Abstract
Background
Deep brain stimulation (DBS) is an effective and approved therapy for advanced Parkinson’s disease (PD), and a recent study suggests efficacy in mid-stage disease. This manuscript reports the results of a pilot trial investigating preliminary safety and tolerability of DBS in early PD.
Methods
Thirty subjects with idiopathic PD (Hoehn & Yahr Stage II off medication), age 50–75, on medication ≥ 6 months but < 4 years, and without motor fluctuations or dyskinesias were randomized to optimal drug therapy (ODT) (n=15) or DBS+ODT (n=15). Co-primary endpoints were the time to reach a 4-point worsening from baseline in the UPDRS-III off therapy and the change in levodopa equivalent daily dose from baseline to 24 months.
Results
As hypothesized, the mean UPDRS total and part III scores were not significantly different on or off therapy at 24 months. The DBS+ODT group took less medication at all time points, and this reached maximum difference at 18 months. With a few exceptions, differences in neuropsychological functioning were not significant. Two subjects in the DBS+ODT group suffered serious adverse events; remaining adverse events were mild or transient.
Conclusions
This study demonstrates that subjects with early stage PD will enroll in and complete trials testing invasive therapies and provides preliminary evidence that DBS is well tolerated in early PD. The results of this trial provide the data necessary to design a large, phase III, double-blind, multicenter trial investigating the safety and efficacy of DBS in early PD.
Keywords: Parkinson’s disease, deep brain stimulation, subthalamic nucleus
INTRODUCTION
Bilateral deep brain stimulation (DBS) of the subthalamic nucleus (STN) is a proven therapy for patients with advanced Parkinson’s disease (PD) [1, 2]. The therapy substantially improves motor symptoms and quality of life while reducing medication requirements [1–3]. Currently, DBS is only approved for advanced PD and thus limited to patients for whom medications are no longer adequate [4, 5]. In the advanced stages of the disease, however, a significant portion of patients experience conditions or co-morbidities that may make them ineligible for DBS.
Significant interest has evolved regarding the possibility of implanting DBS earlier in the disease course [6–9]. A recent study focused on implanting patients with mid-stage PD indicates that the combination of DBS and oral medications is superior to oral medications alone when DBS is applied after the onset of motor fluctuations [7, 9]. Application of the therapy in early disease, before quality of life and functional independence are compromised, may yield superior clinical benefit and delay functional decline [6].
We report here the results of a pilot clinical trial of DBS in the earliest stage of PD studied thus far. The purpose of this trial was to gather the necessary information to inform the design of a large, phase III, multicenter trial investigating bilateral STN-DBS in early stage PD. We report here our experiences with subject recruitment and retention, the tolerability of stimulation over time, and adverse events.
METHODS
This prospective, randomized, parallel-group, single-blind clinical trial comparing bilateral STN-DBS plus optimal drug therapy (DBS+ODT) to ODT alone received approval from the Vanderbilt University Institutional Review Board (IRB# 040797) and was granted an Investigational Device Exemption from the United States Food and Drug Administration limited to 30 subjects (#G050016, clinicaltrials.gov NCT00282152). Complete descriptions of the trial design, methods, recruitment of subjects, study procedures, consent process, surgical technique, targeting procedure, intraoperative microelectrode recording procedure, and baseline subject characteristics have been previously reported and are briefly reviewed as follows [10–14].
Subjects age 50–75 years with idiopathic PD, Hoehn and Yahr Stage II when off medication, treated with antiparkinsonian medications for greater than six months but less than four years, and with no current or prior history of motor fluctuations were prospectively enrolled between August, 2006 and April, 2009. Subjects younger than 50 years of age were excluded in an effort to only enroll subjects with a typical age of onset. Complete inclusion and exclusion criteria are listed in Appendix A. Because this study exposed subjects to surgical risks and was not designed to provide therapeutic benefit, an expanded informed consent process was utilized to help potential subjects understand the goals and risks of the study [13, 15]. Furthermore, an independent data safety monitoring board (DSMB) was formed prior to enrollment and met at least every six months to review interim reports tracking each group’s adverse events and PD progression [13].
Subjects providing informed consent were enrolled and underwent a detailed screening assessment. Subjects who passed screening were scheduled for an 8 day inpatient baseline assessment, which included a 7 day medication washout. [16]. Upon admission, subjects were assessed in the on state using the Hoehn & Yahr, Schwab & England, and Unified Parkinson’s Disease Rating Scale (UPDRS) by the principal investigator. Part III of the UPDRS was videotaped prior to medication withdrawal. Subjects discontinued all antiparkinsonian medications for the next seven days, during which they received daily UPDRS-III ratings scored by the principal investigator. Subjects also completed additional secondary measures, including an 8 hour diary completed on Day 1, stand-walk-sit test, finger taps, global assessments completed on Days 1 and 8, and daily autonomic testing. On the final day of the washout, the UPDRS-III was videotaped in the off state and subjects were randomized to ODT or bilateral STN DBS+ODT.
All subjects randomized to DBS+ODT were implanted in three stages using the same methodology used as standard of care at Vanderbilt University Medical Center [11]. Four weeks after lead implantation, subjects presented off medication for at least 36 hours for evaluation of the clinical response to stimulation. Programming was performed in a standardized fashion using the same methods used for patients with advanced PD. Pulse width was fixed at 60 µsec and frequency at 130 Hz. Modest stimulation increases were performed over three subsequent visits within six months based on clinical response. Stimulation was optimized throughout the trial to maximize clinical benefit while minimizing adverse effects. In order to minimize investigator bias, medication management was performed by each subject’s original treating neurologist. Subjects returned to the CRC every six months over the next two years for repeated inpatient evaluations identical to the baseline visit (Figure 1). For visits after baseline, the washout included discontinuation of both medication and stimulation, if present. “On” was operationally defined as on both medication and stimulation, if applicable, and “off” was defined as off both medication and stimulation, if applicable, for all follow-up visits. A complete list of medications, stimulation parameters, and adverse events was collected at each visit. Levodopa equivalent dose was calculated using the formula presented in Tomlinson et al., 2010 [17]. Adverse events were coded using the Medical Dictionary for Regulatory Activities, version 13.1 [18], and were classified based on severity, relation to the study, surgery, and device, and treatment phase during which they occurred. The active treatment phase was defined as the time from randomization through the 24-month visit for the ODT group and from the first programming visit through the 24-month visit for the DBS+ODT group.
