of these cells, their different gene expression profiles (Dong et al., 2007), their sequential appearance, and the observation that there are few FLCs that remain in the adult testis, suggest that the FLCs and ALCs represent distinct populations of cells.
Although FLCs, which produce high levels of testosterone, ultimately express the LHR and respond to LH stimulation (Baker and O’Shaughnessy, 2001; Migrenne et al., 2001), LH is not required for their development or their initial formation of testosterone. Thus, during the prenatal period, testosterone levels do not differ between LHR null and wild-type mice (Zhang et al., 2001). In the rat, the FLCs become competent to produce testosterone by gestational day (GD) 15.5, with production increasing markedly thereafter (Habert and Picon, 1984) and reaching a peak just prior to birth, on GD19 (Habert and Brignaschi, 1991). Testosterone secreted by the FLCs is required for the differentiation of the male urogenital system late in gestation (Huhtaniemi and Pelliniemi, 1992). FLCs also play a role in the scrotal descent of the testis through their synthesis of insulin-like growth factor 3 (Zimmermann et al., 1999).
Development of Adult Leydig cells (ALCs)
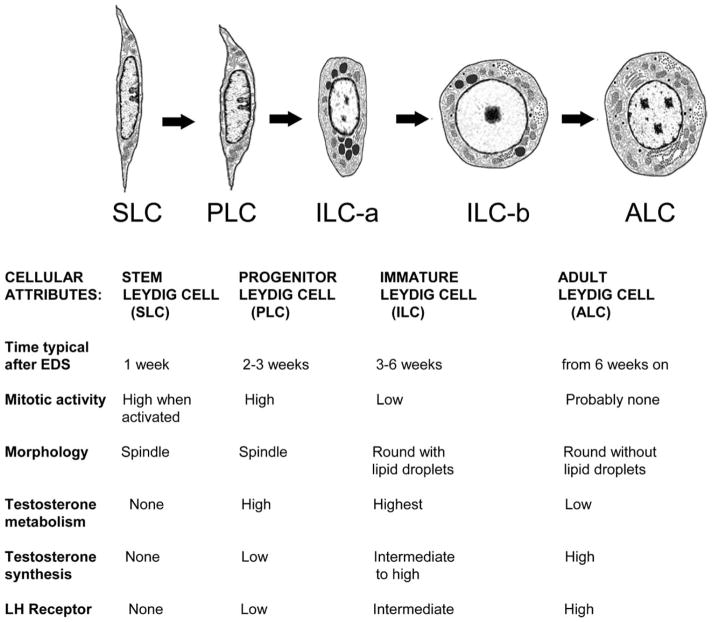
In the rat, testosterone production declines with the loss of the FLCs, reaching a nadir at 2–4 weeks postpartum. Testosterone production then gradually increases to high levels with the development of ALCs (Hardy et al., 1989; Benton et al., 1995; Mendis-Handagama and Ariyaratne, 2001). Though some FLCs apparently persist in the adult testis, they are unlikely to contribute significantly to the testosterone production in the adult (Benton et al., 1995). The ALCs develop from stem cells in the neonate. Four distinct stages of ALC development have been identified, each characterized by the cell types that are involved: SLCs, progenitor Leydig cells (PLCs), immature Leydig cells (ILCs), and ALCs. The time-course of postnatal Leydig cell differentiation and proliferation, and the distinguishing characteristics of SLCs, PLCs, ILCs, and ALCs, are shown in Figure 1. In brief, the SLCs are capable of indefinite self-renewal but also of differentiation to steroidogenic cells. By postnatal days 10–14, some SLCs commit to the Leydig cell lineage, giving rise to the PLCs. These cells proliferate and continue to differentiate to produce the ILCs, which produce high levels of testosterone metabolites. This transition, completed by day 28, accounts for half of the 25 million Leydig cells in the adult rat testis. ILCs then undergo 1–2 divisions and further differentiate, producing the full complement of 25 million ALCs by postnatal day 56. The ALCs are terminally differentiated cells that are characterized by their production of high levels of testosterone.
Figure 1.
Differentiation of the adult Leydig cell population in the rat from stem Leydig cells postnatally. (SLCs) in EDS treated rats. The diagrams depict SLCs, PLCs, ILCs, and ALCs. The cells are depicted as they would appear in situ after EDS treatment on weeks 1, 2–3, 3–6, and after 6. The characteristics of each cell type are listed and described in text (Modified from Benton et al., 1995 and Chen et al., 2009).
Stem Leydig cells (SLCs)
At postnatal day 7, spindle-shaped cells are seen in the rat testicular interstitum, primarily in the peritubular layer but also surrounding the vasculature (Davidoff et al., 2004, 2009). Hardy and his colleagues hypothesized that at least some among these cells in the neonatal rat testis are SLCs (Ge et al., 2006). The putative SLCs were isolated, along with FLCs, from the testes of rat pups on postnatal day 7 by Percoll gradient centrifugation (Ge et al., 2006). By immunoselecting for LHR-negative cells, the FLCS were removed. The remaining cells, putative SLCs, were further purified by immunoselection for the stem cell marker platelet-derived growth factor receptor α (PDGFRα). Over 99% of the cells thus selected were found to be 3β-Hydroxysteroid Dehydrogenase (3β-HSD) negative, LHR negative, and PDGFR-α positive. The cells also were c-kit (83.8%) and leukemia inhibitory factor receptor (97.3%) positive. When cultured in expansion medium that contained various growth factors, the cells maintained a stable 3β-HSD−/LHR−/PDGFRα+ phenotype for more than 6 months. However, when the cells were switched to differentiation medium containing PDGF β homo-dimer (PDGF-BB), LH, thyroid hormone and IGF-1, the cells were induced to express the Leydig cell steroidogenic enzymes, cholesterol side-chain cleavage (P450scc), 3β-HSD and 17α-hydroxylase (P450c17), as well as LHR and steroidogenic acute regulatory protein (StAR). These cells also began to produce testosterone (Ge et al., 2006).
In addition to the ability to self-renew and differentiate, another important characteristic of stem cells is the ability to replenish their niche. To examine the potential of the putative SLCs to differentiate into Leydig cells in vivo, the cells were first tagged with a fluorescent tracking dye, carboxyfluorescein diacetate, and then injected into the adult rat testis from which Leydig cells had been eliminated by administering the Leydig cell toxin EDS to the rats. Ten days after the putative SLCs were injected into the Leydig cell-depleted testes, significant numbers of fluorescently labeled cells were found in the interstitial compartment. Many among the injected cells became 3β-HSD positive, indicating that the injected cells had begun to differentiate in the testis. These results, taken together, indicated that the 3β-HSD−/LHR−/PDGFRα+ cells that had been isolated from postnatal day 7 testes indeed were SLCs because they are capable of self-renewal in vitro without showing signs of differentiation, differentiation into testosterone producing Leydig cells in vitro, and replenishment of their niche in vivo (Ge et al., 2006).
Progenitor Leydig cells (PLCs)
Commitment of some SLCs to the Leydig cell lineage occurs by postnatal days 10 to 14, giving rise to the PLCs. These cells also are spindle-shaped. In contrast to the SLCs, the PLCs are recognizable as members of the Leydig cell lineage by their expression of Leydig cell markers 3β-HSD and LHR, and by their production of low levels of testosterone and relatively high testosterone metabolism. The PLCs have high mitotic activity. Their proliferation forms the cells that ultimately become ALCs.