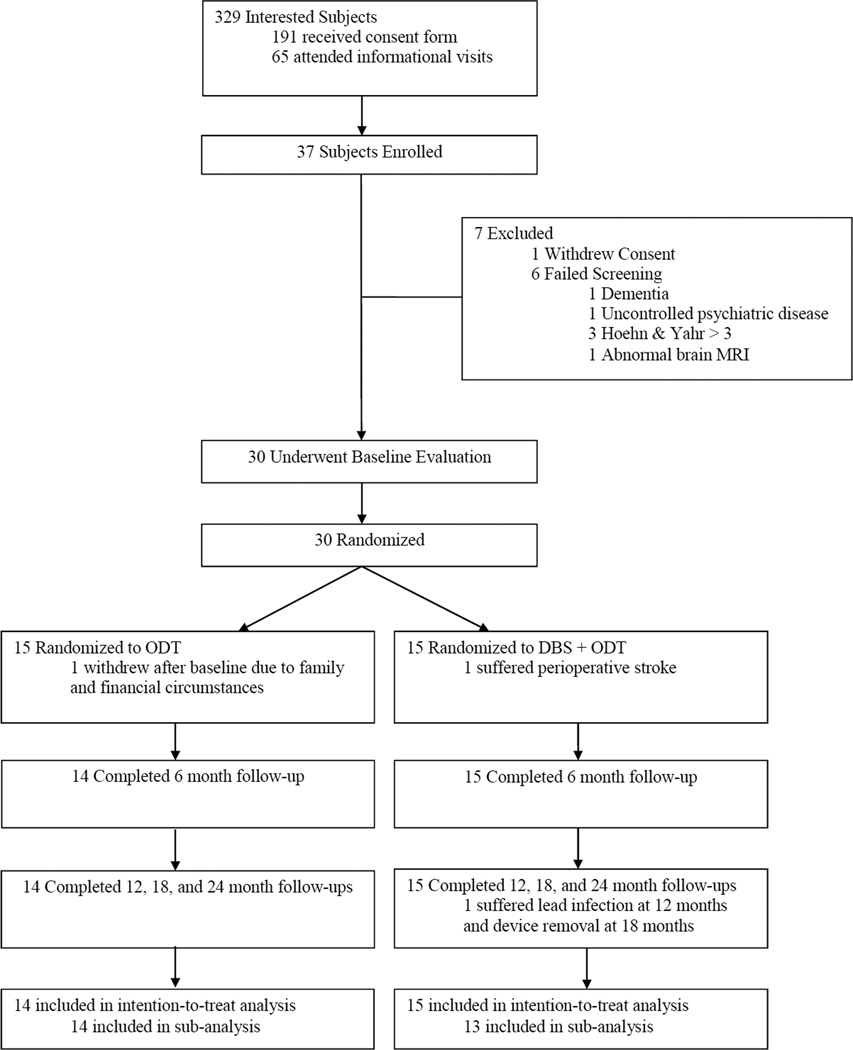
Figure 1. Study design.
Study design indicating study visit duration and frequency. The baseline visit and all visits thereafter included 7 day medication washouts. Adapted from Charles et al., 2012 [13].
At the conclusion of the study, all UPDRS-III videos were scored by an independent, blinded PD expert certified in scoring the UPDRS. To ensure proper blinding, 291 videos were randomized across all time points, study groups, and subjects. Without informing the rater, an additional 78 videos were randomly selected by the statistician and were presented to the rater twice. Twenty-nine of these videos were used to establish scoring consistency and were presented to the rater at the beginning of the scoring period; these scores were later discarded. The remaining 49 videos were randomly interspersed in the scoring order and were scored twice to determine the rater’s test-retest reliability and level of blindness. All questions on the UPDRSIII were scored, with the exception of rigidity [19].
STATISTICAL ANALYSIS
Because the purpose of this trial was to provide preliminary safety and tolerability data, the primary hypothesis was that the DBS+ODT group would not worsen more quickly than the ODT group. The primary endpoint was defined as the time to reach a four-point worsening of the UPDRS-III score following a one week treatment washout as assessed by the blinded rater [13]. The co-primary endpoint was the change from baseline to 24 months in the use of antiparkinsonian medications expressed as a levodopa equivalent dose. Secondary endpoints included changes from baseline in each of the four subscales of the UPDRS and total UPDRS scores.
For the primary analysis, survival curves were estimated by the life table technique and compared using the log-rank test. Medication usage and secondary endpoints were analyzed by comparing changes in the outcomes for the two groups using mixed models that included fixed effects treatment, time and treatment × time interaction, along with an autoregressive covariance structure to account for repeated measures at baseline, 12-month and 24-month evaluations for each subject. Parameters were estimated from this model and were used to compare group differences at each time point as well as change scores from baseline. To evaluate the success of blinding, we estimated James Blinding Index on the video assessments given by the blinded rater for the set of randomized videos [20].