Immature Leydig cells (ILCs)
By postnatal day 28, the PLCs transform from spindle-shaped to round, acquire numerous lipid inclusions and form a population of ILCs. The number of such cells increases to ~13–14 million (Hardy et al., 1989). During the transformation from PLCs to ILCs, the smooth endoplasmic reticulum (smooth ER) content of the cells expands greatly, conferring an ultrastructure similar to that of ALCs. Concurrent with the expansion of the smooth ER, the levels of 3β-HSD, P450scc, and P450c17 increase (Haider et al., 1986; Dupont et al., 1993; Shan et al., 1993), and the cells develop an increased capacity for testosterone production and testosterone metabolism (Zirkin and Ewing, 1987). Due to the presence of testosterone metabolizing enzymes (3α-hydroxysteroid dehydrogenase, 3α-HSD; and 5α-reductase), testosterone is not the major steroid produced. Rather, the primary steroid product of ILCs is the androgen metabolite, 5α-andro-stane-3α, 17β-diol. The ability to produce testosterone not only involves an increase in biosynthetic activity but also a decrease in the activities of the testosterone-metabolizing enzymes (Inano and Tamaoki, 1966; Steinberger and Ficher, 1969). Consistent with the enzyme activities, mRNA levels for 3α-HSD (Shan et al., 1993) and 5α-reductase are higher in ILCs than the ALCs (Murono, 1989). The mitotic activity of these cells is relatively low, and the ILC population is thought, on average, to divide only once between day 28 and day 56.
Adult Leydig cells (ALCs)
By day 56, the ILCs have divided to produce the total adult population of 25 million ALCs per testis (Benton et al., 1995). The activities of androgen metabolizing enzymes decline by day 56 (Inano and Tamaoki, 1966; Steinberger and Ficher, 1969) as ILCs differentiate into ALCs. The decrease in androgen metabolism and continued increase in levels of testosterone biosynthetic enzymes culminate in the predominance of testosterone over 5α-reduced products in the ALCs. In Leydig cells from 90 day-old adults, testosterone production is 150 times greater than that by PLCs at 21 days of age, and five times greater than that by ILCs at 35 days of age (Shan et al., 1993). Compared with ILCs, the ALCs have a greater abundance of smooth ER and are largely devoid of lipid inclusions (Zirkin and Ewing, 1987). ALCs do not normally proliferate (Keeney et al., 1988) but will be regenerated if the original population is eliminated. Thus, the adult population of Leydig cells completely regenerates within 6–10 weeks of the elimination of ALCs by EDS administration (Kerr et al., 1987; Sharpe et al., 1990). This regeneration may involve the same progression as occurs during normal development of ALCs; as is the case during the normal developmental sequence, there is a period after EDS administration in which 5α-reduced androgens predominates before testosterone becomes the major product (Vreeburg et al., 1988; Risbridger and Davies, 1994; Chen et al., 1996).
With aging, both serum and testicular testosterone concentrations progressively decline (Harman et al., 2001). Testosterone decline in the human is associated with alterations in body composition, diminished energy, muscle strength and physical function, reduced sexual function, depressed mood, and decreased cognitive function (Matsumoto, 2002). The age-related decrease in serum testosterone results from the loss of steroidogenic function of the old Leydig cells, not from a reduction of cell numbers (Chen et al., 1994).
STEM LEYDIG CELLS IN ADULT ANIMALS
Evidence for the Presence of Stem Leydig Cells in the Adult Testis
Leydig cells in the adult testis rarely turn over (Keeney et al., 1988; Teerds et al., 1989c). Nevertheless, numerous studies have shown that if the ALC population is depleted experimentally by a single injection of the alkylating agent EDS, a completely new generation of Leydig cells subsequently is restored in the testis. Following the regeneration of Leydig cells, serum testosterone, which becomes undetectable during the first week after EDS treatment, is restored to the pretreatment levels within 4–8 weeks. Such studies have suggested that there must be a stem/progenitor population in the adult testis from which the new population of Leydig cells can be regenerated.
There are other studies that also suggest that adult rodent testis may contain SLCs. One such study involved the transplantation of a “Hoechst dim” population of cells isolated from the testes of cryptorchid mice (Lo et al., 2004). Within bone marrow the “Hoechst dim” side population (SP) cells represents about 0.05–0.1% of the total nucleated cells (Goodell et al., 1997). By the use of transplantation assays, it was confirmed that these bone marrow SP cells are capable of restoring the hematopoietic system and therefore are hematopoietic stem cells (Goodell et al., 1996). SP cells found in other tissues, e.g., skeletal muscle, brain, mammary gland, and liver, also have been suggested to be stem cells (de Rooij and van Bragt, 2004). Such cells isolated from the testes of cryptorchid mice have been transplanted into the interstitium of mice with targeted deletion of the LHR gene (Lo et al., 2004). The recipient mice were hypogonadal and infertile, but following transplantation there was a significant increase in cells within the interstitial space, increased circulating testosterone levels, and restoration of spermatogenesis. Moreover, the cells were positive for the Leydig cell maker protein cyto-chrome, P450scc. Such studies suggest that Leydig stem cells have properties in common with stem cells from other renewing sources.
The Dynamics of Leydig Cell Regeneration After EDS
A single dose of EDS can eliminate all Leydig cells in the adult rat testis (Kerr et al., 1985; Molenaar et al., 1985; Jackson et al., 1986; Morris et al., 1986). In the normal rat testis, there are about 25 million Leydig cells. In the first week after EDS, Leydig cells are depleted and, as a consequence, serum testosterone becomes undetectable. At 2–3 weeks postEDS, the spindle-shaped cells around seminiferous tubules and blood vasculature begin to proliferate and to differentiate into 3β-HSD-expressing progenitor cells. By ~4 weeks after EDS, ~15 million ILCs form clusters in perivascular and peritubular locations. Between 6 and 10 weeks after EDS treatment, the ILCs are transformed into adult-type Leydig cells, assuming positions within the interstitium, with numbers restored to pretreatment levels (Kerr et al., 1987; Sharpe et al., 1990).
Interestingly, the proliferation of precursor cells during the early regeneration process may not occur in a continuous manner. Up to three distinct proliferation periods were identified (Teerds et al., 1990; Myers and Abney, 1991; Gaytan et al., 1992; Miyano et al., 1997; Yan et al., 2000). The first period, immediately following EDS treatment, may represent the proliferation of stem cells. The second, at 10 days, coincides with the appearance of the first 3β-HSD positive spindle-shaped cell at 10–14 days post EDS (Bakalska et al., 2002), and thus may represent differentiation of stem into progenitor cells and their subsequent proliferation. The third period, occurring around 20 days, may represent the appearance of the ILCs, cells that have the appearance of Leydig cells and have increased 5α-reductase activity (Vreeburg et al., 1988; Risbridger and Davies, 1994). Overall, these results suggest that the sequence of events following ALC depletion with EDS are reminiscent of the sequence of events beginning at postnatal day 7 that result in the formation of ALCs (Fig. 1). The process includes the division of spindle-shaped mesenchymal stem cells, appearance of 3β-HSD positive progenitors, division of round ILCs with their increased 5α-reductase activity (Vreeburg et al., 1988; Myers and Abney, 1991; Chen et al., 1996), and finally the appearance of testosterone-producing ALCs.
The Identity and Location of Stem Leydig Cells in the Adult Testis
Early morphological studies of EDS-treated rats suggested that the Leydig cells may arise from connective tissue cells that includes fibroblasts, lymphatic endothelial cells, and pericytes (Jackson et al., 1986; Kerr et al., 1987). Subsequent studies have indicated that there are spindle-shaped cells in both perivascular and peritubular locations in the interstitial compartment that could be involved in ALC development (Haider et al., 1995; Ariyaratne et al., 2003; Davidoff et al., 2004, 2009). However, the relative contribution of cells from these different locations to the establishment of the ALC population remains uncertain. There are studies suggesting that cells located along the outer surface of the seminiferous tubules (peritubular cells) are the precursor cells to Leydig cell regeneration after EDS, while other studies have suggested that the predominant cells involved are those associated with testicular blood vessels, namely vascular smooth muscle cells and pericytes (Davidoff et al., 2004, 2009).
The peritubular origin hypothesis, which is consistent with the studies of normal Leydig cell development during the prepubertal period (Haider et al., 1995; Russell et al., 1995; Haider and Sevros, 1998; Ariyaratne and Mendis-Handagama, 2000), suggests that Leydig cells differentiate during postnatal days 10–15 from fibro-blast-like cells of the multilayered tubule wall, and that the products (PLCs or ILCs) move to areas within the interstitial compartment where they continue to divide and differentiate to achieve the final population of ALCs (Russell et al., 1995). Proponents of the vascular origin hypothesis have suggested that Leydig cells differentiate from perivascular smooth muscle cells and/or pericytes (Davidoff et al., 2004, 2009). This is based on the observation that cells expressing nestin, a stem cell marker in the nervous system, appear to differentiate from the perivascular region of the testis after EDS and then go on to form ALCs. Indeed, it is possible that cells from both locations contribute to ALC development after EDS treatment, depending on the local conditions. For example, a number of labs have reported that Leydig cell regeneration occurs more rapidly around regressed tubules than around tubules with normal spermatogenesis (Kerr and Donachie, 1986; O’Leary et al., 1986; Sharpe et al., 1990; O’Shaughnessy et al., 2008). In testes with normal spermatogenesis, however, Leydig cell regeneration appears to occur both around the tubules and around blood vessels (Haider and Sevros, 1998; Yan et al., 2000; Davidoff et al., 2004). Whether or not the cells from which the ALCs develop are stem cells is not clear. For example, it is possible that the cells from which Leydig cells arise after EDS may be differentiated cells such as peritubular myoid cells, vascular smooth muscle cells, or pericytes which, after the loss of the ALCs, are capable of transdifferentiation. As yet, there is no good way to reliably recognize SLCs and/or to trace their lineage.
Factors that May Play a Role in the Regulation of Stem Cell Differentiation in the Adult Testis
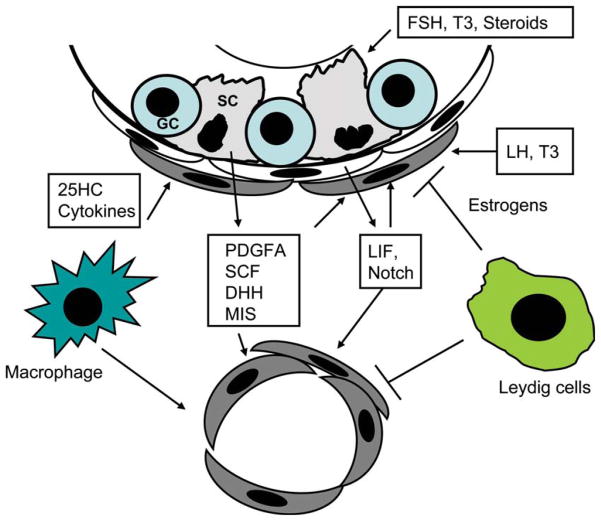
Based on the studies of Leydig cell development during the fetal and prepubertal periods, various hormones and growth factors have been identified that may affect Leydig cell differentiation. These factors are summarized in Figure 2.
Figure 2.
Factors and cells that may affect Leydig cell development in the interstitium of adult rat testis after the rats are administered EDS. These factors/cells constitute the “niche” in which the adult cells develop, presumably from stem cells.
Luteinizing hormone (LH)
The involvement of gonadotrophins in the regeneration of Leydig cells is suggested by the failure of Ledyig cell regeneration in EDS-treated hypophysectomized animals unless the animals were administered human chorionic gonadotropin (hCG) (Veldhuizen-Tsoerkan et al., 1994; Savage and Kerr, 1995; Molenaar et al., 1986; Teerds et al., 1989b). Consistent with this, in EDS-treated animals that received exogenous testosterone to suppress LH, or LH is neutralized by administration of LH antibody, Leydig cell regenerations were prevented completely (Sharpe et al., 1988, 1990; Sriraman et al., 2003) unless hCG was administered (Teerds et al., 1989a).
Although LH is required for the establishment of a normal ALC population in postnatal and adult testes, its role in the initiation of SLC differentiation remains an open question. In mice with extremely low serum LH (hypogonadal mice, for example; Baker and O’shaughnessy, 2001), or in LHR knockout mice (Zhang et al., 2001), ALC development was affected but not completely inhibited. Ariyaratne et al. (2000) reported that the steroidogenic enzymes, 3β-HSD, P450scc and P45017α, appeared before LHRs could be detected in progenitor cells, suggesting that LH signaling may not be required for the initial differentiation of SLCs. However, this has been disputed in a more recent study (Teerds et al., 2007). These results, taken together, suggest that LH may or may not be absolutely required for the initiation of stem cell differentiation but clearly is required for progression through the lineage and thus for the establishment of a normal adult population (Teerds et al., 1989a, Teerds et al., 1989b; Ariyaratne et al., 2000; Mendis-Handagama and Ariyaratne, 2001; Baker et al., 2003; Sriraman et al., 2003).
Thyroid hormone (T3)
Transient neonatal hypothyroidism has been shown to result in increased numbers of Leydig cells in the adult rat testis (Hardy et al., 1993; Mendis-Handagama and Sharma, 1994), functioning by arresting Leydig cell differentiation and thus promoting the continuous proliferation of precursor mesenchymal cells. These cells, in turn, accumulate and then differentiate in the interstitium when euthyroidism is restored (Hardy et al., 1996; Mendis-Handagama et al., 1998; Teerds et al., 1998). Consistent with this, hyperthyroidism was shown to stimulate the differentiation of mesenchymal cells into PLCs (Teerds et al., 1998; Ariyaratne et al., 2000; Wagner et al., 2008). The importance of thyroid hormone in Leydig cell regeneration in adult animals, however, is still controversial. When hypothyroidism was induced by either 6-propyl-2-thiouracil or by thyroidectomy, Leydig cell regeneration after EDS treatment was severely affected (Ariyaratne et al., 2000). However, EDS treatment of rats made hypothyroid by a dietary approach had very little effect on Leydig cell regeneration (Rijntjes et al., 2010). The difference between the two studies is not clear. One possibility could be the difference in the severity of induced thyroid deficiency. Thus, severe thyroid deficiency, such as that induced by thyroidectomy, may result in changes in the hypothalamic-pituitary–testicular axis (Chandrasekhar et al., 1986; Antony et al., 1995; Chiao et al., 1999; Maran et al., 2000). Changes in LH undoubtedly would affect Leydig cell regeneration. It is also possible that the regeneration of Leydig cells in the adult testis is affected differently by thyroid hormone than during the pubertal period, during which the maturation of Leydig cells takes place side-by-side with that of the seminiferous tubules. For example, reduction in thyroid hormone during puberty might delay the maturation of Sertoli cells, which in turn might affect Leydig cell differentiation indirectly.
Stem cell factor (SCF)
In addition to hormones, growth factors may affect Leydig cell regeneration in the adult testis after EDS. One such factor is stem cell factor (SCF). In the rat testis, Sertoli cells produce two forms of SCF, soluble SCF and membrane-associated SCF. The interaction of SCF with its receptor, c-kit, plays an important role in germ cell proliferation, differentiation, and apoptosis during germ cell development. The significance of SCF/c-kit interaction on Leydig cell development also was examined in EDS-treated adult rats (Yan et al., 2000). A function-blocking anti-c-kit antibody was found to inhibit the proliferation of precursor Leydig cells after EDS treatment, suggesting that the soluble SCF might act as a growth factor for precursor Leydig cells. The effect of SCF/c-kit signaling on Leydig cell development was also examined in a c-kit-deficient rat model (Ws/Ws) (Tsuchida et al., 2003). After EDS treatment, there were no significant differences in androgen sensitive organ weights (testis, prostate, and seminal vesicle) between the wild-type and knockout rats, suggesting that SCF/c-kit may not play a critical role in Leydig cell development. The issue has not yet been resolved.