The FDA limited enrollment in this pilot trial to 30 subjects. Given this constraint, the power of the study was calculated based on the amount of antiparkinsonian medication consumed. We anticipated that the control group (ODT) would have a baseline value of 400 which would increase to 600, and that the treated group (DBS+ODT) would decrease from 400 to 300 [21]. A sample size of 12 patients (15 patients assuming 20% drop out) in each group would have 80% power to detect a difference in means of 300 (the difference between a Group 1 mean of 200 and a Group 2 mean of -100) assuming that the common standard deviation is 250 using a two group t-test with a 0.05 two-sided significance level. Because adjusting for multiplicity can be counterproductive for safety considerations, we did not pursue any formal correction for multiple comparisons for the primary endpoints. For the secondary endpoints, a total of 90 tests were conducted, therefore p-values of less than 5.6 × 10−4 (0.05/90) were considered statistically significant based on Bonferroni correction. All tests were two-tailed. All analyses were performed using the statistical software SAS (version 9.3; SAS Institute, Cary, NC) and R (http://www.r-project.org/).
All 29 subjects who completed at least one follow-up visit were included in the primary analysis following the intention-to-treat principle. A secondary analysis was completed that excluded the two subjects in the DBS+ODT group who experienced surgical or device-related adverse events in order to isolate the effects of stimulation in early PD. Data for the subject who experienced a perioperative infarction was excluded from the secondary analysis because this perioperative adverse event affected the subject’s motor and cognitive output throughout the duration of the study. Data for the 24-month follow-up visit for the subject who experienced a lead infection was also excluded from the secondary analysis because he stopped receiving stimulation on the right side after his 18-month follow-up visit. This subject’s 18-month data was thus carried forward through 24 months. The secondary analysis revealed no significant differences in any of the measures in Table 2 compared to the primary analysis. Therefore, all data reported in Table 2 is for all 29 patients who completed the trial.
Table 2. Outcomes at Baseline and 24 Months by Treatment Group.
Mean and mean change scores from baseline to 24 months for primary and secondary endpoints in the trial. All on assessments were completed on Day 1 of the washout with subjects on medicine and stimulation, if applicable. All off assessments were completed on Day 8 with subjects off medicine and stimulation if applicable. Adapted from Weaver et al., 2009 [2]
| ODT (n = 14) | DBS (n = 15) | ODT minus DBS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Baseline | 24 month | Mean Difference (95% CI) |
Baseline | 24 month | Mean Difference (95% CI) |
Mean Difference (95% CI) |
P Value |
|
| Hoehn & Yahr | |||||||||
| ON | 1.8 (0.4) | 2.1 (0.4) | 0.25 (0.0 to 0.5) | 1.7 (0.5) | 2.2 (0.3) | 0.53 (0.29 to 0.77) | 0.28 (−0.06 to 0.63) | 0.11 | |
| OFF | 2.0 (0.0) | 2.1 (0.2) | 0.11 (−0.0 to 0.2) | 2.0 (0.1) | 2.1 (0.2) | 0.1 (−0.0 to 0.2) | −0.0 (−0.2 to 0.2) | 0.94 | |
| Schwab & England | |||||||||
| ON | 90.7 (5.5) | 86.8 (8.9) | −3.9 (−8.14 to 0.28) | 92.3 (4.17) | 90.0 (6.3) | −2.3 (−6.4 to 1.7) | 1.6 (−4.3 to 7.5) | 0.60 | |
| OFF | 88.6 (5.7) | 86.2 (6.2) | −2.3(−6.1 to 1.5) | 89.3 (2.6) | 87.7 (5.9) | −1.7 (−5.3 to 2.0) | 0.6 (−4.7 to 5.8) | 0.82 | |
| UPDRS | |||||||||
| I | 1.8 (1.2) | 2.9 (2.0) | 1.1 (−0.1 to 2.3) | 1.7 (1.4) | 2.9 (2.0) | 1.2 (0.1 to 2.3) | 0.1 (−1.5 to 1.7) | 0.91 | |
| II | |||||||||
| “ON” medication | 7.9 (4.6) | 10.2 (5.9) | 2.3(−1.2 to 5.8) | 8.0 (4.8) | 12.1 (6.4) | 4.1 (0.7 to 7.5) | 1.8 (−3.1 to 6.7) | 0.46 | |
| “OFF” medication | 9.5 (4.0) | 13.9 (5.1) | 4.4 (0.9 to 7.9) | 11.4 (4.9) | 14.7 (5.8) | 3.2 (−0.1 to 6.6) | −1.2 (−6.1 to 3.7) | 0.63 | |
| IIIa | |||||||||
| ON | 21.3 (9.2) | 24.4 (9.5) | 3.4 (−2.9 to 9.8) | 23.7 (12.3) | 23.7 (13.7) | 0.1 (−6.0 to 6.1) | −3.4 (−12.1 to 5.4) | 0.45 | |
| OFF | 29.5 (8.7) | 39.1 (9.1) | 9.6(3.7 to 15.5) | 28.0 (10.2) | 36.1 (13.3) | 8.2 (2.5 to 13.9) | −1.37 (−9.6 to 6.9) | 0.74 | |
| IV | 1.9 (1.9) | 3.8 (2.2) | 1.9 (0.4 to 3.4) | 2.1 (2.1) | 2.4 (1.6) | 0.3 (−1.2 to 1.7) | −1.59 (−3.7 to 0.5) | 0.14 | |
| Totalb | 33.4 (13.3) | 41.2 (16.2) | 8.4 (−0.3 to 17.0) | 35.47 (15.9) | 41.1 (18.0) | 5.63 (−2.6 to 13.9) | −2.7 (−14.7 to 9.3) | 0.65 | |
| ON/OFF Diariesc | |||||||||
| ON with dyskinesias | 0.0 (0.0) | 2.7 (10.0) | 2.7 (−0.6 to 5.9) | 0.0 (0.0) | 1.9 (6.9) | 2.0 (−1.2 to 5.2) | −0.7 (−5.2 to 3.9) | 0.77 | |
| OFF | 6.4 (14.5) | 14.3 (36.3) | 8.2 (−11.3 to 27.7) | 7.5 (25.8) | 12.0 (24.5) | 3.6 (−15.5 to 22.7) | −4.6 (−31.8 to 22.7) | 0.74 | |
| Finger Taps | |||||||||
| Dominant Hand | |||||||||
| ON | 69.1 (15.6) | 70.6 (18.8) | 1.5 (−7.5 to 10.5) | 66.8 (14.0) | 65.7 (21.1) | −1.1 (−9.8 to 7.6) | −2.6 (−15.2 to 9.9) | 0.68 | |
| OFF | 60.0 (13.1) | 57.1 (20.0) | −2.9 (−12.4, 6.7) | 57.2 (13.8) | 53.5 (21.7) | −3.7 (−12.9 to 5.5) | −0.8 (−14.0 to 12.4) | 0.90 | |
| Non-dominant Hand | |||||||||
| ON | 62.0 (11.0) | 65.1 (17.1) | 3.1 (−4.0 to 10.3) | 64.8 (12.0) | 63.7 (17.3) | −1.1 (−8.1 to 5.8) | −4.3 (−14.3 to 5.7) | 0.40 | |
| OFF | 55.0 (11.5) | 55.6 (18.2) | 0.6 (−6.3 to 7.4) | 59.1 (9.6) | 55.1 (10.6) | −3.9 (−10.6 to 2.7) | −4.5 (−14.0 to 5.0) | 0.35 | |
| Stand-walk-sit, s | |||||||||
| ON | 11.7 (2.8) | 11.8 (3.1) | 0.1 (−2.1 to 2.2) | 12.4 (2.6) | 13.7 (3.5) | 1.3 (−0.8 to 3.3) | 1.2 (−1.8 to 4.2) | 0.43 | |
| OFF | 13.1 (3.7) | 12.9 (3.0) | −0.3 (−4.0 to 3.3) | 13.5 (2.9) | 17.1 (6.1) | 3.7 (0.1 to 7.2) | 4.0 (−1.1 to 9.1) | 0.12 | |
| Levodopa equivalents, mgd | 490.7 (216.2) | 705.2 (377.1) | 214.5(16.9 to 412.1) | 417.2 (306.6) | 514.9 (305.1) | 97.7 (−93.2 to 288.7) | −116.8 (−391.6 to 158.0) | 0.40 | |
| Quality of life | |||||||||
| PDQ-39 | |||||||||
| Mobility | 11.3 (14.9) | 18.9 (23.6) | 7.7(−3.3 to 18.6) | 12.7 (14.4) | 24.8 (22.6) | 12.2 (1.6 to 22.8) | 4.5 (−10.8 to 19.7) | 0.56 | |
| Activities of Daily Living | 15.8 (16.1) | 26.2 (27.4) | 10.4(−0.4 to 21.3) | 18.3 (14.5) | 23.6 (18.1) | 5.3 (−5.2 to 15.7) | −5.1 (−20.2 to 9.9) | 0.50 | |
| Emotion Well Being | 13.1 (12.1) | 23.8 (21.7) | 10.7 (2.4 to 19.1) | 11.7 (13.2) | 17.5 (15.5) | 5.8 (−2.2 to 13.9) | −4.9 (−16.5 to 6.7) | 0.40 | |
| Stigma | 13.0 (18.4) | 22.3 (27.1) | 9.4 (−0.0 to 18.8) | 7.9 (11.9) | 9.2 (13.8) | 1.3 (−7.8 to 10.3) | −8.1 (−21.2 to 5.0) | 0.22 | |
| Social Support | 3.9 (5.8) | 7.1 (11.7) | 3.3 (−4.0 to 10.5) | 8.9 (15.3) | 8.3 (14.1) | −0.6 (−7.6 to 6.5) | −3.8 (−13.9 to 6.3) | 0.45 | |
| Cognition | 18.8 (12.7) | 25.0 (16.1) | 6.25 (−3.1 to 15.6) | 13.8 (10.6) | 23.3 (17.8) | 9.6 (0.5 to 18.6) | 3.3 (−9.7 to 16.4) | 0.61 | |
| Communication | 10.1 (5.8) | 23.2 (21.7) | 13.1 (3.1 to 23.1) | 12.8 (11.3) | 25.0 (18.1) | 12.3 (2.6 to 21.9) | −0.85 (−14.7 to 13.0) | 0.90 | |
| Bodily Discomfort | 21.4 (16.3) | 29.8 (22.8) | 8.33 (−4.4 to 21.1) | 24.4 (20.0) | 27.8 (20.3) | 3.3 (−9.0 to 15.7) | −5.0 (−22.8 to 12.8) | 0.58 | |
| Single Index | 13.4 (9.1) | 22.0 (18.1) | 8.6 (2.6 to 14.7) | 13.8 (10.3) | 20.0 (13.9) | 6.2 (0.4 to 12.0) | −2.4(−10.8 to 6.0) | 0.57 | |
UPDRS-III scores rated by a blinded rater following the conclusion of the study. Rigidity was not included in the UPDRS-III scores. On scores were obtained in the typically treated state (medications and DBS if applicable) and were not performed following a medication challenge. The UPDRS-III on score reported in Table 1 is the on score following a structured medication challenge administered at the screening visit after a 36 hour medication washout.
Total UPDRS-III score with blinded rater’s UPDRS-III score. Does not include rigidity.
Eight hour diaries completed on Day 1 of the washouts at baseline and all follow-up visits prior to medication withdrawal.
100 mg of Levodopa with a dopa-decarboxylase inhibitor = 130 mg of controlled-release Levodopa preparations = 83 mg of Levodopa with dopadecarboxylase and COMT inhibitors = 1 mg of Pergolide, Pramipexole, or Lisuride = 3 mg of Ropinirole [17]. A more current levodopa equivalent conversion was used in this calculation, which resulted in different levodopa equivalent values than what is reported in Charles et al., 2012 [10]
RESULTS
Figure 1 illustrates the number of subjects who participated in each stage of the enrollment process and reasons for discontinuation. Of the 30 randomized subjects, 29 completed the study and all study visits within the allotted time frames. One subject withdrew from the study after baseline due to family circumstances and financial hardship.