Müllerian-inhibiting substance
Recent studies suggest that Mullerian inhibiting substance (MIS), a Sertoli cell product, acts in a paracrine manner to inhibit Leydig cell proliferation and differentiation after birth. Mice null for MIS or its receptor gene develop Leydig cell hyperplasia (Behringer et al., 1994; Mishina et al., 1996), whereas the differentiation of Leydig cell precursors is blocked in mice that over express MIS, the latter resulting in reduced Leydig cell numbers and serum testosterone concentrations (Behringer et al., 1990; Lyet et al., 1995; Racine et al., 1998). Consistent with this, in rats that received recombinant MIS after EDS treatment, there were fewer mesenchymal precursors and, subsequently fewer differentiated Leydig cells (Salva et al., 2004).
Other factors?
In addition to the factors discussed above, there are other growth factors and signaling molecules that have been reported to affect Leydig cell development during the fetal and/or pubertal periods. Among these are IGF-1, PDGFAA, TGF-α, LIF, DHH and Notch signaling pathways (Clark et al., 2000; Pierucci-Alves et al., 2001; Ge et al., 2006; Tang et al., 2008; Basciani et al., 2010; Hu et al., 2010). Although it has been found that two of these factors, PDGFAA and LIF, were significantly increased in the testis following Leydig cell ablation by EDS treatment (O’Shaughnessy et al., 2008), it remains unclear whether any of these factors are involved in Leydig cell differentiation in the adult testis after EDS.
The Stem Cell Niche
Adult tissue-specific stem cells have the capacity to self-renew and generate functional differentiated cells throughout an organism’s lifetime. Studies on stem cells from diverse systems have shown that stem cell function is controlled by extracellular cues and by intrinsic genetic programs within the stem cell itself (Underhill and Bhatia, 2007). The “niche” is functionally defined as an anatomic region that regulates stem cell renewal and differentiation, and thus how the stem cells function in tissue regeneration, maintenance, and repair (Li and Xie, 2005).
In the rat testis, ALCs rarely turn over under normal circumstances, and few die. However, under certain conditions, such as the loss of ALCs (see above), there are cells within the interstitial compartment that are capable of dividing and replacing the lost cells. The cells that initiate this process may be stem cells, though this has not been proven. The putative adult SLCs, unlike those in high- turnover lineages such as hematopoietic and spermatogonial stem cells, normally remain quiescent because the ALCs do not themselves turn over. As yet, we know little about the putative SLC niche, and in particular about the environment involved in regulating SLC division and differentiation. However, there is evidence to suggest that several cell types may contribute. First, the loss of Leydig cells after EDS might affect the niche environment directly through loss of steroid hormones (testosterone, estradiol) or growth factors. For example, it has been shown that treatment of rats with estradiol (E2) between days 5 and day 30 post-EDS blocked the regeneration of Leydig cells (Abney and Myers, 1991), suggesting a role for estradiol in Leydig cell regeneration after EDS and perhaps in SLC function (Zhai et al., 1996). Alternatively, the loss of Leydig cells might affect the seminiferous tubules and/or LH secretion by the pituitary which, in turn, might affect the Leydig cell niche. Indeed, there is evidence that the seminiferous tubules affect the regeneration of Leydig cells in EDS-treated rats (Kerr and Donachie, 1986; O’Leary et al., 1986; Sharpe et al., 1990). It has been reported, for example, that seminiferous tubule damage induced by cryptorchidism, x-irradiation, or busulfan treatment at the time of EDS treatment accelerates Leydig cell regeneration. Although it is possible that higher LH in these systems may contribute (Morris and Jackson, 1978; Molenaar et al., 1986; Teerds et al., 1989a), there is strong evidence for local involvement. For example, EDS treatment of rats in which the testes contained atrophic tubules resulted in more rapid Leydig cell regeneration around the atrophic tubules than around tubules containing a normal complement of germ cells (Sharpe et al., 1990). This is consistent with the findings in unilaterally cryptorchid animals in which more rapid Leydig cell regeneration occurred after EDS in the cryptorchid testis than in the contralateral scrotal testis (Kerr and Donachie, 1986; O’Leary et al., 1986), though both testis were exposed to the same hypothame-pituitary environment.
How might seminiferous tubules with different germ cell contents affect Leydig cell regeneration? It is possible that the differences are due to the inhibitory effect of germ cell-containing tubules or to the stimulatory effects of tubules without germ cells. Because cells within the seminiferous tubules do not physically contact SLCs on the surface of tubules or elsewhere in the interstitial compartment, any effect of seminiferous tubule cells must be through paracrine factors. As discussed above, there are signaling molecules that have been shown to affect Leydig cell differentiation, including DHH, Notch, PDGF, SCF, IGF-1, and LIF (Clark et al., 2000; Pierucci-Alves et al., 2001; Ge et al., 2006; Tang et al., 2008). It is well known that Sertoli cells secrete these factors. It would be of interest to know whether tubules with different germ cell contents produce different factors or different amounts of each factor. Another possibility could be that the regression of tubules in some way affects peritubular myoid cells which, in turn, affects Leydig cell differentiation by modifying either paracrine factors (e.g., LIF) (Dong et al., 2007) or signaling molecules.
In addition to the germ and Sertoli cells within the seminiferous tubules, other testicular cells may also have an effect on Leydig cell regeneration. One may be macrophages. There are studies reporting that macrophages are required for Leydig cell regeneration in EDS-treated rats (Gaytan et al., 1994a, b). Testicular macrophages can be selectively eliminated by intratesticular injection of dichloromethylene diphosphonate-containing liposomes. When EDS was administered to rats in which macrophages had been selectively eliminated from one testis, Leydig cell regeneration was almost completely inhibited in that testis (Gaytan et al., 1994a, b). These studies further emphasize the significance of paracrine regulatory mechanisms in Leydig cell differentiation and suggest that testicular macrophages may contribute to the niche environment of SLCs. Though the mechanism by which macrophages might affect Leydig cell regeneration is still unclear, it has been suggested that macrophage effects maybe mediated at least in part through their secretion of 25-hydroxycholesterol (Chen et al., 2002), growth factors, and/or cytokines (Gaytan et al., 1994a, b).
Stem Leydig Cell Aging
As indicated above, Leydig cells from old rats (20 months or more) produce significantly less testosterone than Leydig cells from young rats (3–6 months) (Chen et al., 1994). To determine whether putative adult SLCs also age, young and old rats were treated with EDS to determine whether a new generation of Leydig cells would subsequently appear in old animals, as they do in young adults, and if so, whether the steroidogenic capacity of the new Leydig cells in old animals would be at the relatively low level of the cells they replaced or at the high level of young ALCs from the young adult testis (Chen et al., 1996). It was found that testosterone production by Leydig cells restored to the testes of EDS-treated old rats exceeded that of age-matched controls, and indeed was at the level of young rats. The enhanced ability of the Leydig cells restored to the aged testes to produce testosterone was not a consequence of exposure to increased levels of LH. Thus, although situated in an aged testis and in the environment of an aged hypothalamic-pituitary axis, the steroidogenic function of the Leydig cells restored to aged rat testes was equivalent to that of young Leydig cells, and suggested that SLCs, presumed to be the cells from which the ALCs were derived, may not age. It may be the general case that stem cells in low turnover tissues age more slowly than stem cells in high turnover tissues.
DIFFERENTIATION OF STEROIDOGENIC CELLS FROM THE STEM CELLS OF NONSTEROIDOGENIC TISSUES
During the past decade, as progress has been made to understand the division and differentiation of SLCs in the testis, even more intriguing progress has been made on the differentiation of steroidogenic cells by stem cells from nonsteroidogenic organs.
Bone Marrow Stem Cells
The adult stem cells from bone marrow, referred to as mesenchymal stem cells or bone marrow stromal cells (BMSC), are pluripotent cells that have the ability to differentiate into cells of all three germ layers (Ratajczak et al., 2008). To examine whether BMSCs can be induced to differentiate into Leydig cells, BMSCs from GFP transgenic rats were injected into the testes of 3 week-old rats, and their fate was tracked (Yazawa et al., 2006). The GFP-positive cells were localized to the interstitium and not within the seminiferous tubules. Immunohistochemical studies showed that most of the GFP-positive cells in the interstitium were positive for three Leydig cell markers, P450scc, 3β-HSD, and P450c17. These results indicated that donor bone marrow cells can be induced to differentiate into Leydig cells in vivo, given the appropriate environment (niche).