The baseline characteristics of randomized subjects were not different between the two groups (Table 1) [10]. All subjects were Caucasian and were primarily drawn from the Southeastern United States. Between-group differences at baseline in antiparkinsonian medication requirements, Hoehn & Yahr, Schwab & England, UPDRS subscales, “ON” time, and the PDQ-39 were not significant. All follow-up visits were well tolerated and no subjects requested early exit from a washout period or developed symptoms mimicking neuroleptic malignant syndrome due to sudden withdrawal of dopaminergic medications.
Table 1. Subject Characteristicsa.
Table 1 lists the characteristics of enrolled subjects. There were no significant between-group differences on any of the measures. Adapted from Charles et al., 2012 [10]
| Characteristic | ODT (n=15) | DBS+ODT (n=15) |
|---|---|---|
| Gender | ||
| Male | 13 | 14 |
| Female | 2 | 1 |
| Age (yrs) at Enrollment | ||
| Mean | 60 ± 7.0 | 60 ± 6.8 |
| Range | 51 – 69 | 52 – 74 |
| Medicine Use | ||
| Mean Duration (yrs) | 2.1 ± 1.1 | 2.2 ± 1.4 |
| Mean L-dopa equivalents (mg/day)b | 494.0 ± 208.7 | 417.2 ± 306.6 |
| UPDRS Scores | ||
| Mean Totalc | 36 ± 15 | 39 ± 14 |
| Mean UPDRS-III offd | 25.6 ± 5.8 | 25.3 ± 9.0 |
| Mean UPDRS-III one | 12.3 ± 6.4 | 11.1 ± 6.9 |
Adapted from PD Charles, RM Dolhun, CE Gill, TL Davis, MJ Bliton, MG Tramontana, RM Salomon, L Wang, P Hedera, FT Phibbs, JS Neimat, PE Konrad, Deep brain stimulation in early Parkinson’s disease: Enrollment experience from a pilot trial, 18 / 3, 268-73, 2012, with permission from Elsevier [10]
100 mg of Levodopa with a dopa-decarboxylase inhibitor = 130 mg of controlled-release Levodopa preparations = 83 mg of Levodopa with dopa-decarboxylase and COMT inhibitors = 1 mg of Pergolide, Pramipexole, or Lisuride = 3 mg of Ropinirole [17]. A more current levodopa equivalent conversion was used in this calculation, which resulted in different levodopa equivalent doses than what is reported in Charles et al., 2012 [10]
Total UPDRS on score, including rigidity, assessed by the principal investigator at baseline.
UPDRS-III off score with rigidity after a 36 hour medication washout at the screening visit.
UPDRS-III on score with rigidity after a medication challenge at the screening visit. Details are provided in Charles et al 2012 [13]. Subjects in the ODT group experienced a 52.0% improvement and subjects in the DBS+ODT group experienced a 56.1% improvement in the UPDRS-III following the medication challenge.
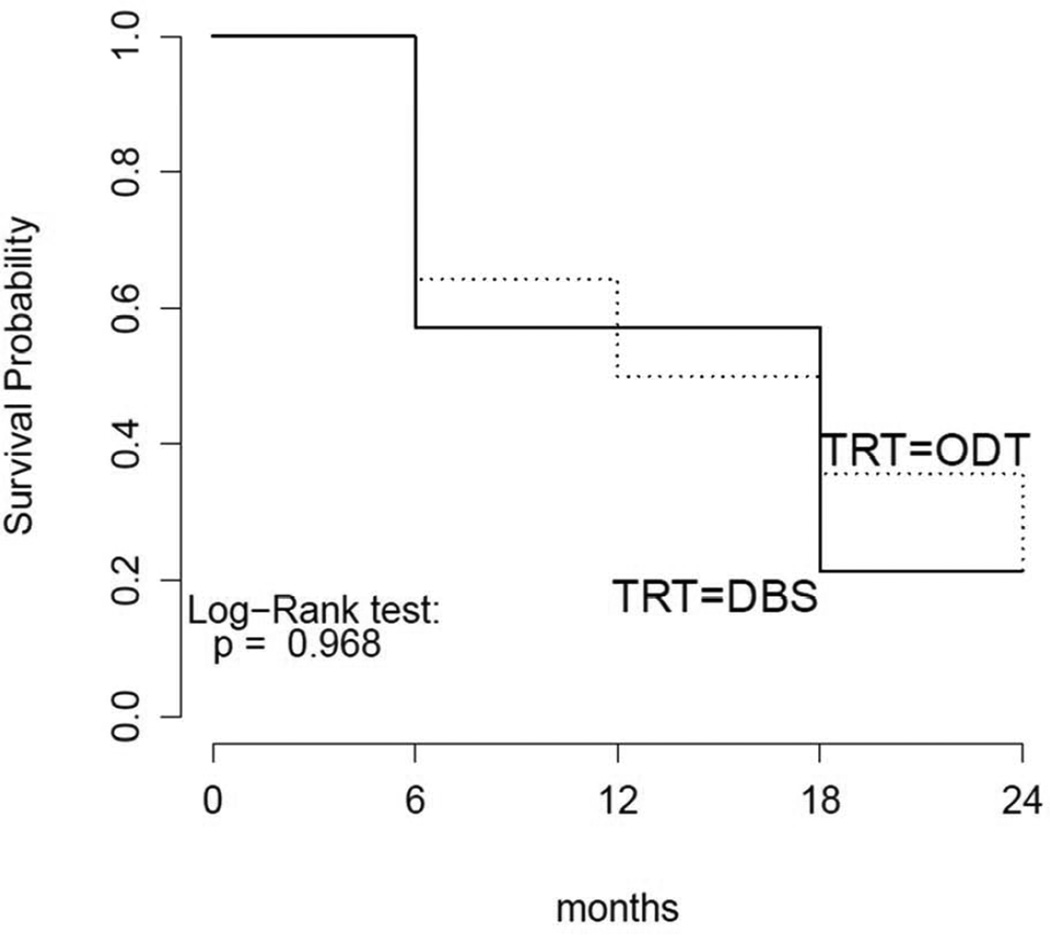
There were few significant differences between the groups at 24 months (Table 2). The study met the primary safety endpoint, demonstrating that the DBS+ODT group did not experience worsening motor function compared to the ODT group (Figure 2). The group’s motor scores, as measured by the blinded UPDRS-III on and off medicine, were not significantly different from the ODT group (p=0.881 and p=0.460, respectively) (Appendices B and C). At 24 months, the UPDRS IV appears to diverge but is not significantly different (p=0.092) (Appendix D). The total UPDRS score was not significantly different between the groups (Appendix E). The DBS group required less antiparkinsonian medication than the ODT group at all time points. The maximal difference was observed at 18 months (p=0.002, but did not reach significance threshold of 5.6 × 10−4 for secondary endpoints); subjects in the DBS+ODT group required approximately 400 levodopa equivalents less than the ODT group (Appendix F). The co-primary endpoint of medication change from baseline to 24 months was not significant.
Figure 2. Kaplan-Meier Curve.
Kaplan-Meier Curve comparing DBS+ODT group (solid line) to ODT group (dashed line). An event was defined as a four point worsening of the UPDRS-III score in the off medication state.
The DBS+ODT group scored approximately 13 points lower than the ODT group at 24 months on the PDQ Stigma score, but this was not statistically significant (p=0.068). Between-group differences in the other subscales and the index score were not significant at any time point. The time taken for the stand walk sit test off treatment was better in the ODT group at 24 months (p=0.019 but did not reach significance threshold of 5.6 × 10−4 for secondary endpoints). Differences in the stand walk sit test on treatment and in the Hoehn & Yahr, Schwab & England, and finger taps on and off treatment were not significant. Stimulation settings remained low in the DBS+ODT group throughout the study. The average amplitude was 1.6 volts (Appendix G) [22].
The results of the annual neuropsychological testing revealed few differences between the two groups at 24 months. Compared to the DBS+ODT group, the ODT group scored better on the Phonemic Fluency task and the Color Naming portion of the Stroop Test (p=0.03 and p=0.01, respectively, but did not reach significance threshold of 5.6 × 10−4 for secondary endpoints on either measure). The change from baseline between the two groups was not significant for either measure. Between-group differences were not significant on any of the remaining measures at 24 months.
The most frequently reported adverse events were insomnia, chest pain, urinary tract infections, neck pain, and extremity pain. No subjects committed or attempted suicide in the trial or in the ongoing extended follow-up period through five years. Adverse events that occurred from enrollment to randomization have been previously reported [10]. Appendix H lists the most frequent moderate and severe adverse events reported in each group during the active treatment phase. A complete list of all adverse events that occurred during the active treatment phase is provided in Appendix I.
There were two serious adverse events related to the surgery or device that occurred in two subjects in the DBS+ODT group. One subject experienced a perioperative infarction in the left basal ganglia, causing permanent cognitive impairment and transient weakness in the right face and hand. The second subject accidently struck the right side of his head on his garage door 12 months postoperatively. A right superior frontal scalp infection subsequently developed along the lead extension and was unsuccessfully treated with oral antibiotics. The right lead, extension, and implantable pulse generator were thus removed 18 months postoperatively. The infection resolved, but the subject did not receive stimulation on the right side for the remainder of the study. Kahn et al 2011 provides a complete list of the perioperative adverse events for this trial [11].
The independent rater had excellent reliability and the blind was maintained successfully. The Spearman correlation coefficient between test and re-test scores was 0.95 and the mean difference of test and re-test scores was 0.43 ± 3.65. Rater blindness was 0.83 with 95% confidence interval (0.69–0.96), which is significantly above the level expected to be due to chance.
DISCUSSION
We present here the earliest prospective controlled clinical trial testing DBS in PD. The results of this study demonstrate that subjects with early stage PD will enroll in, provide meaningful informed consent for, and complete clinical trials testing innovative and invasive therapies that involve significant risks without offered benefit. Subjects who were randomized to the control group remained engaged and compliant with all study procedures and follow-up visits. This finding is remarkable given the prolonged therapy washouts and the considerable time commitment required for participation in the study.
The results of this study also suggest the initial safety and tolerability of DBS in early stage PD. The study met its primary endpoint related to safety, demonstrating that chronic stimulation does not produce greater declines in motor function compared to the control group. While a larger trial is necessary to fully elucidate the safety of prolonged stimulation in early PD, in this pilot trial, both stimulation and the follow-up visits were well tolerated.
This study also suggests that it is likely possible to accurately target and implant bilateral STN deep brain stimulating electrodes in patients with early stage PD and very mild symptomatology [12, 14]. In addition to intraoperative microelectrode recordings and post-operative imaging verification of lead placement, all implanted subjects underwent lead interrogation of all four contacts on each side of the brain in the off medication state and all subjects had at least one efficacious contact. Finally, subjects in the DBS+ODT group experienced a reduction in medication requirements, reaching maximum at 18 months, and achieved this with modest levels of stimulation. Compared to stimulation in subjects with advanced PD, subjects in this trial were managed with approximately 1.5 volts less stimulation [2].
Adverse events occurred at a rate similar to that reported in subjects with advanced PD, and surgical and device related adverse events did not significantly differ from nationally reported rates for DBS surgery. It is important to note, however, that two subjects in the DBS+ODT group experienced serious adverse events. The occurrence of these adverse events must be factored into the risk-benefit ratio and ethical considerations of future studies of DBS in early stage PD.