A similar approach tested the possibility that bone marrow-derived stem cells would differentiate into somatic and/or germ cells when transplanted into the mouse testis. In these studies (Lue et al., 2007), bone marrow cells from adult GFP mice were injected into the seminiferous tubules and the testicular interstitium of busulfan-treated wild-type or c-kit mutant (W/Wv) mice. Ten to 12 weeks later, it was found that in both the busulfan-treated and W/Wv mice, some of the GFP-positive donor cells were situated within the seminiferous tubules and others in the interstitum. The cells localized within the seminiferous tubules had Sertoli cell appearance and expressed follicle-stimulating hormone receptors, whereas the cells in the interstitium expressed the Leydig cell marker protein P450scc. In the seminiferous tubules of busulfan-treated mice, there also were GFP-positive donor cells that had the appearance of spermatogonia or spermatocytes and expressed the germ cell marker protein, VASA. Such cells were not found in the seminiferous tubules of W/Wv mice. These results demonstrated that bone marrow-derived stem cells can also be induced to differentiate into somatic or germ cells, and point to the important role played by the environment (niche) to which these cells were exposed.
The possibility of differentiating BMSCs into Leydig cells was also tested in vitro. SF-1 is a transcription factor that is essential for the expression of steroidogenic enzymes in all steroidogenic cells during their development. To test the possibility of inducing the differentiation of BMSCs into Leydig cells in vitro, BMSCs from mouse and human were transfected with SF-1 (Yazawa et al., 2006). Although SF-1 transfection induced morphological changes in the cells, such as the accumulation of numerous lipid droplets, the transfected cells did not express steroidogenic enzyme nor produce steroid hormone. However, when the transfected cells were treated with 8-bromoadenosine-cAMP (8br-cAMP), steroidogenic enzymes were induced and testosterone was detected in the medium. Interestingly, transfection of human BMSCs with SF-1 induced their differentiation into steroidogenic cells, but the cells produced glucocorticoid rather than testosterone. In subsequent studies, the same group found that SF-1 is not the only factor that is capable of inducing BMSCs to differentiate into steroidogenic cells; transfection with liver receptor homolog-1 (LRH-1), another member of the Nuclear receptor subfamily 5A (NR5A), also induced the human BMSCs into steroid-producing cells (Yazawa et al., 2009). These in vitro results not only confirmed the in vivo observations that BMSCs have potential to become Leydig cells but also suggested critical roles played by NR5A members and cAMP signaling in the reprogramming process. The future challenge could be to program these cells into testosterone-producing cells by hormones or growth factors instead of by forced expression of NR5A nuclear receptors by transfection.
Adipose Tissue Stem Cells
BMSCs are among the best studied adult stem cells and, as indicated above, have the potential to be induced to become a variety of cell types. Consequently, BMSCs have been proposed as having promise for treatment of such male reproductive diseases as erectile dysfunction and male infertility (Lin et al., 2008). More recently, however, it was discovered that adipose tissue-derived mesenchymal cells (AMSC) are virtually identical to bone marrow stem cells in differentiation and therapeutic potential, but are more easily and safely obtained and can be harvested in larger quantities, with the associated benefit of reducing obesity. Therefore, AMSCs appear to be a better choice for future clinical applications (Lin et al., 2008).
The potential of AMSCs to become steroidogenic cells was also tested in vitro. As discussed above, forced expression of SF-1 plus cAMP stimulation can induce BMSCs into steroidogenic cells. Such an approach was also applied to AMSCs and its effectiveness was compared with that of BMSCs prepared from the same mice (Gondo et al., 2008). Several cell surface markers, including potential mesenchymal cell markers, were identical in both cell types and, as expected, forced expression of SF-1 caused AMSCs to be transformed into ACTH-responsive steroidogenic cells. However, the properties of the cells resulting from manipulating AMSCs were found to differ from those of BMSCs. Thus, in response to increased SF-1 expression and/or treatment with retinoic acid, AMSCs were much more prone to produce the adrenal steroid corticosterone rather than the gonadal steroid testosterone, whereas the opposite was true of BMSCs. These novel results suggest the possibility of using AMSCs for autologous cell regeneration therapy for patients with steroid insufficiency, and point to the need for appropriate tissue selection in preparing mesenchymal stem cells. The different steroidogenic potencies of the cells from two sources might provide a way to understand the mechanism of tissue- or cell-specific adrenal and gonadal steroidogenic cell differentiation.
Umbilical Cord Blood-Derived Mesenchymal Stem Cells
In addition to pluripotent embryonic stem cells and the less potent adult stem cells, there is a group of cells in between. These are umbilical cord blood-derived mesenchymal stem cells (UMSCs). The potential of the UMSCs to become steroidogenic cells also was tested in vitro by forced expression of SF-1 plus cAMP stimulation (Yazawa et al., 2010). Very similar to BMSCs and AMSCs, the UMSCs can also be induced to differentiate into steroidogenic cells. However, the induced cells did not resemble the cell products either of BMSCs (Leydig cells) or AMSCs (adrenocortical-like cells). Instead, they exhibited characteristics of granulosaluteal-like cells. The significantly higher progesterone and estrogen production by these cells may be related to the expression of peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α). These results, again, indicated that although the mesenchymal cells from different sources share the ability to be induced to become steroidogenic cells, they have intrinsic differences (Wagner et al., 2005).
SUMMARY
Leydig cells are the testosterone-producing cells of the testis. In the rat, the ALC population develops from undifferentiated mesenchymal-like stem cells present in the interstitial compartment of the neonatal testis. Four developmental stages have been shown to be involved in the development of ALCs, including the formation of SLCs and then the differentiation of SLCs to form PLCs, PLCs to form ILCs, and ILCs to form ALCs. Putative SLCs apparently occur in the adult testis. These cells, which normally are quiescent due to slow turnover of ALCs, are capable of regenerating new Leydig cells upon the loss of the adult cells. The regeneration process is very similar to the development of ALCs during the pubertal period. With aging, ALCs lose about 50% of their steroidogenic capacity. However, after elimination of the aged ALCs, the putative SLCs in the aged testis are able to restore a full complement of Leydig cells with steroidogenic capacity at the level of young adults, suggesting that in contrast to the ALCs, SLCs may not age. The factors that initiate the divisions, self-renewal, and differentiation of SLCs upon the loss of ALCs are uncertain. Apparently both pituitary (LH) and local factors (niche) are involved. The importance of the niche in the development of ALCs is shown by the transplantation of hematopoietic stem cells into the testis. These cells, upon exposure to the interstitial compartment of the testis, become Leydig cells. Forced expression of NR5A nuclear receptor family members in vitro also can induce stem cells to enter steroidogenic cell lineages even without providing an in vivo niche, suggesting that members of this family may be critical in initiating the differentiation of SLCs to PLCs. Such reprogramming of the stem cells from other organs, particularly from fat tissue, provides the possibility of a stem cell-based therapeutic approach to androgen insufficiency in disease states and in the elderly.