A recently completed clinical trial testing DBS in mid-stage PD demonstrated improved quality of life, reduction in medication usage, and improved motor scores [7, 9]. These findings will likely be transformative to the clinical application of DBS for PD. There are several important differences between the recent study of mid-stage disease and the pilot trial reported here, which are outlined in Appendix J. The objective of this pilot trial is to explore the application of DBS in early-stage PD when the disease typically occurs. Given that the average age of onset of PD is approximately 60 years, subjects between the ages of 50 and 75 were eligible to enroll in the trial, and the average age of enrolled subjects was 60.0 [10]. In contrast, the study of mid-stage disease required that subjects be age 60 or younger to be considered for enrollment, and the average age of subjects enrolled in that trial was 52.6 [7]. Finally, the average disease duration for subjects in the mid-stage study was 7.5 years [7], as compared to subjects in this pilot trial who had been on antiparkinsonian medications for 2.2 years [10]. Both studies investigated DBS in earlier stages of PD than has previously been studied. However, a direct comparison of the results is not possible because the study of mid-stage PD is a large scale efficacy trial and the pilot trial reported here was designed to evaluate preliminary safety and tolerability in a small cohort appropriately limited by the FDA to 30 subjects.
The trial design presented here represents what the investigators considered the best balance of scientific design and ethics, but there are several limitations of this study. One is that all measures, except the UPDRS-III, which was a single-blind measure, were open-label. However, the DBS+ODT group was managed with modest stimulation settings and medication management was not performed by the principal investigator. Other limitations include the small sample size, lack of gender and racial diversity, and possibility for alternative diagnoses. Furthermore, despite our efforts to enroll subjects of all genders and races, this study enrolled all Caucasian subjects, the majority of whom were male. In the absence of a biomarker, subjects with secondary parkinsonism or alternative diagnoses may have been enrolled in our efforts to enroll subjects with early stage idiopathic PD. However, the study’s strict enrollment criteria were intended to exclude patients with alternative diagnoses. All 29 subjects have enrolled in an extension phase of the study that closely follows subjects through 5 years of therapy. To date, no subjects have received revised diagnoses indicative of conditions other than idiopathic PD.
Methods from this pilot should be considered for future investigations, as they were an important component of the success of the trial. Specifically, the expanded informed consent process is likely responsible for the exceptionally low dropout rate. Future discussions should focus on the necessary length and frequency of therapy washouts. As expected, a one-week medication washout is not sufficient to fully eliminate the therapeutic effects of antiparkinsonian medications. Although no subjects developed symptoms mimicking neuroleptic malignant syndrome due to sudden withdrawal of dopaminergic medications or requested early exit from a washout period, the investigators noted that the washouts were at times challenging for subjects to endure. Future trials must balance scientific design with what is a reasonable burden to request of research subjects.
The success and results of this pilot trial suggest that a large-scale, phase III, multicenter, double-blind investigation of the safety and efficacy of bilateral STN-DBS in early stage PD is feasible. The purpose of such a study would be to determine if DBS plus medication compared to standard medical therapy would provide improved quality of life, delayed onset of motor fluctuations and dyskinesias, and delayed functional disability. Although there is no perfect trial design, a double-blind study remains the best option for the next trial. How to best achieve blinding for subjects receiving DBS is challenging, but this should not deter investigators from attempting to conduct the most scientifically rigorous trial possible. The next trial should be designed to provide conclusive data regarding the safety and efficacy of offering DBS to patients with early stage PD and inform clinicians regarding the appropriate stage to offer DBS to patients with PD.
AUTHORS’ ROLES
David Charles made substantial contributions to the conception and design; acquisition of data; interpretation of data; drafting and critical revision of the manuscript; obtaining funding; supervision. Dr. Charles has full access to the study data and takes full responsibility for the integrity and accuracy of data analysis. Peter Konrad made substantial contributions to the conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support; supervision. Thomas Davis made substantial contributions to the conception and design; critical revision of the manuscript; administrative, technical or material support. Joseph Neimat made substantial contributions to the interpretation of data; critical revision of the manuscript; administrative, technical or material support; supervision. Anna Molinari made substantial contributions to the acquisition of data; interpretation of data; drafting of the manuscript; critical revision of the manuscript; administrative or technical support. Michael Tramontana made substantial contributions to the conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support. Stuart Finder made substantial contributions to the conception and design; the acquisition of data; critical revision of the manuscript; administrative, technical or material support. Chandler Gill made substantial contributions to the acquisition of data; interpretation of data; critical revision of the manuscript; administrative or technical support. Mark Bliton made substantial contributions to the acquisition of data; critical revision of the manuscript; administrative, technical or material support. Chris Kao made substantial contributions to the conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support. Fenna Phibbs made substantial contributions to the interpretation of data; critical revision of the manuscript; administrative, technical or material support. Peter Hedera made substantial contributions to the interpretation of data; critical revision of the manuscript; administrative, technical or material support. Ronald Salomon made substantial contributions to the conception and design; acquisition of data; interpretation of data; critical revision of the manuscript; administrative, technical or material support. Kevin Cannard made substantial contributions to the conception and design; the acquisition of data; critical revision of the manuscript; administrative, technical or material support. Lily Wang made substantial contributions to the conception and design; interpretation of data; statistical analysis; critical revision of the manuscript. Yanna Song made substantial contributions to the interpretation of data; statistical analysis; critical revision of the manuscript.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by Medtronic, Inc., by Vanderbilt CTSA grant UL1TR000445 from the National Center for Advancing Translational Sciences (NCATS), by NCATS/NIH award UL1TR000011, by NIH R01 EB006136, and by private donations. Medtronic representatives did not take part in data collection, management, analysis, or interpretation of the data or in preparation, review, or approval of the manuscript.