References
- Abney TO, Myers RB. 17 beta-estradiol inhibition of Leydig cell regeneration in the ethane dimethylsulfonate-treated mature rat. J Androl. 1991;12:295–304. [PubMed] [Google Scholar]
- Antony FF, Aruldhas MM, Udhayakumar RC, et al. Inhibition of Leydig cell activity in vivo and in vitro in hypothyroid rats. J Endocrinol. 1995;144:293–300. doi: 10.1677/joe.0.1440293. [DOI] [PubMed] [Google Scholar]
- Ariyaratne HB, Mendis-Handagama SM. Changes in the testis interstitium of Sprague Dawley rats from birth to sexual maturity. Biol Reprod. 2000;62:680–690. doi: 10.1095/biolreprod62.3.680. [DOI] [PubMed] [Google Scholar]
- Ariyaratne HB, Ian Mason J, Mendis-Handagama SM. Effects of thyroid and luteinizing hormones on the onset of precursor cell differentiation into leydig progenitor cells in the pre-pubertal rat testis. Biol Reprod. 2000;63:898–904. doi: 10.1095/biolreprod63.3.898. [DOI] [PubMed] [Google Scholar]
- Ariyaratne HB, Kim I, Mills N, et al. Effects of ethane dimethane sulfonate on the functional structure of the adult rat testis. Arch Androl. 2003;49:313–326. doi: 10.1080/01485010390204922. [DOI] [PubMed] [Google Scholar]
- Baker PJ, O’shaughnessy PJ. Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction. 2001;122:227–234. doi: 10.1530/rep.0.1220227. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Johnston H, Abel M, et al. Differentiation of adult-type Leydig cells occurs in gonadotrophin-deficient mice. Reprod Biol Endocrinol. 2003;1:4–12. doi: 10.1186/1477-7827-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalska M, Koeva I, Atanassova N, et al. Steroidogenic and structural differentiation of new Leydig cell population following exposure of adult rats to ethane dimethanesulphonate. Folia Biol (Praha) 2002;48:205–209. [PubMed] [Google Scholar]
- Barsoum I, Yao HH. The road to maleness: from testis to Wolffian duct. Trends Endocrinol Metab. 2006;17:223–228. doi: 10.1016/j.tem.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basciani S, Mariani S, Spera G, Gnessi L. Role of platelet-derived growth factors in the testis. Endocr Rev. 2011;32:0000–0000. doi: 10.1210/er.2010-0004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Cate RL, Froelick GJ, et al. Abnormal sexual development in transgenic mice chronically expressing Mullerian inhibiting substance. Nature. 1990;345:167–170. doi: 10.1038/345167a0. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Finegold MJ, Cate RL. Müllerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Benton LX, Shan LX, Hardy MP. Differentiation of adult Leydig cells. J Steroid Biochem Mol Biol. 1995;53:61–68. doi: 10.1016/0960-0760(95)00022-r. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germ-line. Curr Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17:800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar Y, D’Occhio MJ, Setchell BP. Reproductive hormone secretion and spermatogenic function in thyroidectomized rams receiving graded doses of exogenous thyroxine. J Endocrinol. 1986;111:245–253. doi: 10.1677/joe.0.1110245. [DOI] [PubMed] [Google Scholar]
- Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl. 1994;15:551–557. [PubMed] [Google Scholar]
- Chen H, Huhtaniemi I, Zirkin BR. Depletion and repopulation of Leydig cells in the testes of aging brown Norway rats. Endocrinology. 1996;137:3447–3452. doi: 10.1210/endo.137.8.8754773. [DOI] [PubMed] [Google Scholar]
- Chen H, Ge RS, Zirkin BR. Leydig cells: from stem cells to aging. Mol Cell Endocrinol. 2009;306:9–16. doi: 10.1016/j.mce.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Lukyanenko Y, Hutson JC. 25-hydroxycholesterol is produced by testicular macrophages during the early postnatal period and influences differentiation of Leydig cells in vitro. Biol Reprod. 2002;66:1336–1341. doi: 10.1095/biolreprod66.5.1336. [DOI] [PubMed] [Google Scholar]
- Chiao YC, Lee HY, Wang SW, et al. Regulation of thyroid hormones on the production of testosterone in rats. Cell Biochem. 1999;73:554–562. [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63:1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, et al. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Müller D, Holstein AF. The neuroendocrine Leydig cells and their stem cell progenitors, the pericytes. Adv Anat Embryol Cell Biol. 2009;205:1–107. [PubMed] [Google Scholar]
- de Rooij DG, van Bragt MPA. Leydig cells: testicular side population harbors transplantable Leydig stem cells. Endocrinology. 2004;145:4009–4010. doi: 10.1210/en.2004-0578. [DOI] [PubMed] [Google Scholar]
- Dong L, Jelinsky SA, Finger JN, et al. Gene expression during development of fetal and adult Leydig cells. Ann NY Acad Sci. 2007;1120:16–35. doi: 10.1196/annals.1411.016. [DOI] [PubMed] [Google Scholar]
- Dupont E, Labrie F, Luu-The V, Pelletier G. Ontogeny of 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD) in rat testis as studied by immunocyto-chemistry. Anat Embryol (Berl) 1993;187:583–589. doi: 10.1007/BF00214437. [DOI] [PubMed] [Google Scholar]
- Forest MG. Role of androgens in fetal and pubertal development. Horm Res. 1983;18:69–83. doi: 10.1159/000179780. [DOI] [PubMed] [Google Scholar]
- Gaytan F, Aceitero J, Lucena C, et al. Simultaneous proliferation and differentiation of mast cells and Leydig cells in the rat testis. Are common regulatory factors involved? J Androl. 1992;13:387–397. [PubMed] [Google Scholar]
- Gaytan F, Bellido C, Morales C, et al. Effects of macrophage depletion at different times after treatment with ethylene dimethane sulfonate (EDS) on the regeneration of Leydig cells in the adult rat. J Androl. 1994a;15:558–564. [PubMed] [Google Scholar]
- Gaytan F, Bellido C, Morales C, et al. Selective depletion of testicular macrophages and prevention of Leydig cell repopulation after treatment with ethylene dimethane sulfonate in rats. J Reprod Fertil. 1994b;101:175–182. doi: 10.1530/jrf.0.1010175. [DOI] [PubMed] [Google Scholar]
- Ge RS, Dong Q, Sottas CM, et al. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc Natl Acad Sci USA. 2006;103:2719–2724. doi: 10.1073/pnas.0507692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo S, Okabe T, Tanaka T, et al. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology. 2008;149:4717–4725. doi: 10.1210/en.2007-1808. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- Habert R, Brignaschi P. Developmental changes in in vitro testosterone production by dispersed Leydig cells during fetal life in rats. Arch Androl. 1991;27:65–71. doi: 10.3109/01485019108987654. [DOI] [PubMed] [Google Scholar]
- Habert R, Picon R. Testosterone, dihydrotestosterone and estradiol-17 beta levels in maternal and fetal plasma and in fetal testes in the rat. J Steroid Biochem. 1984;21:193–198. doi: 10.1016/0022-4731(84)90383-2. [DOI] [PubMed] [Google Scholar]
- Haider SG, Passia D, Overmeyer G. Studies on the fetal and post-natal development of rat Leydig cells employing 3 beta-hydroxysteroid de-hydrogenase activity. Acta Histochem. 1986;32:197–202. [PubMed] [Google Scholar]
- Haider SG, Laue D, Schwochau G, Hilscher B. Morphological studies on the origin of adult-type Leydig cells in rat testis. Ital J Anat Embryol. 1995;100:535–41. [PubMed] [Google Scholar]
- Haider SG, Servos G. Ultracyto-chemistry of 3beta-hydroxysteroid dehydrogenase in Leydig cell precursors and vascular endothelial cells of the postnatal rat testis. Anat Embryol (Berl) 1998;198:101–110. doi: 10.1007/s004290050168. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Zirkin BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology. 1989;124:762–770. doi: 10.1210/endo-124-2-762. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Kirby JD, Hess RA, Cooke PS. Leydig cells increase their numbers but decline in steroidogenic function in the adult rat after neonatal hypothyroidism. Endocrinology. 1993;132:2417–2420. doi: 10.1210/endo.132.6.8504746. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Sharma RS, Arambepola NK, et al. Increased proliferation of Leydig cells induced by neonatal hypothyroidism in the rat. J Androl. 1996;17:231–238. [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men, Baltimore Longitudinal Study of Aging. J Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hu GX, Lin H, Chen GR, et al. Deletion of the IGF-1 gene: suppressive effects on adult Leydig cell development. J Androl. 2010;31:379–387. doi: 10.2164/jandrol.109.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I, Pelliniemi LJ. Fetal Leydig cells: cellular origin, morphology, life span, and special functional features. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY) 1992;201:125–140. doi: 10.3181/00379727-201-43493. [DOI] [PubMed] [Google Scholar]
- Inano H, Tamaoki BI. Bioconversion of steroids in immature rat testes in vitro. Endocrinology. 1966;79:579–590. doi: 10.1210/endo-79-3-579. [DOI] [PubMed] [Google Scholar]
- Jackson AE, O’Leary PC, Ayers MM, de Kretser DM. The effects of ethylene dimethane sulphonate (EDS) on rat Leydig cells: evidence to support a connective tissue origin of Leydig cells. Biol Reprod. 1986;35:425–437. doi: 10.1095/biolreprod35.2.425. [DOI] [PubMed] [Google Scholar]
- Keeney DS, Mendis-Handagama SM, Zirkin BR, Ewing LL. Effect of long term deprivation of luteinizing hormone on Leydig cell volume, Leydig cell number, and steroidogenic capacity of the rat testis. Endocrinology. 1988;123:2906–2915. doi: 10.1210/endo-123-6-2906. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Donachie K, Rommerts FF. Selective destruction and regeneration of rat Leydig cells in vivo. A new method for the study of seminiferous tubular-interstitial tissue interaction. Cell Tissue Res. 1985;242:145–156. doi: 10.1007/BF00225571. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Donachie K. Regeneration of Leydig cells in unilaterally cryptorchid rats: evidence for stimulation by local testicular factors. Cell Tissue Res. 1986;245:649–655. doi: 10.1007/BF00218568. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Bartlett JM, Donachie K, Sharpe RM. Origin of regenerating Leydig cells in the testis of the adult rat. An ultrastructural, morpho-metric and hormonal assay study. Cell Tissue Res. 1987;249:367–377. doi: 10.1007/BF00215521. [DOI] [PubMed] [Google Scholar]
- Lin CS, Xin ZC, Deng CH, et al. Recent advances in andrology-related stem cell research. Asian J Androl. 2008;10:171–175. doi: 10.1111/j.1745-7262.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem Cell Niche: Structure and Function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Lo KC, Lei Z, Rao CV, et al. De novo testosterone production in luteinizing hormone receptor knockout mice after transplantation of Leydig stem cells. Endocrinology. 2004;145:4011–4015. doi: 10.1210/en.2003-1729. [DOI] [PubMed] [Google Scholar]
- Lue Y, Erkkila K, Liu PY, et al. Fate of bone marrow stem cells transplanted into the testis: potential implication for men with testicular failure. Am J Pathol. 2007;170:899–908. doi: 10.2353/ajpath.2007.060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyet L, Louis F, Forest MG, et al. Ontogeny of reproductive abnormalities induced by deregulation of anti-Mullerian hormone expression in transgenic mice. Biol Reprod. 1995;52:444–454. doi: 10.1095/biolreprod52.2.444. [DOI] [PubMed] [Google Scholar]
- Maran RR, Arunakaran J, Jeyaraj DA, et al. Transient neonatal hypothyroidism alters plasma and testicular sex steroid concentration in puberal rats. Endocr Res. 2000;26:411–429. doi: 10.3109/07435800009066177. [DOI] [PubMed] [Google Scholar]
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57:M76–M99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- Mendis-Handagama SM, Sharma OP. Effects of neonatal administration of the reversible goitrogen pro-pylthiouracil on the testis interstitium in adult rats. J Reprod Fertil. 1994;100:85–92. doi: 10.1530/jrf.0.1000085. [DOI] [PubMed] [Google Scholar]
- Mendis-Handagama SM, Watkins PA, Gelber SJ, Scallen TJ. The effect of chronic luteinizing hormone treatment on adult rat Leydig cells. J Androl. 1998;30:64–73. doi: 10.1016/s0040-8166(98)80007-4. [DOI] [PubMed] [Google Scholar]
- Mendis-Handagama SM, Ariyaratne HBC. Differentiation of the adult Leydig cell population in the postnatal testis. Biol Reprod. 2001;65:660–671. doi: 10.1095/biolreprod65.3.660. [DOI] [PubMed] [Google Scholar]
- Migrenne S, Pairault C, Racine C, et al. Luteinizing hormone-dependent activity and luteinizing hormone-independent differentiation of rat fetal Leydig cells. Mol Cell Endocrinol. 2001;172:193–202. doi: 10.1016/s0303-7207(00)00339-7. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Rey R, Finegold MJ, et al. Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10:2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- Miyano M, Ito Y, Fujihira S, et al. Restoration of Leydig cells after repeated administration of ethane dimethanesulfonate in adult rats. Pathol Int. 1997;47:478–488. doi: 10.1111/j.1440-1827.1997.tb04527.x. [DOI] [PubMed] [Google Scholar]
- Molenaar R, de Rooij DG, Rommerts FF, et al. Specific destruction of Leydig cells in mature rats after in vivo administration of ethane dimethyl sulfonate. Biol Reprod. 1985;33:1213–1222. doi: 10.1095/biolreprod33.5.1213. [DOI] [PubMed] [Google Scholar]
- Molenaar R, de Rooij DG, Rommerts FF, van der Molen HJ. Repopulation of Leydig cells in mature rats after selective destruction of the existent Leydig cells with ethylene dimethane sulfonate is dependent on luteinizing hormone and not follicle-stimulating hormone. Endocrinology. 1986;118:2546–2554. doi: 10.1210/endo-118-6-2546. [DOI] [PubMed] [Google Scholar]
- Morris ID, Jackson CM. Gonadotrophin response after castration and selective destruction of the testicular interstitium in the normal and asper-matogenic rat. Acta Endocrinol (Copenh) 1978;88:38–47. doi: 10.1530/acta.0.0880038. [DOI] [PubMed] [Google Scholar]
- Morris ID, Phillips DM, Bardin CW. Ethylene dimethanesulfonate destroys Leydig cells in the rat testis. Endocrinology. 1986;118:709–719. doi: 10.1210/endo-118-2-709. [DOI] [PubMed] [Google Scholar]
- Murono EP. Maturational changes in steroidogenic enzyme activities metabolizing testosterone and dihydrotestosterone in two populations of testicular interstitial cells. Acta Endocrinol. 1989;121:477–483. doi: 10.1530/acta.0.1210477. [DOI] [PubMed] [Google Scholar]
- Myers RB, Abney TO. Interstitial cell proliferation in the testis of the ethylene dimethane sulfonate-treated rat. Steroids. 1991;56:91–96. doi: 10.1016/0039-128x(91)90130-n. [DOI] [PubMed] [Google Scholar]
- O’Leary P, Jackson AE, Averill S, de Kretser DM. The effects of ethane dimethane sulphonate (EDS) on bilaterally cryptorchid rat testes. Mol Cell Endocrinol. 1986;45:183–90. doi: 10.1016/0303-7207(86)90146-2. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Morris ID, Baker PJ. Leydig cell re-generation and expression of cell signaling molecules in the germ cell-free testis. Reproduction. 2008;135:851–858. doi: 10.1530/REP-07-0529. [DOI] [PubMed] [Google Scholar]
- Pierucci-Alves F, Clark AM, Russell LD. A developmental study of the Desert hedgehog-null mouse testis. Biol Reprod. 2001;65:1392–1402. doi: 10.1095/biolreprod65.5.1392. [DOI] [PubMed] [Google Scholar]
- Racine C, Rey R, Forest M, et al. Receptors for anti-Mullerian hormone on Leydig cells are responsible for its effects on steroidogenesis and cell differentiation. Proc Natl Acad Sci USA. 1998;95:594–599. doi: 10.1073/pnas.95.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Zuba-Surma EK, Machalinski B, et al. Very small embryonic-like (VSEL) stem cells: purification from adult organs, characterization, and biological significance. Stem Cell Rev. 2008;4:89–99. doi: 10.1007/s12015-008-9018-0. [DOI] [PubMed] [Google Scholar]