Chris C. Kao receives partial salary support from Sentient Medical Services. Peter E. Konrad receives research funding by Medtronic and the NIH, is on the speaker’s bureau for Medtronic and FHC, and also holds a fiduciary position (Board of Directors) with Neurotargeting, the American Society for Stereotactic and Functional Neurosurgery, and the North American Neuromodulation Society. Fenna T. Phibbs has done consulting work for Medtronic and has received speaking honoraria from Teva. Peter Hedera has received speaking honoraria from Teva. Joseph S. Neimat has done consulting work for Medtronic and has received research funding from Medtronic and the NIH. Vanderbilt University has received income from a grant from Shire for research, nonrelated to this study, led by Michael Tramontana. Michael Tramontana also receives book royalties from Springer, Plenum, and McGraw Hill. Vanderbilt University has received income from a St. Jude’s grant for research, nonrelated to this study, led by Ronald Salomon. Vanderbilt University has received income in excess of $10,000 from grants or contracts with Medtronic, Allergan, Ipsen, Merz, UCB, and Teva for educational or research programs led by David Charles. David Charles receives income in excess of $10,000 from Medtronic, Allergan, Ipsen, and the Alliance for Patient Access for education and consulting services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FULL FINANCIAL DISCLOSURES/CONFLICT OF INTEREST
Mark J. Bliton, Kevin R. Cannard, Thomas L. Davis, Stuart G. Finder, Chandler E. Gill, Anna L. Molinari, Yanna Song, and Lily Wang, do not have conflicts of interest.
REFERENCES
- 1.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 2.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 2010;9:581–591. doi: 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21(Suppl 14):S290–S304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- 5.Okun MS. Deep-brain stimulation for Parkinson's disease. N Engl J Med. 2013;368:483–484. doi: 10.1056/NEJMc1214078. [DOI] [PubMed] [Google Scholar]
- 6.Charles PD, Gill CE, Davis TL, Konrad PE, Benabid AL. Is deep brain stimulation neuroprotective if applied early in the course of PD? Nat Clin Pract Neurol. 2008;4:424–426. doi: 10.1038/ncpneuro0848. [DOI] [PubMed] [Google Scholar]
- 7.Deuschl G, Schupbach M, Knudsen K, Pinsker MO, Cornu P, Rau J, et al. Stimulation of the subthalamic nucleus at an earlier disease stage of Parkinson's disease: concept and standards of the EARLYSTIM-study. Parkinsonism Relat Disord. 2013;19:56–61. doi: 10.1016/j.parkreldis.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Schupbach WM, Maltete D, Houeto JL, du Montcel ST, Mallet L, Welter ML, et al. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology. 2007;68:267–271. doi: 10.1212/01.wnl.0000250253.03919.fb. [DOI] [PubMed] [Google Scholar]
- 9.Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013;368:610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- 10.Charles PD, Dolhun RM, Gill CE, Davis TL, Bliton MJ, Tramontana MG, et al. Deep brain stimulation in early Parkinson's disease: enrollment experience from a pilot trial. Parkinsonism Relat Disord. 2012;18:268–273. doi: 10.1016/j.parkreldis.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn E, D'Haese PF, Dawant B, Allen L, Kao C, Charles PD, et al. Deep brain stimulation in early stage Parkinson's disease: operative experience from a prospective randomised clinical trial. J Neurol Neurosurg Psychiatry. 2012;83:164–170. doi: 10.1136/jnnp-2011-300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remple MS, Bradenham CH, Kao CC, Charles PD, Neimat JS, Konrad PE. Subthalamic nucleus neuronal firing rate increases with Parkinson's disease progression. Mov Disord. 2011;26:1657–1662. doi: 10.1002/mds.23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charles PD, Tolleson C, Davis TL, Gill CE, Molinari AL, Bliton MJ, et al. Pilot Study Assessing the Feasibility of Applying Bilateral Subthalamic Nucleus Deep Brain Stimulation in Very Early Stage Parkinson’s Disease: Study Design and Rationale. Journal of Parkinson's Disease. 2012;2:215–223. doi: 10.3233/JPD-2012-012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camalier CR, Konrad PE, Kao CC, Remple MR, Davis TL, Hedera P, et al. Methods for surgical targeting of the STN in early-stage Parkinson's disease. Frontiers in Neurology. 2014;5 doi: 10.3389/fneur.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finder SG, Bliton MJ, Gill CE, Davis TL, Konrad PE, Charles PD. Potential Subjects' Responses to an Ethics Questionnaire in a Phase I Study of Deep Brain Stimulation in Early Parkinson's Disease. The Journal of Clinical Ethics. 2012;23:207–216. [PubMed] [Google Scholar]
- 16.Hauser RA, Friedlander J, Zesiewicz TA, Adler CH, Seeberger LC, O'Brien CF, et al. A home diary to assess functional status in patients with Parkinson's disease with motor fluctuations and dyskinesia. Clinical neuropharmacology. 2000;23:75–81. doi: 10.1097/00002826-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 18.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug safety : an international journal of medical toxicology and drug experience. 1999;20:109–117. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 19.Louis ED, Levy G, Cote LJ, Mejia H, Fahn S, Marder K. Diagnosing Parkinson's disease using videotaped neurological examinations: validity and factors that contribute to incorrect diagnoses. Mov Disord. 2002;17:513–517. doi: 10.1002/mds.10119. [DOI] [PubMed] [Google Scholar]
- 20.James KE, Bloch DA, Lee KK, Kraemer HC, Fuller RK. An index for assessing blindness in a multi-centre clinical trial: disulfiram for alcohol cessation--a VA cooperative study. Statistics in medicine. 1996;15:1421–1434. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1421::AID-SIM266>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 21.Vingerhoets FJ, Villemure JG, Temperli P, Pollo C, Pralong E, Ghika J. Subthalamic DBS replaces levodopa in Parkinson's disease: two-year follow-up. Neurology. 2002;58:396–401. doi: 10.1212/wnl.58.3.396. [DOI] [PubMed] [Google Scholar]
- 22.Cooper SE, McIntyre CC, Fernandez HH, Vitek JL. Association of deep brain stimulation washout effects with Parkinson disease duration. JAMA neurology. 2013;70:95–99. doi: 10.1001/jamaneurol.2013.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.