- Rijntjes E, van Kesteren-Buiting A, Keijer J, Teerds KJ. Chronic hypothyroidism only marginally affects adult-type Leydig cell regeneration after EDS administration. Int J Androl. 2010;33:123–131. doi: 10.1111/j.1365-2605.2009.00955.x. [DOI] [PubMed] [Google Scholar]
- Risbridger GP, Davies A. Isolation of rat Leydig cells and precursor forms after administration of ethane dimethane sulfonate. Am J Physiol. 1994;266:E975–E979. doi: 10.1152/ajpendo.1994.266.6.E975. [DOI] [PubMed] [Google Scholar]
- Ross AJ, Capel B. Signaling at the crossroads of gonad development. Trends Endocrinol Metab. 2005;16:19–25. doi: 10.1016/j.tem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Russell LD, de França LR, Hess R, Cooke P. Characteristics of mitotic cells in developing and adult testes with observations on cell lineages. Tissue Cell. 1995;27:105–128. doi: 10.1016/s0040-8166(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Salva A, Hardy MP, Wu XF, et al. Müllerian-inhibiting substance inhibits rat Leydig cell regeneration after ethylene dimethanesulphonate ablation. Biol Reprod. 2004;70:600–607. doi: 10.1095/biolreprod.103.021550. [DOI] [PubMed] [Google Scholar]
- Savage GN, Kerr JB. Effect of seminiferous tubule size on hCG-induced regeneration of peritubular Leydig cells in hypophysectomized, EDS-treated rats. Int J Androl. 1995;18:35–45. doi: 10.1111/j.1365-2605.1995.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- Shan LX, Phillips DM, Bardin CW, Hardy MP. Differential regulation of steroidogenic enzymes during differentiation optimizes testosterone production by adult rat Leydig cells. Endocrinology. 1993;133:2277–2283. doi: 10.1210/endo.133.5.8404681. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Fraser HM, Ratnasooriya WD. Assessment of the role of Leydig cell products other than testosterone in spermatogenesis and fertility in adult rats. Int J Androl. 1988;11:507–523. doi: 10.1111/j.1365-2605.1988.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Maddocks S, Kerr JB. Cell-cell interactions in the control of spermatogenesis as studied using Leydig cell destruction and testosterone replacement. Am J Anat. 1990;188:3–20. doi: 10.1002/aja.1001880103. [DOI] [PubMed] [Google Scholar]
- Sriraman V, Sairam MR, Rao AJ. Evaluation of relative roles of LH and FSH in regulation of differentiation of Leydig cells using an ethane 1,2-dimethylsulfonate-treated adult rat model. J Endocrinol. 2003;176:151–161. doi: 10.1677/joe.0.1760151. [DOI] [PubMed] [Google Scholar]
- Steinberger E, Ficher M. Differentiation of steroid biosynthetic pathways in developing testes. Biol Reprod. 1969;1:119–133. doi: 10.1095/biolreprod1.supplement_1.119. [DOI] [PubMed] [Google Scholar]
- Tang H, Brennan J, Karl J, et al. Notch signaling maintains Leydig progenitor cells in the mouse testis. Development. 2008;135:3745–3753. doi: 10.1242/dev.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teerds KJ, de Rooij DG, Rommerts FF, et al. Proliferation and differentiation of possible Leydig cell precursors after destruction of the existing Leydig cells with ethane dimethyl sulphonate: the role of LH/human chorionic gonadotrophin. J Endocrinol. 1989a;122:689–696. doi: 10.1677/joe.0.1220689. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, De Rooij DG, Rommerts FF, et al. Stimulation of the proliferation and differentiation of Leydig cell precursors after the destruction of existing Leydig cells with ethane dimethyl sulphonate (EDS) can take place in the absence of LH. J Androl. 1989b;10:472–477. doi: 10.1002/j.1939-4640.1989.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, De Rooij DG, Rommerts FF, et al. Turnover time of Leydig cells and other interstitial cells in testes of adult rats. Arch Androl. 1989c;23:105–111. doi: 10.3109/01485018908986831. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, de Rooij DG, Rommerts FF, Wensing CJ. Development of a new Leydig cell population after the destruction of existing Leydig cells by ethane dimethane sulphonate in rats: an autoradiographic study. J Endocrinol. 1990;126:229–236. doi: 10.1677/joe.0.1260229. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, de Rooij DG, de Jong FH, van Haaster LH. Development of the adult-type Leydig cell population in the rat is affected by neonatal thyroid hormone levels. Biol Reprod. 1998;59:344–350. doi: 10.1095/biolreprod59.2.344. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, Rijntjes E, Veldhuizen-Tsoerkan MB, et al. The development of rat Leydig cell progenitors in vitro: how essential is luteinising hormone? J Endocrinol. 2007;194:579–593. doi: 10.1677/JOE-06-0084. [DOI] [PubMed] [Google Scholar]
- Tsuchida J, Dohmae K, Kitamura Y, Nishimune Y. The role of the c-kit receptor in the regenerative differentiation of rat Leydig cells. Int J Androl. 2003;26:121–125. doi: 10.1046/j.1365-2605.2003.00412.x. [DOI] [PubMed] [Google Scholar]
- Underhill GH, Bhatia SN. High-throughput analysis of signals regulating stem cell fate and function. Curr Opin Chem Biol. 2007;11:357–366. doi: 10.1016/j.cbpa.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen-Tsoerkan MB, Ivell R, Teerds KJ. hCG-induced changes in LH/CG receptor mRNA transcript levels in the testis of adult hypophysectomized, ethane dimethyl sulphonate-treated rats. Mol Cell Endocrinol. 1994;105:37–44. doi: 10.1016/0303-7207(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Vreeburg JT, Ooms MP, Rommerts FF, Teerds KJ. Functional properties of developing rat Leydig cells after treatment with ethylene dimethanesulphonate (EDS) J Reprod Fertil. 1988;84:63–69. doi: 10.1530/jrf.0.0840063. [DOI] [PubMed] [Google Scholar]
- Wagner MS, Wajner SM, Maia AL. The role of thyroid hormone in testicular development and function. J Endocrinol. 2008;199:351–365. doi: 10.1677/JOE-08-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Yan W, Kero J, Huhtaniemi I, Toppari J. Stem cell factor functions as a survival factor for mature Leydig cells and a growth factor for precursor Leydig cells after ethylene dimethane sulfonate treatment: implication of a role of the stem cell factor/c-Kit system in Leydig cell development. Dev Biol. 2000;227:169–182. doi: 10.1006/dbio.2000.9885. [DOI] [PubMed] [Google Scholar]
- Yazawa T, Mizutani T, Yamada K, et al. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006;147:4104–4111. doi: 10.1210/en.2006-0162. [DOI] [PubMed] [Google Scholar]
- Yazawa T, Inanoka Y, Mizutani T, et al. Liver receptor homolog-1 regulates the transcription of steroidogenic enzymes and induces the differentiation of mesenchymal stem cells into steroidogenic cells. Endocrinology. 2009;150:3885–3893. doi: 10.1210/en.2008-1310. [DOI] [PubMed] [Google Scholar]
- Yazawa T, Inaoka Y, Okada R, et al. PPAR-gamma coactivator-1alpha regulates progesterone production in ovarian granulosa cells with SF-1 and LRH-1. Mol Endocrinol. 2010;24:485–496. doi: 10.1210/me.2009-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J, Lanclos KD, Abney TO. Estrogen receptor messenger ribonucleic acid changes during Leydig cell development. Biol Reprod. 1996;55:782–788. doi: 10.1095/biolreprod55.4.782. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Steding G, Emmen JM, et al. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol. 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Ewing LL. Leydig cell differentiation during maturation of the rat testis: a stereological study of cell number and ultrastructure. Anat Rec. 1987;219:157–163. doi: 10.1002/ar.1092190208. [DOI] [PubMed] [Google Scholar]